Abstract
BACKGROUND
Minimally invasive component separation (CS) with inlay bioprosthetic mesh (MICSIB) is a recently developed technique for abdominal wall reconstruction that preserves the rectus abdominis perforators and minimizes subcutaneous dead space using limited-access tunneled incisions. We hypothesized that MICSIB would result in better surgical outcomes than would conventional open CS.
STUDY DESIGN
All consecutive patients who underwent CS (open or minimally invasive) with inlay bioprosthetic mesh for ventral hernia repair from 2005 to 2010 were included in a retrospective analysis of prospectively collected data. Surgical outcomes including wound-healing complications, hernia recurrences, and abdominal bulge/laxity rates were compared between patient groups based on the type of CS repair: MICSIB or open.
RESULTS
Fifty-seven patients who underwent MICSIB and 50 who underwent open CS were included. The mean follow-ups were 15.2±7.7 months and 20.7±14.3 months, respectively. The mean fascial defect size was significantly larger in the MICSIB group (405.4±193.6 cm2 vs. 273.8±186.8 cm2; p =0.002). The incidences of skin dehiscence (11% vs. 28%; p=0.011), all wound-healing complications (14% vs. 32%; p=0.026), abdominal wall laxity/bulge (4% vs. 14%; p=0.056), and hernia recurrence (4% vs. 8%; p=0.3) were lower in the MICSIB group than in the open CS group.
CONCLUSIONS
MICSIB resulted in fewer wound-healing complications than did open CS used for complex abdominal wall reconstructions. These findings are likely attributable to the preservation of paramedian skin vascularity and reduction in subcutaneous dead space with MICSIB. MICSIB should be considered for complex abdominal wall reconstructions, particularly in patients at increased risk of wound-healing complications.
INTRODUCTION
The incidence of ventral hernias following primary laparotomy ranges from 2% to 11%1–3 and hernia recurrence after initial repair is far more prevalent, with reported rates after primary suture repair as high as 63%.4–8
Patients with complex ventral hernias and risk factors for surgical complications-- such as bacterial wound contamination, prior failed hernia repair, loss of domain, abdominal wall irradiation, adverse prior incision patterns, prior or current ostomies, and unstable skin coverage--are a particular challenge to the reconstructive surgeon. Within the past 20 years, major reconstructive innovations including component separation (CS) and the introduction of bioprosthetic mesh materials have advanced the field of complex hernia repair and improved outcomes but further advances are needed, particularly for patients at high risk of complications.
The open “components separation” technique, introduced in 1990 by Ramirez and colleagues, involves release of the bilateral external oblique aponeuroses to enable medial advancement of the rectus abdominis musculofascial complex while preserving the motor nerve supply to the rectus abdominis muscles.9 This technique is well suited to large ventral hernia defects for which primary fascial closure with or without mesh reinforcement could result in excessive tension leading to failure of the hernia repair, abdominal compartment syndrome, or ventilatory compromise.10
Synthetic or bioprosthetic mesh materials are frequently used in addition to CS to reinforce hernia repairs.5, 11 Despite the advantages of synthetic mesh, including its high tensile strength and relatively low cost, it has several drawbacks, including risks of visceral adhesions to the mesh, fistula formation, persistent seromas, and skin breakdown or surgical site infection necessitating mesh removal.12, 13 In patients with significant comorbidities placing them at higher risk for postoperative complications, bioprosthetic mesh materials are frequently used instead because of their ability to support rapid tissue regeneration through revascularization and cellular infiltration.14–16 This revascularization allows the bioprosthetic mesh to tolerate bacterial contamination and wound exposure without the need for explantation. However, the use of bioprosthetic mesh cannot avoid all of the complications associated with synthetic mesh; many complications are related more to patients’ conditions and the surgical techniques used than to the choice of mesh material.
Open CS involves dissection of subcutaneous skin flaps from the anterior rectus sheath to expose the linea semilunaris for division of the external oblique aponeurosis. This technique divides the musculocutaneous perforators overlying the rectus sheath, thus reducing perfusion to the paramedian skin from that of a musculocutaneous flap to that of a random flap. This has considerable consequences for midline skin perfusion and wound healing, particularly in contaminated, irradiated, and/or previously surgically altered abdominal walls. In addition, the widespread elevation of skin flaps in open CS creates a large subcutaneous dead space, which increases the risk for seroma formation, wound infection, and wound dehiscence. In an attempt to minimize the morbidity of CS, the senior author (CEB) has developed a modified technique,17 termed “minimally invasive component separation with inlay bioprosthetic mesh” (MICSIB). This technique preserves the integrity of the bilateral rectus abdominis myocutaneous perforator vessels that supply the overlying skin and maintains the connection of the subcutaneous fat to the anterior rectus sheath with the goals of improving vascularity to the overlying skin flaps and reducing subcutaneous dead space.18
This study aimed to evaluate outcomes of complex ventral hernia repair with MICSIB versus traditional open CS with bioprosthetic mesh. We hypothesized that the MICSIB group would have fewer early wound-healing complications and better long-term outcomes than the open CS group.
METHODS
We evaluated all patients who underwent ventral hernia repair with open CS and bioprosthetic mesh reinforcement or with MICSIB at The University of Texas MD Anderson Cancer Center between March 2005 and October 2010. This study was approved by MD Anderson Cancer Center’s Institutional Review Board.
The indication for open CS or MICSIB in ventral hernia repair was the inability to approximate fascial edges at all or without what the attending surgeon believed would be excessive tension that placed the closure at an extremely high risk of failure. The choice of open CS or MICSIB was at the discretion of the attending surgeon; all attending surgeons who treated patients in this study were familiar with both techniques. In general, surgeons opted to use MICSIB in cases they thought were more complex and at higher risk for wound complications. The indications for using bioprosthetic mesh in hernia repair have been previously reported and include bacterial contamination, unreliable overlying skin coverage, and unavoidable placement of mesh directly on the bowel.16
We retrospectively reviewed a departmental database into which data had been prospectively entered and patients’ medical records to extract details on patient, wound, and treatment characteristics; hernia repair technique; surgical complications; and surgical outcomes. Preoperative potential risk factors for postoperative surgical complications that were evaluated included wound contamination (via surgical site infection or intestinal fluid leakage), body mass index (BMI) of ≥25 kg/m2, diabetes mellitus, current smoker, previous abdominal surgery, previous ventral hernia repair, preoperative radiotherapy or chemotherapy, presence of an ostomy, and fascial defect size of ≥500 cm2.
Surgical complications that were evaluated included abscess, seroma, hematoma, skin necrosis, skin dehiscence, office-based complications (non-operative debridement or outpatient wound care), complications requiring reoperation, laxity or bulge, and hernia recurrence. Abdominal wall skin necrosis involved clearly demarcated necrotic skin edges greater than 1 cm in width. Skin dehiscence was defined as a wound breakdown with full-thickness skin separation extending greater than 2 cm with or without infection. Wound-healing complications were defined as skin necrosis or dehiscence. A seroma was defined as a non-infected subcutaneous fluid collection that required aspiration with or without drain placement. An abscess was defined as an infected subcutaneous collection that required open drainage. Fascial dehiscence was defined as separated fascial edges that could be visualized through an open wound. A recurrent hernia was defined as a fascial defect palpable on physical examination and/or visible on computed tomography (CT). Laxity (bulge) was defined as a focal contour abnormality without an underlying fascial defect appreciable on physical examination and/or CT.
MICSIB Technique
The MICSIB technique has been previously described in detail.18 After exploratory laparotomy, lysis of adhesions, and definition of fascial edges, 3-cm-wide subcutaneous access tunnels are created bilaterally over the anterior rectus abdominis sheath from the midline to the linea semilunaris at the level of the costal margin. Through these access tunnels, the external oblique aponeurosis is vertically incised 1.5 cm lateral to the linea semilunaris. The tip of a metal Yankauer suction handle (Cardinal Health, Dublin, OH), without suction, is inserted through the opening in the avascular plane between the internal and external oblique aponeuroses to separate them at their junction with the rectus sheath. The suction tip is advanced inferiorly to the pubis and superiorly to above the costal margin. The internal and external oblique muscles are bluntly separated using the suction handle in a sweeping motion in the avascular plane between the muscles. A narrow (2.5 cm wide) subcutaneous tunnel is created with electrocautery superficial to the external oblique aponeurosis over the planned release location using a narrow retractor and a headlight. The external oblique aponeurosis is then released approximately 1.5 cm lateral to the lateral edge of the rectus sheath from 12 cm above the costal margin superiorly to near the pubis inferiorly. Next, lateral dissection between the internal and external oblique muscles is completed to the midaxillary line.
Subcutaneous skin flaps are then elevated over the anterior rectus sheath circumferentially to the medial row of rectus abdominis perforator vessels. The preperitoneal fat is dissected from the posterior sheath circumferentially to allow the bioprosthetic mesh to be inlaid directly against the posterior sheath or rectus muscle (inferior to the arcuate line).
During our early experience with this technique, we used human acellular dermal matrix (HADM) in the majority of cases. In early 2007, xenograft dermal matrices became available and became frequently used in abdominal wall reconstruction because of their ability to resist attenuation over time.16 The decision to use one bioprosthetic mesh over another was further influenced by insurance carrier coverage of the product and surgeon preference on a case-by-case basis. Types of mesh used in MICSIB included HADM (AlloDerm; LifeCell Corp, Branchburg, NJ), porcine acellular dermal matrix (PADM) (Strattice; LifeCell), and bovine acellular dermal matrix (BADM) (SurgiMend; TEI BioSciences Inc., Boston, MA).
Regardless of which mesh is used, the mesh is inset using a preperitoneal inlay technique with interrupted, #1 polypropylene sutures placed 3 to 5 cm peripheral to the true fascial edge, through the bioprosthetic mesh, and back through the musculofascia to create “U” stitches. All sutures are preplaced and tagged with hemostats to allow assessment, and potentially adjustment, of the inset tension before they are tied.
Interrupted resorbable quilting 3-0 sutures are placed to affix the posterior sheath to the mesh, thus reducing dead space and potential fluid collection. A #15 round channeled closed-suction drain is placed between the mesh and musculofascial closure. The fascial edges are closed with interrupted resorbable #1 monofilament sutures. If complete musculofascial midline closure is not possible, the musculofascial edges are sutured to the mesh using interrupted #1 monofilament sutures to create a “bridged” repair, with the mesh spanning the defect between the musculofascial edges.
The redundant medial aspects of the skin flaps are carefully excised as a vertical panniculectomy. Closed-suction #19 round channeled drainage catheters are placed in each CS donor site area, ventral to the bioprosthetic mesh, and in the midline subcutaneous space. The remaining undermined skin flaps are quilted to the musculofascia with resorbable 3-0 quilting sutures to reduce subcutaneous dead space and potential shear between the subcutaneous tissue and musculofascia. The midline skin incision is then closed in layers.
Postoperative care includes gradual diet advancement based on intestinal function, epidural pain management (which is transitioned to oral analgesics), and early ambulation. Patients are generally discharged from the hospital on postoperative day 4 to 7. Drains are removed when the output is ≤25 mL over 24 hours, and heavy physical exercise is avoided for 8 weeks.
Patient follow-up includes physical examinations every 1 to 3 months for 1 year and then every 6 months, unless complications necessitated interim evaluation. CT is generally performed at 6-month intervals for oncologic surveillance; these scans are also evaluated for hernia recurrence.
Open CS Technique
The open CS technique has been previously described.9 Briefly, after the abdominal cavity is entered, bilateral subcutaneous skin flaps are elevated from the midline fascial edge to the linea semilunaris. Cutaneous perforators emerging from the anterior rectus sheath are ligated and divided to facilitate exposure of the linea semilunaris in its entirety. The external oblique aponeurosis is identified and released approximately 1.5 cm lateral to the lateral edge of the rectus sheath from 12 cm above the costal margin superiorly to near the pubis inferiorly. The open CS dissection continues in the plane between the external oblique and internal oblique muscles laterally to the midaxillary line. The bioprosthetic mesh selection and inset then proceeds as described for MICSIB. Once the mesh is inset and fascial closure is performed, the subcutaneous skin flaps are advanced with multiple quilting sutures placed over the oblique and rectus muscle complexes to minimize subcutaneous dead space and resuspend the skin flaps to the musculofascial abdominal wall. Paramedian skin perfusion is critically assessed, and a vertical panniculectomy is performed to find the balance between acceptable skin perfusion and tension across the midline closure. The drain management, postoperative care, and follow-up are identical to those with the MICSIB technique.
Statistical Analysis
Descriptive statistics were calculated for the open CS and MICSIB groups, and the groups were compared. Pearson’s chi-square or Fisher’s exact test, with Bonferroni method to adjust for multiple comparisons, when appropriate, was used to assess differences in categorical variables between groups (diabetes, radiation, chemotherapy, wound contamination, high BMI, previous ventral hernia repair, previous abdominal surgery, ostomy, HADM use, fascial defect of ≥500 cm2, and current smoker), ratios of MICSIB to open CS cases over time, complication rates between surgeons, and surgical outcomes (between groups and by type of mesh used). Univariate logistic regression was used to evaluate differences in overall complication rates over time. Student’s t-test or the Mann Whitney U test was used to assess differences in continuous variables (age, BMI, fascial defect size, bridge size, and mesh size).
To evaluate differences in case volume (open CS vs. MICSIB) and complication rates over time, we divided the study period into 3 time periods: early (2005–2006), middle (2007–2008), and late (2009–2010). Ratios of MICSIB to open CS cases and overall complication rates by technique were compared for the 3 periods. We also compared complication rates between the 10 surgeons whose cases were included in the study.
Univariate and multivariate logistic regression analysis was used to evaluate the association between patient, defect, and treatment characteristics and surgical complications and outcomes. Variables were included in multivariate analysis if p<0.1 on univariate analysis. For all analyses, a p value of < 0.05 was considered significant. All analyses were performed using SPSS Statistical software (SPSS Statistics, version 19; IBM Corporation, Armonk, NY).
RESULTS
Patient, Wound, and Treatment Characteristics
Open CS was performed on 50 patients (26 males), while 57 patients (31 males) underwent MICSIB. Ten surgeons contributed cases to this series. Four surgeons performed both open CS and MICSIB and contributed 86% of the total cases (35 open CS and 57 MICSIB). The remaining 14% of cases (15 open CS) were performed by 6 surgeons.
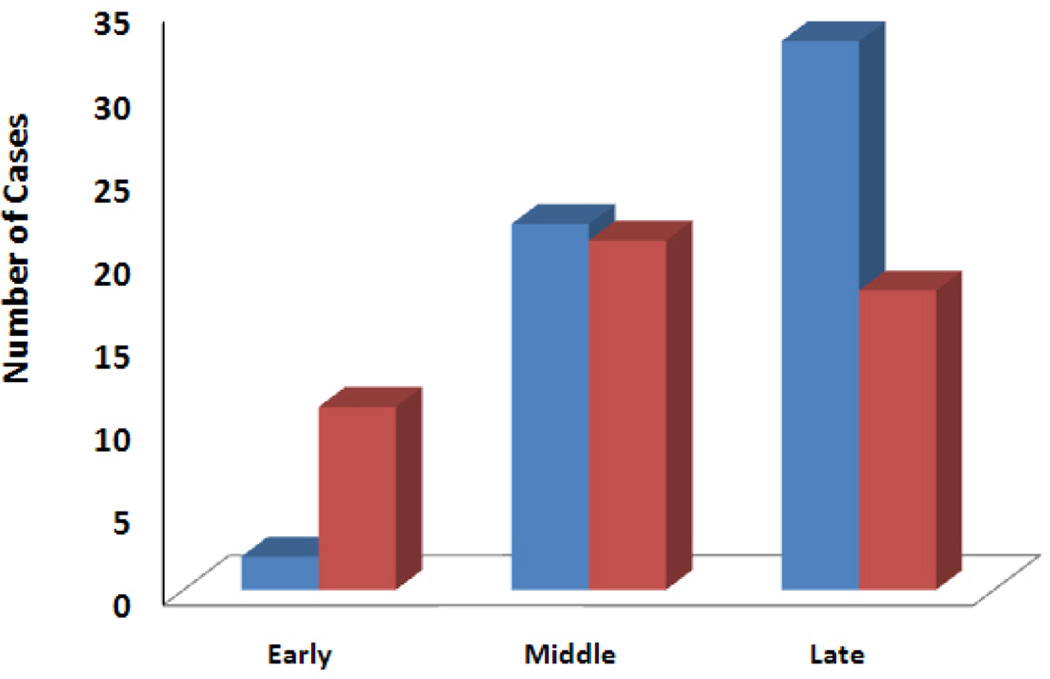
Patient, defect, and treatment variables are summarized in Table 1. Patient demographics and potential risk factors for complications were similar in the 2 groups. Eighty-two percent (47/57) of MICSIB patients and 86% (43/50) of open CS patients had at least one pre-existing medical comorbidity. The relative ratio of MICSIB to open CS cases (0.18, 1.05 and 1.83 in the early, middle, and late time periods, respectively) increased significantly over time (p=0.005; Fig. 1).
Table 1.
Patient, Wound and Treatment Characteristics in Open Component Separation or Minimally Invasive Component Separation with Inlay Bioprosthetic Mesh for Ventral Hernia Repair
| Characteristic | Open CS, n=50 | MICSIB, n=57 | p Value |
|---|---|---|---|
| Smoker (current), n (%) | 16 (32) | 13 (23) | 0.3 |
| Diabetes, n (%) | 7 (14) | 14 (25) | 0.2 |
| Preoperative radiotherapy, n (%) | 16 (32) | 18 (32) | 0.9 |
| Preoperative chemotherapy, n (%) | 26 (52) | 32 (56) | 0.7 |
| Wound contamination, n (%) | 18 (36) | 17 (30) | 0.5 |
| Ostomy, n (%) | 16 (32) | 13 (23) | 0.3 |
| BMI ≥25 kg/m2, n (%) | 40 (80) | 44 (77) | 0.7 |
| Previous abdominal surgery, n (%) | 44 (88) | 53 (93) | 0.4 |
| Previous ventral hernia repair, n (%) | 11 (22) | 13 (23) | 0.9 |
| Simultaneous intra-abdominal surgery n (%) | 11 (22) | 17 (30) | |
| Bridged repair, n (%) | 6 (12) | 8 (14) | 0.57 |
| HADM use, n (%) | 18 (36) | 9 (16) | 0.03 |
| Fascial defect ≥500 cm2, n (%) | 2 (4) | 14 (25) | 0.003 |
| HADM, n (%) | 18 (36) | 9 (16) | 0.03 |
| PADM, n (%) | 22 (44) | 44 (77) | 0.0009 |
| BADM, n (%) | 10 (20) | 4 (7) | 0.08 |
| Age, y, mean ± SD | 60.5 ± 13 | 63.4 ± 11.3 | 0.3 |
| BMI, kg/m2, mean ± SD | 29.5 ± 5.7 | 31.2 ± 9.0 | 0.2 |
| Fascial defect size, cm2, mean ± SD | 273.8 ± 186.8 | 405.4 ± 193.6 | 0.002 |
| Bioprosthetic size, cm2, mean ± SD | 301.4 ± 113.0 | 393.0 ± 124.6 | 0.0003 |
| Follow-up, mo, mean ± SD | 20.7 ± 14.3 | 15.2 ± 7.7 | 0.014 |
BMI, body mass index; CS, component separation; HADM, human acellular dermal matrix; MICSIB, minimally invasive component separation with inlay bioprosthetic mesh; SD, standard deviation; BADM, bovine acellular dermal matrix; HADM, human acellular dermal matrix; PADM, porcine acellular dermal matrix.
Fig. 1.
Number of cases performed in the early (2005–2006), middle (2007–2008), and late (2009–2010) time periods of the study. The ratio of minimally invasive component separation with inlay bioprosthetic mesh (blue bars) to open component separation (red bars) cases increased significantly over time (p=0.005).
The mean follow-up (± standard deviation) was 20.7 ± 14.3 months for open CS patients versus 15.2 ± 7.7 months for MICSIB patients (p = 0.014). Twenty-two percent of the open CS group and 30% of the MICSIB group underwent simultaneous intra-abdominal surgery with gastrointestinal tract violation (p = 0.4). The mean fascial defect size (± standard deviation) was significantly smaller in the open CS group (273.8 ± 186.8 cm2) than in the MICSIB group (405.4 ± 193.6 cm2; p = 0.002) (Table 1). The mean surface area of the bioprosthetic mesh used was 301.4 ± 113.0 cm2 for open CS and 393.0 ± 124.6 cm2 for MICSIB (p = 0.0003). Only 12% and 14% of patients underwent an interposition/bridged repair rather than a reinforced repair in the open CS and MICSIB groups, respectively. There was no significant difference in the bridging technique or size of the bridged defect between groups (Table 1). The percentage of patients who received HADM was significantly greater in the open CS group than in the MICSIB group, while the percentage of patients who received PADM was significantly greater in the MICSIB group than in the open CS. These differences reflect the temporal evolution of surgical technique and availability of xenograft bioprosthetic mesh materials. No significant difference was observed in the frequency of BADM use between groups (Table 1).
Complications and Outcomes
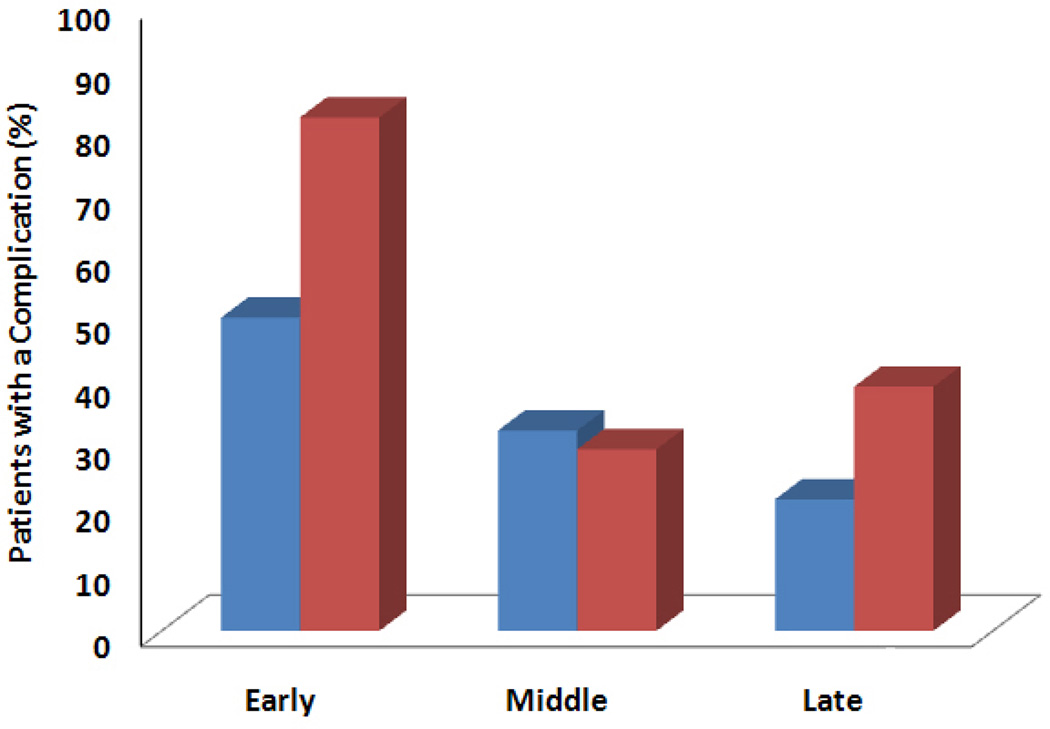
Postoperative complications occurred in 44% of open CS patients and 26% of MICSIB patients (p = 0.055) (Table 2). Five open CS patients (10%) versus 1 MICSIB patient (2%) had more than 1 postoperative complication, but this difference was not significant. Overall, significantly more patients had wound-healing complications in the open CS group (32%) than in the MICSIB group (14%; p = 0.026). Skin dehiscences occurred more frequently in the open CS group than in the MICSIB group (28% vs. 11%; p = 0.011). Hernia recurrence rates were similarly low in the 2 groups (8% vs 4%; p=0.3). The open CS group had a non-significantly greater rate of laxity/bulge (14% vs. 4%; p = 0.056). The rate of overall complications over time is shown in Fig. 2. The complication rate was lower in each successive time period in the MICSIB group; however, this difference was not statistically significant (p=0.26). In the open CS group, the difference in complication rates over time also was not significant (p=0.06). There were no differences in the rate of any complication between any of the participating surgeons (p=1.0).
Table 2.
Surgical Complications and Outcomes in Open CS and MICSIB Groups
| Complication/outcomes | Open CS, n=50, n (%) |
MICSIB, n=57, n (%) | p Value |
|---|---|---|---|
| Any complication | 22 (44) | 15 (26) | 0.055 |
| All wound-healing complication | 16 (32) | 8 (14) | 0.026 |
| Skin necrosis | 2 (4) | 2 (4) | 0.9 |
| Skin dehiscence | 14 (28) | 6 (11) | 0.011 |
| Reoperation | 8 (16) | 4 (7) | 0.1 |
| Abscess | 2 (4) | 2 (4) | 0.89 |
| Hematoma | 1 (2) | 1 (2) | 0.9 |
| Seroma | 3 (6) | 1 (2) | 0.25 |
| Office-based complications | 11 (22) | 10 (18) | 0.56 |
| Laxity/bulge | 7 (14) | 2 (4) | 0.056 |
| Hernia recurrence | 4 (8) | 2 (4) | 0.3 |
CS, component separation; MICSIB, minimally invasive component separation with inlay bioprosthetic mesh.
Fig. 2.
Overall complication rates over time, by technique. Overall complication rates were lower in later time periods, but these differences were not significant in the minimally invasive component separation with inlay bioprosthetic mesh (blue bars) (p=0.26) or open component separation (red bars) (p=0.059) group.
We also examined whether the type of mesh used affected outcomes (Table 3). No significant differences were found between the open CS and MICSIB groups in the incidence of wound-healing complications, laxity/bulge, or hernia recurrence for any type of mesh. There were also no significant differences in the incidence of any individual complication between groups for any type of mesh (data not shown).
Table 3.
Surgical Complications by Bioprosthetic Mesh Used
| Patients with complication | |||
|---|---|---|---|
| Complication/mesh used |
Open CS, n=50, n (%) |
MICSIB, n=57, n (%) |
p Value |
| All wound-healing complication with HADM | 8 (16) | 1 (2) | 0.192 |
| All wound-healing complication with PADM | 4 (8) | 6 (11) | 0.72 |
| All wound-healing complication with BADM | 4 (8) | 1 (2) | 1.0 |
| Total | 16 (32) | 8 (14) | |
| Hernia recurrence with HADM | 2 (4) | 1 (2) | 1.0 |
| Hernia recurrence with PADM | 0 | 1 (2) | 1.0 |
| Hernia recurrence with BADM | 2 (4) | 0 | 1.0 |
| Total | 4 (8) | 2 (4) | |
| Laxity/Bulge with HADM | 5 (10) | 1(2) | 0.62 |
| Laxity/Bulge with PADM | 2(4) | 1(2) | 0.25 |
| Laxity/Bulge with BADM | 0 | 0 | 1.0 |
| Total | 7 (14) | 2 (4) | |
CS, component separation; HADM, human acellular dermal matrix; MICSIB, minimally invasive component separation with inlay bioprosthetic mesh; PADM, porcine acellular dermal matrix; BADM, bovine acellular dermal matrix.
In addition to serial physical examination, postoperative CT scans were utilized to evaluate hernia recurrence in 84% (42/50) of open CS patients and 82% (47/57) of MICSIB patients. The open CS group had a higher incidence of laxity/bulge than the MICSIB group (14% vs. 4%; p = 0.056) (Table 2). Five of 7 patients in the open CS group who developed laxity/bulge had received HADM; laxity/bulge occurred at a mean of 10.2 months (range 1 to 38 months) postoperatively in these patients. The remaining 2 open CS patients who had laxity/bulge had received PADM; laxity/bulge developed at 6 and 7 months postoperatively in these patients. Two MICSIB patients developed laxity/bulge: 1 who received PADM developed laxity/bulge at 6 months postoperatively that was complicated by overlying skin necrosis, and 1 who received HADM developed laxity/bulge at 9 months.
A total of 12 patients underwent reoperation: 8 patients (16%) in the open CS group and 4 patients (7%) in the MICSIB group (Table 4).
Table 4.
Indications for Reoperation in 12 Patients
| Initial surgery type |
Indication for reoperation | Time after initial operation |
|---|---|---|
| Open CS | Mesenteric hematoma | 1 d |
| Open CS | Debridement – Abscess (MRSA) + musculofascial necrosis | 30 d |
| Open CS | Debridement – Necrotic fascia and fat | 54 d |
| Open CS | Debridement – Superficial tissue | 56 d |
| Open CS | Debridement – Necrotic fascia and fat | 58 d |
| Open CS | Recurrent ventral hernia | 6.5 mo |
| Open CS | Abdominal wall bulge/laxity | 12 mo |
| Open CS | Recurrent ventral hernia | 29 mo |
| MICSIB | Subcutaneous hematoma | 2 d |
| MICSIB | Debridement – Soft tissue, fascia, and muscle | 24 d |
| MICSIB | (1) Debridement – Soft tissue / (2) Debridement – Abscess / (3) Hernia | 49/78/249 d |
| MICSIB | Abdominal wall bulge/laxity | 11 mo |
CS, component separation; MICSIB, minimally invasive component separation with inlay bioprosthetic mesh; MRSA, methicillin-resistant Staphylococcus aureus
There was no significant difference in reoperation rates between the 2 groups (p = 0.22). There were 5 deaths during follow-up in this series: 2 in the open CS group and 3 in the MICSIB group, at a mean of 13.4 months postoperatively (range 4 to 29 months). All deaths were due to tumor progression or causes unrelated to the abdominal wall reconstructions.
Logistic Regression Analysis
Univariate logistic regression analysis demonstrated 3 factors associated with wound-healing complications: open (rather than minimally invasive) CS (odds ratio [OR] 2.8, 95% confidence interval [CI] 1.1 to 7.5; p = 0.03), contamination of the surgical wound (OR 2.6, 95% CI 1.03 to 6.6; p = 0.04), and presence of an ostomy (OR 3, 95% CI 1.17 to 7.9; p = 0.02). Open CS was associated with skin dehiscence (OR 3.6, CI 1.3 to 10.3; p=0.015). The association between smoking and skin necrosis was not quite significant (OR 8.9, CI 0.89 to 89.2; p=0.063). The only factor significantly associated with laxity/bulge on univariate logistic regression analysis was use of HADM (OR 7.33, CI 1.69 to 31.81; p = 0.008). Of note, none of the risk factors were associated with hernia recurrence on the univariate analysis.
Multivariate logistic regression analysis demonstrated that open CS independently increased the risk of skin dehiscence (OR 3.6, 95% CI 1.2 to 10.2; p=0.02). There was a non-significant trend between open CS and the risks of all wound-healing complications (OR 2.6, 95% CI 0.97 to 6.9; p = 0.057). The use of HADM was independently predictive for laxity/bulge (OR 7.3, 95% CI 1.7 to 31.8; p=0.008). Current smoking approached significance as a risk factor for skin necrosis (OR 9.8, 95% CI 0.74 to 129.3; p=0.08).
DISCUSSION
Our study is the first to compare surgical outcomes of open versus minimally invasive CS with bioprosthetic mesh reinforcement in patients with complex ventral hernias and comorbidities. Clear advantages of MICSIB were demonstrated in this direct comparative study. Despite the general similarity of the 2 groups, MICSIB patients had significantly larger defects than did the open CS group and required a significantly larger surface area of bioprosthetic mesh for repair. Nevertheless, the MICSIB group had significantly fewer wound-healing complications (32% vs. 14%; p = 0.026) and skin dehiscences (28% vs. 11%; p = 0.011) than the open CS group. There was an almost 3 times greater incidence of laxity/bulge in the open CS group than in the MICSIB group (14% vs. 4%; p = 0.056), although the difference was not quite significant. Multivariate logistic regression analysis demonstrated that open CS conferred a 3-fold greater risk of skin dehiscence, a complication that MICSIB was designed to reduce. The improved wound-healing outcomes with MICSIB are likely due to preservation of the vascularity of the overlying skin flaps and reduction of paramedian dead space, which are the surgical principles highlighted in the MICSIB procedure.
The goal of rectus perforator preservation in MICSIB is to improve the blood supply to the abdominal skin flaps, which is paramount to primary midline wound healing. Saulis and Dumanian, in 2002, reported on the use of rectus perforator preservation to decrease the morbidity of open CS.19 The MICSIB technique, first introduced by the senior author, builds on that advance and attempts to maximize the number of perforators spared and preserve the subcutaneous tissue’s adherence to the rectus complex in order to maximize vascularity while minimizing paramedian subcutaneous dead space.17 Judicious placement of multiple drainage catheters and the use of quilting sutures to secure the skin flaps to the underlying musculofascia are other technical aspects of MICSIB that likely contributed to the decreased wound-healing complications demonstrated in this study.
A low seroma rate was seen in both groups (6% in open CS vs 2% in MICSIB; p = 0.25); the lack of a significant difference may be credited to the great care taken to place quilting sutures and drains and to resect skin in open CS. Literature reports of seroma rates vary; with the use of PADM and open CS, a seroma rate of 10% has been reported.20
Our experience with bioprosthetic materials has evolved over time, with increasing use of xenograft dermis replacing the initial use of HADM in abdominal wall reconstructions. This shift is supported by a growing body of evidence that allografts become attenuated over time, resulting in unacceptably high incidences of bulge and/or hernia--up to 78% within 2 years for HADM repairs.10, 20, 21 Fewer wound-healing complications and markedly greater cell and vascular infiltration have been reported in animal studies with PADM versus cross-linked PADM.22 In addition, a recent prospective trial evaluating repair of infected and contaminated ventral hernias (RICH trial) using PADM, often with CS, demonstrated that 80% of patients had successful single-stage reconstruction of their hernia defects.23
Type of surgery (open CS vs. MICSIB) was nearly significant as a risk factor for laxity/bulge on univariate (p = 0.07) logistic regression analysis. This may be a consequence of technique differences but might be accounted for by the significantly greater use of HADM in the open CS patients since HADM use was an independent predictor of laxity/bulge in the entire cohort. The MICSIB and open CS patients had similarly low long-term hernia recurrence rates (4% and 8%; p=0.3). There was no difference in hernia recurrence rates between the open CS and MICSIB groups, attesting to the durability of the MICSIB technique despite a significantly larger defect size. These results compare favorably with those of other studies of open CS perforator preservation techniques, which had hernia recurrence rates of 7%.19 Also of note, the patients in earlier series presented with a smaller mean defect size (14.5 cm wide) compared with our MICSIB group (405.4 ± 193.6 cm2), as well as a lower rate of prior irradiation (7% vs. 32%).19
There are inherent limitations of this retrospective study, including the potential for selection bias in performing open CS or MICSIB. It appears, however, MICSIB was more likely to be used for more challenging hernias with significantly larger fascial defects, rendering the improved outcomes associated with MICSIB more compelling. Various bioprosthetic meshes were used in the patients; the surgeons preferred to move away from HADM for abdominal wall reconstruction once xenograft alternatives became available. Meshes used were also influenced by patients’ health insurance coverage. The longer follow-up period in the open CS group could represent a potential confounding factor for outcomes, particularly hernia and bulge/laxity. Long-term data are not yet possible for MICSIB because of the technique’s relatively recent development compared to open CS. Strengths of this study include a defined, consistent technique developed by the senior author and used by other experienced surgeons who were all experienced with the technique at a single institution, comparison of outcomes in 2 similar groups, thorough follow-up with physical examination, and CT evaluation for hernia recurrence in more than 80% of patients in both groups.
Further study will be needed to address patient selection for MICSIB versus open CS. Thus far, we have not identified any absolute contraindications for MICSIB when a CS is indicated. Our group has demonstrated that CS can be safe and effective even when one or both rectus complexes have been violated (e.g., by prior surgery or ostomy placement).24 We find that MICSIB is extremely valuable in these cases because it avoids dissection over the anterior rectus sheath; this is particularly beneficial when a previous incision or ostomy is present. We also believe that MICSIB has benefits over open CS in cases of multiply recurrent hernias where skin flaps have previously been raised and perforators sacrificed. We feel that limiting the dead space and preserving the integrity of any newly formed vessels between the rectus complexes and skin flaps may reduce complications in these cases.
At our institution, the MICSIB technique has largely replaced the open CS technique for abdominal wall reconstructions. As noted in this study, MICSIB complication rates were non-significantly lower as more experience with the technique was gained over time. The learning curve for MICSIB appears to be similar to that for open CS, and good outcomes can be achieved early, though they may improve with experience. The MICSIB technique represents the next step in the ongoing evolution from maximally invasive open surgical procedures to minimally invasive procedures that not only reduce donor site morbidity but also improve the primary outcome measures of the procedures. Additional studies and longer follow-up screening for hernia recurrence will help identify which patients would benefit most from the MICSIB technique.
In conclusion, we found that MICSIB for complex ventral hernia repair results in fewer wound-healing complications than open CS. Preserving the rectus abdominis myocutaneous perforators improves skin vascularity and minimizes subcutaneous dead space. This technique should be considered when CS is planned as a component of complex ventral hernia repair.
Acknowledgments
The University of Texas MD Anderson Cancer Center is funded in part by a cancer center support grant from the National Institutes of Health (CA16672).
Abbreviations and Acronyms
- BADM
bovine acellular dermal matrix
- BMI
body mass index
- CI
confidence interval
- CS
component separation
- CT
computed tomography
- HADM
human acellular dermal matrix
- MICSIB
minimally invasive component separation with inlay bioprosthetic mesh
- OR
odds ratio
- PADM
porcine acellular dermal matrix
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Dr. Butler receives an honorarium as a consultant to LifeCell Corporation. All other authors have nothing to declare.
Abstract presented at the American Society of Plastic Surgeons’ Annual Scientific Meeting, Denver CO, September 2011.
REFERENCES
- 1.Poole GV. Mechanical factors in abdominal wound closure: the prevention of fascial dehiscence. Surgery. 1985;97:631–640. [PubMed] [Google Scholar]
- 2.Mudge M, Huges LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985;72:70–71. doi: 10.1002/bjs.1800720127. [DOI] [PubMed] [Google Scholar]
- 3.Santora TA, Roslyn JJ. Incisional hernia. Surg Clin North Am. 1993;73:557–570. doi: 10.1016/s0039-6109(16)46037-8. [DOI] [PubMed] [Google Scholar]
- 4.Luijendijk RW, Hop WCJ, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398. doi: 10.1056/NEJM200008103430603. [DOI] [PubMed] [Google Scholar]
- 5.Burger JW, Luijendijk RW, Hop WCJ, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–585. doi: 10.1097/01.sla.0000141193.08524.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer S, Christiansen J. Long-term results after incisional hernia repair. Acta Chir Scand. 1985;151:217–219. [PubMed] [Google Scholar]
- 7.George CD, Ellis H. The results of incisional hernia repair: a twelve year review. Ann R Coll Surg Engl. 1986;68:185–187. [PMC free article] [PubMed] [Google Scholar]
- 8.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population based analysis. Ann Surg. 2003;237:129–135. doi: 10.1097/00000658-200301000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez OM, Ruas E, Dellon AL. "Components separation" method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519–526. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Agnew SP, Small W, Wang E, et al. Prospective measurement of intra-abdominal volume and pulmonary function after repair of massive ventral hernias with the components separation technique. Ann Surg. 2010;251:981–988. doi: 10.1097/SLA.0b013e3181d7707b. [DOI] [PubMed] [Google Scholar]
- 11.Group MR. Millennium Research Group Inc. US markets for soft tissue repair. Toronto, ON: 2009. [Google Scholar]
- 12.Leber GE, Gard JL, Alexander A, et al. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133:378–382. doi: 10.1001/archsurg.133.4.378. [DOI] [PubMed] [Google Scholar]
- 13.Bauer JJ, Harris MT, Kreel I, et al. Twelve-year experience with expanded polytetrafluoroethylene in the repair of abdominal wall defects. Mt. Sinai J Med. 1999;66:20–25. [PubMed] [Google Scholar]
- 14.Holton LH, Kim D, Silverman RP, et al. Human acellular dermal matrix for repair of abdominal wall defects: a review of clinical experience and experimental data. J Long Term Eff Med Implants. 2005;15:547–558. doi: 10.1615/jlongtermeffmedimplants.v15.i5.70. [DOI] [PubMed] [Google Scholar]
- 15.Milburn ML, Holton LH, Chung TL, et al. Acellular dermal matrix compared with synthetic implant material for repair of ventral hernia in the setting of peri-operative Staphylococcus aureus implant contamination: a rabbit model. Surg Infect (Larchmt) 2008;9:433–442. doi: 10.1089/sur.2007.044. [DOI] [PubMed] [Google Scholar]
- 16.Nemeth NL, Butler CE. Complex torso reconstruction with human acellular dermal matrix: long-term clinical follow-up. Plast Reconstr Surg. 2009;123:192–196. doi: 10.1097/PRS.0b013e3181934812. [DOI] [PubMed] [Google Scholar]
- 17.Butler CE. Minimally invasive component separation with inlay bioprosthetic mesh (MICSIB) (CC-2041). Proceedings of the American College of Surgeons, Cine-Med; San Francisco, CA. October 2008. [Google Scholar]
- 18.Butler CE, Campbell KT. Minimally invasive component separation with inlay bioprosthetic mesh (MICSIB) for complex abdominal wall reconstruction. Plast Reconstr Surg. 2011;128:698–709. doi: 10.1097/PRS.0b013e318221dcce. [DOI] [PubMed] [Google Scholar]
- 19.Saulis A, Dumanian G. Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in “separation of parts” hernia repairs. Plast Reconstr Surg. 2002;109:2275–2280. doi: 10.1097/00006534-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Breuing K, Butler CE, Ferzoco S, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Ko JH, Wang EC, Salvay DM, et al. Abdominal wall reconstruction: lessons learned from 200 “components separation” procedures. Arch Surg. 2009;144:1047–1055. doi: 10.1001/archsurg.2009.192. [DOI] [PubMed] [Google Scholar]
- 22.Butler CE, Burns NK, Campbell KT, et al. Comparison of cross-linked and non-cross-linked porcine acellular dermal matrices for ventral hernia repair. J Am Coll Surg. 2010;211:368–376. doi: 10.1016/j.jamcollsurg.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Itani KM, Rosen M, Vargo D, et al. NP2010-9938: prospective multicenter clinical study of single-stage repair of infected or contaminated abdominal incisional hernias using Strattice reconstructive tissue matrix. Proceedings of the American College of Surgeons 96th Annual Clinical Congress; Washington, DC. October 2010. [Google Scholar]
- 24.Garvey PB, Bailey C, Baumann DP, Liu J, Butler CE. Violation of the rectus complex is not a contraindication to component separation for abdominal wall reconstruction. J Am Coll Surg. 2012;214:131–139. doi: 10.1016/j.jamcollsurg.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]