Abstract
Cisplatin is one of the commonly used chemotherapeutic drugs for the treatment of head and neck squamous cell carcinoma (HNSCC). However, acquisition of cisplatin resistance is common in patients with HNSCC and it often leads to local and distant failure. In this study, we demonstrate that survivin expression is significantly upregulated in HNSCC primary tumors and cell lines. In addition, survivin levels were significantly higher in HPV negative patients that normally respond poorly to cisplatin treatment. Survivin expression was further increased in cisplatin resistant cells (CAL27-CisR) as compared to its parent cells (CAL27). Therefore, we hypothesize that targeting of survivin in HNSCC could reverse the resistant phenotype in tumor cells thereby enhancing the therapeutic efficacy of cisplatin. We used both in vitro and in vivo models to test the efficacy of YM155, a small molecule survivin inhibitor, either as a single agent or in combination with cisplatin. YM155 significantly decreased survivin levels and cell proliferation in a dose-dependent manner. In addition, YM155 pretreatment significantly reversed cisplatin resistance in cancer cells. Interestingly, YM155 treatment altered the dynamic localization of survivin in cells by inducing a rapid reduction in cytoplasmic survivin, which plays a critical role in its anti-apoptotic function. In a SCID mouse xenograft model, YM155 significantly enhanced the anti-tumor and anti-angiogenic effects of cisplatin with no added systemic toxicity. Taken together, our results suggest a potentially novel strategy to use YM155 to overcome the resistance in tumor cells thereby enhancing the effectiveness of the chemotherapy in HNSCC.
Keywords: Head and neck cancer, survivin, cisplatin resistance, YM155, angiogenesis
INTRODUCTION
Head and neck cancer is the sixth leading cancer by incidence worldwide and approximately 600,000 cases are diagnosed worldwide every year. In the United States, an estimated 40,250 new cases of head and neck cancer were expected in 2012 (1). Although advancements in the techniques for surgery, radiation and chemotherapy have increased the local control of HNSCC, the overall survival rates have not improved significantly over the last three decades. This poor outcome becomes even worse (20% 5-year survival rate) for advanced stage HNSCC patients whose tumors are not amenable for surgery (2). Cisplatin is one of the most commonly used chemotherapeutic agents used for the treatment of head and neck cancers (3). However, many patients acquire resistance to chemotherapeutic agents leading to treatment failures. Prognosis of such patients who have to undergo late salvage surgery is very poor (4). Therefore, it is important to understand the molecular mechanisms that contribute to drug resistance in order to identify novel therapeutic targets for head and neck cancer.
One such protein that has been identified to be deregulated in a number of human tumors is survivin. It is a bifunctional protein that acts as a suppressor of apoptosis and has an essential role in mitosis (5). It is a nuclear and cytoplasmic shuttling protein that is predominantly cytoplasmic in part because of an active nuclear export signal (NES) in its linker region (6). Cytoplasmic survivin predominantly mediates the anti-apoptotic function; whereas nuclear survivin mediates the mitotic function and is significantly less stable (6–8). Survivin is largely undetected or expressed at very low levels in normal tissues (9), whereas it is overexpressed in many malignancies including breast, lung, colon, pancreas, liver and head and neck cancer and has also been linked to poor patient survival (10, 11). In addition, growing evidence suggest that survivin expression is associated with drug-resistance in cancer cells and cancer associated endothelial cells (12–17). Therefore, we hypothesize that targeting of survivin in head and neck cancer may enhance the therapeutic efficacy of cisplatin by inhibiting the acquisition of chemoresistance by tumor and tumor-associated endothelial cells.
Several therapeutic approaches for targeting survivin protein using immunotherapy or small molecule antagonists either as single agents or in combination with conventional chemotherapeutic agents are currently in clinical trials (18). Recently, a novel small molecule inhibitor of survivin, YM155, was identified by cell-based high-throughput screening (19). It has been shown to exhibit potent anti-tumor activity in vitro, and induced tumor regression in established non-small cell lung cancer, non-Hodgkin’s lymphoma, melanoma and hormone refractory prostate cancer xenografts (19–22). In addition, Phase I and Phase II trials with YM155 have demonstrated its safety and tolerability in patients with unresectable melanoma and advanced refractory NSCLC (23, 24).
The objective of this study was to determine the in vitro and in vivo efficacy of YM155 alone or in combination with cisplatin in preclinical head and neck cancer models. YM155 treatment significantly down-regulated survivin expression in head and neck cancer cells, in a dose dependent manner, in vitro as well as in a preclinical in vivo model. In addition, YM155 treatment was able to reverse cisplatin resistance in a naturally occurring cisplatin resistant HNSCC cell line (UM-SCC-74A) as well as in a cisplatin resistant cell line (CAL27-CisR) with acquired cisplatin resistance. YM155 and cisplatin combination regimen was very well tolerated in vivo and significantly inhibited tumor growth and tumor angiogenesis. Taken together, our results demonstrate that YM155 could be a useful adjuvant for the treatment of head and neck cancer, particularly for the ones that are resistant to cisplatin and provides a scientific rationale to evaluate this or a similar combination strategy for clinical trials.
MATERIALS AND METHODS
Patient samples, cell culture and reagents
We used two patient sample groups for this study. Use of patient samples was approved by the Ohio State University institutional review board. A board certified pathologist diagnosed all tumor tissue as HNSCC. For group 1, tumor and adjacent normal tissue samples were collected from head and neck cancer patients undergoing surgical resection at the James Comprehensive Cancer Center at The Ohio State University. Normal samples were collected from areas adjacent to the tumor but outside the tumor margins (patient tumor characteristics are presented in Supplementary Table S1). Group 2 consisted of 225 patients with oropharyngeal SCC treated at our institution from 2002–2009. Patients underwent complete resection or had a biopsy and neck dissection with or without adjuvant chemotherapy/radiation therapy (patient tumor characteristics are presented in Supplementary Table S2). HNSCC cell lines (UM-SCC-38, UM-SCC-74A, UM-SCC-49, UM-SCC-47, UM-SCC-11B and UM-SCC-25) were obtained from Dr. Thomas E. Carey (University of Michigan). CAL27 was purchased from ATCC (Manassas, VA). The identity of all cell lines was confirmed by STR genotyping (Identifier Kit, Applied Biosystems, Carlsband, CA). The characteristic of cell lines (25, 26) (origin, p53 status, HPV status) are presented in Supplementary Table S3. Normal human oral keratinocytes (HOK) were purchased from ScienCell (Carlsbad, CA). Human epidermal keratinocytes, adult (HEKa) and human epidermal keratinocytes, neonatal (HEKn) were purchased from (Invitrogen, Carlsbad, CA). All HNSCC cell lines were cultured in DMEM supplemented with 10% fetal bovine serum. HOK, HEKa and HEKn were grown in keratinocyte growth medium (Invitrogen). YM155 was obtained from Selleck Chemicals (Houston, TX). Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against survivin, β-catenin, lamin A/C and GAPDH were obtained from Cell Signaling Technology (Danvers, MA). Survivin antibody for immunefluorescence was purchased from Novus Biologicals (Littleton, CO). CD31 antibody was from Dianova (Hamburg, Germany).
Induction of cisplatin resistance in a head and neck cancer cell line
CAL27 cells were initially cultured in DMEM containing 0.2μM cisplatin and the cells that proliferated were repeatedly sub-cultured in DMEM containing increasing concentrations of cisplatin over a 6 month period. Cells that grew in 20μM cisplatin were designated as CAL27-CisR. They were maintained in DMEM containing 3μM cisplatin.
Quantitative real time PCR analysis
RNA from the HNSCC tumors, adjacent normal controls, HNSCC cell lines was extracted using TRIZOL reagent (Invitrogen). RNA from paraffin embedded xenograft tumors was extracted using the Recover All mammalian RNA extraction kit (Ambion, Austin, TX). Survivin RNA was transcribed into cDNA and amplified with TaqMan primer/probe Hs03043576_m1. Survivin mRNA expression was normalized to RNU48 and OAZ1, respectively using the 2−ΔΔCt method (27).
Cell Proliferation Assay
Cell proliferation was measured using the MTT proliferation kit (Roche Applied Science, Mannheim, Germany) as described previously (28). Cells were treated with cisplatin or YM155 for 72 hrs. For combination treatment, cells were pretreated with YM155 for 6 hrs and then treated with cisplatin for additional 72 hrs. The percentage cell growth inhibition for each treatment group was calculated by adjusting the untreated control group to 100%.
Apoptosis Assay
Cells were plated in 6 cm dishes and treated with YM155, cisplatin or in combination. Seventy two hours post treatment; cells were stained with Annexin V (Molecular Probes) and Propidium Iodide (Sigma-Aldrich) and analyzed by flow cytometry (BD Biosciences, San Jose, CA).
Nuclear and cytoplasmic protein extraction
Cells were cultured in 6 cm plates and treated with YM155 for different time points. At the end of each treatment, cells were harvested and nuclear and cytoplasmic protein fractions were separated by NE-PER nuclear and cytoplasmic extraction kit (Pierce Biotechnology, Rockford, IL) according to manufacturer’s instructions.
Western blot analysis
Whole cell lysates or nuclear and cytoplasmic fractions were separated by 4–12% NuPAGE Bis-Tris gels (Invitrogen) as described previously (28). Protein loading in all the experiments was normalized by stripping the blots and then re-probing with anti-GAPDH antibody.
Colony formation assay
Tumor cells were plated in 6 cm dishes and treated with cisplatin, YM155 or a combination of both. After 72 hours, 4×103 viable cells from each group were plated in 6 cm dishes and cultured for additional 10 days. The colonies were fixed with methanol and stained with crystal violet. Photomicrographs were taken and the number of colonies was counted by Alpha Innotech imaging software (San Leandro, CA).
Immunofluorescent staining
CAL27-CisR cells were cultured in 4-well labtech chambers. CAL27-CisR cells were treated with YM155 (10 nM) for different time points. At the end of incubation, survivin localization was analyzed by immunofluorescent staining as described previously (29). The fluorescent images were captured using Nikon Eclipse 80i microscope with DS-Ri1 camera at 600X magnification and overlaid using NIS-Elements-Basic Research software (Nikon, Melville, NY).
Matrigel in vitro endothelial tube formation assay
Endothelial tube formation was assayed using Matrigel coated 8-well chamber slides as described previously (30). Each chamber was photographed (Nikon Eclipse Ti microscope with DS-Fi1 camera) at 100X magnification and total area occupied by endothelial cell derived tubes in each chamber was calculated using software (NIS-Elements-Basic Research, Nikon, Melville, NY) and expressed as an angiogenic score.
SCID mouse xenograft model
6–8 week old SCID mice (NCI) were used in all the in vivo experiments (31). Tumor cells (1 × 106) were mixed with 100 μl of Matrigel and injected in the flanks of SCID mice. After 8 days, mice were stratified into different groups (n=5), so that the mean tumor volume in each group was comparable. At days 8, 11, 14, 17, 21, 24 and 28 animals were treated with YM155 (3 mg/kg) or cisplatin (5 mg/kg) via I.P. injections. Tumor volume measurements [volume (mm3) = L × W2/2 (length L, mm; width W, mm)] began on day 6 and continued twice a week until the end of the study. After 36 days, primary tumors were carefully removed, photographed and analyzed for survivin expression and tumor angiogenesis.
Tissue Microarrays and Immunohistochemistry
Paraffin embedded tissue sections from patients in group 2 were represented on tissue microarrays (TMA). The slides were stained with survivin and p16, an established surrogate marker for HPV status (Roche mtm AG Heidelberg, Germany) as described previously (32). HPV16 status was determined using in-situ hybridization method (GenPoint™ HPV DNA Probe, Dako, Carpinteria, CA) (33). Survivin intensity was scored as 1: none, 2: low, 3: moderate or 4: high. p16 expression was scored as positive if ≥50% of tumor cells showed positive staining. A tumor was scored as HPV16 positive when specific staining was observed in tumor-cell nuclei. Xenograft tumor sections were stained for angiogenesis (CD31) as described previously (28). Microvessel density was calculated by counting 5 random high power fields (200X).
Statistical Analysis
Data from all the in vitro experiments are expressed as mean ± SEM from a minimum of 3 independent experiments. The survivin expression in patient samples from group 1 and HNSCC cell lines was analyzed by Mann-Whitney test. To assess survivin stain intensity in patient samples from group 2, core samples were averaged across each patient to create one survivin stain intensity score. p16 staining was defined as positive if any core had a p16 stain proportion ≥ 50%. The smoking status group of ≤ 10 pack-years includes patients who reported pack-years of zero. Two-sample t tests were used in comparing mean survivin stain intensity between two independent groups. One-way ANOVA, or Kruskal-Wallis if assumptions were not met, were used to compare mean survivin stain intensity between three or more groups. If significant main effects were found, post hoc tests using a Bonferroni adjustment were performed. All statistical analyses for TMA data were conducted in SAS version 9.2, Cary, North Carolina. The rest of the data was statistically analyzed by two-way analysis of variance (ANOVA) or Student’s t test and a p value of <0.05 was considered significant.
RESULTS
Survivin expression is significantly higher in primary tumor samples and cancer cell lines from head and neck cancer patients
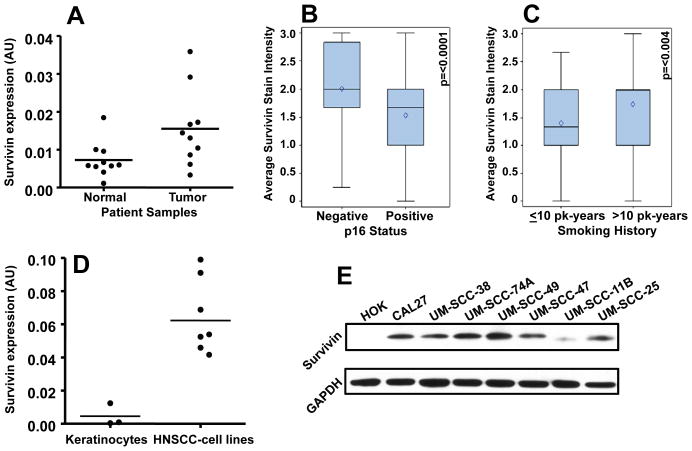
Our results show that survivin expression was significantly higher (Mann-Whitney test; p=0.028) in HNSCC tumor samples (group 1, Supplementary Table 1) as compared to adjacent normal tissue (Fig. 1A). Analysis of group 2 patients showed that patients whose tumors were p16 negative had a significantly higher survivin stain intensity as compared to patients with p16 positive tumors (p<0.0001; Fig. 1B). Similar results were obtained when HPV16 status was taken into consideration (p<0.021, Supplementary Table 2). Tumors from patients who smoked >10 pack-years had higher survivin stain intensity as compared to those with ≤10 pack-years smoking history (p<0.004, Fig. 1C). In addition, patients with T4 stage tumors had significantly higher mean survivin stain intensity than those with T1 stage (p<0.01). We next examined if survivin expression is also elevated in head and neck tumor cell lines. Indeed, both survivin mRNA and protein levels were significantly higher in all the head and neck cancer cell lines as compared to normal keratinocytes (Mann-Whitney test; p=0.009 for RT-PCR data, Fig. 1D–E).
Figure 1. Survivin expression is upregulated in HNSCC cell lines and patient’s primary tumor samples.
A: Survivin expression in primary tumor samples (n=10) and in adjacent normal mucosa (n=10) of head and neck cancer patients. B: Survivin stain intensity was analyzed in p16 negative (n=56) and p16 positive primary tumor samples (n=169). C: Survivin stain intensity was analyzed in tumors from patients with ≤10 pack (pk)-years smoking history (n=58) and compared with survivin stain intensity in tumors from patients with >10 pack-years smoking history (n=157). Blue box, interquartile range (25th to 75th percentile); black diamond, mean; black horizontal bar, median; top and bottom whiskers, maximum and minimum values, respectively. D–E: Survivin expression was analyzed in normal keratinocytes and head and neck cancer cell lines by RT-PCR (D) and Western blotting (E). For survivin Western blot, equal protein loading was verified by stripping the blots and reprobing with GAPDH antibody.
Survivin expression is markedly elevated in cisplatin resistant head and neck cancer cell line and treatment with YM155 significantly down-regulates survivin expression in a dose-dependent manner
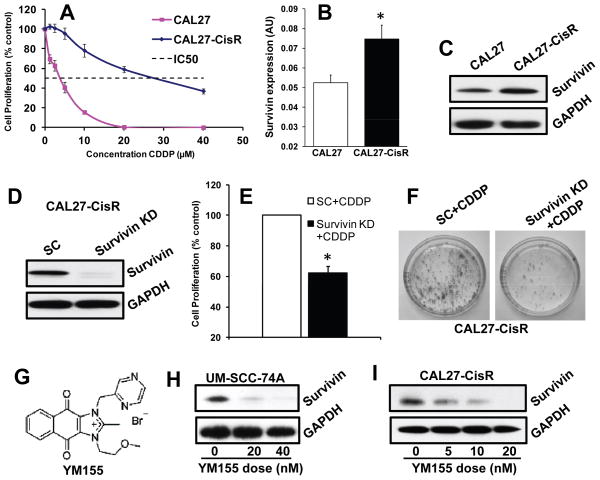
To examine the role of survivin in the acquisition of cisplatin resistance, we took a head and neck cancer cell line (CAL27) that is sensitive to cisplatin treatment (IC50 3 μM) and induced cisplatin resistance by culturing this cell line in increasing doses of cisplatin over an extended period of time. This new cell line, designated CAL27-CisR (IC50 28 μM) was found to be significantly more resistant to cisplatin treatment (> 7 folds) as compared to its parental cell line (Fig. 2A). Interestingly, CAL27-CisR cells also showed a significant increase in survivin levels as compared to CAL27 cells (Fig. 2B–C). Survivin knockdown in CAL27-CisR cells significantly reversed cisplatin resistance in these cells (Fig. 2D–E).
Figure 2. Survivin expression is upregulated in cisplatin resistant cells and YM155 inhibits survivin expression in a dose dependent manner.
A: CAL27-CisR and its parental cell line CAL27 were treated with different concentrations of cisplatin (CDDP) and cell proliferation was assessed by MTT assay. B–C: Survivin expression in CAL27-CisR and its parental cell line CAL27 was examined by RT-PCR (B) and Western blotting (C). *, represents a significant difference (p<0.05). D–F: Survivin expression was knocked down in CAL27-CisR cells by siRNA. D: Survivin knockdown was verified by Western blotting. E–F: CAL27-CisR cells with survivin knockdown or treated with scrambled RNA (SC) were treated with cisplatin (CDDP) and examined for cell proliferation (E; MTT assay) or colony formation (F). G: Chemical structure of YM155. H–I: UM-SCC-74A and CAL27-CisR cells were treated with different doses of YM155 and survivin expression was examined by Western Blotting. Equal protein loading was verified by stripping the blots and reprobing with GAPDH antibody.
Recently, a novel small molecule inhibitor of survivin (YM155, Fig. 2G) was identified by cell-based high-throughput screening (19). We next examined if YM155 is effective in inhibiting survivin protein expression in head and neck cancer cell lines. We selected two cisplatin resistant cell lines UM-SCC-74A (naturally cisplatin resistant) and CAL27-CisR (generated in our laboratory) for our in vitro and in vivo work. YM155 significantly downregulated survivin expression in both the cell lines in a dose dependent manner (Fig. 2H–I). In addition, YM155 was very effective in inhibiting tumor cell proliferation (nano-molar concentrations) in HNSCC cell lines (Supplementary Fig. S1). For all the subsequent in vitro experiments using UM-SCC-74A and CAL27-CisR, we used the respective IC50 doses of YM155 (15 and 10 nM for UM-SCC-74A and CAL27-CisR, respectively) and cisplatin (10 and 28 μM for UM-SCC-74A and CAL27-CisR, respectively) as a single agent or in combination.
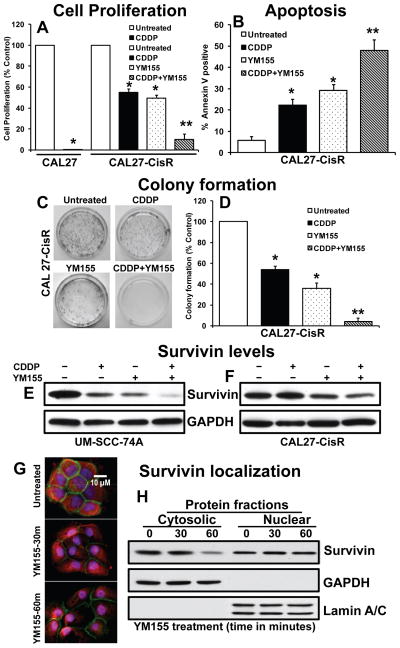
YM155 significantly reverses cisplatin resistance in head and neck cancer cells by rapidly decreasing cytoplasmic survivin levels
Recent studies have highlighted the role of survivin in the acquisition of drug-resistance in cancer cells (12, 15, 16). We next examined if YM155 could reverse the cisplatin resistance in head and neck cancer cells and enhance its anti-tumor effects. Treatment of CAL27-CisR cells with cisplatin showed 51% inhibition of cell proliferation, whereas it completely inhibited cell proliferation in CAL27 cells (Fig. 3A). Interestingly, pretreatment of CAL27-CisR cells with YM155 (10 nM) significantly reversed cisplatin resistance (90%) in CAL27-CisR cells (Fig. 3A). Similarly, treatment with YM155 significantly reversed cisplatin resistance in naturally cisplatin resistant UM-SCC-74A cells (Supplementary Fig. S2). We next investigated the effect of YM155 alone or in combination with cisplatin on tumor cell colony formation. As observed with cell proliferation assay, YM155 and cisplatin combination treatment was very effective showing 96% inhibition of tumor cell colony formation, whereas YM155 and cisplatin alone showed 64% and 47% inhibition of tumor cell colony formation, respectively (Fig. 3C–D). In the next set of experiments, we examined if YM155-induced inhibition of cell proliferation and colony formation is mediated via tumor cell apoptosis. Indeed, YM155 treatment in combination with cisplatin showed significantly higher tumor cell apoptosis (Fig. 3B). In addition, YM155 and cisplatin combination treatment markedly reduced survivin levels in both UM-SCC-74A and CAL27-CisR cells (Fig. 3E–F). Recent studies have highlighted the role of cytoplasmic survivin in mediating the anti-apoptotic function (7). We further performed immunofluorescence and sub-cellular fractionation studies to examine if YM155 treatment decreases cytoplasmic survivin levels in cancer cells. Our results clearly demonstrate that YM155 treatment rapidly decreases (within one hour) survivin levels in cytoplasmic cellular compartment, whereas nuclear survivin levels were not significantly decreased in the same cells (Fig. 3G–H and Supplementary Fig. S3).
Figure 3. YM155 enhances the anti-proliferative effects of cisplatin treatment.
A: CAL27, CAL27-CisR cells were treated with YM155 or cisplatin (CDDP) alone or in combination and cell proliferation was assessed by MTT assay. *, represents a significant difference (p<0.05) as compared to no treatment group and **, represents a significant difference (p<0.05) as compared to single treatment groups. B: CAL27-CisR cells were treated with cisplatin (CDDP) or YM155 alone or in combination. After 72 hours, cells were stained with annexin V and analyzed by flow cytometry. C–D: CAL27-CisR cells were treated with YM155 or cisplatin (CDDP) alone or in combination for 72 hours. Four thousand viable cells from each group were cultured for additional 10 days 6 cm plates. Each assay was photographed and the number of colonies analyzed. E–F: UM-SCC-74A and CAL27-CisR cells were treated with YM155 or cisplatin (CDDP) alone or in combination and survivin expression was examined by Western Blotting. Equal protein loading was verified by stripping the blots and reprobing with GAPDH antibody. G: CAL27-CisR cells were cultured in 4-well labtech chambers and treated with YM155 (10 nM) for different time points. At the end of YM155 treatment, cells were fixed and stained with survivin (red), β–catenin (green, membrane staining), and DAPI (blue, nucleus). H: CAL27-CisR cells were treated with YM155 (10 nM) for different time points and cytosolic and nuclear protein fractions were isolated and probed for survivin expression by Western Blot. Purity of cytosolic and nuclear fractions was verified by the presence of GAPDH and lamin A/C, respectively.
Survivin has also been shown to regulate cell motility (34). We next examined if YM155 treatment alone or in combination with cisplatin inhibits tumor cell motility. YM155 and cisplatin treatment alone showed 36% and 13% inhibition of CAL27-CisR cell motility and 39% and 14% inhibition of UM-SCC-74A cell motility respectively (Supplementary Fig. S4). YM155 and cisplatin combination treatment showed 74% and 70% inhibition of CAL27-CisR and UM-SCC-74A cell motility, respectively.
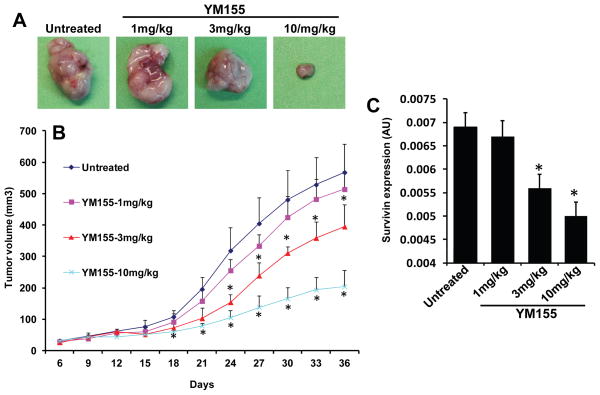
YM155 inhibits tumor growth in a dose-dependent manner
To confirm the anti-tumor effects of YM155 in vivo, we used a SCID mouse xenograft model. Animals bearing UM-SCC-74A tumors were treated with different doses of YM155. Animals treated with YM155 at 10 mg/kg dose showed the maximal tumor growth inhibition (65% at day 36), whereas YM 155 at 3 mg/kg and 1 mg/kg showed 31% and 10% tumor growth inhibition, respectively, at day 36 (Fig. 4A–B). We next examined the effectiveness of different YM155 doses in downregulating survivin expression in vivo. Survivin expression in tumor samples at the end of the in vivo study (day 36) was examined by quantitative RT-PCR. Similar to tumor growth inhibition results, YM155 treatment at 1 mg/kg did not significantly decrease survivin levels. However, YM155 treatment at 3 mg/kg and 10 mg/kg doses significantly decreased survivin expression in UM-SCC-74A tumors (Fig. 4C).
Figure 4. YM155 inhibits tumor growth in a dose-dependent manner.
A–B: Tumor bearing animals (n=5) were treated with YM155 at different doses (1 mg/kg, 3 mg/kg or 10 mg/kg) as described in methods. A: Representative photomicrographs of tumors from untreated, YM155 1 mg/kg, YM155 3 mg/kg and YM155 10 mg/kg groups. B: Tumor growth curves for UM-SCC-74A tumors treated with different doses of YM155. C: Survivin levels in UM-SCC-74A tumors at the end of the in vivo experiments. *, represent a significant difference (p<0.05).
YM155 significantly enhances the therapeutic efficacy of cisplatin in cisplatin resistant head and neck cancers
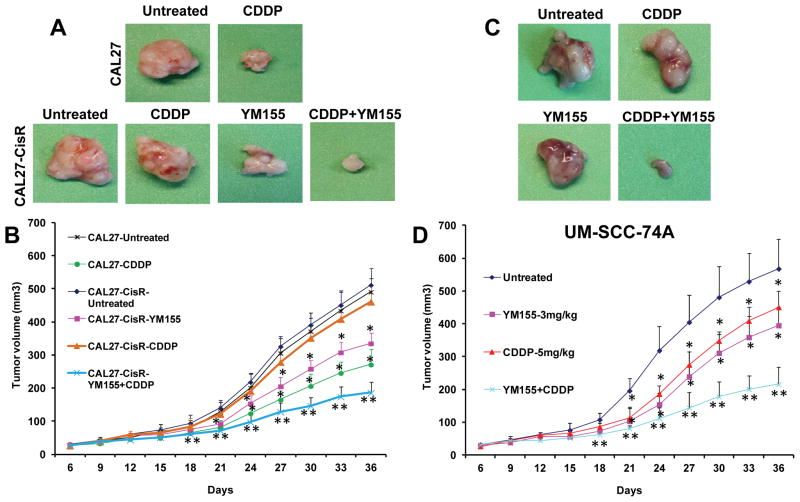
Our in vitro data suggest that YM155 significantly reverses cisplatin resistance in head and neck cancer cells. To further validate our in vitro results, we performed YM155 and cisplatin combination treatment study in a SCID mouse xenograft model. Animals bearing cisplatin resistant cells (CAL27-CisR) as well as its parental cisplatin sensitive cells (CAL27) showed a similar tumor growth profile (Fig. 5A–B). As observed in our in vitro studies, cisplatin treatment (5 mg/kg) of animal bearing CAL27-CisR tumors did not significantly affect tumor growth (11% inhibition at day 36) whereas cisplatin treatment of CAL27 markedly decreased tumor growth (55% inhibition at day 36). YM155 (3 mg/kg) treatment of CAL27-CisR tumors was significantly more effective in reducing tumor burden (38% inhibition at day 36). YM155 in combination with cisplatin was most effective in inhibiting tumor growth of CAL27-CisR (66% inhibition at day 36).
Figure 5. YM155 and cisplatin combination significantly inhibits tumor growth.
Animals bearing CAL27, CAL27-CisR or UM-SCC-74A were treated with YM155 (3 mg/kg) or cisplatin (CDDP, 5 mg/kg) alone or in combination. A: Representative photomicrographs of tumors from untreated, cisplatin (CDDP), YM155, or CDDP and YM155 treated groups of CAL27 or CAL27-CisR. B: B: Tumor growth curves for CAL27 and CAL27-CisR tumors treated with cisplatin (CDDP), YM155, or CDDP and YM155. C: Representative photomicrographs of tumors from untreated, cisplatin (CDDP), YM155, or CDDP and YM155 treated groups of UM-SCC-74A. D: Tumor growth curves for UM-SCC-74A tumors treated with cisplatin (CDDP), YM155, or CDDP and YM155. *, represents a significant difference (p<0.05) as compared to no treatment group and **, represents a significant difference (p<0.05) as compared to single treatment groups.
We next tested the efficacy of YM155 and cisplatin combination treatment in a naturally cisplatin resistant head and neck cell line (UM-SCC-74A). Cisplatin (5 mg/kg) and YM155 (3 mg/kg) treatment alone showed 19% and 31% tumor growth inhibition (Fig. 5C–D), where YM155 and cisplatin in combination showed significantly higher tumor growth inhibition (64%). In addition, the combination treatment was very well tolerated and it did not cause any animal mortality or induce significant decrease in body weight (Supplementary Fig. S5).
YM155 and cisplatin combination treatment significantly inhibits tumor angiogenesis
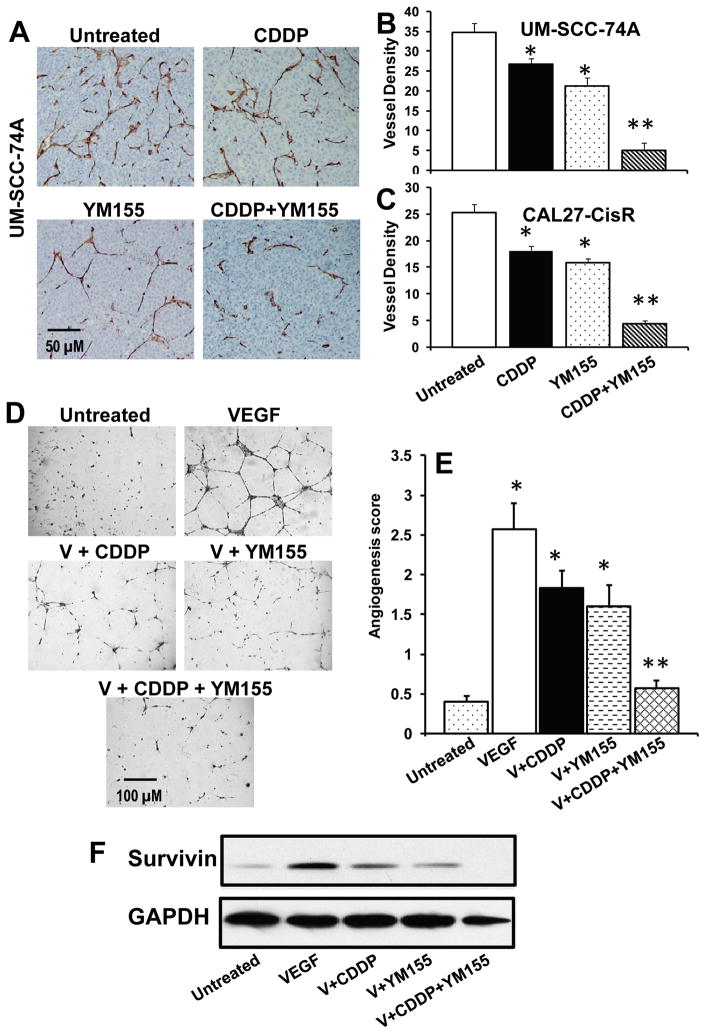
We have previously shown that VEGF, a key angiogenic factor, upregulated Bcl-2 proteins in endothelial cells via the PI3K/Akt pathway (35) and Bcl-2, in turn, protected endothelial cells by upregulating survivin via the Raf-MEK-ERK signaling cascade (36). Recently, Virrey et al, have also shown that increased survivin expression confers chemoresistance to tumor-associated endothelial cells (37). We therefore examined if YM155 treatment alone or in combination with cisplatin inhibits tumor angiogenesis. YM155 and cisplatin treatment alone showed 40% and 24% inhibition of tumor angiogenesis in UM-SCC-74A (Fig. 6A–B) and 38% and 29% inhibition in CAL27-CisR, respectively (Fig. 6C), whereas YM155 and cisplatin combination treatment showed 86% and 83% inhibition of tumor angiogenesis in UM-SCC-74A and CAL27-CisR tumors, respectively.
Figure 6. YM155 and cisplatin combination treatment significantly inhibits tumor angiogenesis and VEGF mediated endothelial cell tube formation.
A: Representative photomicrographs of tumor blood vessel staining for untreated, cisplatin (CDDP) or YM155 alone or combination groups for UM-SCC-74A tumors. B–C: Microvessel density in the tumor samples was calculated by counting 5 random fields (200x) and expressed as vessel density ± SE. *, represents a significant difference (p<0.05) as compared to no treatment group and **, represents a significant difference (p<0.05) as compared to single treatment groups. D: Representative photomicrographs of in vitro tube formation assay for untreated, VEGF, VEGF + cisplatin (V + CDDP), VEGF + YM155 (V + YM155), and VEFG + CDDP + YM155. E: Quantitative data for tube formation expressed as angiogenic score ± SE from three independent experiments. E: Endothelial cells were treated with VEGF and cisplatin (5 μM) or YM155 (10 nM) alone or in combination for 48 hour. Whole cell lysates from each group were Western blotted and probed for survivin. Equal sample loading was verified by stripping the blots and reprobing with anti-GAPDH antibody.
We next examined if YM155 combination treatment mediates its anti-angiogenesis effects by inhibiting VEGF-mediated angiogenesis. VEGF treatment of endothelial cells significantly enhanced the tube formation on growth factor reduced Matrigel (Fig. 6D–E). Low dose combination of YM155 (10 nM) and cisplatin (5 μM) significantly inhibited (92%) VEGF-mediated tube formation (Fig. 6D–E), whereas YM155 and cisplatin treatment alone showed 45% and 34% inhibition of endothelial cell tube formation, respectively. VEGF treatment of endothelial cells markedly upregulated survivin levels, whereas YM155 treatment alone or in combination with cisplatin significantly inhibited survivin levels (Fig. 6F).
DISCUSSION
Head and neck squamous cell carcinoma (HNSCC) remains a challenging clinical problem due to the persistent high rate of local and distant failure which is in turn due to the acquisition of chemo and radio-resistance (38). Therefore, there is an urgent need to identify new therapeutic targets so that novel treatment regimens can be developed to improve the therapeutic efficacy while minimizing the toxic side effects. One such target molecule for head and neck cancer is survivin protein. Recent studies have shown that survivin is largely undetectable in normal mucosa, but it is highly expressed in most head and neck cancers correlating with poor survival and resistance against chemotherapy and radiotherapy (10, 39, 40). In our study, we also observed significantly higher levels of survivin in primary tumors from head and neck cancer patients as compared to surrounding normal tissue. In addition, survivin expression was also significantly upregulated in all head and neck cancer cell lines, both at the mRNA and protein level as compared to normal keratinocytes. More importantly, survivin levels were further elevated in cisplatin resistant cells as compared to their parental cisplatin sensitive cells. Therefore, we hypothesized that targeting of survivin in advanced HNSCC could reverse the resistant phenotype in tumor cells, thereby enhancing the therapeutic efficacy of cisplatin.
Patients with head and neck cancer encompass a heterogeneous group and can be further subdivided into two distinct tumor subtypes; human papillomavirus (HPV)-negative and HPV-positive tumors. Interestingly, majority of the head and neck cancer patients with HPV positive tumors respond very well to traditional chemotherapy with cisplatin and demonstrate significantly favorable clinical outcome (32, 38). It is the patients with HPV negative tumors that show markedly poor clinical outcome and often develop resistance to chemotherapy. These HPV negative HNSCC patients are usually smokers, have more aggressive disease, are older in age and unable to tolerate the comorbidities normally associated with toxic chemotherapeutic agents. Therefore, this non-HPV associated patient population could tremendously benefit from the addition of targeted therapies to currently used treatment regimens. In this study, we found that tumors from HPV negative patients express significantly higher levels of survivin as compared to tumors from HPV positive patients. We also found that survivin expression is higher in patients with a >10 pack-years of tobacco smoking history. This data gave us a strong rationale to test the anti-tumor effects of a novel survivin inhibitor YM155 in HNSCC. In this study, we have used 2 HPV negative HNSCC cell lines (UM-SCC-74A and CAL27-CisR). UM-SCC-74A cell line is derived from a head and neck cancer patient with base of tongue tumor and is highly resistant to both chemotherapy and radiation treatment (41, 42). In addition, we generated a cisplatin resistant cell line (CAL27-CisR, IC50 28 μM) in our laboratory by culturing a cisplatin sensitive tongue SCC cell line CAL27 (IC50 3 μM) in increasing doses of cisplatin over a period of time.
YM155 treatment was very effective in inhibiting tumor cell proliferation in nano-molar concentrations in all HNSCC cell lines that we tested. In addition, YM155 pretreatment significantly reversed cisplatin resistance in a naturally resistant head and neck cell line (UM-SCC-74A) as well as in cell line with acquired cisplatin resistance (CAL27-CisR). YM155 treatment also significantly downregulated survivin expression in both of these cisplatin resistant cell lines in a dose dependent manner. Survivin has been shown to mediate its cytoprotective function predominantly at the initiation of mitochondrial apoptosis to prevent caspase-9 activation by forming a survivin–caspase-9 complex and preventing caspase-9 incorporation in a functional apoptosome complex (43, 44). Interestingly, YM155 treatment induced a rapid reduction of cytoplasmic survivin levels in cancer cells. These results suggest that YM155 is not only able to downregulate survivin expression at the transcriptional level, but it may also be able to reduce cytoplasmic survivin levels by shuttling survivin from cytoplasm to nucleus and mediating its degradation (7, 45, 46). This assumption is supported by recent studies demonstrating that only export competent survivin was able to efficiently inhibit chemo- and radiotherapy induced cell death (5, 47). In line with this hypothesis, Engels et al showed that in patients with oral squamous cell carcinoma, increased cytoplasmic survivin levels were associated with significantly shorter disease free survival (8). Therefore, these results highlight the importance of regulating not only the total levels, but also the localization of survivin in cancer cells.
To determine if the observed in vitro synergy between YM155 and cisplatin extends to the in vivo setting, we used a SCID mouse model to study the effect of combination treatment on tumor growth and tumor angiogenesis. Indeed, low dose combination of YM155 (3 mg/kg) and cisplatin (5 mg/kg) induced significant reduction in tumor burden. This marked inhibition of tumor growth by combination therapy, particularly in chemoresistant cell line could be due to YM155-mediated downregulation of survivin levels, notably cytoplasmic survivin (7), as well as reduction in the formation of new blood vessels by inhibiting VEGF function (28) as observed in tube formation assay. YM155 and cisplatin combination treatment was very well tolerated in the animals. It did not cause any animal mortality, induce significant weight loss or cause any major systemic toxicity such as dry scaly skin or respiratory distress which has been reported in animals treated with high doses of chemo-radiation treatment with other small molecular weight inhibitors (48).
In conclusion, we have demonstrated that YM155 significantly enhances the therapeutic efficacy of cisplatin treatment by inhibiting tumor growth and tumor angiogenesis. These results suggest a potentially novel strategy to reverse cisplatin resistance in head and neck cancers. Moreover, this strategy of using a combination of low doses of YM155 and cisplatin has the potential of significantly decreasing side effects associated with the concurrent chemo-radiation treatment while maintaining the therapeutic efficacy.
Supplementary Material
Acknowledgments
Financial support: NIH/NCI-CA133250 (P. Kumar) and Joan’s fund Research Grant (B. Kumar and P. Kumar).
Footnotes
Conflict of interest: The authors declare that they have no competing interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kalavrezos N, Bhandari R. Current trends and future perspectives in the surgical management of oral cancer. Oral Oncol. 2010;46:429–32. doi: 10.1016/j.oraloncology.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 4.Worden FP, Kumar B, Lee JS, Wolf GT, Cordell KG, Taylor JMG, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: Response and survival positively associated with HPV16 copy number. Journal of Clinical Oncology. 2008;26:3138–46. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colnaghi R, Connell CM, Barrett RM, Wheatley SP. Separating the anti-apoptotic and mitotic roles of survivin. J Biol Chem. 2006;281:33450–6. doi: 10.1074/jbc.C600164200. [DOI] [PubMed] [Google Scholar]
- 6.Knauer SK, Mann W, Stauber RH. Survivin’s dual role: an export’s view. Cell Cycle. 2007;6:518–21. doi: 10.4161/cc.6.5.3902. [DOI] [PubMed] [Google Scholar]
- 7.Connell CM, Colnaghi R, Wheatley SP. Nuclear survivin has reduced stability and is not cytoprotective. J Biol Chem. 2008;283:3289–96. doi: 10.1074/jbc.M704461200. [DOI] [PubMed] [Google Scholar]
- 8.Engels K, Knauer SK, Metzler D, Simf C, Struschka O, Bier C, et al. Dynamic intracellular survivin in oral squamous cell carcinoma: underlying molecular mechanism and potential as an early prognostic marker. J Pathol. 2007;211:532–40. doi: 10.1002/path.2134. [DOI] [PubMed] [Google Scholar]
- 9.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 10.Su L, Wang Y, Xiao M, Lin Y, Yu L. Up-regulation of survivin in oral squamous cell carcinoma correlates with poor prognosis and chemoresistance. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:484–91. doi: 10.1016/j.tripleo.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Duffy MJ, O’Donovan N, Brennan DJ, Gallagher WM, Ryan BM. Survivin: a promising tumor biomarker. Cancer Lett. 2007;249:49–60. doi: 10.1016/j.canlet.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Asechi H, Hatano E, Nitta T, Tada M, Iwaisako K, Tamaki N, et al. Resistance to cisplatin-induced apoptosis via PI3K-dependent survivin expression in a rat hepatoma cell line. Int J Oncol. 2010;37:89–96. [PubMed] [Google Scholar]
- 13.Chandele A, Prasad V, Jagtap JC, Shukla R, Shastry PR. Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine-induced apoptosis. Neoplasia. 2004;6:29–40. doi: 10.1016/s1476-5586(04)80051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem. 2006;281:25903–14. doi: 10.1074/jbc.M603414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirro E, Consoli ML, Massimino M, Manzella L, Frasca F, Sciacca L, et al. Altered expression of c-IAP1, survivin, and Smac contributes to chemotherapy resistance in thyroid cancer cells. Cancer Res. 2006;66:4263–72. doi: 10.1158/0008-5472.CAN-05-3248. [DOI] [PubMed] [Google Scholar]
- 16.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, et al. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci. 2002;59:1406–12. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgillo F, Martinelli E, Troiani T, Orditura M, De Vita F, Ciardiello F. Antitumor Activity of Sorafenib in Human Cancer Cell Lines with Acquired Resistance to EGFR and VEGFR Tyrosine Kinase Inhibitors. PLoS One. 2011;6:e28841. doi: 10.1371/journal.pone.0028841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Church DN, Talbot DC. Survivin in Solid Tumors: Rationale for Development of Inhibitors. Curr Oncol Rep. 2012 doi: 10.1007/s11912-012-0215-2. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara T, Takeuchi M, Kinoyama I, Minematsu T, Shirasuna K, Matsuhisa A, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–21. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 20.Kita A, Nakahara T, Yamanaka K, Nakano K, Nakata M, Mori M, et al. Antitumor effects of YM155, a novel survivin suppressant, against human aggressive non-Hodgkin lymphoma. Leuk Res. 2011;35:787–92. doi: 10.1016/j.leukres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Nakahara T, Yamanaka K, Hatakeyama S, Kita A, Takeuchi M, Kinoyama I, et al. YM155, a novel survivin suppressant, enhances taxane-induced apoptosis and tumor regression in a human Calu 6 lung cancer xenograft model. Anticancer Drugs. 2011;22:454–62. doi: 10.1097/CAD.0b013e328344ac68. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka K, Nakahara T, Yamauchi T, Kita A, Takeuchi M, Kiyonaga F, et al. Antitumor activity of YM155, a selective small-molecule survivin suppressant, alone and in combination with docetaxel in human malignant melanoma models. Clin Cancer Res. 2011;17:5423–31. doi: 10.1158/1078-0432.CCR-10-3410. [DOI] [PubMed] [Google Scholar]
- 23.Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, et al. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–6. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 24.Lewis KD, Samlowski W, Ward J, Catlett J, Cranmer L, Kirkwood J, et al. A multi-center phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Invest New Drugs. 2011;29:161–6. doi: 10.1007/s10637-009-9333-6. [DOI] [PubMed] [Google Scholar]
- 25.Gioanni J, Fischel JL, Lambert JC, Demard F, Mazeau C, Zanghellini E, et al. Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: establishment, characterization and response to cytotoxic treatment. Eur J Cancer Clin Oncol. 1988;24:1445–55. doi: 10.1016/0277-5379(88)90335-5. [DOI] [PubMed] [Google Scholar]
- 26.Lansford CD, Grénman R, Bier H, Somers KD, Kim SY, Whiteside TL, et al. Head and neck cancers. In: Masters JPB, editor. Human Cell Culture. 1999. pp. 185–255. [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−[Delta][Delta]CT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Yadav A, Kumar B, Teknos TN, Kumar P. Sorafenib enhances the antitumor effects of chemoradiation treatment by downregulating ERCC-1 and XRCC-1 DNA repair proteins. Mol Cancer Ther. 2011;10:1241–51. doi: 10.1158/1535-7163.MCT-11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 Promotes Head and Neck Tumor Metastasis by Inducing Epithelial-Mesenchymal Transition via the JAK-STAT3-SNAIL Signaling Pathway. Mol Cancer Res. 2011;9:1658–67. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar P, Benedict R, Urzua F, Fischbach C, Mooney D, Polverini P. Combination treatment significantly enhances the efficacy of antitumor therapy by preferentially targeting angiogenesis. Lab Invest. 2005;85:756–67. doi: 10.1038/labinvest.3700272. [DOI] [PubMed] [Google Scholar]
- 31.Kumar P, Ning Y, Polverini PJ. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Invest. 2008;88:740–9. doi: 10.1038/labinvest.2008.46. [DOI] [PubMed] [Google Scholar]
- 32.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. New England Journal of Medicine. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKenzie JA, Liu T, Goodson AG, Grossman D. Survivin Enhances Motility of Melanoma Cells by Supporting Akt Activation and α5 Integrin Upregulation. Cancer Research. 2010;70:7927–37. doi: 10.1158/0008-5472.CAN-10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar P, Miller AI, Polverini PJ. p38 MAPK mediates gamma-irradiation-induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol Chem. 2004;279:43352–60. doi: 10.1074/jbc.M405777200. [DOI] [PubMed] [Google Scholar]
- 36.Kumar P, Coltas IK, Kumar B, Chepeha DB, Bradford CR, Polverini PJ. Bcl-2 protects endothelial cells against gamma-radiation via a Raf-MEK-ERK-survivin signaling pathway that is independent of cytochrome c release. Cancer Res. 2007;67:1193–202. doi: 10.1158/0008-5472.CAN-06-2265. [DOI] [PubMed] [Google Scholar]
- 37.Virrey JJ, Guan S, Li W, Schonthal AH, Chen TC, Hofman FM. Increased survivin expression confers chemoresistance to tumor-associated endothelial cells. Am J Pathol. 2008;173:575–85. doi: 10.2353/ajpath.2008.071079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 39.Khan Z, Khan N, Tiwari RP, Patro IK, Prasad GBKS, Bisen PS. Down-regulation of survivin by oxaliplatin diminishes radioresistance of head and neck squamous carcinoma cells. Radiotherapy and Oncology. 2010;96:267–73. doi: 10.1016/j.radonc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Marioni G, Bedogni A, Giacomelli L, Ferraro SM, Bertolin A, Facco E, et al. Survivin expression is significantly higher in pN+ oral and oropharyngeal primary squamous cell carcinomas than in pN0 carcinomas. Acta Otolaryngol. 2005;125:1218–23. doi: 10.1080/00016480510038194. [DOI] [PubMed] [Google Scholar]
- 41.Bradford CR, Zhu S, Ogawa H, Ogawa T, Ubell M, Narayan A, et al. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck. 2003;25:654–61. doi: 10.1002/hed.10274. [DOI] [PubMed] [Google Scholar]
- 42.Kumar P, Gao Q, Ning Y, Wang Z, Krebsbach PH, Polverini PJ. Arsenic trioxide enhances the therapeutic efficacy of radiation treatment of oral squamous carcinoma while protecting bone. Mol Cancer Ther. 2008;7:2060–9. doi: 10.1158/1535-7163.MCT-08-0287. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proceedings of the National Academy of Sciences. 2000;97:13103–7. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marusawa H, Matsuzawa S-i, Welsh K, Zou H, Armstrong R, Tamm I, et al. HBXIP functions as a cofactor of survivin in apoptosis suppression. Embo J. 2003;22:2729–40. doi: 10.1093/emboj/cdg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez JA, Lens SMA, Span SW, Vader G, Medema RH, Kruyt FAE, et al. Subcellular localization and nucleocytoplasmic transport of the chromosomal passenger proteins before nuclear envelope breakdown. Oncogene. 2006;25:4867–79. doi: 10.1038/sj.onc.1209499. [DOI] [PubMed] [Google Scholar]
- 46.Stauber RH, Rabenhorst U, Rekik A, Engels K, Bier C, Knauer SK. Nucleocytoplasmic Shuttling and the Biological Activity of Mouse Survivin are Regulated by an Active Nuclear Export Signal. Traffic. 2006;7:1461–72. doi: 10.1111/j.1600-0854.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- 47.Knauer SK, Kramer OH, Knosel T, Engels K, Rodel F, Kovacs AF, et al. Nuclear export is essential for the tumor-promoting activity of survivin. Faseb J. 2007;21:207–16. doi: 10.1096/fj.06-5741com. [DOI] [PubMed] [Google Scholar]
- 48.Gupta AK, Cerniglia GJ, Mick R, Ahmed MS, Bakanauskas VJ, Muschel RJ, et al. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys. 2003;56:846–53. doi: 10.1016/s0360-3016(03)00214-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.