Abstract
Hypoxia-inducible transcription factors (HIFs) have long been linked to malignant tumor phenotypes in various cancer types, and several downstream mediators of HIF action have been identified in metastatic carcinomas. A new study links hypoxia-induced collagen remodeling to sarcoma progression, providing evidence for unifying mechanisms of carcinoma and sarcoma metastasis.
Sarcomas are rare malignancies that can arise in several different tissues of mesenchymal origin, such as bone, muscle, cartilage, and fat. They can be classified into numerous subtypes, ranging from indolent to aggressive metastatic tumors, and they affect about 14,000 individuals in the United States each year (1, 2). Even though each individual subtype is rare in comparison to the most common cancer types, combined their impact on human cancer burden is significant. Thus, new insights into sarcoma biology are desperately needed.
From a biological perspective, understanding the details of sarcoma metastasis is also of interest. Much of our insight into the molecular mechanisms of metastasis comes from studies on carcinomas, which originate from epithelial tissues such as breast, lung, or colon. An essential structural denominator of these tissues is the presence of cell-cell junctions that keep the epithelia intact. In order for invasive and metastatic carcinomas to emerge, carcinoma cells must shed these epithelial qualities in a process that is often termed epithelial-to-mesenchymal transition (EMT) (3). EMT allows the epithelial cells to adopt a ‘mesenchymal’ phenotype that is thought to support motility, invasion, and metastasis. Sarcomas, on the other hand, arise directly in cells of the mesenchymal lineage. It is therefore interesting to compare the mechanisms of metastasis between these two classes of tumors.
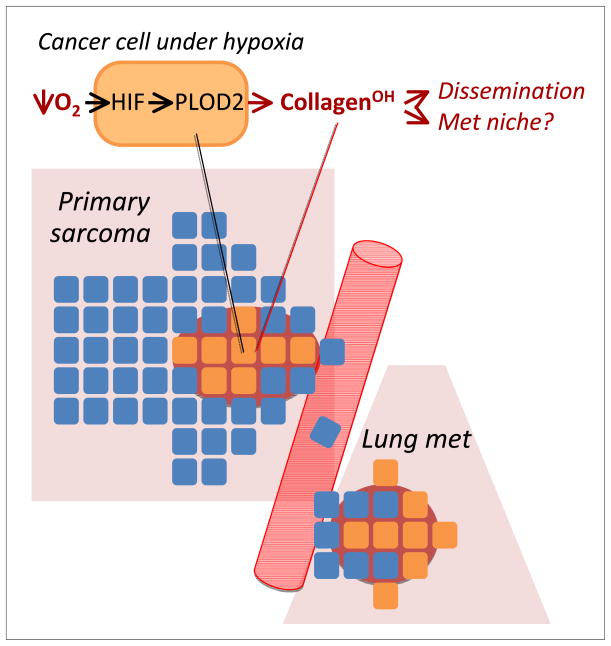
In their study, Eisinger-Mathason et al. (4) followed up on an old observation that had linked poor prognosis of sarcoma patients with tumor hypoxia. They focused on undifferentiated pleomorphic sarcoma (UPS), an aggressive subtype of sarcoma for which an elegant mouse model was available. This conditional system, driven by KrasG12D and loss of Trp53, recapitulates many of the clinical features of human UPS, including pulmonary metastasis. Using a variety of tools in both genetically engineered mice and mouse and human cell lines, Eisinger-Mathason et al. demonstrated that in UPS, tumor hypoxia-driven HIF1α expression results in increased expression of PLOD2, a procollagen lysyl hydroxylase. This led to aberrant collagen deposition that was crucial for metastatic dissemination, even though it played no role in the growth of primary tumors (Figure 1). Thus, the hypoxic microenvironment in the primary tumor can induce a reactive change in cancer cell gene expression, which in turn results in the acquisition of a pro-metastatic local extracellular matrix. The authors attribute the increased metastatic tumor spread to an enhancement of tumor cell migration within the primary tumor as well as from the distant micro capillaries into the lung parenchyma.
Figure.
In undifferentiated pleomorphic sarcomas, hypoxia activates HIF signaling, which increases the expression of many genes among which is the procollagen lysyl hydroxylase PLOD2. The resulting alteration in collagen deposition in the extracellular matrix somehow paves the way for cancer cell emigration from the primary tumor and the establishment of metastatic colonies in the lungs. Metastatic lesions might also benefit from further contributions of PLOD2 to forming a pro-metastatic niche.
Hypoxia inducible factors, most notably HIF1α and in some tissues also HIF2α, are powerful transcriptional regulators that are constitutively expressed but also rapidly degraded at the protein level. Under hypoxic conditions, however, their degradation is inhibited, leading to the activation of a transcriptional program with several target genes of various functions (5). In principle, this program can be co-opted for metastasis through two distinct but mutually compatible routes. First, HIFs can be stabilized by a reactive response to low oxygen pressure in the tumor microenvironment. This reflects the physiological function of the HIF axis in regulating tissue oxygenation in metazoans, but the same mechanism is often hijacked by tumor cells in order to promote metastatic cancer progression (6). Second, HIFs can be stabilized by genetic mechanisms. This is most prominent in renal cancer where genetic inactivation of the VHL tumor suppressor leads to HIF stabilization and tumor initiation. However, epigenomic alterations can allow HIFs to acquire additional target genes, which promote renal cancer progression and metastasis through increased cancer cell survival and chemotactic migration (7).
The pro-metastatic downstream effects of an activated HIF program are diverse and mediated by several transcriptional target genes (6). Eisinger-Mathason et al. describe a mechanism whereby hypoxia induced PLOD2-dependent changes in extacellular collagen deposition facilitate tumor cell migration in support of sarcoma metastasis. Very similar results have been obtained earlier in breast cancer where HIF-driven metastasis has also been shown to be dependent on collagen remodeling by the lysyl hydroxylase PLOD2 as well as the prolyl hydroxylases P4HA1 and P4HA2 (8, 9). Hence, at least regarding the role of extracellular matrix collagen, both carcinomas and sarcomas resort to similar mechanisms of metastasis. An interesting possibility that remains to be explored is, however, that instead of only supporting tumor cell migration, HIF-dependent collagen modifications may also be required for the formation of a supportive microenvironmental niche for disseminating metastasis-initiating cells. Recent reports have demonstrated that such mechanisms are essential for breast cancer metastasis where the extracellular matrix proteins tenascin C and periostin provide survival signals for micrometastatic lesions (10). HIFs have also been shown to modulate the pre-metastatic niche in the lung through lysyl oxidase secretion (11). Thus, further studies could expand the relevance of HIF-dependent collagen modification for metastatic cancer.
From the point of view of therapeutic interventions for metastatic cancer, HIFs are obvious targets of interest. Accordingly, efforts are under way to develop inhibitors of the hypoxia machinery. In this regard, focusing on individual target genes seems too narrow, as HIFs activate a number of known and potential mediators of metastasis. Genetic experiments in mouse models of metastases, as demonstrated nicely by Eisinger-Mathason et al. (4), are powerful tools in determining the relevance of HIF inhibition in the metastatic process. In addition to testing for effects on tumor dissemination only, what should be done, however, is a thorough assessment of HIF-dependency at various steps of the metastatic process. For example, whether or not existing metastatic nodules are sensitive to HIF inhibition needs to be clarified. While the finding of HIF-dependent cancer cell dissemination is of biological interest and it adds to our understanding of the adaptive mechanisms that induce metastatic cancer cell traits, inhibiting the process of tumor cell dissemination in the clinic does not seem feasible due to the narrow and unpredictable therapeutic window of opportunity. Targeting disseminated cells and their reliance on a supporting microenvironment would seem a more promising avenue.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Taylor BS, Barretina J, Maki RG, Antonescu CR, Singer S, Ladanyi M. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer. 2011;11:541–57. doi: 10.1038/nrc3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisinger-Mathason TS, Zhang M, Qiu Q, Skuli N, Nakazawa MS, Karakasheva T, et al. Hypoxia-Dependent Modification of Collagen Networks Promotes Sarcoma Metastasis. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–14. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanharanta S, Shu W, Brenet F, Hakimi AA, Heguy A, Viale A, et al. Epigenetic expansion of VHL-HIF signal output drives multiorgan metastasis in renal cancer. Nat Med. 2013;19:50–6. doi: 10.1038/nm.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73:3285–96. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilkes DM, Bajpai S, Wong CC, Chaturvedi P, Hubbi ME, Wirtz D, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res. 2013;11:456–66. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Oskarsson T, Massague J. Extracellular matrix players in metastatic niches. EMBO J. 2012;31:254–6. doi: 10.1038/emboj.2011.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]