Abstract
A significant clinical need exists to differentiate human pluripotent stem cells (hPSCs) into cardiomyocytes, enabling tissue modeling for in vitro discovery of new drugs or cell-based therapies for heart repair in vivo. Chemical and mechanical microenvironmental factors are known to impact efficiency of stem cell differentiation, but cardiac differentiation protocols in hPSCs are typically performed on rigid tissue culture polystyrene (TCPS) surfaces which do not present a physiological mechanical setting. To investigate the temporal effects of mechanics on cardiac differentiation, we cultured human embryonic stem cells (hESCs) and their derivatives on polyacrylamide hydrogel substrates with a physiologically relevant range of stiffnesses. In directed differentiation and embryoid body culture systems, differentiation of hESCs to cardiac Troponin T-expressing (cTnT+) cardiomyocytes peaked on hydrogels of intermediate stiffness. Brachyury expression also peaked on intermediate stiffness hydrogels at day 1 of directed differentiation, suggesting that stiffness impacted the initial differentiation trajectory of hESCs to mesendoderm. To investigate the impact of substrate mechanics during cardiac specification of mesodermal progenitors, we initiated directed cardiomyocyte differentiation on TCPS and transferred cells to hydrogels at the Nkx2.5/Isl1+ cardiac progenitor cell stage. No differences in cardiomyocyte purity with stiffness were observed on day 15. These experiments indicate that differentiation of hESCs is sensitive to substrate mechanics at early stages of mesodermal induction, and proper application of substrate mechanics can increase the propensity of hESCs to differentiate to cardiomyocytes.
Keywords: Human embryonic stem cells, cardiomyocytes, differentiation, substrate mechanics
1. Introduction
Cues from the mechanical microenvironment play a critical role in many cell fate decisions. Adherent cells interact with their substrate through integrin adhesion receptors, and they are capable of sensing the stiffness of their substrate and responding through alterations in contractility, spreading, and migration [1, 2]. In recent years, several studies have demonstrated the influence of physiologically-relevant (1–100 kPa) substrate stiffness on differentiation of mesenchymal stem cells and neural stem cells [3, 4] and maintenance of mouse and human pluripotent stem cells [5–8]. Less is known about the effects of stiffness on differentiation of pluripotent stem cells to specific lineages.
As heart failure is the leading cause of death in the United States, there is great interest in deriving cardiomyocytes from human pluripotent stem cells (hPSCs) to explore novel strategies for heart repair [9, 10]. Early methods for differentiation of human embryonic stem cells (hESCs) [11] and human induced pluripotent stem cells (hiPSCs) [12, 13] to cardiomyocytes employed embryoid bodies (EBs), spherical aggregates formed in suspension culture with serum [14, 15]. Recent defined methods involve application of small molecules or proteins to monolayers of hPSCs to efficiently direct differentiation to cardiomyocytes [16–19].
EB and monolayer-based differentiation to cardiomyocytes typically occur on rigid tissue culture polystyrene (TCPS) surfaces, which are not representative of physiological tissue mechanics in the developing or adult heart. Culture systems which incorporate elements representing the mechanical microenvironment of the heart may benefit cardiogenesis. For example, applying mechanical strain to mimic cyclic contractions increased expression of early cardiac markers in mouse ESCs [20]. A hydrogel designed to stiffen 9-fold, representing the transition from mesoderm tissue to myocardium, enhanced maturation of chicken pre-cardiac cells [21]. In contrast, the absence of biophysical signals is detrimental for cardiogenesis; blocking blood flow and its resultant shear stresses led to phenotypic defects in zebrafish hearts [22]. These examples illustrate that developing cardiomyocytes can sense mechanical cues, and their temporal presentation is significant.
In this study, we used polyacrylamide hydrogels to present physiologically-relevant (4–80 kPa) substrate stiffnesses to hESCs subjected to directed differentiation, EBs, and cardiac progenitor cells (CPCs), and we quantified effects of stiffness on cardiomyocyte purity in each culture context. Taken together, our results demonstrate that hESC differentiation to mesendoderm progenitors is sensitive to mechanical modulation, but cardiac progenitor differentiation to cardiomyocytes is not affected by matrix mechanics during multiple distinct cardiac differentiation protocols.
2. Experimental
2.1 hESC maintenance
For embryoid body experiments, tissue culture polystyrene (TCPS) 6-well plates (Corning) were coated with 0.1% gelatin (Sigma) and irradiated mouse embryonic fibroblasts (MEFs) were seeded at a density of 19,500 cells/cm2 in MEF medium. MEF medium consisted of DMEM supplemented with 10% heat-inactivated FBS and 1% MEM non-essential amino acid solution (all components from Life Technologies). hESCs (H9 or H9-hTnnTZ-pGZ-D2 cTnT reporter) were passaged onto the feeder layers every 5 days by exposure to 1 mg/mL collagenase type IV (Life Technologies) in DMEM/F12 (Life Technologies) for 3 minutes at 37°C, followed by mechanical dissociation and centrifugation. hESCs were maintained in UM/F+, which consisted of DMEM/F12 culture medium supplemented with 20% KnockOut serum replacer (Life Technologies), 1% MEM non-essential amino acid solution, 1 mM L-glutamine (Life Technologies), 0.1 mM β-mercaptoethanol (Sigma) and 4 ng/mL human recombinant bFGF (Waisman Biomanufacturing).
For directed differentiation experiments, TCPS 6-well plates were coated with 8.3 µg/cm2 growth factor reduced Matrigel (BD Biosciences) by resuspending 0.5 mg of Matrigel in 6 mL cold DMEM/F12, adding 1 mL to each well of a 6-well plate, and incubating overnight at 37°C. hESCs (H9) were passaged every 4 days by exposure to Versene (Life Technologies) for 3 minutes at 37°C, followed by mechanical dissociation. hESCs were maintained in mTeSR1 medium (STEMCELL Technologies).
2.2 Polyacrylamide hydrogel substrate fabrication and characterization
Polyacrylamide (PA) substrates were fabricated as previously described, with minor modifications [17]. Stock solutions of 10% acrylamide (Acros Organics) and 0.03–0.6% bisacrylamide (Fisher) in deionized water were generated and stored at 4°C in amber glass vials. Prior to polymerization, aliquots of each stock solution were brought to room temperature and degassed under vacuum. Polymerization was initiated by 1:100 addition of 5% (w/v) ammonium persulfate (APS, Fisher) in deionized water and 5% (v/v) N, N, N’, N’, - tetramethyletylenediamine (TEMED, Sigma) in deionized water. 2500 µL of pre-polymer was pipetted onto the inverted lid of a glass petri dish (Pyrex) and covered with its base. Both faces of the glass petri dish which contacted the pre-polymer were coated with Rain-X (ITW Global Brands), and 1 mm PDMS (Dow Corning) spacers were employed to control gel thickness. After 75 minutes, polymerization was halted by flooding each dish with 50 mM HEPES (Sigma) buffer, pH 8.5. Gels were allowed to swell in HEPES buffer for 1–3 days before continuing.
Circular gels with 1.59 cm diameters were generated from the polymer slabs using a punch cutter (McMaster-Carr). To functionalize the gels for protein adhesion, 60 µL of 1 mM N-sulfosuccinimidyl- 6-[4’-azido-2’-nitrophenylamino] (Sulfo-SANPAH, Pierce) in HEPES buffer was dried onto all gel surfaces in a 60°C oven for 1.5 hours. Gels were exposed to UV light (OmniCure) at 365 nm, 90 mW/cm2 for 2 minutes. The Sulfo-SANPAH addition, drying, and UV exposure steps were repeated once. Gels were transferred to individual wells of 12-well plates, hydrated in PBS, and exposed to germicidal UV light for 20 minutes to sterilize. Gels were coated with 0.6 µg/cm2 fibronectin (Life Technologies) or 8.3 µg/cm2 growth factor reduced Matrigel at 37°C overnight. If not used the next day, gels were transferred to 4°C. Methods for characterizing the elastic moduli of these hydrogels were previously reported [17] and are duplicated here.
Pre-polymer of each bis-acrylamide concentration was prepared as described above, and 400 µL of pre-polymer was pipetted to fill a dogbone-shaped Teflon mold. Glass beads with diameters of 30–50 µm (Polysciences, Inc.) were sprinkled over the pre-polymer to allow for optical strain measurement during the test, and the pre-polymer was covered with a polyethylene terephthalate transparency film. After 75 minutes of polymerization, the mold was disassembled and samples were stored in HEPES buffer for 1–3 days before mechanical testing to allow the gels to reach hydrostatic equilibrium.
Prior to testing, additional glass beads with diameters of 30–50 µm were adhered to the surface of the samples. The samples were tested in an Instron 5548 MicroTester mechanical testing machine. The samples were secured using self-aligning grips with an abrasive surface at either end to prevent slipping of the sample during testing. The displacement rate of the test was 1 mm/min, which correlated to a strain rate of approximately 0.0025 sec−1. This rate was fast enough that evaporation of the surrounding PBS was negligible, but slow enough to reduce inertial and viscous effects. A 10 N load cell was used to measure load data at a rate of 1 Hz. The entire system was placed on a pneumatic air table to eliminate noise caused by environmental vibrations.
A temperature-controlled environmental chamber was used during the tests to match in vivo conditions as closely as possible. The temperature during the tests was maintained at a constant 37°C via a water jacket surrounding the chamber. The samples were also fully hydrated prior to testing and submerged in PBS during the test to simulate the salinity that the gels would typically experience in vivo. Corrections were made to account for the buoyancy of the submerged portions of the testing apparatus. A “buoyancy test” was conducted after each real test to measure the amount of buoyant force that the Instron experienced. This was done by simply removing the sample and running an identical test without any tension between the grips.
Due to the compliant nature of the samples, an optical strain measurement technique was chosen in which the relative displacements of small glass beads embedded within the material or on its surface were measured and used to calculate the strain experienced by the samples. Previous studies have shown that embedding beads within the samples has no effect on the measured modulus of the samples, provided that the beads are small enough, comprise below 1% of the volume, and are evenly distributed within the material [23]. Time-lapse microscopy was used to observe the locations of the beads at designated increments during the tensile test. By measuring the vertical (axial) distance between pairs of beads during these increments, the strains at these times were calculated with Matlab using particle-tracking software developed by Prof. John C. Crocker of the University of Pennsylvania.
The rectangular cross-sections of the samples were measured before and after testing. Before the test, they were measured in multiple places using calipers. After the test, cross-sections were cut from the neck region of the samples and measured optically using a 1.25× microscope objective. Both tests resulted in nearly identical cross-sectional areas, which were then used to calculate stress data. The elastic modulus for each sample is the slope of its stress vs. strain curve.
2.3 Directed differentiation of hESCs to cardiomyocytes with GiWi protocol
A previously described protocol was employed for directed differentiation with slight modifications for hydrogel culture of hESCs [18, 24]. hESCs were dissociated by exposure to Accutase (Innovative Cell Technologies) for 5 minutes at 37°C to generate single cells. Cells were seeded onto Matrigel-coated polyacrylamide hydrogels on day −3 at a density of 2.5 × 105 cells within a Teflon Raschig ring with 10.5 mm inner diameter to restrict cell attachment (seeding density ~2.9 × 105 cells/cm2) in mTeSR1 medium supplemented with 5 µM Y27632 ROCK inhibitor (Tocris). Cells were maintained in mTeSR1 until reaching confluency. On day 0, medium was exchanged with RPMI/B27 without insulin supplemented with 10–12 µM CHIR99021 (Selleck). 24 hours later, medium was exchanged with RPMI/B27 without insulin. On day 3, medium was exchanged with RPMI/B27 without insulin supplemented with 5 µM IWP4 (Stemgent). On day 5, medium was exchanged with RPMI/B27 without insulin. On day 7 and every 3 days following, medium was exchanged with RPMI/B27. In some experiments, media were supplemented with 1% Antibiotic-Antimycotic fluid (A-A, Life Technologies). This protocol is known as GiWi since it employs a Gsk3 inhibitor (CHIR99021) and a Wnt inhibitor (IWP4) for cardiac differentiation.
For directed differentiation on TCPS, hESCs were seeded onto Matrigel-coated TCPS at a density of 1.0–1.25 × 105 cells/cm2 in mTeSR1 supplemented with 5 µM Y27632. Four days later, differentiation was initiated as described above.
2.4 Embryoid body formation
hESCs were passaged onto low-density MEFs (~13,000 cells/cm2) on day −5 and maintained in UM/F+. On day −4 through day −1, UM/F+ was supplemented with 2 µM 6- bromoindirubin-3’-oxime (BIO, Sigma). On day 0, hPSCs were exposed to 1 mg/mL dispase (Life Technologies) in DMEM/F12 for 10 minutes at 37°C. An equal volume of UM/F- was added, and colonies were removed from the plate with gentle pipetting and pooled into a conical tube. UM/F- consisted of DMEM/F12 culture medium supplemented with 20% KnockOut serum replacer, 1% MEM non-essential amino acid solution, 1 mM L-glutamine, and 0.1 mM β- mercaptoethanol. Colonies were rinsed with UM/F- three times, settling by gravity for 5 minutes between each rinse. Colonies were evenly distributed into ultra-low attachment 6-well plates (Corning) in UM/F-. The following day medium was exchanged with EB20, which consisted of DMEM/F12 culture medium supplemented with 20% fetal bovine serum (FBS, Life Technologies, lot tested for cardiogenic potential), 1% MEM non-essential amino acid solution, 1 mM L-glutamine, and 0.1 mM β-mercaptoethanol. Colonies transitioned into embryoid bodies (EBs) during maintenance in suspension with no medium change until day 5.
2.5 Embryoid body culture on polyacrylamide hydrogels
Fibronectin-coated polyacrylamide hydrogels were washed with PBS, and a Teflon Raschig ring with 10.5 mm inner diameter (Sigma) was placed onto the center of each well to restrict EB attachment to the gels. 150 µL of fresh EB20 was added to each ring. Day 5 EBs were pooled into a conical tube, excess medium was aspirated, and 150 µL of old EB20 + EBs was added to each ring (about 25–50 EBs). The following day, 200 µL of EB20 was added to each ring. The following day, the rings were removed and medium was exchanged with EB20. Medium was exchanged daily with EB20 through day 10. Starting from day 11, medium was exchanged every other day with EB2. EB2 consisted of DMEM/F12 culture medium supplemented with 2% FBS (lot tested for cardiogenic potential), 1% MEM non-essential amino acid solution, 1 mM L-glutamine, and 0.1 mM β-mercaptoethanol. EB20 and EB2 were supplemented with 1% A-A. Beating EBs were counted using a 4× microscope objective on days 9, 12, 15, 20, and 30 of culture.
2.6 Cardiac progenitor cell culture on polyacrylamide hydrogels
Directed differentiation of hESCs to cardiomyocytes was begun on TCPS using the GiWi protocol. On day 6 of differentiation, cardiac progenitor cells (CPCs) were dissociated by exposure to Accutase for 5 minutes at 37°C. Cells were resuspended in RPMI + 20% FBS + 1% A-A + 5 µM Y27632 and seeded onto TCPS or gels at a density of 1.0 × 105 cells/cm2. Gels were seeded with a 100 µL drop of cell suspension and flooded with 1 mL/well medium 45 minutes later in order to restrict cell attachment to the hydrogels. The next day and every 3 days following, medium was exchanged with RPMI/B27 + 1% A-A.
2.7 Flow cytometry
Cells were washed twice with PBS and singularized with Accutase (< day 15 cells) or 0.25% trypsin-EDTA (>/= day 15 cells, Life Technologies) for 5 minutes at 37°C. Cells were fixed with 1% paraformaldehyde (Electron Microscopy Sciences) for 15 minutes at 37°C and permeabilized with ice cold 90% methanol (Fisher) and stored at −20°C. 2.0 × 105 cells were aliquotted per sample, and samples were washed twice with FACS buffer. FACS buffer consisted of 0.5% bovine serum albumin (BSA, Sigma) and 0.1% Triton-X 100 (Fisher) in PBS. Samples were stained with primary antibodies overnight at 4°C, washed once with FACS buffer, and stained with secondary antibodies at 1:1000 for 30 minutes at room temperature. Antibodies used are listed in Supplementary Table 1. Samples were washed once with FACS buffer and filtered through nylon mesh with 37 µm square openings (Small Parts) into flow tubes (Beckton, Dickinson, and Company, BD). Samples were analyzed on a FACSCalibur flow cytometer (BD) with Cell Quest software, with samples stained only with secondary antibody serving as negative controls. Data were analyzed using FlowJo software.
2.8 Immunocytochemistry and image analysis
Cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) for 15 minutes at room temperature. Cells were stored at 4°C in PBS. Cells were permeabilized with 0.4% Triton-X 100 (Fisher) in PBS for 1 hour at room temperature, and then blocked with 5% non-fat dry milk (Bio-Rad) in PBS for 1 hour at room temperature. Cells were stained with primary antibodies in 0.4% Triton X-100 in PBS overnight at 4°C. Antibodies used are listed in Supplementary Table 1. Cells were washed 4 times in PBS, then stained with secondary antibodies at 1:1000 in 0.4% Triton X-100 in PBS for 1 hour at room temperature. Cells were washed 3 times in PBS, and nuclei were stained with Hoechst 33342 (Life Technologies) at 1:5000 in PBS for 5 minutes at room temperature. Prior to imaging, cells were inverted onto a glass coverslip containing one drop of SlowFade Gold antifade reagent (Life Technologies) and sandwiched with another glass coverslip on top. Images were collected on a Nikon A1R confocal microscope with a 20× objective.
To characterize the area and sum of H9 cTnT-GFP+ regions, cells were fixed and stained as described above. Whole-well images were collected on a Nikon Eclipse fluorescent microscope with a 4× objective. Images were processed by employing the “analyze particles” feature in ImageJ. All regions greater than 250 µm2 were included in our analysis.
2.9 Statistical analysis
Statistical significance was determined using one-way or two-way ANOVA followed by post-hoc Tukey tests. Comparisons with p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) were determined to be significant. Statistics were performed for all comparisons; when statistics are not shown, the comparison was determined to be not significant (p > 0.05). All error bars represent SEM.
3. Results
3.1 Small molecule-induced directed differentiation to cardiomyocytes peaked at an intermediate stiffness
Initial experiments employed a protocol developed for efficient directed differentiation of hESCs to cardiomyocytes by temporal modulation of canonical Wnt signaling [18, 24]. We singularized hESCs and seeded them onto Matrigel-coated polyacrylamide hydrogels of varying elastic modulus (Table 1) or TCPS. The seeding density of 2.9 × 105 cells/cm2 resulted in consistent attachment efficiency across all hydrogel stiffnesses, as measured by cell counts 1 day after seeding (Supplemental Fig. 1A). We expanded the cells for an additional 2 days in mTeSR1 medium to achieve multilayer structures prior to initiating differentiation, with similar cell densities present on all hydrogel stiffnesses at this time (Supplemental Fig. 1B). We employed the GiWi small-molecule based cardiac differentiation protocol, activating Wnt signaling with the Gsk3 inhibitor CHIR99021 at day 0 and inhibiting Wnt signaling at day 3 with IWP4 [18, 24]. Beating was first observed between days 7–9 and plateaued at day 12. Because the cells beat as coordinated sheets rather than individual foci, it was not possible to quantify the extent of spontaneous contraction in these cultures. Differentiation efficiency was instead quantified by flow cytometry as the fraction of cells expressing cTnT at day 15. When comparing the different hydrogel stiffnesses relative to one another, cTnT expression was greatest on the 50 kPa hydrogel, and was significantly higher than on the 4 and 80 kPa hydrogels (Fig. 1A, Supplementary Fig. 2). When comparing the hydrogel stiffnesses relative to TCPS, cTnT expression of cells on TCPS was significantly higher than on the 4, 20, and 80 kPa hydrogels. Immunocytochemistry of H9-derived cardiomyocytes revealed organized sarcomeres on hydrogels at all hydrogel stiffnesses, with α-actinin bands perpendicular to cardiac Troponin I (cTnI) (Fig. 1B).
Table 1.
Elastic moduli of polyacrylamide hydrogels used in this study. All hydrogels were 10% acrylamide and bisacrylamide content varied from 0.03–0.5%. Values shown are average +/− SEM (n = 6–13).
| Bisacrylamide (%) | E (kPa) |
|---|---|
| 0.03 | 4.4 ±0.7 |
| 0.1 | 18.4 ± 0.8 |
| 0.3 | 49.4 ± 3.1 |
| 0.5 | 76.0 ± 5.8 |
Fig. 1.
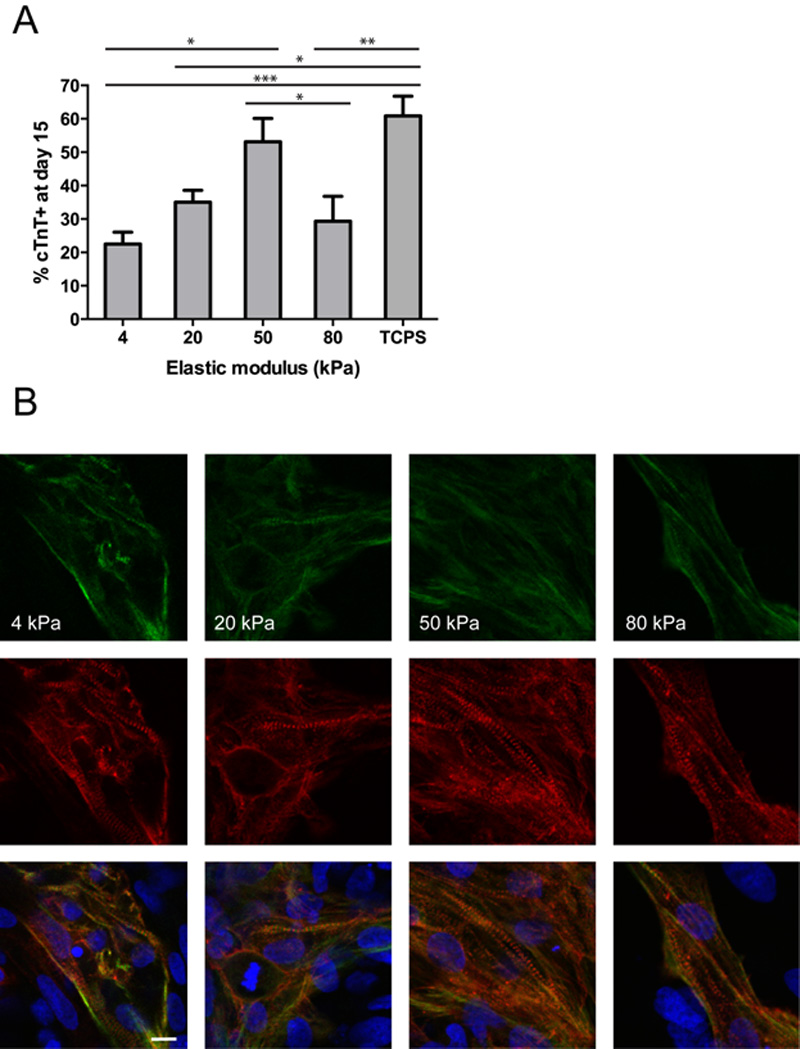
Cardiomyocyte differentiation on TCPS and polyacrylamide substrates of different stiffness during directed cardiomyocyte differentiation. (A) H9 cells were seeded onto hydrogels or TCPS on day −3, subjected to directed differentiation with the GiWi protocol starting from day 0, and fixed on day 15. cTnT expression on the 50 kPa hydrogel was significantly higher than cTnT expression on the 4 (p < 0.01) and 80 kPa (p < 0.05) hydrogels. cTnT expression on TCPS was significantly higher than cTnT expression on the 4 (p < 0.001), 20 (p < 0.01), and 80 kPa (p < 0.05) hydrogels. n = 9 for all hydrogel stiffnesses (3 wells each from 3 independent experiments) and n = 6 for TCPS (3 wells each from 2 independent experiments). (B) Representative images depicting organized sarcomeres in H9-derived cardiomyocytes on hydrogels of the indicated stiffness on day 15. Green = cardiac Troponin I, red = α-actinin, and blue = nuclei (Hoechst 33342). Scale bar = 10 µm.
3.2 Differentiation of EBs to cardiomyocytes peaked at intermediate stiffness
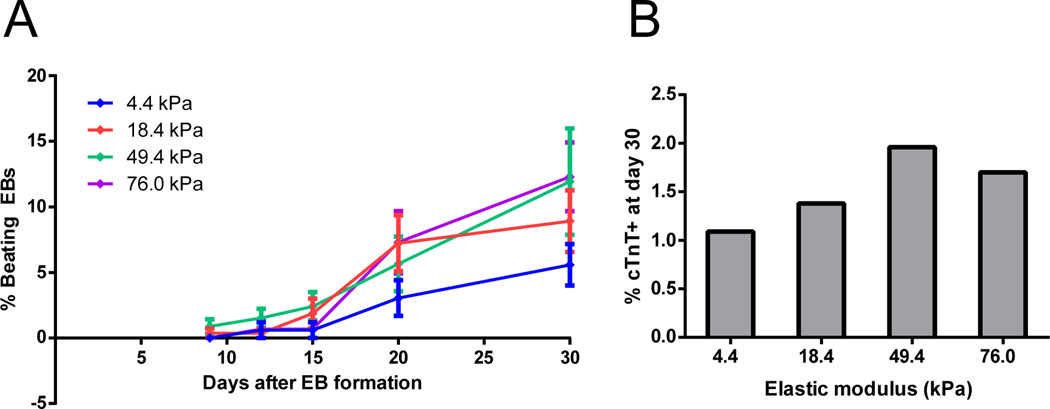
To address whether substrate mechanics similarly impacted cardiomyocyte generation in another differentiation context, we employed the classical embryoid body (EB) method for cardiac differentiation. We harvested hESC colonies with dispase treatment and cultured them in suspension in EB20 medium, which includes DMEM/F12 basal medium and 20% fetal bovine serum (FBS), for 5 days to form EBs. On day 5, EBs were seeded onto fibronectin-coated polyacrylamide hydrogels of varying elastic modulus. Supplementary Fig. 3 shows the morphology of representative EBs plated on hydrogels of different stiffness. We visually monitored EBs throughout differentiation to quantify the percentage of EBs containing regions that spontaneously contracted, indicative of cardiomyocyte differentiation. The percentage of contracting EBs appeared greatest on 50 and 80 kPa hydrogels, reaching a maximum of ~12% beating EBs at day 30 (Fig. 2A). On day 30, we dissociated the EBs and performed flow cytometry for cardiac Troponin T (cTnT), a protein expressed in cardiomyocytes. The percentage of cells expressing cTnT appeared to reach a maximum on the 50 kPa hydrogel, suggesting that beating areas may be larger or enriched in cardiomyocytes on this stiffness as compared to the 80 kPa hydrogel (Fig. 2B).
Fig. 2.
Timecourse of cardiomyocyte differentiation in EBs cultured on polyacrylamide substrates of different stiffness. (A) H9-derived EBs were cultured in suspension for 5 days in DMEM/F12 basal media, then seeded onto polyacrylamide hydrogels. The baseline number of EBs attached was counted on day 8, and beating regions were counted on days 9, 12, 15, 20, and 30. The percentage of beating EBs at each day represents the number of beating EBs on each day normalized to the number attached in the same well on day 8. 6 wells at each elastic modulus were averaged, with each well containing 129–210 EBs. (B) Flow cytometric analysis of cTnT expression on day 30. EBs from 6 wells containing hydrogels at each stiffness were pooled together to ensure that each sample contained sufficient cells for flow cytometry, thus the data represent an average % cTnT+ for EBs on each stiffness.
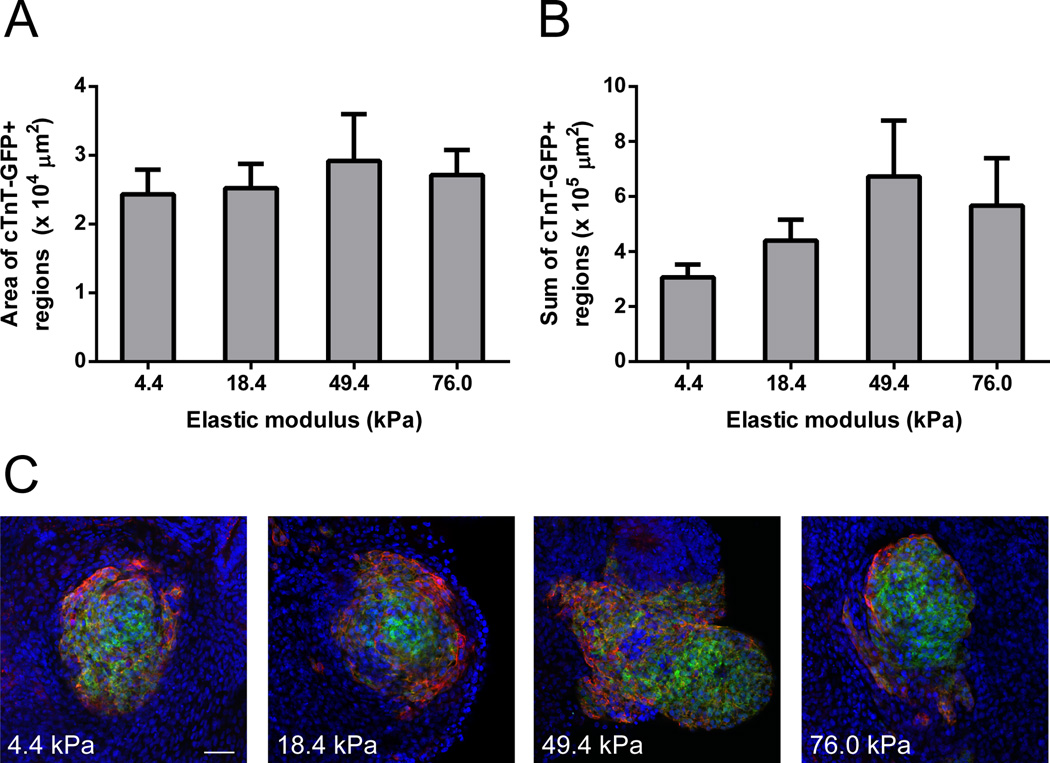
To characterize the morphology of cardiomyocyte regions in EBs cultured on hydrogels with different elastic moduli, we generated EBs from H9-hTnnTZ-pGZ-D2 cells, which express GFP under control of the cTnT promoter. On day 30, we fixed these cells and performed whole-well imaging to quantify the average area of cTnT-GFP+ regions and the average sum of cTnTGFP+ regions for each well. Both average area (Fig. 3A) and average sum (Fig. 3B) of cTnT-GFP+ regions in each well appeared greater on the 50 kPa hydrogel than on softer and stiffer hydrogels, suggesting that the 50 kPa stiffness generates larger and more numerous cardiomyocyte regions. Representative images depicting this trend are presented in Fig. 3C, where EBs were stained with α-actinin to show its co-localization with cTnT-GFP.
Fig. 3.
Quantification and representative images of cTnT+ regions in EBs derived from H9- hTnnTZ-pGZ-D2 cells. (A) Average area and (B) average sum of cTnT-GFP+ regions present on hydrogels of the indicated stiffnesses. EBs were seeded onto the hydrogels on day 5 and fixed on day 30. Whole-well images were obtained and analyzed using ImageJ to quantify GFP+ regions. Data from 2 independent experiments were averaged together (n = 3 wells/stiffness in experiment 1, n = 6 wells/stiffness in experiment 2). (C) Representative images of EBs on each stiffness on day 30. EBs were stained with α-actinin (red) to show its co-localization with cTnT-GFP (green). Nuclei stained with Hoechst 33342 are shown in blue. Scale bar = 50 µm.
Basal medium has been shown to impact efficiency of EB formation, and RPMI 1640 is generally preferred over DMEM/F12 in directed cardiomyocyte differentiation protocols [16–19, 24, 25]. Thus, we also generated EBs in RPMI 1640 + 20% FBS to test the dependence of our observations of a preferred intermediate stiffness for cardiomyocyte differentiation in EBs on basal medium. The percentage of beating EBs and the size of cTnT+ regions appeared to reach a maximum on 50 kPa hydrogels when EBs were generated in RPMI 1640 in a similar fashion to DMEM/F12 (Supplementary Fig. 4). Taken together, these results demonstrate that, relative to softer and stiffer hydrogel substrates, 50 kPa polyacrylamide hydrogel substrates enhance cardiac differentiation in multiple differentiation contexts.
3.3 Substrate stiffness impacted the transition of pluripotent cells to mesendoderm
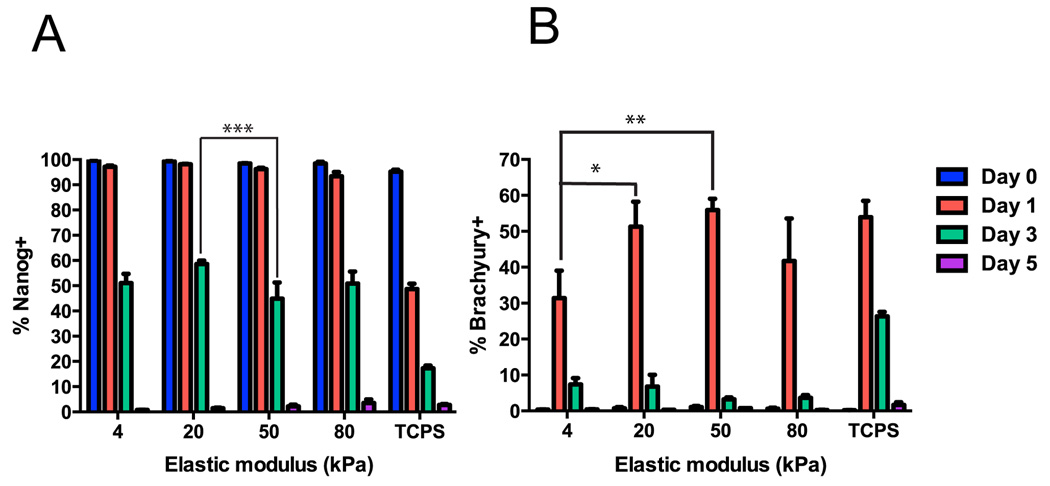
We next sought to explore the temporal effects of substrate stiffness on cardiac differentiation. For these experiments, we employed directed differentiation to avoid the heterogeneity and low purities associated with EB-based differentiation. To explore early differentiation fates, we fixed cells at days 0 (pre-differentiation), 1, 3, and 5 of differentiation. Expression of the pluripotency marker Nanog decreased with time on hydrogels of all stiffnesses and TCPS, and was significantly lower at day 3 on the 50 kPa hydrogel than on the 20 kPa hydrogel (Fig. 4A, Supplementary Fig. 5). Nanog expression was significantly higher on all hydrogel stiffnesses than TCPS on day 1 and day 3, suggesting more rapid initial differentiation on TCPS than polyacrylamide. By day 5 of directed differentiation, essentially no Nanog+ pluripotent cells remained on hydrogels any stiffness. Expression of the mesendoderm marker brachyury peaked at day 1 on hydrogels of all stiffnesses and TCPS, and was significantly higher on TCPS and the 50 kPa and 20 kPa hydrogels than the 4 kPa hydrogel at this timepoint (Fig. 4B, Supplementary Fig. 6). Brachyury expression was maintained at day 3 on TCPS, but had disappeared from cells on hydrogels by that time. The greatest fraction of brachyuryexpressing cells was achieved on the 50 kPa hydrogel, consistent with the highest percentage of cTnT+ cardiomyocytes observed on this stiffness. This suggests that effects of stiffness on cardiomyocyte purity in the directed differentiation system are, at least to some extent, a manifestation of effects of stiffness on the transition from pluripotent cells to mesendoderm.
Fig. 4.
Timecourse of marker expression during early directed differentiation on hydrogels. H9 cells were seeded onto hydrogels or TCPS on day −3 and fixed for flow cytometry on days 0, 1, 3, and 5. (A) Nanog expression decreased over time on hydrogels of all stiffnesses and TCPS. On day 3, Nanog expression was significantly lower on the 50 kPa hydrogel versus the 20 kPa hydrogel (***, p < 0.001). On day 1 and day 3, Nanog expression was significantly lower on TCPS than all hydrogel stiffnesses (#, p < 0.001). (B) Brachyury expression peaked on day 1 on all stiffnesses and was significantly higher on the 20 (*, p < 0.05) and 50 (**, p < 0.01) kPa hydrogels than the 4 kPa hydrogel on day 1. Brachyury expression was significantly higher on TCPS than all hydrogels on day 3 (#, p < 0.01). For each marker, 3 wells per stiffness were analyzed on days 0, 1, and 3 and 2 or 3 wells per stiffness were analyzed on day 5 for hydrogels and TCPS respectively.
3.4 Substrate stiffness did not impact differentiation of cardiac progenitor cells to cardiomyocytes
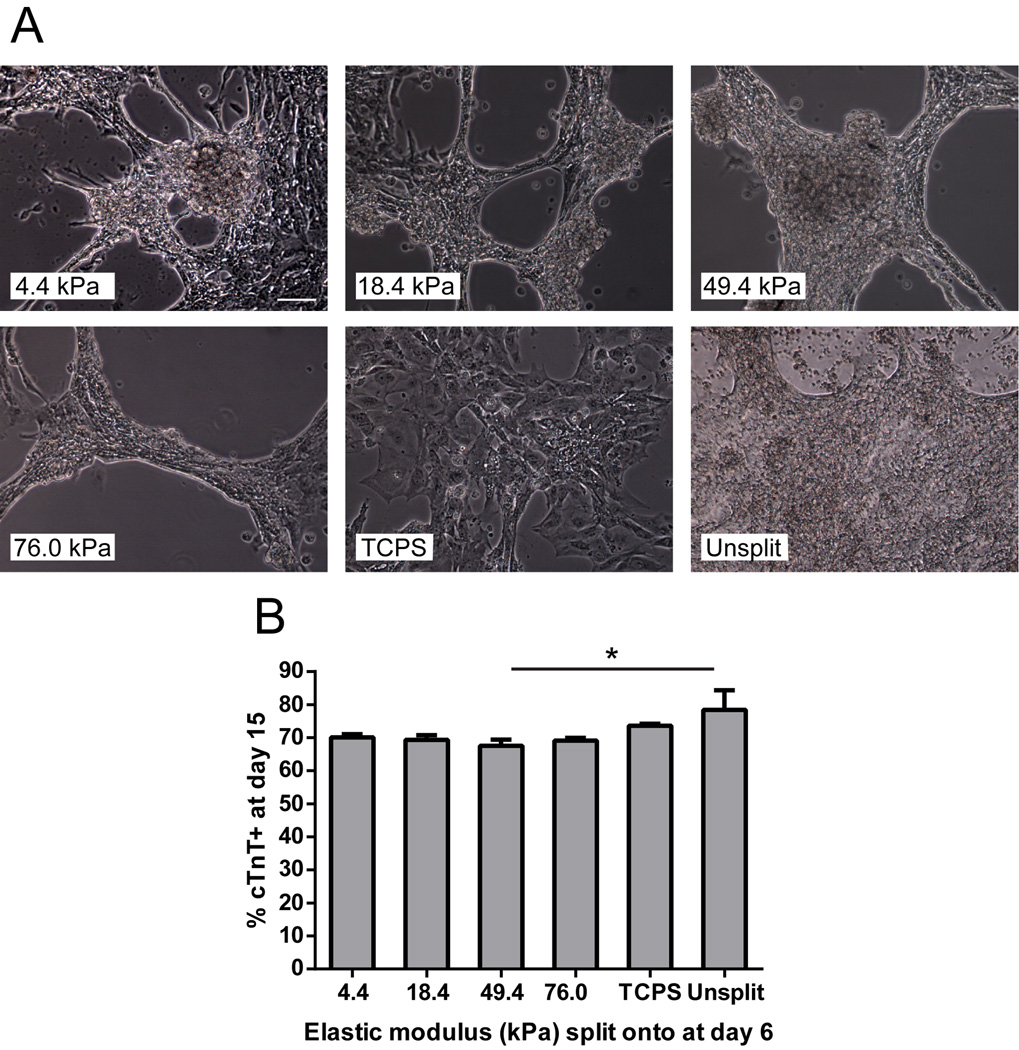
We next investigated whether varying stiffness cues later in cardiac differentiation could impact commitment of hESC-derived cardiac progenitor cells (CPCs) to cardiomyocytes. To address this, we initiated hESC differentiation on TCPS and split cells to Matrigel-coated hydrogels or TCPS at day 6, when cells robustly expressed Nkx2.5 and Isl1+ (Supplemental Fig. 7). After several days of culture on hydrogels, but not TCPS, cells aggregated into beating, tissue-like structures (Fig. 5A, Supplemental Videos 1–6). Flow cytometric analysis at day 15 revealed that introducing stiffness cues at day 6 did not impact cardiomyocyte purity, as significant differences were not observed among cells split onto any hydrogel stiffness or TCPS (Fig. 5B). In all samples, however, splitting CPCs at day 6 led to a reduction in cTnT purity at day 15 relative to the unsplit control, and this difference was significant between cells split to the 50 kPa hydrogel and the unsplit control. These results demonstrate that commitment of embryonic stem cell-derived CPCs to cardiomyocytes between days 6 and 15 is not sensitive to substrate mechanics during directed differentiation employing Wnt pathway modulation. Taken together, our results demonstrate that differentiation of hESCs to mesendoderm progenitors is sensitive to mechanics, and substrate mechanics can significantly alter the propensity of hESCs to become cardiomyocytes.
Fig. 5.
Morphology and flow cytometry data for hESC-derived CPCs seeded onto hydrogels on day 6. (A) Differentiation was initiated in H9 cells on TCPS on day 0, and Nkx2.5/Isl1+ CPCs were singularized and replated onto hydrogels or TCPS on day 6. Representative images from day 13 show that H9-derived CPCs self-aggregated into tissue-like structures on all hydrogel stiffnesses, but not TCPS. For comparison, an unsplit well on day 13 is shown. Scale bar = 100 µm. (B) Flow cytometric analysis of replated CPCs on day 15. % cTnT+ did not significantly differ among hydrogel stiffnesses or TCPS; however, the percentage of cTnT+ cells on the 50 kPa hydrogel was significantly lower than the unsplit control (p < 0.05). 3 wells of each hydrogel stiffness, 3 wells of TCPS, and 2 unsplit wells were measured.
4. Discussion
When making the decision to differentiate, hESCs monitor numerous signals from their chemical and mechanical microenvironment [26]. While researchers have identified chemical factors to direct hESC differentiation to cardiomyocytes, including Activin A/BMP4 and small molecule modulators of Wnt signaling [16, 18], less is known about the roles of mechanical factors. Developmental studies in zebrafish have demonstrated that forces from proximal blood flow are essential for cardiogenesis [22]. In vitro culture systems incorporating mechanical strain or shear stress increased cardiogenesis in pluripotent stem cells, suggesting the sensitivity of these cells to mechanical modulation and its significance in differentiation [20, 27]. Here we explored the role of substrate mechanics at different stages of hESC differentiation to cardiomyocytes, using polyacrylamide hydrogel substrates to modulate extracellular mechanical cues. Polyacrylamide gels permit independent tuning of chemical and mechanical properties, and resist protein adsorption and gel degradation during cell culture [2, 28]. Our results demonstrate that hESCs are sensitive to mechanical modulation during early specification of undifferentiated hESCs to mesendoderm progenitors, while mechanical modulation does not influence the later transition from cardiac progenitor cells to spontaneously-contracting cardiomyocytes.
In both the directed differentiation and EB culture platforms, differentiation to cardiomyocytes was most efficient on hydrogels of intermediate stiffness. Although EBs were seeded on day 5 of differentiation following a compulsory suspension culture phase while cells for directed differentiation were seeded on day −3, exposure to substrates with varying elastic moduli occurred early in both of these protocols. We previously showed that Wnt activation, a precursor to brachyury expression, peaked on day 4 in EB, while brachyury expression peaked on day 1 in directed differentiation [18, 29]. Our results from both differentiation platforms suggest that an intermediate stiffness promotes the formation of cTnT+ cardiomyocytes in hESCs. The preferred modulus for cardiogenesis that we identified, 50 kPa, is near the reported values for elastic moduli of adult rat heart tissue (11.9–46.2 kPa), perhaps indicating that physiologically relevant substrate stiffness enhances differentiation efficiency [3, 30]. Reported values for elastic moduli of developing heart tissue (4.0–11.4 kPa), however, are lower than the preferred modulus of 50 kPa found in our in vitro study. In vivo, the dynamic extracellular matrix profile and presence of other cell types in the heart are among factors which may impact the contextual presentation of mechanical cues and explain this discrepancy [31, 32]. Also of note, it is possible that a true peak of cardiogenesis occurs at an intermediate modulus between 20 and 80 kPa.
Since hESCs were seeded on day −3 in the directed differentiation platform, this enabled us to observe effects of stiffness during the transition from pluripotent cell to mesendoderm. Through a flow cytometry timecourse, we determined that stiffness induced differences in brachyury expression at day 1 that paralleled eventual cTnT+ expression. This observation suggested that early effects of stiffness on the transition of hPSCs to mesendoderm during directed differentiation are responsible to some extent for later differences in cardiomyocyte purity observed across stiffness.
It is important to note that stiff substrates do not preclude differentiation of hESCs to cardiomyocytes. We found that differentiation on very rigid TCPS was as efficient as on the 50 kPa hydrogel, which produced the greatest purity of cardiomyocytes. Also, efficient cardiomyocyte differentiation protocols have been developed on TCPS substrates [16, 18]. In addition we observed differences in the kinetics of early differentiation on TCPS and polyacrylamide, indicated by loss of Nanog and expression of brachyury. Direct comparison of the effects of mechanics on TCPS and hydrogel substrates is complicated by differences in surface chemistry, however. For example, TCPS is less resistant to protein adsorption so the ECM composition and density throughout differentiation on TCPS is likely very different than on hydrogels. Mechanistic studies will be necessary to identify how material chemistry and mechanics synergize to regulate cardiomyocyte differentiation.
To understand the role of mechanical modulation in later differentiation events, we split cells at the Nkx2.5/Isl1+ CPC stage from TCPS to hydrogels. After 9 days of culture on hydrogels, these cells did not exhibit differences in cardiomyocyte purity with stiffness, suggesting that hESCs are not sensitive to mechanics during the transition from CPC to cardiomyocyte. We observed some decrease in cardiomyoycte purity in all wells which underwent a split at day 6 relative to an unsplit control, suggesting that disrupting native connections at this timepoint may be mildly detrimental to cardiomyocyte commitment of CPCs. Although we demonstrated that the directed differentiation protocol yielded Nkx2.5/Isl1+ cells on day 6, it is worth noting that our split procedure did not involve elimination of heterogeneous cells.
Moving forward, the true utility of physiologically relevant stiffnesses in cardiac differentiation may lie in their ability to bias phenotype of hESC-derived cardiomyocytes rather than the differentiation process. Current cardiomyocyte differentiation protocols yield immature cardiomyocytes, a significant barrier for their usefulness in transplantation therapies [33]. Recent studies have shown that rat embryonic cardiomyocytes and hESC-derived cardiomyocytes generate greater forces on stiffer substrates than soft substrates, maintaining a constant contraction amplitude [17, 34]. Furthermore, we observed organized sarcomeres in hESC-derived cardiomyocytes on hydrogels across the stiffness range analyzed, but rat adult cardiomyocytes were reported to form well-organized sarcomeres on stiff (255 kPa) and soft (7 kPa) substrates but not substrates with an intermediate elastic modulus [35]. Another study reported greater maturity, as assessed by sarcomere assembly and gene expression, in neonatal murine cardiomyocytes cultured on 1 MPa substrates than on 50–130 MPa substrates [36]. Clearly, substrate mechanics play a role in regulating specification and maturation of cardiomyocytes, and mechanisms of this regulation have yet to be elucidated.
5. Conclusions
In summary, we used polyacrylamide hydrogels to demonstrate that hPSCs are sensitive to substrate stiffness during early mesendoderm specification, but not late fate choices during cardiac differentiation. An intermediate, physiologic stiffness was found to promote more efficient cardiomyocyte differentiation than soft or stiff substrates in both directed differentiation and EB protocols. When cells were split from TCPS to hydrogels at the Nks2.5+/Isl1+ cardiac progenitor stage, substrate stiffness did not impact the efficiency of their transition to cTnT+ cardiomyocytes. This supports the notion that hESCs can sense and respond to their mechanical microenvironment with biased differentiation decisions in a developmental stage-specific manner.
Supplementary Material
Acknowledgements
This work was supported by funding from NIH grant R01 EB007534, NSF grant EFRI-0735903, InvivoSciences LLC, and the State of Wisconsin. The authors would like to thank Lance Rodenkirch of the W.M. Keck Laboratory for Biological Imaging at the University of Wisconsin-Madison for imaging assistance and the University of Wisconsin-Madison Carbone Cancer Center for use of flow cytometry facilities. We thank WiCell Research Institute for hPSC culture reagents and support and use of the Vi-CELL automated cell counter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Discher D, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Pelham R, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engler A, Sen S, Sweeney H, Discher D. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Saha K, Keung A, Irwin E, Li Y, Little L, Schaffer D, et al. Substrate Modulus Directs Neural Stem Cell Behavior. Biophysical Journal. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury F, Li Y, Poh Y, Yokohama-Tamaki T, Wang N, Tanaka T. Soft Substrates Promote Homogeneous Self-Renewal of Embryonic Stem Cells via Downregulating Cell-Matrix Tractions. Plos One. 2010;5 doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu T, Ueda J, Ho J, Iwasaki K, Poellinger L, Harada I, et al. Dual Inhibition of Src and GSK3 Maintains Mouse Embryonic Stem Cells Whose Differentiation is Mechanically Regulated by Src Signaling. Stem Cells. 2012;30:1394–1404. doi: 10.1002/stem.1119. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Villa-Diaz L, Lam R, Chen W, Krebsbach P, Fu J. Mechanics Regulates Fate Decisions of Human Embryonic Stem Cells. Plos One. 2012;7 doi: 10.1371/journal.pone.0037178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musah S, Morin SA, Wrighton PJ, Zwick DB, Jin S, Kiessling LL. Glycosaminoglycan-Binding Hydrogels Enable Mechanical Control of Human Pluripotent Stem Cell Self-Renewal. ACS nano. 2012 doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones Heart Disease and Stroke Statistics-2010 Update: A Report From the American Heart Association. Circulation. 2010;121:E260-E. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 10.Mummery C, Zhang J, Ng E, Elliott D, Elefanty A, Kamp T. Differentiation of Human Embryonic Stem Cells and Induced Pluripotent Stem Cells to Cardiomyocytes A Methods Overview. Circulation Research. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Vodyanik M, Smuga-Otto K, Antosiewicz-Bourget J, Frane J, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 14.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. Journal of Clinical Investigation. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Wilson G, Soerens A, Koonce C, Yu J, Palecek S, et al. Functional Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells. Circulation Research. 2009;104:E30–E41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laflamme M, Chen K, Naumova A, Muskheli V, Fugate J, Dupras S, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 17.Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, et al. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012;2012:508294. doi: 10.1155/2012/508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine L, Azarin S, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1848-E57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. Faseb Journal. 2006;20:1182-+. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- 21.Young J, Engler A. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hove J, Koster R, Forouhar A, Acevedo-Bolton G, Fraser S, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 23.Johnson B, Bauer JM, Niedermaier DJ, Crone WC, Beebe DJ. Experimental techniques for mechanical characterization of hydrogels at the microscale. Experimental Mechanics. 2004;44:21–28. [Google Scholar]
- 24.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protocols. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burridge P, Thompson S, Millrod M, Weinberg S, Yuan X, Peters A, et al. A Universal System for Highly Efficient Cardiac Differentiation of Human Induced Pluripotent Stem Cells That Eliminates Interline Variability. Plos One. 2011;6 doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metallo C, Mohr J, Detzel C, de Pablo J, Van Wie B, Palecek S. Engineering the stem cell microenvironment. Biotechnology Progress. 2007;23:18–23. doi: 10.1021/bp060350a. [DOI] [PubMed] [Google Scholar]
- 27.Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, et al. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circulation Research. 2005;96:501–508. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- 28.Nelson CM, Raghavan S, Tan JL, Chen CS. Degradation of micropatterned surfaces by cell-dependent and -independent processes. Langmuir. 2003;19:1493–1499. [Google Scholar]
- 29.Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. Modulation of Wnt/β-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials. 2012;33:2041–2049. doi: 10.1016/j.biomaterials.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhana B, Iyer R, Chen W, Zhao R, Sider K, Likhitpanichkul M, et al. Influence of Substrate Stiffness on the Phenotype of Heart Cells. Biotechnology and Bioengineering. 2010;105:1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 31.Corda S, Samuel JL, Rappaport L. Extracellular matrix and growth factors during heart growth. Heart failure reviews. 2000;5:119–130. doi: 10.1023/A:1009806403194. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee I, Fuseler J, Price R, Borg T, Baudino T. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Kim C, Mercola M. Electrophysiological Challenges of Cell-Based Myocardial Repair. Circulation. 2009;120:2496–2508. doi: 10.1161/CIRCULATIONAHA.107.751412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hersch N, Wolters B, Dreissen G, Springer R, Kirchgessner N, Merkel R, et al. The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening. Biol Open. 2013;2:351–361. doi: 10.1242/bio.20133830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galie PA, Khalid N, Carnahan KE, Westfall MV, Stegemann JP. Substrate stiffness affects sarcomere and costamere structure and electrophysiological function of isolated adult cardiomyocytes. Cardiovasc Pathol. 2013;22:219–227. doi: 10.1016/j.carpath.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forte G, Pagliari S, Ebara M, Uto K, Tam JK, Romanazzo S, et al. Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Eng Part A. 2012;18:1837–1848. doi: 10.1089/ten.TEA.2011.0707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.