Introduction
Multimodality treatment guidelines for colon cancer have been revised frequently as data accumulate on the optimal selection and timing of treatment. The National Comprehensive Cancer Network (NCCN) is a nonprofit organization that has established working, expert consensus and evidence-based guidelines for organ-specific cancer care, including care of patients with colon cancer.1 Stage-based treatment for colon cancer has been subjected to significant scrutiny over the last decade, and several large trials have examined the benefits of multimodality care for this disease.2-7 The current NCCN guidelines for colon cancer recommend that patients with stage I and low-risk stage II disease be treated with surgery alone; that patients with high-risk stage II disease and all patients with stage III disease be treated with surgical resection and adjuvant chemotherapy unless there are obvious contraindications; and that patients with stage IV disease be offered some combination of chemotherapy with or without surgical resection.1
There is clear evidence that adjuvant chemotherapy in patients with stage III colon cancer is associated with improved survival outcomes.3,4,8-15 However, the optimal treatment of patients with high-risk stage II disease (defined by tumor depth, histologic grade, margin status, and number of nodes retrieved)has been the subject of considerable study and is a subject of ongoing controversy. Many studies have shown an overall survival benefit from the addition of adjuvant chemotherapy to surgery, but some have not. Thus, although the NCCN guidelines recommend adjuvant chemotherapy for patients with high-risk stage II disease, a significant proportion of such patients do not receive adjuvant chemotherapy.
The goal of the current study, which expands on a previous study by our group that evaluated practice variation with respect to adherence to NCCN recommendations within the National Cancer Data Base (NCDB),16 was to examine the impact of adherence to guidelines on stage-specific survival outcomes in patients with stage III and high-risk stage II colon cancer. We examined factors associated with survival in order to identify subgroups of patients who may benefit from improved access to or delivery of cancer care.
Materials and Methods
Data Sources
The NCDB is a program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society that accumulates outcomes data from more than 1500 Commission-accredited cancer programs in the US and Puerto Rico. Reporting hospitals range from small community hospitals to National Cancer Institute-designated comprehensive cancer centers. The database captures approximately 70% of all cancer diagnoses within the US, offering a large hospital-based sampling of current clinical practice.17-19
Definition of Patient Cohort
The International Classification of Disease for Oncology (ICD-O-3) codes associated with a diagnosis of adenocarcinoma of the colon (ICD-O-3 topography codes C180 and C182-199 and morphology codes 8140-8144, 8210, 8211, 8220, 8221, 8260-8263, 8480-8481, 8490, and 8550) were used to select patients from within the National Cancer Data Base who were diagnosed with colon adenocarcinoma between 1998 and 2002. The cohort was limited to patients who received their first course of treatment at the reporting facility. Pathologic variables, including tumor depth, nodal status, and evidence of metastatic disease, were used to re-stage patients according to the American Joint Committee on Cancer staging system (6th edition).
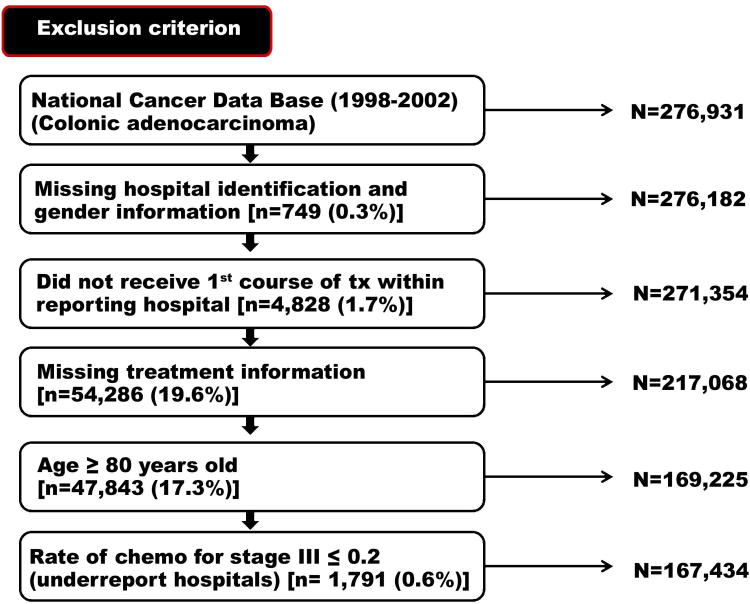
Patients with stage II disease were subdivided into low- and high-risk categories on the basis of criteria defined by the NCCN: patients were considered to have high-risk disease if they had T4 depth of invasion, histologic grade of 3 or greater, R1-R2 margin status, or fewer than 12 nodes were retrieved. Several other selection criteria were also used to restrict the cohort to minimize the potential for selection or reporting bias. Patients were excluded if they were older than 80 years or treated at an institution where the overall rate for recommending chemotherapy for stage III cancer was less than 20%, suggesting that an institution may have underreported this treatment variable (as previously described16). Patients with missing or incomplete treatment information were excluded from the analysis (Figure 1).
Figure 1.
Selection of patient cohort.
The definition of adherence or non-adherence to NCCN guidelines in this study is based upon stage-based NCCN recommendations for colon cancer. The current NCCN guidelines for colon cancer recommend that patients with stage I and low-risk stage II disease be treated with surgery alone; that patients with high-risk stage II disease and all patients with stage III disease be treated with surgical resection and adjuvant chemotherapy unless there are obvious contraindications; and that patients with stage IV disease be offered some combination of chemotherapy with or without surgical resection.1 Therefore, patients treated in accordance with these recommendations were categorized as “adherent” and those not treated according to these recommendations were categorized as “non-adherent”.16
Statistical Analyses
To assess cancer-specific causes of death, relative survival was used as a means of estimating disease-specific survival.20 Relative survival analysis is a validated method for performing survival studies that gives an objective measure of survival of cancer patients when comorbidities or issues complicating treatment do not exist. Relative survival is best defined as the ratio of the observed survival rate (including all causes of death) among an established cohort of cancer patients to the expected survival rate of a similar cohort of people who do not have cancer. Five-year relative survival was presented according to cancer stage and whether treatment adhered or did not adhere to NCCN guidelines. Propensity scores were also used to adjust for potential confounding in the analysis of treatment outcome.21,22 Specifically, several models were created to compare the effects of adherent versus nonadherent treatment on relative survival within groups stratified by propensity scores incorporating gender, age, race, insurance, facility type, year of diagnosis, and class of case; pooled stratum-specific estimates were computed for groups with similar propensity scores. We also included the propensity score, as a summary measure of all potential confounders, as one of the covariates in the multivariable regression models.
Two-level, stage-specific, hierarchical generalized linear regression models were used to analyze the influence of multiple factors on relative survival for patients with stage III and high-risk stage II disease to evaluate the influence of various demographic factors. All the reported p values were 2-sided, and values less than 0.05 were considered statistically significant. Statistical analyses were performed using Stata 10 software (Stata Corp, College Station, TX. Forest plots were generated using SigmaPlot 10 for Windows.
Results
Following the application of defined inclusion and exclusion criteria (Figure 1), the final study cohort consisted of 167,434 patients. The baseline patient characteristics are shown in Table 1. The gender distribution did not differ by stage, and 50.7% of patients overall were male. Younger patients were more likely to present with more advanced disease (51.1% of patients with stage IV disease were < 65 years old). The bulk of patients across all stages were Caucasian (81.2%), and most patients had Medicare (47.4%) or private insurance (43.0%). Most patients (81.2%) had a median household income (as measured per zip code) greater than $30,000 per year. Patients most commonly received treatment at community cancer centers (52.0%); the remaining patients were treated at academic hospitals (29.2%) or community hospitals (18.8%). Most patients received their treatment at the hospital of initial diagnosis (81.4%).
Table 1. Patient Characteristics Stratified by Cancer Stage.
| Characteristic | % of Patients | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Stage I (n=34,476) | Stage II Low Risk (n=18,192) | Stage II High Risk (n=28,592) | Stage III (n=48,758) | Stage IV (n=37,416) | Total (n=167,434) | |
| Male | 51.2 | 49.8 | 49.5 | 50.3 | 52.2 | 50.7 |
| Age, years | ||||||
| <50 | 8.4 | 11.9 | 8.7 | 13.5 | 15.2 | 11.8 |
| 50-64 | 31.8 | 31.6 | 29.3 | 34.0 | 35.9 | 32.9 |
| 65-74 | 38.3 | 35.7 | 38.4 | 34.3 | 32.8 | 35.6 |
| ≥75 | 21.5 | 20.8 | 23.7 | 18.2 | 16.2 | 19.6 |
| Race/ethnicity | ||||||
| Caucasian | 83.8 | 80.7 | 82.9 | 80.6 | 78.6 | 81.2 |
| African American | 9.7 | 11.7 | 10.1 | 11.5 | 14.2 | 11.5 |
| Hispanic | 2.9 | 3.9 | 3.8 | 3.8 | 3.7 | 3.6 |
| Other | 3.6 | 3.7 | 3.2 | 4.2 | 3.5 | 3.7 |
| Insurance status | ||||||
| Uninsured | 1.5 | 3.1 | 2.8 | 3.0 | 4.4 | 3.0 |
| Medicaid | 2.0 | 3.0 | 3.1 | 3.4 | 4.5 | 3.3 |
| Medicare | 51.0 | 47.9 | 52.7 | 45.0 | 42.7 | 47.4 |
| Other government* | 0.4 | 0.5 | 0.4 | 0.4 | 0.5 | 0.4 |
| Private | 42.7 | 42.6 | 38.2 | 45.1 | 44.4 | 43.0 |
| Unknown | 2.5 | 3.0 | 2.8 | 3.1 | 3.5 | 3.0 |
| Median household income | ||||||
| <$30,000/year | 12.2 | 13.3 | 14.1 | 13.8 | 15.1 | 13.7 |
| $30-45.999,000/year | 43.3 | 44.1 | 45.7 | 43.7 | 44.0 | 44.1 |
| ≥$46,000/year | 39.4 | 37.6 | 35.6 | 37.4 | 35.7 | 37.1 |
| Unknown | 5.1 | 5.0 | 4.6 | 5.1 | 5.2 | 5.0 |
| Year of diagnosis | ||||||
| 1998 | 17.4 | 18.0 | 19.8 | 18.9 | 18.5 | 18.5 |
| 1999 | 18.5 | 18.2 | 19.3 | 19.0 | 18.9 | 18.8 |
| 2000 | 19.8 | 19.4 | 20.1 | 19.7 | 19.2 | 19.7 |
| 2001 | 22.1 | 21.4 | 21.0 | 21.3 | 21.7 | 21.5 |
| 2002 | 22.2 | 23.0 | 19.8 | 21.1 | 21.7 | 21.5 |
| Treatment facility | ||||||
| Community hospital | 18.7 | 17.2 | 21.0 | 18.9 | 17.8 | 18.8 |
| Community cancer center | 54.0 | 51.0 | 54.0 | 52.5 | 48.5 | 52.0 |
| Academic hospital | 27.3 | 31.8 | 25.0 | 28.6 | 33.8 | 29.2 |
| Treatment facility and facility where disease diagnosed | ||||||
| Same facility | 83.1 | 82.3 | 83.7 | 80.4 | 78.9 | 81.4 |
| Different facilities | 16.9 | 17.7 | 16.3 | 19.6 | 21.1 | 18.6 |
| Adherent treatment | 96.4 | 67.0 | 35.7 | 73.6 | 63.4 | 68.8 |
Other government includes Federal insurance programs such as Veterans Affairs, TRICARE/Military, and Public Health Service.
Rates of adherence to NCCN treatment guidelines were 96.4% for patients with stage I disease, 67.0% for patients with low-risk stage II disease, 35.7% for patients with high-risk stage II disease, 73.6% for patients with stage III disease, and 63.4% for patients with stage IV disease. The highest rate of nonadherence occurred in the high-risk stage II group (undertreatment), followed by stage IV (undertreatment), low-risk stage II (overtreatment), and stage III (undertreatment); the treatment of stage I disease was highly compliant with guidelines.16
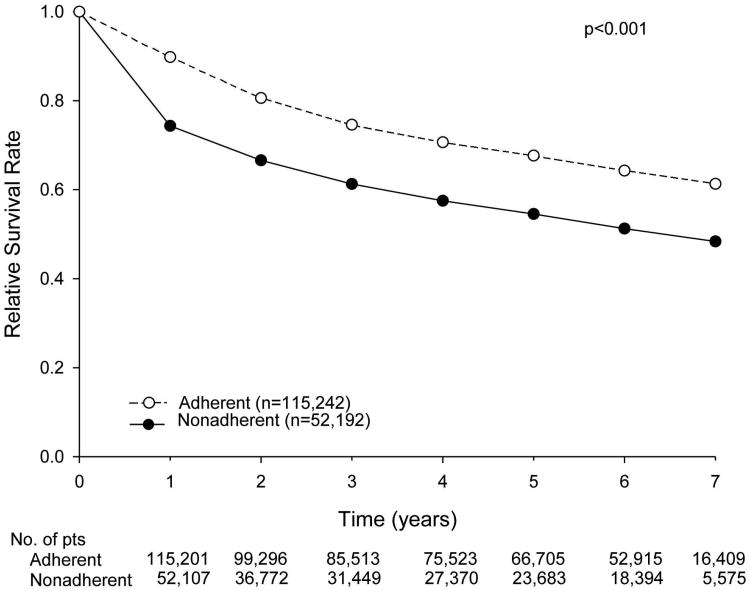
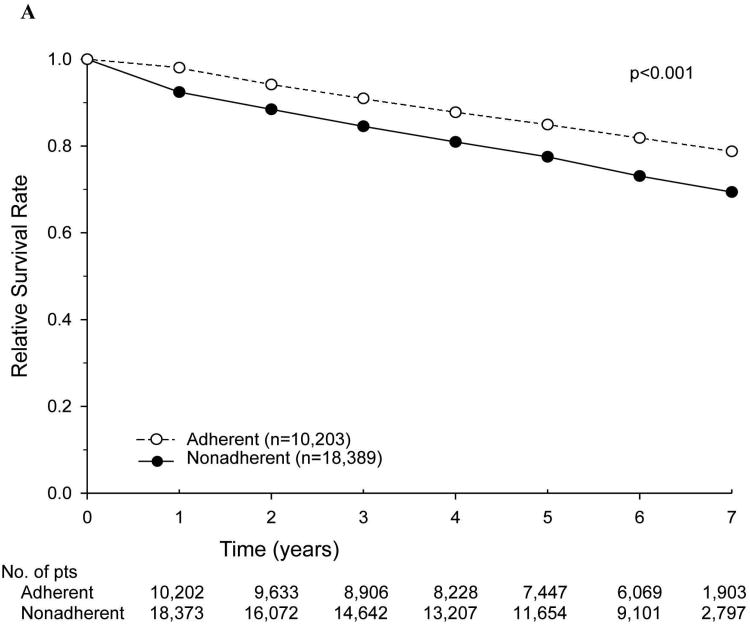
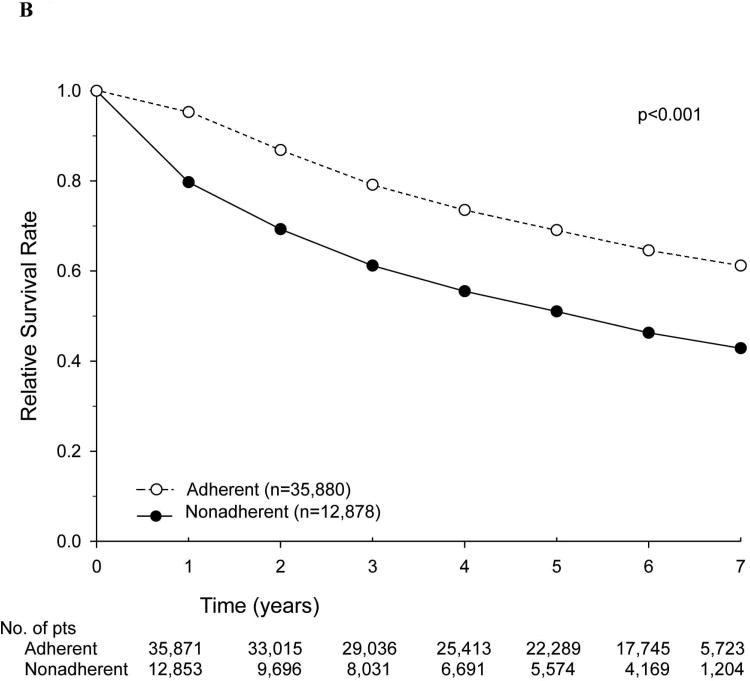
The relative survival rates of patients for the entire cohort, stratified according to adherence versus nonadherence to NCCN treatment guidelines, are shown in Figure 2. Nonadherence to current NCCN guidelines was associated with a decreased 5-year relative survival rate (54.5% vs. 67.7%). We then examined stage-specific relative survival rates stratified by adherence to current NCCN guidelines, specifically focusing on patients with stage III and high-risk stage II disease. Among patients with high-risk stage II disease, the 5-year relative survival rate was lower with nonadherence (i.e., no adjuvant chemotherapy) (77.5% vs. 84.9%) (Figure 3A). A similar trend was noted for patients with stage III disease who did not receive adherent treatment (5-year relative survival rate of 50.9% vs. 68.8%) (Figure 3B). Overall survival was also calculated with identical stage-specific survival trends observed, as well as similar hazard ratios in multivariate analyses (data not shown).
Figure 2.
Relative survival of patients with colon cancer treated in accordance with (adherent) or not in accordance with (nonadherent) National Comprehensive Cancer Network guidelines.
Figure 3.
Relative survival of patients with colon cancer treated in accordance with (adherent) or not in accordance with (nonadherent) National Comprehensive Cancer Network guidelines, by stage. (A) Patients with high-risk stage II disease. (B) Patients with stage III disease.
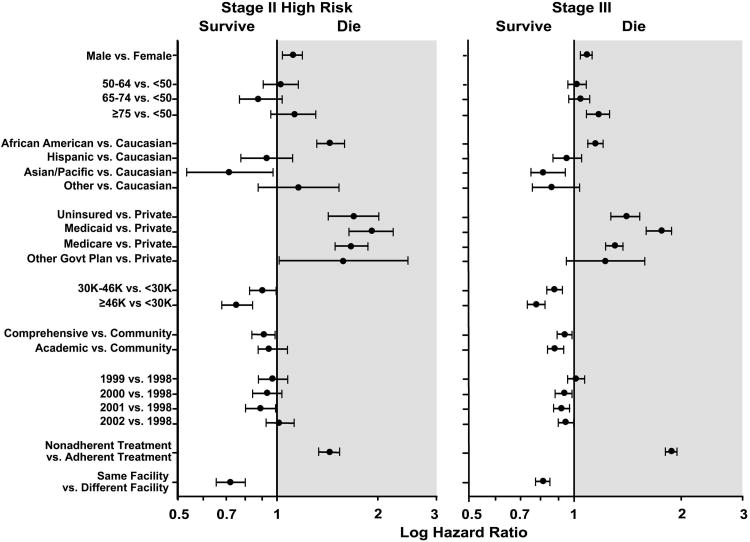
On multivariate analysis of relative survival in patients with high-risk stage II disease and patients with stage III disease (Table 2 and Figure 4), multiple factors were associated with differences in relative survival. In both patient subgroups, factors associated with decreased survival included male gender, African American ethnicity, lack of insurance, Medicaid coverage, and Medicare coverage. Age 75 years or greater was also associated with decreased relative survival in patients with stage III disease. In both patient subgroups, residence in area code with median income of $30,000/year or greater was associated with improved relative survival, as was treatment at a comprehensive cancer center and treatment at the same facility where the colon cancer was initially diagnosed. There was a trend towards increased relative survival in later years compared to earlier years of diagnosis in both patients with high-risk stage II disease and patients with stage III disease, most likely representing the evolution of practice patterns and available treatment options over time. In both groups, patients not treated in accordance with NCCN guidelines (i.e., “undertreated”) had an increased risk of death.
Table 2. Multivariate Relative Survival Analysis by Disease Stage.
| Variable | High-Risk Stage II | Stage III | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Male vs. female | 1.11 | 1.04-1.18 | 1.09 | 1.05-1.12 |
| Age, years | ||||
| <50 | 1.00 | Referent | 1.00 | Referent |
| 50-64 | 1.02 | 0.91-1.15 | 1.02 | 0.96-1.07 |
| 65-74 | 0.89 | 0.77-1.03 | 1.04 | 0.97-1.11 |
| ≥75 | 1.12 | 0.95-1.30 | 1.17 | 1.08-1.26 |
| Race/ethnicity | ||||
| Caucasian | 1.00 | Referent | 1.00 | Referent |
| African American | 1.44 | 1.31-1.59 | 1.15 | 1.09-1.21 |
| Hispanic | 0.93 | 0.78-1.11 | 0.95 | 0.87-1.04 |
| Asian/Pacific Islander | 0.72 | 0.54-0.97 | 0.84 | 0.75-0.94 |
| Insurance | ||||
| Private | 1.00 | Referent | 1.00 | Referent |
| Uninsured | 1.70 | 1.43-2.01 | 1.39 | 1.27-1.53 |
| Medicaid | 1.91 | 1.64-2.23 | 1.73 | 1.60-1.88 |
| Medicare | 1.67 | 1.49-1.87 | 1.30 | 1.23-1.37 |
| Other government* | 1.58 | 1.01-2.47 | 1.22 | 0.95-1.57 |
| Income | ||||
| <$30,000/year | 1.00 | Referent | 1.00 | Referent |
| $30,000-46,000/year | 0.90 | 0.83-0.99 | 0.88 | 0.83-0.92 |
| >$46,000/year | 0.76 | 0.68-0.84 | 0.78 | 0.74-0.82 |
| Treatment facility | ||||
| Community hospital | 1.00 | Referent | 1.00 | Referent |
| Community cancer center | 0.91 | 0.84-0.99 | 0.94 | 0.90-0.98 |
| Academic hospital | 0.94 | 0.85-1.03 | 0.89 | 0.84-0.93 |
| Year of diagnosis | ||||
| 1998 | 1.00 | Referent | 1.00 | Referent |
| 1999 | 0.97 | 0.88-1.07 | 1.01 | 0.96-1.07 |
| 2000 | 0.93 | 0.84-1.03 | 0.93 | 0.89-0.99 |
| 2001 | 0.89 | 0.80-0.98 | 0.92 | 0.87-0.97 |
| 2002 | 1.02 | 0.92-1.13 | 0.95 | 0.90-0.99 |
| Treatment | ||||
| Adherent | 1.00 | Referent | 1.00 | Referent |
| Nonadherent | 1.43 | 1.32-1.54 | 1.88 | 1.82-1.95 |
| Treatment facility and facility where disease diagnosed | ||||
| Different facility | 1.00 | Referent | 1.00 | Referent |
| Same facilities | 0.73 | 0.66-0.80 | 0.82 | 0.78-0.85 |
HR indicates hazard ratio. CI indicates confidence interval.
Bold font indicates statistical significance.
Other government includes Federal Insurance Programs such as Veterans Affairs, TRICARE/Military, and Public Health Service.
Figure 4.
Multivariate analysis of factors affecting relative survival in patients with high-risk stage II colon cancer and patients with stage III colon cancer.
To elucidate the specific factors underlying the trends in adherence to treatment recommendations, univariate analysis was used to analyze adherence patterns in both patient subgroups (high-risk stage II and stage III). Several factors were associated with a higher rate of nonadherence in both groups, including older age (p<0.001); Medicaid, Medicare, or uninsured status versus private insurance (p<0.001); and subsequent treatment at a different facility than the facility where the cancer was first diagnosed(p<0.001).
The hazard ratios for relative survival associated with nonadherent versus adherent treatment were noted to be similar regardless of the specific propensity-adjusted application examined (Table 3). In addition, the propensity-adjusted estimates were similar to the multivariate hazard ratios for relative survival which were estimated for nonadherent versus adherent treatment without propensity scores, HR=1.43 (95%CI: 1.33-1.54) for stage II high risk and HR=1.88 (95%CI: 1.82-1.95) for stage III patients.
Table 3.
Hazard Ratios for Relative Survival Associated with Nonadherent versus Adherent Treatment With and Without Adjustment by Propensity Scores.
| Stage II High | Stage III | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Univariate | 1.66 | 1.54-1.80 | <0.001 | 1.57 | 1.46-1.69 | <0.001 |
| Multivariate | 1.43 | 1.33-1.54 | <0.001 | 1.88 | 1.82-1.95 | <0.001 |
| Propensity scores | ||||||
| as a covariate | 1.44 | 1.33-1.55 | <0.001 | 1.88 | 1.82-1.95 | <0.001 |
| strata | 1.44 | 1.33-1.55 | <0.001 | 1.89 | 1.82-1.95 | <0.001 |
| weight (reverse) | 1.49 | 1.39-1.61 | <0.001 | 1.82 | 1.76-1.89 | <0.001 |
| weight (SMR) | 1.49 | 1.39-1.61 | <0.001 | 1.82 | 1.76-1.89 | <0.001 |
HR indicates hazard ratio. CI indicates confidence interval.
Discussion
The findings of this study, a large hospital-based analysis, demonstrate a relative survival benefit for patients with stage III and high-risk stage II colon cancer treated according to the NCCN guidelines. While a number of trials support the survival benefits of adjuvant chemotherapy in patients with stage III disease,3,8-12 the recommendations in favor of adjuvant chemotherapy in patients with high-risk stage II disease are based on compiled data from several studies suggesting that there is a subset of such patients who may benefit from adjuvant therapy.2,8-10,23,24 Our data offer additional evidence to support the current NCCN recommendations on the effectiveness of adjuvant chemotherapy in heterogeneous populations that extend the results of well-controlled clinical trials. The current analysis also identified several other factors associated with an increased risk of death in both patients with stage III disease and those with high-risk stage II disease, including male gender, African American race, insurance status other than private insurance (i.e., Medicare, Medicaid, other government insurance, or lack of insurance), lower household income, treatment at a community hospital, and treatment at an institution other than the hospital of diagnosis.
The current recommendations for adjuvant treatment of high-risk stage II colon cancer are based on composite data from a number of different studies. These include the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-04,6 NSABP C-05,25 and NSABP C-077 trials as well as the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC trial), all of which showed a benefit from adjuvant chemotherapy with 5-fluorouracil and leucovorin.3 The Intergroup 0035 study demonstrated a trend towards a lower rate of recurrence in patients with stage II colon cancer treated with adjuvant fluorouracil plus levamisole but did not show improvements in overall survival.5 A Surveillance, Epidemiology, and End Results (SEER) Medicare study of patients with stage II disease did not show a significant increase in survival with adjuvant treatment but did show a trend in that direction.15 The Quick and Simple and Reliable (QUASAR) study is the most contemporary study to specifically evaluate the benefit of adjuvant chemotherapy in patients with stage II colon cancer and identified a small but measurable benefit in overall survival and a clinically significant reduction in recurrence risk with the addition of 5-fluorouracil-based adjuvant chemotherapy.23 Pooled analysis of the NSABP C-01 through C-05 trials demonstrated improved overall survival in patients with both stage II and stage III disease treated with adjuvant 5-fluorouracil and leucovorin.10 The combined observations from NSABP C-07 10 as well as the International Multicentre Pooled Analysis of B2 Colon Cancer (IMPACT B-2) trial, 2contributed to the NCCN adoption of their current recommendations for adjuvant treatment in both patients with stage III and patients with high-risk stage II disease when possible. However, the formal NCCN recommendations for the treatment of high risk stage II patients with adjuvant chemotherapy is based upon several pooled analyses of the literature 26, 27, 28.
The recently published pooled analysis of the NSABP C-01 through C-05 trials, including 2273 patients with colon cancer (rectal cancer excluded), showed outcomes consistent with our study findings24. In the pooled analysis, improved overall survival was seen with adjuvant chemotherapy in both patients with stage II disease (HR=0.65, 95% CI: 0.48-0.71) and patients with stage III disease (HR=0.65, 95% CI: 0.55-0.75). These results, as well as our own findings, demonstrate a much greater effect than that seen in the ACCENT study group, which compiled data from 18 trials of adjuvant chemotherapy in patients with stage II and III colon cancer.29 These differences may reflect differences in patient populations or selection criteria. In the NSABP pooled analysis24, factors associated with poor outcomes were age greater than 60 years, male gender, African-American race, and fewer than 12 lymph nodes examined. Our findings are similar, with consistent trends in relative survival demonstrating an increased risk of death associated with age greater than 75 years, male gender, and African American race. Additionally, our analysis noted an increased risk of death in patients with insurance status other than private insurance among patients with stage III and high-risk stage II disease not treated in adherence to NCCN guidelines (i.e., with surgery alone). Similar survival trends relating to insurance status and demographic factors have been noted in several other studies.30-34
These survival benefits associated with adherence to NCCN guidelines, which call for patients with high-risk stage II disease to receive adjuvant chemotherapy, are in contrast to results reported by O'Connor et al,35 who examined data from the SEER-Medicare database from the same time period and reported no survival benefit associated with chemotherapy in patients with high-risk stage II disease (HR=1.03; 95% CI: 0.94-1.13).35 SEER accumulates data from specific geographic areas covering 26% of the US population and documents cancer incidence and survival in 15 population-based registries.36 An earlier analysis of SEER-Medicare data (1991-1996), which examined outcomes from 3151 patients with low-risk stage II colon cancer (T3N0M0), reported a 5-year survival rate of 75% for patients treated with surgery, but without adjuvant chemotherapy and 78% for patients treated with adjuvant chemotherapy. In this older SEER analysis, the hazard ratio for survival was 0.91 (95% CI: 0.77-1.09), and the survival advantage from adjuvant chemotherapy did not reach statistical significance despite a trend toward improved outcomes.15 Our current findings demonstrating a survival benefit from adjuvant chemotherapy in patients with high-risk stage II disease may reflect cohort differences and/or differences in patient selection practices between the SEER-Medicare and the National Cancer Data Base. The most obvious difference is patient age, as 45% of patients in the National Cancer Data Base analysis were under the age of 65 years and our selection criteria excluded all patients over the age of 80 years. In addition, our data demonstrated poorer survival outcomes in patients with Medicare than in patients with private insurance, a group excluded from the previous SEER analysis. These differences in patient selection may contribute to our overall improved survival statistics for each stage of disease as compared to the survival statistics in previously reported studies.
When we examined factors influencing adherence to NCCN treatment recommendations, our findings on univariate analysis suggested that in both patients with high-risk stage II disease and those with stage III disease, adherence to NCCN guidelines was worse with increasing age, insurance status other than private insurance, and treatment at a different facility from the one where colon cancer was diagnosed.
Recently, several groups have examined trends in racial disparities in colon cancer patients. A study of the SEER database by Robbins et al.37 spanning the same timeframe as our current study demonstrated increasing disparities between blacks and whites in colon cancer survival, relating to differences in the stage of diagnosis and resultant outcomes. These differences may be related to differences in access to and receipt of adjuvant chemotherapy. While referral rates may be the same for black and white patients, differences in these 2 groups in terms of actual treatment with adjuvant therapy have previously been reported.37 The current analysis is not an intention-to-treat analysis and reflects the actual treatment received by patients, which may also contribute to the racial disparities identified.
In terms of access to care, other groups have previously documented differences in cancer outcomes based on insurance status.38,39 A recent evaluation of the Ohio Cancer Incidence Surveillance System demonstrated higher mortality and unfavorable survival outcomes in Medicaid patients compared to non-Medicaid patients with various malignancies, including colon cancer.40 These studies support the hypothesis that multiple factors influence the utilization of and access to health resources and that these factors may be associated with measurable survival outcomes.
The current study has a number of limitations, including the possibility of variations in practice patterns and chemotherapy regimens administered over the time period covered by the study (1998 – 2002). However, multivariate analyses which included year of diagnosis did not demonstrate significant differences over time (Table 2). Additionally, specific pathologic data fields were absent (e.g., lymphovascular invasion and microsatellite stability/instability) as well as clinical data noting whether the tumors were obstructing and/or emergent surgery was required. Therefore, the low and high risk categorization of patients with stage II disease in this analysis does not include all of the criteria currently used in the current clinical setting. However such misclassification of high risk patients as low risk would likely result in minimizing differences in outcomes. In addition, the NCDB is a hospital-based database that includes a younger cohort of patients with various types of insurance not previously included in the other studies and spans a broad range of hospital settings. Our selection criteria created an analytic cohort limited to patients less than 80 years old, almost half of whom were younger than 65 years, which may have influenced the magnitude of the treatment effects seen in our analysis. Given that measures of baseline health indices including co-morbidities were not available, it is possible that baseline health factors may have contributed to treatment decisions and be reflected in the relative survival differences.
Furthermore, our analysis was not an intention-to-treat analysis, and outcomes relating to adjuvant chemotherapy reflect situations in which adjuvant chemotherapy was actually received by the patient.
Overall, the current analysis documents practice patterns in a heterogeneous population of patients with colon cancer and demonstrates a survival benefit for patients with stage III and high-risk stage II colon cancer who received treatment that adhered to NCCN guidelines. These data validate the current NCCN practice guidelines for colon cancer and support the concept of guideline-based metrics that can be compared across institutions to assess the quality of cancer care and compare the quality of cancer care among institutions.
Acknowledgments
The authors would like to thank Stephanie Deming for editorial assistance.
This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant, CA016672.
Footnotes
Financial Disclosures/Conflicts of Interest: There are no financial disclosures or conflicts of interest to report.
References
- 1.National Comprehensive Cancer Center N. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. doi: 10.6004/jnccn.2009.0056. [online] http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 2.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17(5):1356–1363. [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 4.Laurie JA, Moertel CG, Fleming TR, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989 Oct;7(10):1447–1456. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. J Clin Oncol. 1995 Dec;13(12):2936–2943. doi: 10.1200/JCO.1995.13.12.2936. [DOI] [PubMed] [Google Scholar]
- 6.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999 Nov;17(11):3553–3559. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 7.Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011 Oct 1;29(28):3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 9.Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23(34):8671–8678. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 10.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 11.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25(23):3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 12.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352(26):2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009 Jul 1;27(19):3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 14.Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802) Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2009 Apr;20(4):674–680. doi: 10.1093/annonc/mdn680. [DOI] [PubMed] [Google Scholar]
- 15.Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20(19):3999–4005. doi: 10.1200/JCO.2002.11.084. [DOI] [PubMed] [Google Scholar]
- 16.Chagpar RXY, Chian YJ, Feig BW, Chang GJ, You NY, Cormier JN. Predicting adherence to stage-specific treatment guidelines for adenocarcinoma of the colon. Journal of ClinicalOncology. 2012 doi: 10.1200/JCO.2011.39.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele GD, Jr, Winchester DP, Menck HR. The National Cancer Data Base. A mechanism for assessment of patient care. Cancer. 1994;73(2):499–504. doi: 10.1002/1097-0142(19940115)73:2<499::aid-cncr2820730241>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Winchester DP, Stewart AK, Phillips JL, Ward EE. The national cancer data base: past, present, and future. Ann Surg Oncol. 2010;17(1):4–7. doi: 10.1245/s10434-009-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23(1):51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 21.Shah BR, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. Journal of clinical epidemiology. 2005 Jun;58(6):550–559. doi: 10.1016/j.jclinepi.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Suh HS, Hay JW, Johnson KA, Doctor JN. Comparative effectiveness of statin plus fibrate combination therapy and statin monotherapy in patients with type 2 diabetes: use of propensity-score and instrumental variable methods to adjust for treatment-selection bias. Pharmacoepidemiology and drug safety. 2012 May;21(5):470–484. doi: 10.1002/pds.3261. [DOI] [PubMed] [Google Scholar]
- 23.Quasar Collaborative G. Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson NW, Yothers G, Lopa S, Costantino JP, Petrelli NJ, Wolmark N. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Ann Surg Oncol. 2010;17(4):959–966. doi: 10.1245/s10434-009-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolmark N, Bryant J, Smith R, et al. Adjuvant 5-fluorouracil and leucovorin with or without interferon alfa-2a in colon carcinoma: National Surgical Adjuvant Breast and Bowel Project protocol C-05. Journal of the National Cancer Institute. 1998 Dec 2;90(23):1810–1816. doi: 10.1093/jnci/90.23.1810. [DOI] [PubMed] [Google Scholar]
- 26.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004 Aug 15;22(16):3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 27.Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care's gastrointestinal cancer disease site group. J Clin Oncol. 2004 Aug 15;22(16):3395–3407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 28.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004 May 15;22(10):1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 29.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009 Feb 20;27(6):872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhoads KF, Cullen J, Ngo JV, Wren SM. Racial and ethnic differences in lymph node examination after colon cancer resection do not completely explain disparities in mortality. Cancer. 2012;118(2):469–477. doi: 10.1002/cncr.26316. [DOI] [PubMed] [Google Scholar]
- 31.Richards CA, Kerker BD, Thorpe L, et al. Increased screening colonoscopy rates and reduced racial disparities in the New York Citywide campaign: an urban model. Am J Gastroenterol. 2011;106(11):1880–1886. doi: 10.1038/ajg.2011.191. [DOI] [PubMed] [Google Scholar]
- 32.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. Journal of the National Cancer Institute. 2010;102(8):538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry J, Caplan L, Davis S, et al. A black-white comparison of the quality of stage-specific colon cancer treatment. Cancer. 2010;116(3):713–722. doi: 10.1002/cncr.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etzioni DA, El-Khoueiry AB, Beart RW., Jr Rates and predictors of chemotherapy use for stage III colon cancer: a systematic review. Cancer. 2008;113(12):3279–3289. doi: 10.1002/cncr.23958. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011 Sep 1;29(25):3381–3388. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SEER Cancer Statistics Review 1975-2008. National Cancer Institute; 1975-2008. SEER Cancer Statistics Review 1975-2008. [Google Scholar]
- 37.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012 Feb 1;30(4):401–405. doi: 10.1200/JCO.2011.37.5527. [DOI] [PubMed] [Google Scholar]
- 38.Robbins AS, Pavluck AL, Fedewa SA, Chen AY, Ward EM. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009 Aug 1;27(22):3627–3633. doi: 10.1200/JCO.2008.20.8025. [DOI] [PubMed] [Google Scholar]
- 39.Kelz RR, Gimotty PA, Polsky D, Norman S, Fraker D, DeMichele A. Morbidity and mortality of colorectal carcinoma surgery differs by insurance status. Cancer. 2004 Nov 15;101(10):2187–2194. doi: 10.1002/cncr.20624. [DOI] [PubMed] [Google Scholar]
- 40.Koroukian SM, Bakaki PM, Raghavan D. Survival disparities by Medicaid status: An analysis of 8 cancers. Cancer. 2011 Dec 27; doi: 10.1002/cncr.27380. [DOI] [PMC free article] [PubMed] [Google Scholar]