Abstract
The present study was conducted to investigate whether colon tumors were capable of metabolizing benzo (a)pyrene (BaP), and fluoranthene (FLA), two toxicants that belong to the polycyclic aromatic hydrocarbon family of compounds. Microsomes were isolated from the colon tumors of ApcMin mice that received subchronic doses of 50 μg/kg BaP and incubated with either BaP or FLA (3 μM each) alone or in combination and appropriate control groups that received nothing. Subsequent to incubation, samples were extracted with ethyl acetate and analyzed for BaP and FLA metabolites by reverse-phase HPLC equipped with fluorescence detection. Microsomes from tumor tissues were found to metabolize BaP to a greater extent than those from the non-tumor tissues. The rate of BaP metabolism (picomoles of metabolite per minute per milligram of protein) was found to be more when microsomes from BaP-pretreated mice were exposed to BaP alone and FLA in combination with BaP, compared to controls. The microsomes from BaP-preexposed mice generated greater proportion of BaP 7,8-diol and BaP 3,6- and 6,12-diones compared to other experimental groups. Additionally, microsomes from BaP-pretreated mice produced greater proportion of FLA 2, 3-diol and 2, 3 D FLA when microsomes were incubated with FLA alone or a combination of BaP and FLA. Our studies revealed that the tumor microsomes were competent to metabolize BaP and FLA either singly or in combination. The biotransformation of BaP and FLA as a consequence of prior and simultaneous exposure to BaP may influence the growth of tumors. Our findings may have relevance to human long-term dietary intake of these toxicants and the consequent acceleration of the colon carcinogenesis process.
Keywords: Benzo(a)pyrene, Fluoranthene, Polycyclic aromatic hydrocarbons, Colon tumors, ApcMin mice
Introduction
The mortality and morbidity statistics attributed to colorectal cancer (CRC) in the USA is a matter of concern [1]. Environmental and dietary factors have been blamed as some of the culprits in the development of sporadic CRC [2]. Strategies to prevent CRC require an understanding of the role played by the tumor microenvironment in advancing the disease. The tumor microenvironment is very unique in that the metastasized tissue harbors preneoplastic and/or neoplastic cells showing various stages of growth harboring quiescent cells and proliferating cells. The conditions in the tumor microenvironment such as hypoxia, increased glycolysis, and other metabolic changes are conducive for metastasis [3]. While solid tumors are rich in quiescent cells, developing tumors contain proliferating cells [4, 5]. Additionally, the tissue architecture of tumors shows a great variation from its normal counterparts in tissue integrity and exhibit differential genetic programming [6-8].
There is a paucity of information on the responsiveness of tumors to continuous exposure to toxicants. In this context, whether the tumors are able to metabolize and accumulate the resulting metabolites of chemical carcinogens that may influence the tumor growth through a cascade of various biochemical events is not known. Given the ability of cancer cells to undergo unregulated growth and cell proliferation, an understanding of toxicant metabolism by tumors is necessary from the perspective of developing appropriate therapeutic strategies [9-11].
Benzo(a)pyrene (BaP), a member of the polycyclic aromatic hydrocarbon (PAH) family of compounds is a well-characterized environmental toxicant and carcinogen [12-15]. This pollutant has been detected in a wide variety of food items and considerable intake through dietary items has been reported (reviewed in 15, 16). Of late, attention has been focused on its ability to cause digestive tract cancers [2, 16, 17]. In this study, we attempted to investigate the metabolism of BaP by microsomes harvested from the colon tumors of an ApcMin mouse model, subsequent to daily oral administration of BaP for 60 days. Additionally, we sought to examine the ability of colon tumor microsomes in metabolizing FLA, another high-volume PAH toxicant that coexists with BaP in environmental, occupational settings [18, 19] and dietary items [20, 21].
Material and methods
Animal dosing and tumor retrieval
Six-week-old male ApcMin mice (Jackson Labs, Bar Harbor, ME, USA) weighing approximately 25 g were used in this study. The mice were housed in groups of two or three per cage, maintained on a 12-h light/dark cycle (lights on at 6:00 a.m.), and allowed free access to rodent chow (2016 Teklad Global 16 % protein rodent diet [3.5 % fat]; Harlan Laboratories, Indianapolis, IN, USA) and water. The mice were kept in polycarbonate cages (Lab Products, Inc., Seaford, DE, USA) with laboratory grade 7089 Teklad Diamond Soft Cellulose (Harlan Laboratories) material as bedding. Mice were housed in an Association for the Assessment and Accreditation of Laboratory Animal Care International-accredited animal care facility (ACF). The ACF is under the oversight of Institutional Animal Care and Use Committee, which ensures that animal care, conforms to the National Institutes of Health (NIH) guidelines for the humane care and use of laboratory animals [22]. All animals were allowed a 7-day acclimation period.
The mice (n=5 for each treatment category) were administered 50 μg/kg of BaP (97 % pure, unlabeled; Sigma Chemical Co., St.Louis, MO) dissolved in peanut oil, daily via oral gavage for 60 days. Mice that received no vehicle (peanut oil) and BaP served as controls. At the end of 60 days of exposure, mice were sacrificed and colons were harvested from control and experimental mice. The colons were opened and gently rinsed with physiological saline to flush the food/excreta. The tissue lesions (0.5 cm size) which stood out from the surrounding mucosa as tumor-like excrescences (adenomas) were used for this study. The tumors were carefully excised from the colon. Non-polypoid mucosae were also excised to evaluate for differences in carcinogen metabolism in tumorous and non-tumorous regions. Both tumorous and non-tumorous tissues were kept frozen at −70 °C until analyses.
Preparation of microsomes
The colon from BaP-exposed mice were harvested following which, they were cleaned and processed as mentioned above. Two to three tumors measuring around 5 mm in diameter were retrieved from each tumor-bearing mouse. Each tissue sample from control and BaP-exposed mice (tumorous and non-tumorous) was individually cut into small pieces using sterile scalpel blades, minced separately with a fine pair of scissors and thoroughly mixed to obtain a homogenous mixture of minced tissue sample per category (tumorous and non-tumorous) per mouse. Pooled tumor tissues were used for this study. Each minced sample was chilled in isotonic saline and microsomal fractions were prepared using the endoplasmic reticulum isolation kit (Sigma–Aldrich Chemicals, St. Louis).
Microsomal incubations and metabolism studies
Before conducting metabolism studies, microsomes were thawed at room temperature and 120 μL of the microsomal pellet resuspended in TKM buffer (final protein concentration 0.5 mg/mL) were added to 5 mL of cocktail containing NADPH (0.72 mM), EDTA (0.1 mM), KPO4 (100 mM), and MgCl2. 6H2O (3.75 mM). Samples per animal were preincubated for 2 min at 37 °C in a tissue shaker (Precision Scientific Instruments, Chicago, IL). Treatment consisted of exposure in vitro to 3 μM BaP (CAS no. 50-32-8; 98 % pure, Sigma) or FLA (CAS No. 206-44-0; 98 % pure, Sigma) dissolved in dimethyl sulfoxide. After a 15-min incubation at 37 °C, the reaction was stopped with 8 mL of ethyl acetate containing butylated hydroxytoluene (0.2 mg/mL). Benzo(a)pyrene or fluoranthene metabolites were extracted twice with ethyl acetate and the extracts were analyzed by reverse-phase HPLC for BaP/FLA metabolites as described previously [23, 24]. Metabolite standards for these two toxicants were obtained from the National Cancer Institute Chemical Carcinogen Repository (Midwest Research Institute, Kansas City, MO).
The microsomal incubation experiments for BaP and FLA (FLA in the presence of BaP and vice versa) were done separately. Controls were processed alongside with experimental samples under identical assay conditions. Dimethyl sulfoxide [vehicle for FLA/BaP] was used as a negative control while reaction mixtures without BaP or FLA or microsomes served as positive controls. The rates of metabolism [formation of BaP/FLA metabolites] were expressed as picomoles of product formed per minute per milligram of microsomal protein. Since FLA or BaP and their metabolites are suspected carcinogens, they were handled in accordance with NIH guidelines [25] for ensuring safety of laboratory personnel during the course of these studies.
Statistical analysis
Data on microsomal mean FLA/BaP metabolite concentrations were analyzed by one-way ANOVA and Bonferroni post hoc test. The statistical significance was set at p values less than 0.05.
Results
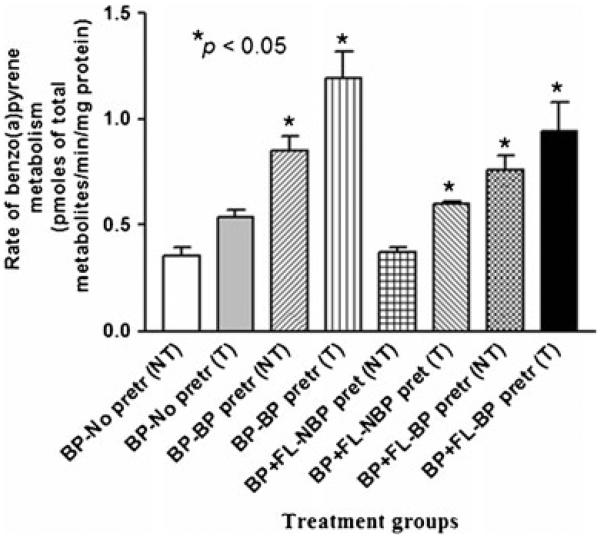
The total concentrations of BaP metabolites produced by the mouse colon tumor microsomes were shown in Fig. 1. The concentration of BaP metabolites from colon tumor tissues was much greater than that from nonmalignant tissues of tumor-bearing BaP-exposed mice (p<0.05).
Fig. 1.
Rate of benzo(a)pyrene metabolism in colon tumor microsomes of ApcMin mice preexposed to benzo(a)pyrene. Explanations of the abbreviations are as follows: NT microsomes from non-tumorous tissues; T microsomes from tumorous tissues; BP-no pretr BaP metabolite concentrations from microsomes of unexposed mice incubated with 3 μM BaP alone; BP-BP pretr BaP metabolite concentrations from microsomes of mice pretreated with 50 μg BaP—incubated with 3 μM of BaP alone; BP + FL-NBP pretr BaP metabolite concentrations from microsomes of unexposed mice incubated with 3 μM FLA+3 μM BaP; BP + FA-BP pretr BaP metabolite concentrations from microsomes of mice pretreated with 50 μg BaP—incubated with 3 μM each of BaP and FLA. Values are expressed as mean concentration of total metabolites ± SE. Data from triplicate determination (variability was <10 %) of five individual animals from each treatment group were compared. Asterisks denote statistical significance (*p<0.05) in BaP metabolite concentrations among the treatment categories
Microsomes from BaP-pretreated mice when incubated with BaP have generated significantly greater (p<0.05) concentrations of BaP metabolites compared to mice that were not preexposed to BaP. Similarly, microsomes from BaP-pretreated mice when incubated with BaP alone generated significantly greater (p<0.05) concentrations of BaP metabolites when compared to other treatment categories (BaP with no BaP pretreatment; BaP + FLA with no BaP pretreatment; FLA + BaP with BaP pretreatment).
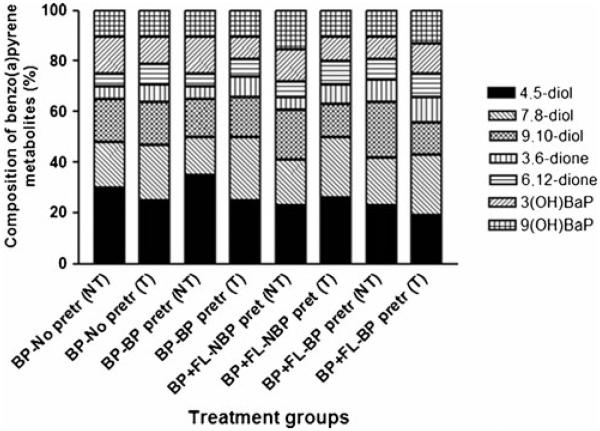
The various BaP metabolites generated from colon tumor microsomes were BaP 9,10-diol, BaP 4,5-diol, BaP 7, 8-diol, 3-hydroxy BaP, and 9-hydroxy BaP. The relative concentrations of individual BaP metabolites among total metabolites are depicted in Fig. 2. Microsomes from mice pretreated with BaP generated relatively higher proportion of BaP diols and diones compared to microsomes from mice that received no prior BaP.
Fig. 2.
Composition of metabolite types generated from colon tumor microsomal metabolism of benzo(a)pyrene. The ApcMin mice were exposed to 50 μg BaP/kg bw through peanut oil Values are expressed as the percentage of individual metabolite types among the total metabolites (sum of individual concentrations of BaP 9,10-diol; BaP 4,5-diol; BaP 7,8-diol; BaP 3,6-dione; BaP 6,12-dione; 3-hydroxy BaP and 9-hydroxy BaP) formed (n=5 for each treatment category). See the legend for Fig. 1 for explanation of abbreviations
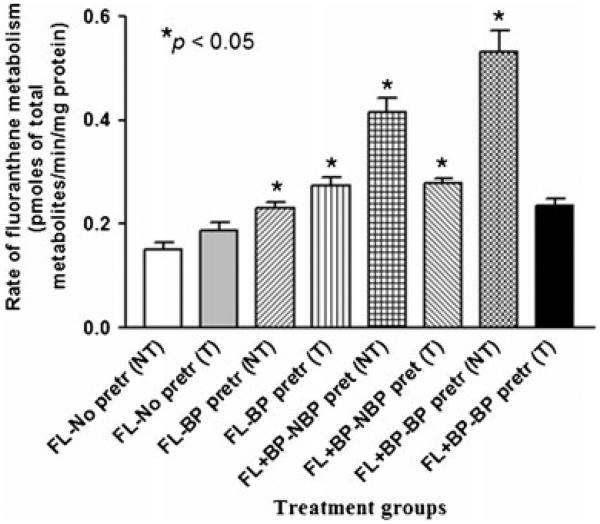
Since colon tumor microsomes from mice that received BaP yielded greater concentrations of metabolites, we have evaluated the ability of these microsomes to metabolize fluoranthene, another PAH compound The total concentrations of FLA metabolites produced by the colon tumor microsomes were shown in Fig. 3. Microsomes retrieved from BaP-pretreated mice when incubated with FLA produced greater (p<0.05) concentrations of FLA metabolites when compared to microsomes from the other treatment groups (FLA with no BaP pretreatment; FLA + BaP with no BaP pretreatment; FLA + BaP with BaP pretreatment).
Fig. 3.
Rate of fluoranthene metabolism in colon tumor microsomes of ApcMin mice preexposed to benzo(a)pyrene. Explanations of the abbreviations are as follows: NT microsomes from non-tumorous tissues; T microsomes from tumorous tissues; FL-no pretr FLA metabolite concentrations from microsomes of unexposed mice incubated with 3 μM FLA alone; FL-BP pretr FLA metabolite concentrations from microsomes of BaP preexposed mice incubated with 3 μM FLA+3 μM BaP; FL + BP-NBP pretr FLA metabolite concentrations from microsomes of unexposed mice incubated with 3 μM each of BaP and FLA; FL + BP-BP pretr FLA metabolite concentrations from microsomes of mice pretreated with 50 μg BaP—incubated with 3 μM each of BaP and FLA. Values are expressed as mean concentration of total metabolites ± SE. Data from triplicate determination (variability was <10 %) of five individual animals from each treatment group were compared. Statistical significance in FLA metabolite concentrations among the treatment categories is indicated by asterisks (*p<0.05)
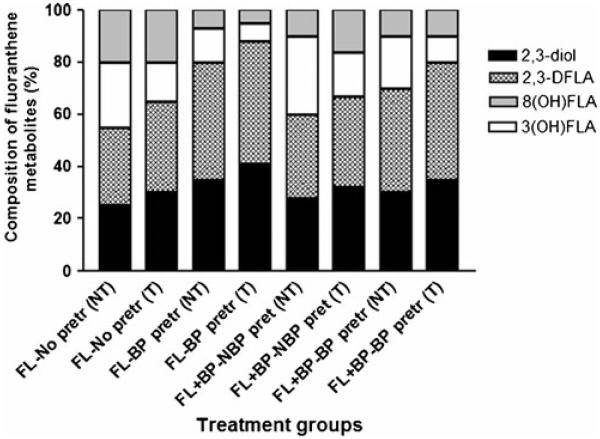
The FLA metabolites generated from colon tumor microsomes were FLA 2, 3-diol, trans-2, 3-dihydroxy-1, 10b-epoxy-1, 2, 3, 10b tetrahydro FLA (2, 3 D FLA), 3-hydroxy FLA, and 8-hydroxy FLA. The concentrations of individual FLA metabolites among total metabolites are shown in Fig. 4. Microsomes from BaP-pretreated mice produced FLA 2, 3-diol and 2, 3 D FLA in greater proportion than microsomes were incubated with FLA alone and FLA in combination with BaP.
Fig. 4.
Metabolite composition of fluoranthene generated from colon tumor microsomal metabolism of fluoranthene. The ApcMin mice were preexposed to benzo(a)pyrene and microsomes were isolated and incubated with fluoranthene alone or in combination with benzo(a)pyrene. Values are expressed as the percentage of individual metabolite types among the total metabolites (sum of individual concentrations of FLA 2, 3-diol, trans-2, 3-dihydroxy-1, 10b-epoxy-1, 2, 3, 10b tetrahydro FLA [2, 3D FLA]; 3-hydroxy FLA; and 8-hydroxy FLA) formed (n=5 for each treatment category). See the legend for Fig. 3 for explanation of abbreviations
Discussion
Some studies cast doubt whether colon tumors have the necessary machinery to activate enzymes that metabolize BaP. Massaad et al. [26] reported the absence of CYP isozymes (CYP1A1, 1A2, 2B1, 2B2, 2C, 2E1, and 3A) in Co38 mice colon tumors. These studies have not probed for CYP1B1, which is also involved in the biotransformation of PAHs in extrahepatic tissues [27, 28]. Sattar et al. [29] reported that adenomas in ApcMin mice were not metabolically proficient when compared to uninvolved mucosa. Their conclusion was based on the fact that CYP1A1/CYP1A2 induction levels were low in adenomas subsequent to BaP treatment. Perhaps, the BaP dose used by Sattar et al. [29] (a single I.P. dose of 100 mg/kg) may not have been sufficient enough to cause a pronounced induction and build up of metabolites as opposed to sustained induction at the subchronic (60 days) daily BaP dose (50 μg/kg) used in the present study.
Induction of CYP enzymes by subchronic exposure to BaP may have modulated its own enzymatic metabolic activation thereby enhancing its carcinogenic potential. Our findings are in agreement with that of Vaca et al. [30] and Huderson et al. [31] who have shown that pretreatment with BaP induces the microsomal metabolism of BaP to precursors of reactive metabolites. Conceivably biotranformation of BaP by tumor cells may lead to localized production of intracellular metabolites [32, 33] and may therefore have a role to play not only in the causation but also in the progression of disease as well. Evidence is available in support of the involvement of metabolism in toxicity-associated inflammatory diseases such as cancer. That BaP through metabolic activation induce reactive oxygen species (ROS) production, which also induces inflammatory responses in colon and other target tissues has been reported [34, 35].
Metabolism of BaP by colon tumor microsomes generate BaP dihydrodiols (trans-4,5-; trans-7,8-; and trans-9,10-diols) along with hydroxyl (3- and 9-OH) metabolites. The trans-dihydrodiols, especially the 7,8-diol is oxidized to bay-region diol epoxides, which bind to DNA strongly and form stable DNA adducts, which perturb nuclear functions such as transcription and replication ultimately leading to neoplasia [13, 36]. The hydroxy metabolites of BaP have been implicated in DNA damage, contributing to unscheduled DNA synthesis [37].
The differences in metabolite concentrations among tumor tissues could be attributed to differences in CYP activities and expression during various phases of tumor growth. Due to the limited mass of tumor tissue availability, we could not evaluate the activities or expression of drug-metabolizing enzymes (DME) in these tumors in order to observe whether the measured BaP metabolite concentrations signified modulation of DME due to BaP exposure. This limitation notwithstanding, we contend that the metabolite concentrations are predictive of enzyme induction and enhanced metabolic activation due to sustained BaP exposure.
The metabolism of FLA by colon tumor microsomes may have been enhanced by prolonged exposure of mice to BaP. Published reports suggest that FLA is not a potent inducer of DME [38, 39]. However, the presence of another PAH compound may have an effect on the metabolism of FLA. Even though individual PAH compounds or mixtures are tumorigenic, mixtures of anthracene, chrysene, pyrene, fluoranthene, and phenanthrene demonstrated a greater tumor latency period for skin tumors, when the PAH mixture was supplemented with BaP, a decreased latency period was seen [40]. Additionally, studies conducted in our laboratory have revealed an enhanced metabolism of FLA when adipose tissue microsomes were exposed to BaP and FLA together [31].
While colon tumor microsomes were able to produce FLA metabolites, the generation of FLA 2, 3-diol in greater proportions in microsomal incubations of FLA with BaP assumes importance given the ability of this metabolite to bind to cellular macromolecules such as DNA and proteins [41, 42]. Therefore, it is reasonable to speculate that BaP pretreatment may enhance the generation of reactive metabolites of FLA which in a dual or sequential exposure scenario may influence tumor progression.
To put our findings into perspective, in natural conditions, BaP and FLA are unlikely to be present in the same proportions or concentrations used in this study, given the variation seen in the levels of PAH residues in food items and dietary intake by humans [15, 20]. Carcinogenesis is a multistage process that encompasses initiation, promotion and progression phases and colon carcinogenesis is no exception. During this process, the body receives a continuous intake of environmental contaminants through diet that can target different stages of the carcinogenesis process simultaneously [43, 44]. Polycyclic aromatic hydrocarbon-induced carcinogenesis requires long-term exposure to an individual PAH compound [45, 46] or mixtures [47]. Regardless of the relative concentrations of BaP and FLA, as a result of enhanced biotransformation of BaP versus FLA or vice versa, the reactive metabolites that were generated by these chemicals may have the potential to modulate inflammatory responses in the tumor microenvironment [48] contributing to tumor promotion. Additionally, the conditions in the tumor microenvironment are conducive for generation of ROS, increased DNA damage, and less efficient DNA repair processes [3]. Thus, chronic exposure to environmental toxicants such as BaP fuels both tumor promotion and progression stages through enhanced biotransformation.
In recent years, several chemopreventive agents have been proposed for reducing carcinogenesis in colon [49]. Studies conducted in our laboratory (unpublished data) have shown that one of these agents, resveratrol, lowers colon tumor burden, and size in BaP-treated ApcMin mouse model. Experiments are in progress in our laboratory to find out whether resveratrol could alter biotransformation pathways in tumorous and non-tumorous tissues of BaP-treated ApcMin mouse.
Acknowledgments
This research was supported by the NIH grants 5R01CA142845-02, 1F31ES017391-01, 5T32HL007735-12, and 5R25GM059994-11. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. Our thanks are also due to the Southern Regional Education Board, Atlanta, Georgia for a doctoral scholar award to Ms. Kelly Harris.
Footnotes
Present Address: D. L. Diggs U.S. Environmental Protection Agency, Office of Research and Development, Toxicity Assessment Division, Research Triangle Park, NC 27711, USA
References
- 1.Whitlock EP, Lin JS, Liles E, et al. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 2.Diggs DL, Huderson AC, Harris KL, Myers JN, Banks LD, Rekhadevi PV, et al. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29:324–57. doi: 10.1080/10590501.2011.629974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laconi E. The evolving concept of tumor microenvironments. Bioessays. 2007;29:738–44. doi: 10.1002/bies.20606. [DOI] [PubMed] [Google Scholar]
- 4.Mellor HR, Snelling S, Hall MD, Modok S, Jaffar M, Hambley TW, et al. The influence of tumour microenvironmental factors on the efficacy of cisplatin and novel platinum(IV) complexes. Biochem Pharmacol. 2005;70:1137–46. doi: 10.1016/j.bcp.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Augsten M, Hägglöf C, Peña C, Ostman A. A digest on the role of the tumor microenvironment in gastrointestinal cancers. Cancer Microenviron. 2010;3:167–76. doi: 10.1007/s12307-010-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer AH, Young KA, DoLollis RA. Incorporating pathologists criteria of malignancy into the evolutionary model for cancer development. J Cell Biochem. 2004;93:28–36. doi: 10.1002/jcb.20105. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenschein C, Soto A. Carcinogenesis and metastasis now in the third dimension—what’s in for the pathologists? Am J Pathol. 2006;168:363–6. doi: 10.2353/ajpath.2006.051084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peddareddigari VG, Wang D, DuBois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3:149–66. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL. Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther. 2001;296:537–41. [PubMed] [Google Scholar]
- 10.Mérida I, Ávila-Flores A. Tumor metabolism: new opportunities for cancer therapy. Clin Transl Oncol. 2006;8:711–6. doi: 10.1007/s12094-006-0117-6. [DOI] [PubMed] [Google Scholar]
- 11.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 12.Knuckles ME, Inyang F, Ramesh A. Acute and subchronic oral toxicity of benzo(a)pyrene in F-344 rats. Toxicol Sci. 2001;61:382–8. doi: 10.1093/toxsci/61.2.382. [DOI] [PubMed] [Google Scholar]
- 13.Luch A. The carcinogenic effects of polycyclic aromatic hydrocarbons. Imperial College Press Eds; London: 2005. [Google Scholar]
- 14.WHO . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 92. International Agency for Research on Cancer; Lyon, France: 2010. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. [PMC free article] [PubMed] [Google Scholar]
- 15.Ramesh A, Archibong A, Hood DB, Guo Z, Loganathan BG. Global environmental distribution and human health effects of polycyclic aromatic hydrocarbons. In: Lam PKS, Loganathan BG, editors. Global contamination trends of persistent organic chemicals. Taylor & Francis; Boca Raton: 2011. pp. 95–124. [Google Scholar]
- 16.Sinha R, Kulldorff M, Gunter MJ, Strickland P, Rothman N. Dietary benzo(a)pyrene intake and risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2005;14:2030–4. doi: 10.1158/1055-9965.EPI-04-0854. [DOI] [PubMed] [Google Scholar]
- 17.Gunter MJ, Probst-Hensch NM, Cortessis VK, Kulldorff M, Haile RW, Sinha R. Meat intake, cooking-related mutagens and risk of colorectal adenoma in a sigmoidoscopy-based case–control study. Carcinogenesis. 2005;26:637–42. doi: 10.1093/carcin/bgh350. [DOI] [PubMed] [Google Scholar]
- 18.Knuckles ME, Inyang F, Ramesh A. Acute and subchronic oral toxicity of fluoranthene in F-344 rats. Ecotoxicol Environ Saf. 2004;59:102–8. doi: 10.1016/S0147-6513(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 19.Walker SA, Addai AB, Mathis M, Ramesh A. Effect of dietary fat on metabolism and DNA adduct formation after acute oral exposure of F-344 rats to fluoranthene. J Nutr Biochem. 2007;18:236–49. doi: 10.1016/j.jnutbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol. 2004;23:301–33. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- 21.Lobscheid AB, Maddalena RL, McKone TE. Contribution of locally grown foods in cumulative exposure assessments. J Expo Anal Environ Epidemiol. 2004;14:60–73. doi: 10.1038/sj.jea.7500306. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council [NRC] Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- 23.Ramesh A, Inyang F, Hood DB, Archibong AE, Knuckles ME, Nyanda AM. Metabolism, bioavailability, and toxicokinetics of benzo(a)pyrene in F344 rats following oral administration. Exp Toxic Pathol. 2001;53:275–90. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- 24.Walker SA, Whitten LB, Seals GB, Lee WE, Archibong AE, Ramesh A. Interspecies comparison of liver and small intestinal microsomal metabolism of fluoranthene. Food Chem Toxicol. 2006;44:380–7. doi: 10.1016/j.fct.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.NIH . Guidelines for the laboratory use of chemical carcinogens. National Institutes of Health, US Government Printing Office; Washington, DC: 1981. NIH Publication No. 81-2385. [Google Scholar]
- 26.Massaad L, de Waziers I, Ribrag V, Janot F, Beaune PH, Morizet J, et al. Comparison of mouse and human colon tumors with regard to phase I and phase II drug metabolizing enzyme systems. Cancer Res. 1992;52:6567–75. [PubMed] [Google Scholar]
- 27.Halberg RB, Larsen MC, Elmergreen TL, Ko AY, Irving AA, Clipson L, et al. Cyp1b1 exerts opposing effects on intestinal tumorigenesis via exogenous and endogenous substrates. Cancer Res. 2008;68:7394–402. doi: 10.1158/0008-5472.CAN-07-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uno S, Dragin N, Miller ML, Dalton TP, Gonzalez FJ, Nebert DW. Basal and inducible Cyp1 mRNA quantitation and protein localization throughout the mouse gastrointestinal tract. Free Radic Biol Med. 2008;44:570–83. doi: 10.1016/j.freeradbiomed.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattar A, Hewer A, Phillips DH, Campbell FC. Metabolic proficiency and benzo(a)pyrene DNA adduct formation in ApcMin mouse adenomas and uninvolved mucosa. Carcinogenesis. 1999;20:1097–101. doi: 10.1093/carcin/20.6.1097. [DOI] [PubMed] [Google Scholar]
- 30.Vaca C, Tornquist M, Rannug U, Lindahl-Kiessling K, Ahnstrom G, Ehrenberg L. On the bioactivation and genotoxic action of fluoranthene. Arch Toxicol. 1992;66:538–45. doi: 10.1007/BF01973383. [DOI] [PubMed] [Google Scholar]
- 31.Huderson AC, Harris DL, Niaz MS, Ramesh A. Effect of benzo(a)pyrene exposure on fluoranthene metabolism by mouse adipose tissue microsomes. Toxicol Mech Methods. 2010;20:53–8. doi: 10.3109/15376510903584677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buesen R, Mock M, Seidel A, Jacob J, Lampen A. Interaction between metabolism and transport of benzo[a]pyrene and its metabolites in enterocytes. Toxicol Appl Pharmacol. 2002;183:168–78. doi: 10.1006/taap.2002.9484. [DOI] [PubMed] [Google Scholar]
- 33.Myers JN, Rekhadevi PV, Ramesh A. Comparative evaluation of different cell lysis and extraction methods for studying benzo(a)pyrene metabolism in HT-29 colon cancer cell cultures. Cell Physiol Biochem. 2011;28:209–18. doi: 10.1159/000331732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uno S, Makishima M. Benzo(a)pyrene toxicity and inflammatory disease. Curr Rheumatol Rev. 2009;5:266–71. [Google Scholar]
- 35.Khalil A, Villard PH, Dao MA, Burcelin R, Champion S, Fouchier F, et al. Polycyclic aromatic hydrocarbons potentiate high-fat diet effects on intestinal inflammation. Toxicol Lett. 2010;196:161–7. doi: 10.1016/j.toxlet.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Weston A, Harris CC. Holland-Frei Cancer Medicine. 5th Hamilton, Ontario: 2000. Chemical carcinogenesis. [Google Scholar]
- 37.Chen JK, Wu ZL, Liu YG, Lei YX. Effects of metabolites of benzo(a)pyrene on unscheduled DNA synthesis in BALB/3T3 cell line. Chemosphere. 2000;41:139–42. doi: 10.1016/s0045-6535(99)00401-4. [DOI] [PubMed] [Google Scholar]
- 38.Piskorska-Pliszczynska J, Keys B, Safe S, Newman MS. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicol Lett. 1986;34:67–74. doi: 10.1016/0378-4274(86)90146-3. [DOI] [PubMed] [Google Scholar]
- 39.Willett KL, Gardinali PR, Sericano JL, Wade TL, Safe SH. Characterization of the rat hepatoma cell bioassay for evaluation of environmental samples containing polynuclear aromatic hydrocarbons (PAHs) Arch Environ Contam Toxicol. 1997;32:442–8. doi: 10.1007/s002449900211. [DOI] [PubMed] [Google Scholar]
- 40.Warshawsky D, Barkley W, Bingham E. Factors affecting carcinogenic potential of mixtures. Fundam Appl Toxicol. 1993;20:376–82. doi: 10.1006/faat.1993.1048. [DOI] [PubMed] [Google Scholar]
- 41.Babson JR, Russo-Rodriguez S, Rastetter WH, Wogan GN. Invitro DNA-binding of microsomally-activated fluoranthene: evidence that the major product is a fluoranthene N2—deoxyguanosine adduct. Carcinogenesis. 1986;7:859–65. doi: 10.1093/carcin/7.6.859. [DOI] [PubMed] [Google Scholar]
- 42.Hutchins DA, Skipper PL, Naylor S, Tannenbaum SR. Isolation and characterization of the major fluoranthene-hemoglobin adducts formed in vivo in the rat. Cancer Res. 1988;48:4756–61. [PubMed] [Google Scholar]
- 43.Hursting SD, Slaga TJ, Fischer SM, DiGiovanni J, Phang JM. Mechanism-based cancer prevention approaches: targets, examples, and the use of transgenic mice. J Natl Cancer Inst. 1999;91:215–20. doi: 10.1093/jnci/91.3.215. [DOI] [PubMed] [Google Scholar]
- 44.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 45.Harris DL, Washington MK, Hood DB, Roberts LJ, II, Ramesh A. Dietary fat-influenced development of colon neoplasia in ApcMin mouse exposed to benzo(a)pyrene. Toxicol Pathol. 2009;37:938–46. doi: 10.1177/0192623309351722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hakura A, Seki Y, Sonoda J, Hosokawa S, Aoki T, Suganuma A, et al. Rapid induction of colonic adenocarcinoma in mice exposed to benzo[a]pyrene and dextran sulfate sodium. Food Chem Toxicol. 2011;49:2997–3001. doi: 10.1016/j.fct.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 47.Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison tar and benzo(a)pyrene in a 2-year bioassay. Carcinogenesis. 1998;19:117–24. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 48.Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012 doi: 10.1007/s00535-011-0523-6. (in press) [DOI] [PubMed] [Google Scholar]
- 49.Pan MH, Lai CS, Wu JC, Ho CT. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res. 2011;55:32–45. doi: 10.1002/mnfr.201000412. [DOI] [PubMed] [Google Scholar]