Abstract
Introduction
Angiogenin is a member of the ribonuclease superfamily and promotes degradation of basement membrane and extracellular matrix. After stroke in Type one diabetes (T1DM) rats, Angiogenin is significantly increased and the Angiogenin is inversely correlated with functional outcome. Neamine, an aminoglycoside antibiotic, blocks nuclear translocation of Angiogenin, thereby abolishing the biological activity of Angiogenin. In this study, we therefore investigated the effect and underlying protective mechanisms of Neamine treatment of stroke in T1DM.
Methods
T1DM was induced in male Wistar rats by streptozotocin (60mg/kg, ip), and T1DM rats were subjected to embolic middle cerebral artery occlusion (MCAo). Neamine (10 mg/kg i.p.) was administered at 2h, 24h and 48h after induction of embolic MCAo. A battery of functional outcome tests was performed. Brain blood barrier (BBB) leakage, and lesion volume were evaluated and immunostaining, and Western blot were performed.
Results
Neamine treatment of stroke in T1DM rats significantly decreased BBB leakage and lesion volume as well as improved functional outcome compared to T1DM-control. Neamine also significantly decreased apoptosis and cleaved caspase-3 in the ischemic brain. Using immunostaining, we found that Neamine treatment significantly decreased nuclear Angiogenin, nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) activity, advanced glycation endproducts receptor (RAGE) number, the positive area of toll-like receptor 4 (TLR4) and increased Angeopoietin-1 expression compared to T1DM-MCAo control rats. Western blot results are consistent with the immunostaining.
Conclusion
Neamine treatment of stroke is neuroprotective in T1DM rats. Inhibition of neuroinflammatory factor expression and decrease of BBB leakage may contribute to Neamine induced neuroprotective effects after stroke in T1DM rats.
Keywords: Angiogenin, Neamine, neuroprotection, stroke, type one diabetes
Introduction
Stroke is a major cause of death and long-term disability with unusually high accompanying social and medical costs. Diabetes mellitus (DM) is a severe health problem associated with both microvascular and macrovascular disease and leads to 3–4 fold higher risk of experiencing ischemic stroke and arteriosclerosis (Mast et al., 1995). Ischemic stroke patients with type one diabetes (T1DM) or type two diabetes (T2DM) exhibit a distinct risk-factor, etiologic profile and a worse vascular prognosis than non-DM patients (Putaala et al., 2011). Treatment of stroke has historically focused on neuroprotection, which has yielded failed trials, except for the NINDS recombinant tissue plasminogen activator (rtPA) trial (Brott et al., 1998). However, even within 3 hours after stroke, tPA treatment of stroke in patients with DM induces an incremental risk of death and spontaneous intracerebral hemorrhage and unfavorable 90-day outcomes with increasing admission hyperglycemia (Alvarez-Sabin et al., 2003, Poppe et al., 2009). Studies by our group and several others have found that tPA treatment within 2 hours after stroke in T1DM rats significantly increases brain hemorrhage and blood-brain barrier (BBB) leakage and failed to improve functional outcome after stroke (Fan et al., 2012, Ning et al., 2012). Thus, there is a compelling need to develop therapeutic approaches to reduce neurological deficits after stroke in the DM population.
Previous studies have found that T1DM significantly increased vascular density, BBB leakage and cerebral hemorrhagic transformation after stroke (Ye et al., 2011a). Angiogenin expression was also increased in the ischemic brain of T1DM rats compared to wild type (WT) non-DM-MCAo rats (Chen et al., 2011). The increased Angiogenin expression is correlated with worse functional outcome and BBB leakage in T1DM stroke rats (Chen et al., 2011). Angiogenin is a small protein with ribonucleolytic activity and is a potent angiogenic factor implicated in angiogenesis and tumor growth (Strydom, 1998, Gao and Xu, 2008). Angiogenin degrades the basement membrane and extracellular matrix (ECM) thereby acting as a stimulus that promotes the invasion and migration of endothelial cells into the surrounding tissue towards the source of stimulus (Hu et al., 1994). Angiogenin also stimulates proliferation of human umbilical artery smooth muscle cells and is associated with inflammation and atherosclerosis (Xu et al., 2001). Levels of Angiogenin are inversely related with ejection fraction and correlated positively with coronary atheroma scores in left ventricular systolic dysfunction patients (Patel et al., 2009). Therefore, we hypothesize that inhibition of Angiogenin activity may provide a neuroprotective effect after stroke in T1DM stroke animals.
To inhibit Angiogenin activity, agents that block nuclear translocation of Angiogenin are a better choice than those that neutralize Angiogenin protein directly, because it is not necessary to neutralize all the circulating Angiogenin (Tsuji et al., 2005). The aminoglycoside antibiotic neomycin (Hu, 1998) has been shown to block nuclear translocation of Angiogenin thereby abolishing the biological activity of Angiogenin (Hirukawa et al., 2005). Neamine is less toxic than neomycin and is an effective inhibitor of nuclear translocation of Angiogenin and has been shown to serve as an inhibitor for Angiogenin-induced angiogenesis and cancer progression (Hirukawa et al., 2005, Tsuji et al., 2005, Ibaragi et al., 2009, Zhao et al., 2010). In this study, we investigate the effects of Neamine treatment of stroke in T1DM rats on functional outcome and neuroprotection.
Materials and Methods
All experiments were strictly conducted in accordance with the Henry Ford Hospital Institutional Animal Care and Use Committee.
Induction of diabetes in rats
Adult (2–3 months) Male Wistar rats (225–250g) purchased from Charles River (Wilmington, MA) were used in this experiment. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ, Sigma Chemical Co., St. Louis, MO) (60mg/kg dissolved in sodium citrate, 0.1mM, pH4.5) to rats (Cossel et al., 1985). The fasting blood glucose level from a tail vein sample was tested by using a glucose analyzer (Accu-Chek Compact System; Roche Diagnostics, Indianapolis, IN). Diabetes was defined by a fasting blood glucose >300mg/dl. Animals were subjected to stroke 2 weeks after diabetes induction.
Embolic middle cerebral artery occlusion (MCAo) model and experimental groups
T1DM rats were initially anesthetized with 4% isoflurane during induction and then maintained with 2% isoflurane in a mixture of 30% O2 and 70% N2O. Body temperature was monitored and maintained at 37°C using a feedback-regulated water heating system. Under the operating microscope (Carl Zeiss), the right common carotid artery (CCA), the right external carotid artery (ECA), and the internal carotid artery (ICA) were isolated via a midline incision. A modified PE-50 catheter with a 0.3 mm outer diameter was gently advanced from the ECA into the lumen of the ICA until the catheter is 2–3mm from the origin of the MCA. Using the catheter, a single clot (~0.8 µL) along with 2 to 3 µL of 0.9% saline was then gently injected. The catheter was withdrawn immediately after injection, and the right ECA was legated (Zhang et al., 1997). Rats were randomized and assigned to different groups and were treated starting 2 hours after embolic MCAo with:
T1DM-MCAo control: phosphate-buffered saline (PBS) for vehicle control in T1DM-MCAo rats (n=8).
T1DM-MCAo+Neamine treatment: Neamine (10mg/kg, ip injection) was injected in T1DM-MCAo rats (n=8). The dose used (i.e. 10 mg/kg i.p.) is well tolerated in mice (Hirukawa et al., 2005, Ibaragi et al., 2009), and converting to the rats, the Neamine dose of 10mg/kg represents a moderate dose for the rats. Therefore, the dose of 10mg/kg (ip injection) was employed at 2h, 24h and 48h after induction of embolic MCAo. A battery of functional outcome tests was performed before MCAo and at 2h, 24h and 48h after induction of embolic MCAo.
Functional tests
A battery functional tests including Foot-fault test, adhesive removal test and a modified neurological severity score (mNSS) test were performed prior to MCAo, and at 2h (before treatment), 24h, 48h after induction of embolic MCAo by an investigator who was blinded to the experimental groups, mNSS is a composite of motor, sensory, balance and reflex tests (Chen et al., 2001a, Chen et al., 2001b). Rats were excluded if modified neurological severity score was <6 or >13 before treatment. The foot-fault test evaluates placement dysfunction of forelimbs (Barth et al., 1990, Schallert et al., 2000). In addition, the adhesive removal test was performed both pre- and postoperatively (Schallert et al., 1982).
Histological and immunohistochemical assessment
At 2 days after induction of embolic MCAo, animals were sacrificed and brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. Seven coronal sections of tissue were processed and stained with hematoxylin and eosin (H&E) for calculation of volume of cerebral infarction (Swanson et al., 1990). The indirect lesion area, in which the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere, was calculated using the Global Lab Image analysis system (Data Translation, Malboro, MA) (Swanson et al., 1990). Lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere.
Blood pressure (BP) and rectal temperature measurements
At 2 days after induction of embolic MCAo, rats (n=4/group) were anesthetized with 2% isoflurane in a jar for pre anesthetic, and spontaneously respired with 1.5% isoflurane in 2:1 N2O:O2 mixture using a facemask connected and regulated with a modified FLUOTEC 3 Vaporizer (Fraser Harlake, Orchard Park, NY 14127). Intra-arterial blood pressure was measured at 2h after last treatment by the insertion of a Mikro-tip pressure catheter (AD Instruments, Colorado Springs, CO, USA) into the internal carotid artery. Arterial pressure was measured using a DC amplifier (AD Instruments) and mean arterial pressure (MAP) and heart rate (HR) were determined using a PowerLab Data Acquisition System (AD Instruments) averaged over 20–30 minutes. Rectal temperature was recorded using a rectal probe (AD instruments).
Quantitative evaluation of Evans blue dye extravasation
To test blood brain barrier (BBB) permeability and Western blot assay, an additional set of rats (n=4/group) were sacrificed at 48h after induction of embolic MCAo. 2% Evans blue dye in saline was injected intravenously at 4h before sacrifice. Evans blue dye fluorescence intensity was measured using a microplate fluorescence reader (excitation 620nm and emission 680nm). The amount of extravasated Evans blue dye was quantified as micrograms per ischemic hemisphere (Zhang et al., 2002).
Immunohistochemical staining
A standard paraffin block was obtained from the center of the lesion (bregma −1 mm to +1 mm). A series of 6 εm thick sections were cut from the block. Every 10th coronal section for a total 5 sections was used for immunohistochemical staining. Antibody against cleaved caspase-3 (rabbit polyclonal IgG; dilution 1:200, Cell Signaling Technology, Danvers, Massachusetts), advanced glycation end products (RAGE, 1:400; Dako, Carpenteria, CA, USA), toll-like receptor 4 (TLR-4) (goat polyclonal IgG; dilution 1:100; Cruz Biotech Inc, Santa Cruz, California), nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) (1:500; Abcam, Cambribridge, MA, USA), Angiogenin (monoclonal, 1:500, Abcam Cambridge, MA) and Angiopoietin-1 (Ang1) (Ang1, 1:2,000, Abcam) immunostaining were performed. Control experiments consisted of staining brain coronal tissue sections as outlined above, but the primary antibodies were omitted, as previously described (Li et al., 1998). The immunostaining analysis was performed by an investigator blinded to the experimental groups.
Terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) for measuring apoptosis was performed using a commercial kit (Apop Tag kit, Chemicon, S7100).
Immunostaining quantitation
For measurement of apoptotic markers, each cleaved caspase-3 and TUNEL immunostained coronal section was digitized using a 40X objective, via the MCID computer imaging analysis system (Imaging Research, St. Catharines, Canada). In order to minimize the potential effect of infarct volume reduction on the tested parameters, five sections from the standard reference coronal section and 8 brain fields within each section were acquired and the total number of cleaved caspase-3 and TUNEL positive cells in the 8 fields of the ischemic border area (IBZ), adjacent to the ischemic core, were counted using the MCID computer imaging analysis system. The total number of positive cells per mm2 area is presented.
For quantitative measurements of Angiogenin, Angiopoietin-1 (Ang1), NFkB, RAGE and TLR-4, five slides from each brain, with each slide containing 8 fields from the IBZ were digitized under a 40x or 20x objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Calza et al., 2001, Chen et al., 2003a, Chen et al., 2003b). Data were analyzed in a blinded manner and presented as percentage of positive area for Ang1, RAGE and TLR-4, percentage number of nuclear positive cells for Angiogenin and NFkB, respectively.
Nuclear and Cytoplasmic Extraction
Nuclear extracts were isolated using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) following standard protocol. Briefly, 40mg of tissue was weighed and resuspended in buffer and a Dounce homogenizer was used to break up the tissue. Afterwards the tissue was treated with lysis reagents for 1min on ice before they were centrifuged (16,000g), providing the cytoplasmic extracts in the supernatant. The pellet was then subjected to a second stage of lysis while being stored on ice for 40min, vortexing for 15sec every 10min. Afterwards the samples were centrifuged (16000g) for 10min and supernatant (nuclear extract) was removed.
Western blot assay
Protein was isolated from samples using Trizol (Invitrogen), following standard protocol. Protein concentration was measured using the BCA (Thermo Scientific) kit. 40ug of protein/lane in a 10% SDS PAGE precast gel (Invitrogen). Gel was transferred to a nitrocellulose membrane (Bio Rad) by running the transfer at 400mA for two hours. Nitrocellulose membrane was blocked in 2% I-Block (Applied Biosystems) in 1× TBS-T for one hour, and then either Ang1 (Abcam 1:1000), Ang (Abcam, 1:1000), RAGE (R&D Biosystems, 1:500), TLR4 (Santa Cruz, 1:500), or NFkB (Abcam, 1:1000) primary antibodies were added in 2% I- Block in TBS-T, and incubated on a shaker overnight at 4°C. Following morning, the membrane was washed three times for 5 minutes with 1× TBS-T. Secondary antibodies, (anti-mouse, anti-rabbit, or anti-rat, (Jackson ImmunoResearch) was added at 1:1000 dilution in 2% I-Block in 1 × TBS-T on a room temperature shaker for one hour. After the incubation the membranes were washed three times for 5 minutes with 1 × TBS-T. After the final wash, Luminol Reagent (Santa Cruz) was added and allowed to react with the membranes for 2 minutes. The membranes were then developed using a FluorChem E Imager system (ProteinSimple) exposing them for 1 – 30 minutes depending on the intensity of the band
Statistical analysis
One-way Analysis of Variance (ANOVA) was used for the evaluation of functional outcome and histology, respectively. “Contract/estimate” statement was used to test the group difference. Spearman partial correlation coefficient analysis was employed for the correlation between functional tests and histology evaluations at two days after induction of embolic MCAo. If an overall treatment group effect was detected at p<0.05, pair-wise comparisons were made. All data are presented as mean ± standard error (SE).
Results
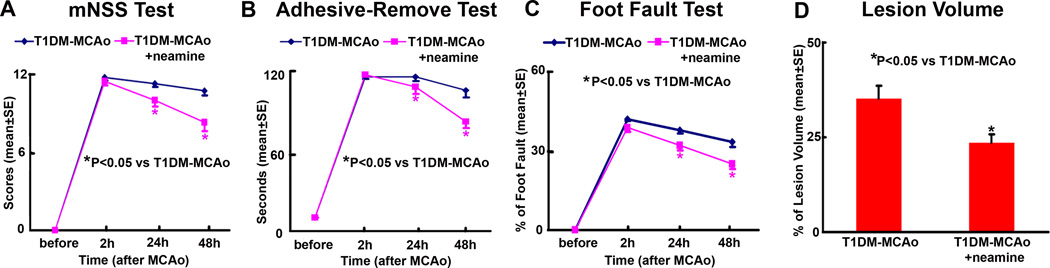
Neamine significantly decreases lesion volume and improves functional outcome after stroke in T1DM rats (Figure 1)
Figure 1. Functional tests and lesion volume measurements.
Neamine treatment of stroke in T1DM rats significantly improved functional outcome and reduces lesion volume compared to PBS treated T1DM-MCAo rats. A: mNSS test. B: Adhesive removal test. C: Foot-fault test. D: Lesion volume measurement.
To test whether Neamine regulates blood pressure (BP) and body temperature (BT), BP and BT were measured at 2h after last treatment before sacrifice. The non-treatment control group recorded a mean systolic/diastolic pressure of 120/97mmHg±3.6mmHg with mean heart rate of 365±13 BPM, while the treatment group recorded a mean systolic/diastolic pressure of 123/90mmHg±4.2mmHg with mean heart rate of 350±11 BPM. The control group exhibited body temperatures of 36±1°C on day 2 after induction of embolic MCAo, while the Neamine treatment group exhibited 36.7±0.3°C on day 2 after induction of embolic MCAo. There are no significant differences in BP, heart rate and rectal temperature between Neamine treatment and non-treatment control (p>0.05)
To test whether Neamine treatment induces a neuroprotective effect, a battery of functional outcomes and lesion volume were measured. Figure 1 shows that Neamine treatment starting 2h after induction of embolic MCAo significantly decreased lesion volume and improved functional outcome after stroke in T1DM rats measured by mNSS, foot-fault and adhesive removal test compared to PBS treated T1DM-MCAo control rats (p<0.05). The data suggest that Neamine treatment promotes neuroprotection after stroke in T1DM rats.
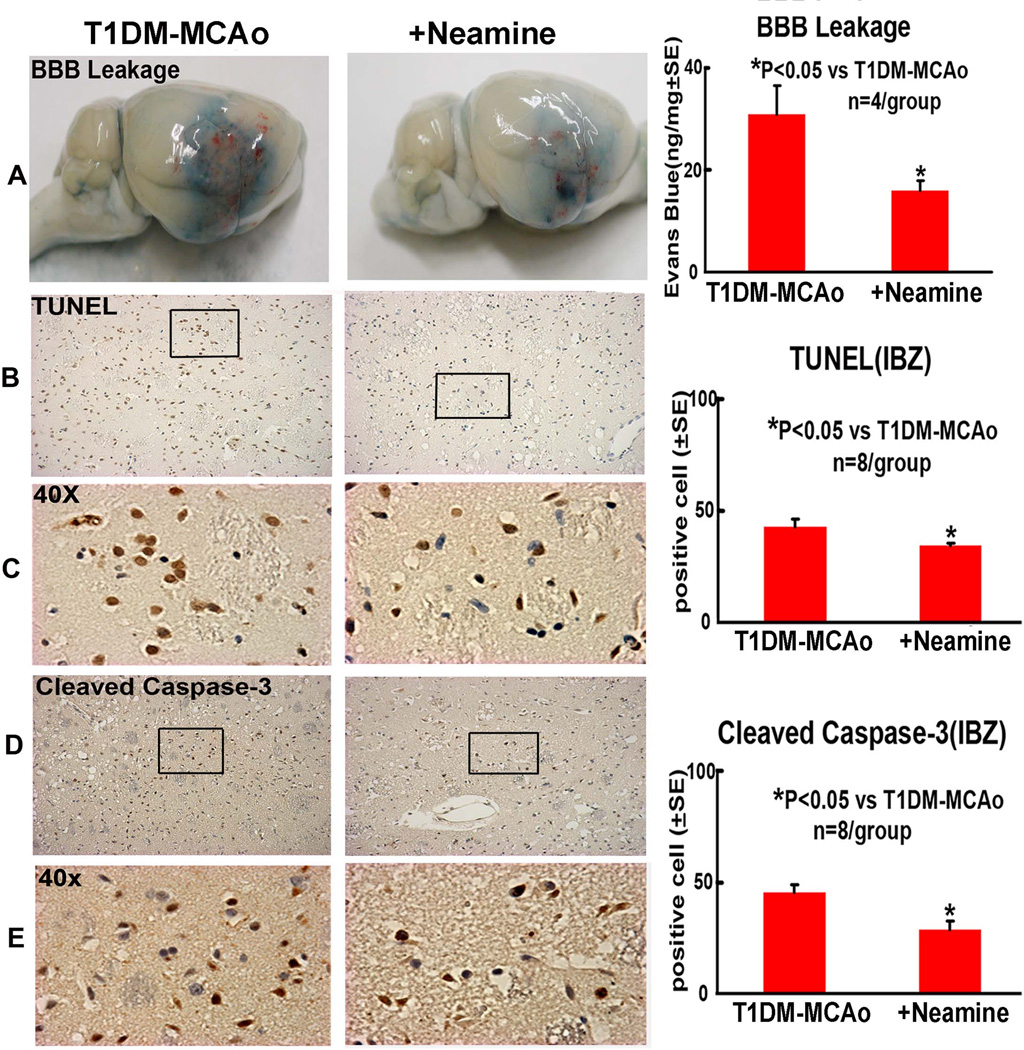
Neamine treatment decreases BBB leakage and the expression of TUNEL and cleaved caspase-3 (Figure 2)
Figure 2. BBB leakage and apoptosis changes in the ischemic brain.
Neamine treatment of stroke in T1DM rats significantly decreased BBB leakage and apoptotic cell numbers in the ischemic brain compared to PBS treated T1DM-MCAo rats. A: Evans blue dye assay for BBB leakage. B-E: TUNEL and cleaved caspase-3 immunostaining and quantitative data in the IBZ.
To test whether Neamine regulates BBB leakage, Evans blue dye assay was employed. Figure 2A shows that Neamine treatment of stroke in T1DM rats significantly decreased BBB leakage in the ischemic brain compared to PBS treated T1DM-MCAo control rats (Figure 2A).
To test whether Neamine treatment regulates apoptosis, two apoptotic markers, TUNEL and cleaved caspase-3, were employed. Figure 2B–E show that Neamine treatment significantly decreased the number of immunoreactive TUNEL-positive cells and cleaved- caspase-3 in the ischemic brain in T1DM rats compared to PBS treated T1DM-MCAo control rats (p<0.05). These data indicate that Neamine treatment has an anti-apoptotic role after stroke in T1DM rats.
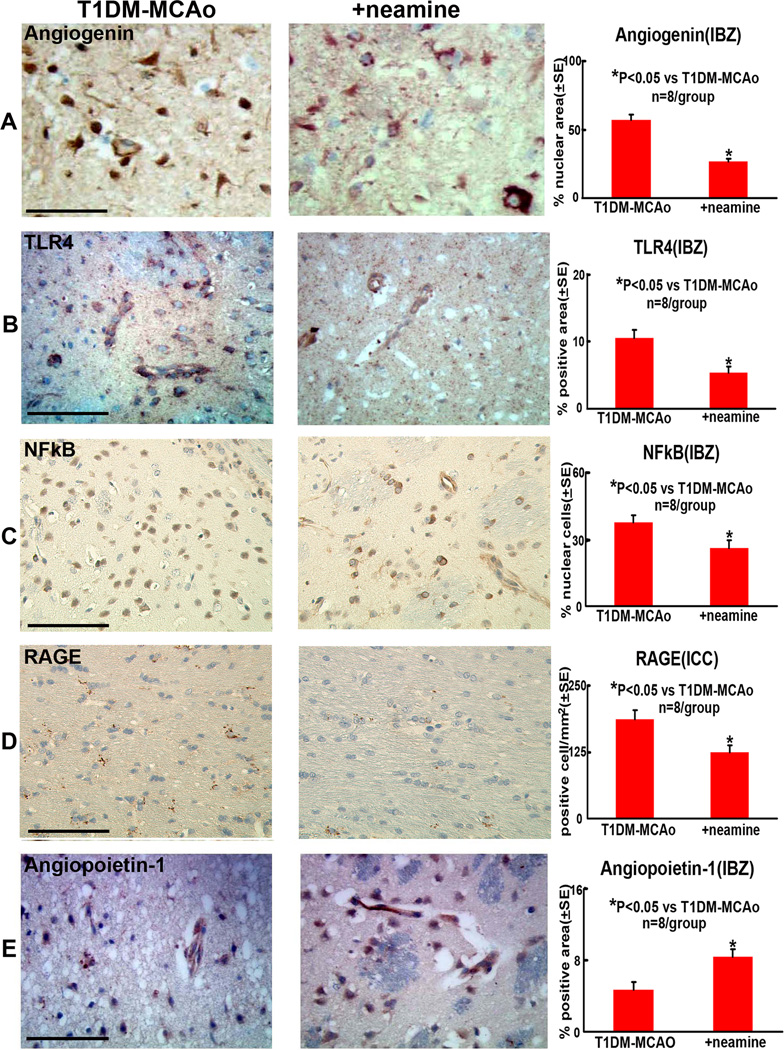
Neamine treatment attenuates expression of RAGE, TLR-4, Angiogenin and NFkB activity and increases Ang1 expression in the ischemic brain (Figure 3A–E)
Figure 3. Angiogenin, Angiopoietin-1, TLR4, NFkB and RAGE expression in the ischemic brain.
Neamine treatment of stroke in T1DM rats significantly increased angiopoietin-1 and decreased TLR4, RAGE, nuclear Angiogenin and NFkB expression in the ischemic brain compared to PBS treated T1DM-MCAo rats. A-E: Angiogenin, TLR4, NFkB, RAGE and Angiopoietin-1, immunostaining and quantitative data in IBZ. Scale bar in A-E=0.1 mm.
To further investigate the molecular mechanisms underlying the Neamine-induced neuroprotection after stroke, RAGE, TLR-4, NFkB and Ang1 expression were measured in the ischemic border. Using immunostaining, Figures 3A–E show that Neamine treatment significantly decreased TLR4 and RAGE expression and increased Ang1 expression compared to PBS T1DM-MCAo control rats (p<0.05). Neamine treatment also significantly decreased activity (nuclear location) of Angiogenin and NFkB compared to PBS treated T1DM-MCAo control rats.
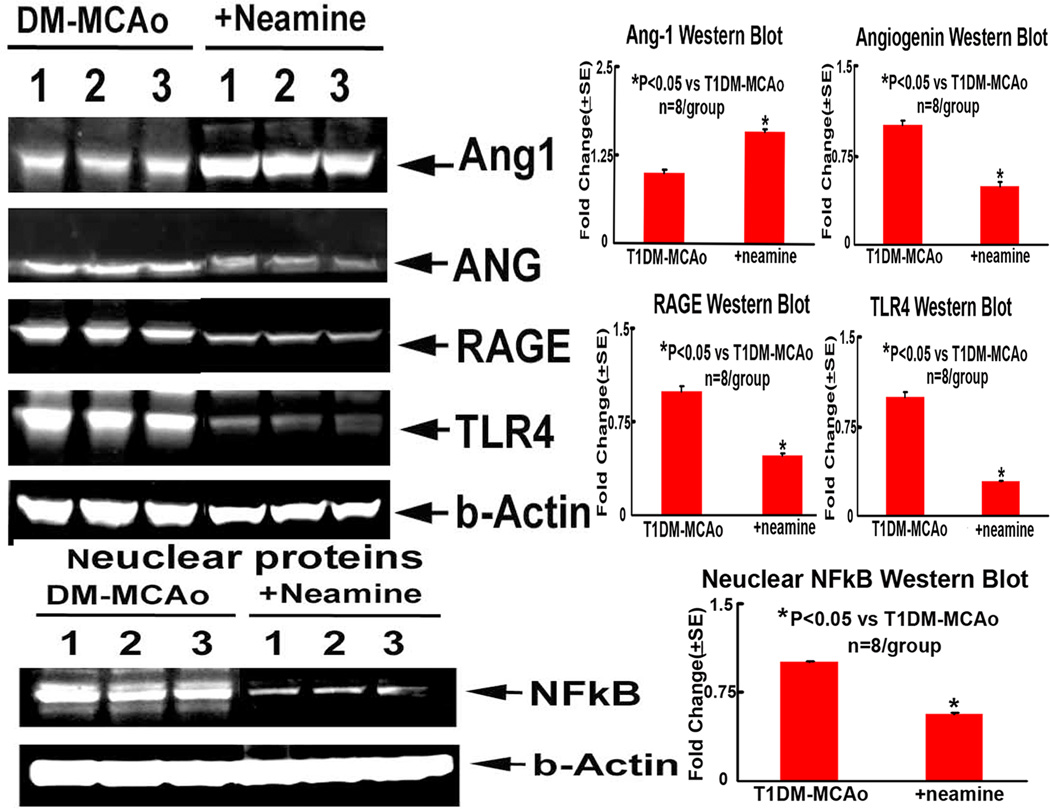
Consistent with immunostaining, Western blot assay also showed that Neamine treatment of T1DM-MCAo rats significantly decreased TLR4, RAGE, Angiogenin and nuclear NFkB and increased Ang1 expression in the ischemic brain compared to PBS treated T1DM-MCAo control rats (Figure 4). These data suggest that Neamine treatment in T1DM-MCAo rats decreases inflammatory mediators in the ischemic brain.
Figure 4. Western blot assay.
Neamine treatment of stroke significantly increased brain tissue Ang-1, but decreased Angiogenin, NFkB, RAGE and TLR4 levels in T1DM rats compared to non-treatment T1DM-MCAo rats (P<0.05). A: Western blot assay; B-E: Ang1 (B), Angiogenin (C), RAGE (D) and TLR4 (E) quantitative data from Western blot assay; F-G: Nuclear NFkB activity measured by Western blot assay (F) and quantitative data (G).
Discussion
In the present study, we demonstrated that treatment of stroke with Neamine in T1 diabetic rats improves functional outcome and significantly decreases brain infarct volume, BBB leakage and the expression of inflammatory mediators RAGE,NFkB, and TLR4 as well as increases Ang1 expression in the ischemic brain. Inhibition of neuroinflammatory factor expression and decrease of BBB leakage may contribute to Neamine induced neuroprotection effects after stroke in T1DM rats.
Neamine decreases cleaved caspase-3 and apoptosis
Cerebral ischemia leads to neuronal damage through complex and interconnected pathophysiological events. Apoptosis is an important contributor to cell death after stroke. Diabetes stroke animals have significantly increased apoptosis compared to no-DM stroke animals (Rizk et al., 2005). Neuronal apoptosis is an important contributing factor to neurological deficiencies associated with diabetes, and these deficiencies are exaggerated following ischemic stroke (Rizk et al., 2006). Apoptosis and inflammation play key roles in the delayed cell death and injury in the penumbra (Becker, 1998, Graham and Chen, 2001). In our previous studies have found that diabetes significantly increases RAGE, TLR4, NFkappaB and Angiogenin expression and increases neuroinflammatory effects in the ischemic brain (Chen et al., 2001b, Ye et al., 2011b). Neuroinflammatory factors upregulate apoptosis (Ding et al., 2013). Downregulation of TLR4/NFkappaB activity inhibits apoptosis and inflammation induced by cerebral ischemic reperfusion injury and improves the neurobehavioral function of rat (Hua et al., 2007, Guo et al., 2010). In the present study, we found that Neamine treatment significantly decreases the neuroinflammatroy factors such as RAGE, TLR4 expression and NFkappaB activity. Therefore, decreases of neuroinflammatoryy factors may contribute to the Neamine treatment induced decrease of apoptosis after stroke in T1DM rats. We also found that Neamine treatment significantly decreased cleaved caspase-3 as well as decreased lesion volume in the ischemic brain. Caspases, a family of cysteinyl-aspartate family, are essential players in apoptotic cell death after focal cerebral ischemia (Endres et al., 1998, Schulz et al., 1999). Administration of broad spectrum caspase inhibitors to ischemic rodents induced neuroprotection (Rabuffetti et al., 2000). Caspase-3 is the most abundant caspase family member in the adult rodent brain (Namura et al., 1998). Neurons are the predominant brain cells that undergo caspase-3-dependent apoptosis after hypoxia-ischemia (Manabat et al., 2003). Therefore, a decrease in cleaved-caspase-3 by Neamine treatment may play an important role in attenuating apoptosis and decreasing lesion volume after stroke in T1DM rats.
Neamine decreases Angiogenin nuclear translocation and attenuates BBB leakage
Angiogenin is a member of the ribonuclease superfamily and promotes degradation of basement membrane and extracellular matrix, allowing endothelial cells to proliferate, penetrate and migrate into the perivascular tissue (Hu et al., 1997). Diabetes is associated with abnormal angiogenesis which may lead to vascular abnormalities of various organs (Stitt et al., 2005). Our previous studies have found that Angiogenin expression was increased in the ischemic brain in T1DM rats compared to wild type (WT) non-DM-MCAo rats (Chen et al., 2011) and the increased Angiogenin was correlated with worse functional outcome in T1DM rats (Chen et al., 2011). In this study, we found that Neamine treatment of stroke in T1DM rats significantly decreases Angiogenin nuclear translocation in the endothelial cell, as well as decreases BBB leakage and improves functional outcome after stroke compared to non-treatment T1DM rats. Angiogenin is rapidly endocytosed and translocated to the endothelial cell nucleus where it accumulates in the nucleolus and binds to the promoter region DNA (Hu et al., 2000) and stimulates ribosomal RNA (rRNA) transcription (Xu et al., 2003, Kishimoto et al., 2005). Nuclear translocation is the key for Angiogenin to exert its biological activity and is a critical step in the process of angiogenesis (Moroianu and Riordan, 1994a, b). Decreasing angiogenin endothelial cell nuclear translocation may contribute to the decreased BBB leakage.
Neamine decreased RAGE, TLR4 and NFkB activity
The neuroinflammatory response is progressive after stroke and leads to the evolution of brain tissue injury (Barone and Feuerstein, 1999) and is associated with vascular disease (Chamorro and Hallenbeck, 2006). In addition, inflammation also plays a key role in the development and progression of diabetic complications. Previous studies have found that diabetic stroke animals significantly increased inflammatory factors high-mobility group box 1(HMGB-1) and its receptors, such as RAGE, and TLR4 expression in the ischemic brain (Ye et al., 2011b). RAGE has been implicated as a pro-inflammatory factor in chronic inflammatory conditions such as diabetes mellitus and it functions as a sensor of necrotic cell death and contributes to inflammation and ischemic brain damage. TLR4, as one of the receptors of HMGB1, is important in the activation of the innate immune system that contributes to inflammation and may increase neuroinflammation and exacerbate stroke injury. Inflammatory response, which is tightly regulated by the TLR4/NFkB pathway, promotes brain damage after cerebral ischemia-reperfusion (I/R) injury, Activation of inflammatory receptors results ultimately in the activation of NFkB, inducing the up-regulation of leukocyte adhesion molecules, production of pro-inflammatory cytokines and angiogenic factors in both hematopoietic and endothelial cells, thereby promoting inflammation (Nogueira-Machado and de Oliveira Volpe, 2012). NFkB, a pivotal player in inflammatory responses is induced in neurons during human stroke. Activation of NFkB in the brain contributes to infarction in permanent MCAo (pMCAO) (Nurmi et al., 2004). The resident inflammatory brain cells, microglia, are especially activated in response to ischemic insults, many of which are regulated by NFkB. Therefore, suppression of RAGE/TLR4/NF-κB signaling has become a promising target for the anti-inflammatory treatment in ischemic stroke (Ceulemans et al., 2010). We found that Neamine treatment of stroke in T1DM rats significantly decreased inflammatory factors such as RAGE and TLR4 expression in the ischemic brain as well as inhibits NFkB activity. Therefore, decreasing RAGE/TLR4/NFkB signaling activity may mediate the neuroprotective effect of Neamine after stroke in T1DM rats.
Neuroinflammatory response has been associated with the deleterious effects of increased temperature during the acute phase of stroke (Ceulemans et al., 2010). Hypothermia has been introduced as a promising neuroprotective strategy (Joseph et al., 2012). Long-term hypothermia may be a viable clinical approach to protect the brain from cerebral injury (Joseph et al., 2012). Hypothermia decreases inflammatory factor gene and protein expression and promotes antiinflammatory effects as well as decreases lesion volume after stroke (Florian et al., 2008, Meybohm et al., 2010, Jenkins et al., 2012, Gu et al., 2013). In the present study, using Neamine, an aminoglycoside antibiotic, treatment of stroke in T1DM rats did not significantly decrease body temperature, but significantly decreases inflammatory factor expression. Therefore, decrease of neuroinflammatory mediators may contribute to the Neamine treatment induced decrease of apoptosis after stroke in T1DM rats.
Angiopoietin-1 signaling promotes angiogenesis and remodeling of blood vessels through its receptor tyrosine kinase Tie2, which is expressed on blood vessel endothelial cells. Angiopoietin-1 reduces endothelial permeability and enhances vascular stabilization and maturation, and stimulates the mural cell recruitment process (Ye et al., 2007). Diabetic stroke animals showed a significantly fdecreased Ang1 expression and Ang1/Tie2 signaling activity as well as increased pericyte damage and BBB leakage that induce worse functional outcome after stroke compared to non-DM animals (Cui et al., 2011). Ang1/Tie2 signaling system not only regulates angiogenesis, but also regulates anti- inflammatory effects. Ang1-Tie2 blocks lipopolysaccharide (LPS)-induced activation of NFkB in macrophages (Gu et al., 2010). Ang1 induces antiinflammatory response by inhibition of endothelial permeability, and neutrophil adherence to endothelial cell (Pizurki et al., 2003). Ang1 also decreases VEGF induced NF-kappaB transcription factor, vascular cell adhesion molecule-1 (VCAM-1), and E-selectin expressions (Ismail H, Mofarrahi, 2012). In this study, we found that treatment of stroke in T1DM rats with Neamine significantly increased ischemic brain Ang1 expression, decreased BBB leakage as well as promoted anti-inflammatory effects identified by the decreased expression of proinflammatory factors and activity in the ischemic brain. Therefore, increasing Ang1 expression may also contribute to Neamine induced neuroprotective effects post stroke in the T1DM rat.
Limitation and caveat
Neamine, an aminoglycoside antibiotic, and has many effects. It not only has antibacterial potency, but also inhibits nuclear translocation of Angiogenin and inhibits tumor cell proliferation, migration, and has anti-tumor effects (Ibaragi et al., 2009, Zhao et al., 2010). In this study, we for the first time demonstrate that Neamine treatment also promotes neuroprotection after stroke in T1DM rats as well as decreases neuroinflammatory factor expression. However, the underlying mechanisms by which Neamine induces neuroprotective effects warrant further study. In the present study, the functional outcome and lesion volume were only measured at 48h after MCAo, and long term functional outcome after Neamine treatment warrant investigation.
Conclusion
We found that Neamine treatment of stroke significantly decreases lesion volume, BBB leakage and promotes functional outcome after stroke in T1DM rats. Neamine induces anti-inflammatory effects identified by the decrease in RAGE, TLR4 and NFkB activity in the ischemic brain as well as an increase Ang1 expression. Increasing Ang1 and anti-inflammatory effects may contribute to Neamine induced neuroprotective effects after stroke in T1DM rats.
Highlights.
Neamine treatment of stroke decreases BBB leakage, lesion volume in T1DM rats.
Neamine treatment of stroke improves functional outcome in T1DM rats.
Neamine treatment of stroke increases Ang1 and decreases Angiogenin, NFkB, RAGE, and TLR4 expression in T1DM rats.
Decreased inflammatory response may contribute to Neamine induced neuroprotection effects on T1DM rats.
Acknowledgments
Sources of Funding:
This work was supported by National Institute on Aging Grants AG031811 (JC), AG037506 (MC) and 1R41NS080329 (JC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, Codina A, Quintana M. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator--treated patients. Stroke; a journal of cerebral circulation. 2003;34:1235–1241. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Barth TM, Grant ML, Schallert T. Effects of MK-801 on recovery from sensorimotor cortex lesions. Stroke; a journal of cerebral circulation. 1990;21:III153–III157. [PubMed] [Google Scholar]
- Becker KJ. Inflammation and acute stroke. Curr Opin Neurol. 1998;11:45–49. doi: 10.1097/00019052-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Brott T, Lu M, Kothari R, Fagan SC, Frankel M, Grotta JC, Broderick J, Kwiatkowski T, Lewandowski C, Haley EC, Marler JR, Tilley BC. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29:1504–1509. doi: 10.1161/01.str.29.8.1504. [DOI] [PubMed] [Google Scholar]
- Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. Journal of neuroinflammation. 2010;7:74. doi: 10.1186/1742-2094-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke; a journal of cerebral circulation. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. Journal of the neurological sciences. 2001a;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke; a journal of cerebral circulation. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, Cui Y, Zacharek A, Roberts C, Liu X, Dai X, Lu M, Chopp M. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke; a journal of cerebral circulation. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circulation research. 2003a;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Annals of neurology. 2003b;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Cossel L, Schneider E, Kuttler B, Schmidt S, Wohlrab F, Schade J, Bochmann C. Low dose streptozotocin induced diabetes in mice. Metabolic, light microscopical, histochemical, immunofluorescence microscopical, electron microscopical and morphometrical findings. Exp Clin Endocrinol. 1985;85:7–26. doi: 10.1055/s-0029-1210415. [DOI] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiology of disease. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HS, Yang J, Chen P, Yang J, Bo SQ, Ding JW, Yu QQ. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene. 2013;527:389–393. doi: 10.1016/j.gene.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, Hyman BT, Moskowitz MA. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- Fan X, Qiu J, Yu Z, Dai H, Singhal AB, Lo EH, Wang X. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke; a journal of cerebral circulation. 2012;43:567–570. doi: 10.1161/STROKEAHA.111.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian B, Vintilescu R, Balseanu AT, Buga AM, Grisk O, Walker LC, Kessler C, Popa-Wagner A. Long-term hypothermia reduces infarct volume in aged rats after focal ischemia. Neuroscience letters. 2008;438:180–185. doi: 10.1016/j.neulet.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Gao X, Xu Z. Mechanisms of action of angiogenin. Acta biochimica et biophysica Sinica. 2008;40:619–624. doi: 10.1111/j.1745-7270.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- Graham SH, Chen J. Programmed cell death in cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Gu H, Cui M, Bai Y, Chen F, Ma K, Zhou C, Guo L. Angiopoietin-1/Tie2 signaling pathway inhibits lipopolysaccharide-induced activation of RAW264.7 macrophage cells. Biochemical and biophysical research communications. 2010;392:178–182. doi: 10.1016/j.bbrc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Gu LJ, Xiong XX, Ito T, Lee J, Xu BH, Krams S, Steinberg GK, Zhao H. Moderate Hypothermia Inhibits Brain Inflammation and Attenuates Stroke-Induced Immunodepression in Rats. CNS neuroscience & therapeutics. 2013 doi: 10.1111/cns.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xu X, Li Q, Li Z, Du F. Anti-inflammation effects of picroside 2 in cerebral ischemic injury rats. Behavioral and brain functions. 2010;BBF6:43. doi: 10.1186/1744-9081-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirukawa S, Olson KA, Tsuji T, Hu GF. Neamine inhibits xenografic human tumor growth and angiogenesis in athymic mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:8745–8752. doi: 10.1158/1078-0432.CCR-05-1495. [DOI] [PubMed] [Google Scholar]
- Hu G, Riordan JF, Vallee BL. Angiogenin promotes invasiveness of cultured endothelial cells by stimulation of cell-associated proteolytic activities. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:12096–12100. doi: 10.1073/pnas.91.25.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Xu C, Riordan JF. Human angiogenin is rapidly translocated to the nucleus of human umbilical vein endothelial cells and binds to DNA. J Cell Biochem. 2000;76:452–462. doi: 10.1002/(sici)1097-4644(20000301)76:3<452::aid-jcb12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hu GF. Neomycin inhibits angiogenin-induced angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9791–9795. doi: 10.1073/pnas.95.17.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu GF, Riordan JF, Vallee BL. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, Li C. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. Journal of neuroimmunology. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaragi S, Yoshioka N, Li S, Hu MG, Hirukawa S, Sadow PM, Hu GF. Neamine inhibits prostate cancer growth by suppressing angiogenin-mediated rRNA transcription. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:1981–1988. doi: 10.1158/1078-0432.CCR-08-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DD, Rollins LG, Perkel JK, Wagner CL, Katikaneni LP, Bass WT, Kaufman DA, Horgan MJ, Languani S, Givelichian L, Sankaran K, Yager JY, Martin RH. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1888–1896. doi: 10.1038/jcbfm.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph C, Buga AM, Vintilescu R, Balseanu AT, Moldovan M, Junker H, Walker L, Lotze M, Popa-Wagner A. Prolonged gaseous hypothermia prevents the upregulation of phagocytosis-specific protein annexin 1 and causes low-amplitude EEG activity in the aged rat brain after cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1632–1642. doi: 10.1038/jcbfm.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–456. doi: 10.1038/sj.onc.1208223. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke; a journal of cerebral circulation. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. discussion 1980-1971. [DOI] [PubMed] [Google Scholar]
- Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke. 2003;34:207–213. doi: 10.1161/01.STR.0000047101.87575.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast H, Thompson JL, Lee SH, Mohr JP, Sacco RL. Hypertension and diabetes mellitus as determinants of multiple lacunar infarcts. Stroke; a journal of cerebral circulation. 1995;26:30–33. doi: 10.1161/01.str.26.1.30. [DOI] [PubMed] [Google Scholar]
- Meybohm P, Gruenewald M, Zacharowski KD, Albrecht M, Lucius R, Fosel N, Hensler J, Zitta K, Bein B. Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Critical care. 2010;14:R21. doi: 10.1186/cc8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Nuclear translocation of angiogenic proteins in endothelial cells: an essential step in angiogenesis. Biochemistry. 1994a;33:12535–12539. doi: 10.1021/bi00208a001. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proceedings of the National Academy of Sciences of the United States of America. 1994b;91:1677–1681. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning R, Chopp M, Yan T, Zacharek A, Zhang C, Roberts C, Cui X, Lu M, Chen J. Tissue plasminogen activator treatment of stroke in type-1 diabetes rats. Neuroscience. 2012;222:326–332. doi: 10.1016/j.neuroscience.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Machado JA, de Oliveira Volpe CM. HMGB-1 as a target for inflammation controlling. Recent patents on endocrine, metabolic & immune drug discovery. 2012;6:201–209. doi: 10.2174/187221412802481784. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke; a journal of cerebral circulation. 2004;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- Patel JV, Abraheem A, Chackathayil J, Gunning M, Creamer J, Hughes EA, Lip GY. Circulating biomarkers of angiogenesis as indicators of left ventricular systolic dysfunction amongst patients with coronary artery disease. J Intern Med. 2009;265:562–567. doi: 10.1111/j.1365-2796.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. British journal of pharmacology. 2003;139:329–336. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD Canadian Alteplase for Stroke Effectiveness Study I. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes care. 2009;32:617–622. doi: 10.2337/dc08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaala J, Liebkind R, Gordin D, Thorn LM, Haapaniemi E, Forsblom C, Groop PH, Kaste M, Tatlisumak T. Diabetes mellitus and ischemic stroke in the young: clinical features and long-term prognosis. Neurology. 2011;76:1831–1837. doi: 10.1212/WNL.0b013e31821cccc2. [DOI] [PubMed] [Google Scholar]
- Rabuffetti M, Sciorati C, Tarozzo G, Clementi E, Manfredi AA, Beltramo M. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:4398–4404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk NN, Rafols J, Dunbar JC. Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain research. 2005;1053:1–9. doi: 10.1016/j.brainres.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain research. 2006;1096:204–212. doi: 10.1016/j.brainres.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, Vaughn D, Wilcox RE. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacology, biochemistry, and behavior. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Weller M, Moskowitz MA. Caspases as treatment targets in stroke and neurodegenerative diseases. Annals of neurology. 1999;45:421–429. doi: 10.1002/1531-8249(199904)45:4<421::aid-ana2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Stitt AW, McGoldrick C, Rice-McCaldin A, McCance DR, Glenn JV, Hsu DK, Liu FT, Thorpe SR, Gardiner TA. Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes. 2005;54:785–794. doi: 10.2337/diabetes.54.3.785. [DOI] [PubMed] [Google Scholar]
- Strydom DJ. The angiogenins. Cellular and molecular life sciences : CMLS. 1998;54:811–824. doi: 10.1007/s000180050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Sun Y, Kishimoto K, Olson KA, Liu S, Hirukawa S, Hu GF. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer research. 2005;65:1352–1360. doi: 10.1158/0008-5472.CAN-04-2058. [DOI] [PubMed] [Google Scholar]
- Xu Z, Monti DM, Hu G. Angiogenin activates human umbilical artery smooth muscle cells. Biochemical and biophysical research communications. 2001;285:909–914. doi: 10.1006/bbrc.2001.5255. [DOI] [PubMed] [Google Scholar]
- Xu ZP, Tsuji T, Riordan JF, Hu GF. Identification and characterization of an angiogenin-binding DNA sequence that stimulates luciferase reporter gene expression. Biochemistry. 2003;42:121–128. doi: 10.1021/bi020465x. [DOI] [PubMed] [Google Scholar]
- Ye L, Haider H, Jiang S, Tan RS, Toh WC, Ge R, Sim EK. Angiopoietin-1 for myocardial angiogenesis: a comparison between delivery strategies. European journal of heart failure. 2007;9:458–465. doi: 10.1016/j.ejheart.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Ye X, Chopp M, Cui X, Zacharek A, Cui Y, Yan T, Shehadah A, Roberts C, Liu X, Lu M, Chen J. Niaspan enhances vascular remodeling after stroke in type 1 diabetic rats. Experimental neurology. 2011a;232:299–308. doi: 10.1016/j.expneurol.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Chopp M, Liu X, Zacharek A, Cui X, Yan T, Roberts C, Chen J. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011b;190:339–345. doi: 10.1016/j.neuroscience.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain research. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang YC, Yang LY, Yu DH, Pan PT, Wang L. Neamine inhibits cell proliferation, migration, and invasion in H7402 human hepatoma cells. Saudi medical journal. 2010;31:1309–1314. [PubMed] [Google Scholar]