Abstract
The purpose of this study was to examine the biodistribution of 99mTc-RAD-Arg-(Arg11)CCMSH in B16/F1 melanoma-bearing C57 mice to determine whether the replacement of the Lys linker with an Arg linker could decrease the renal uptake of 99mTc-RAD-Arg-(Arg11)CCMSH. 99mTc-RAD-Arg-(Arg11)CCMSH exhibited rapid and high tumor uptake (17.98 ± 4.96% ID/g at 2 h post-injection) in B16/F1 melanoma-bearing C57 mice. As compared to 99mTc-RAD-Lys-(Arg11)CCMSH, the replacement of the Lys linker with an Arg linker dramatically decreased the renal uptake of 99mTc-RAD-Arg-(Arg11)CCMSH by 68, 62, 73 and 64% at 0.5, 2, 4 and 24 h post-injection, respectively. Flank B16/F1 melanoma lesions were clearly imaged at 2 h post-injection using 99mTc-RAD-Arg-(Arg11)CCMSH as an imaging probe.
Keywords: Alpha-melanocyte stimulating hormone peptide, melanocortin-1 receptor, renal uptake, melanoma imaging
Melanocortin-1 (MC1) receptor is a G protein-coupled receptor which is over-expressed on human and mouse melanoma cells.1–5 Over the past several years, we have been developing a novel class of radiolabeled alpha-melanocyte stimulating hormone (α-MSH) peptides to target MC1 receptors for melanoma imaging.5–8 Specifically, the cyclic RXD {Arg-X-Asp-dTyr-Asp} motif (X = Gly, Ala, Ser, Val, Thr, Nle, Phe or dPhe) was conjugated to [Cys3,4,10, d-Phe7, Arg11]α-MSH3-13 {(Arg11)CCMSH} peptide via a lysine linker to generate a series of RXD-Lys-(Arg11)CCMSH peptides. Interestingly, single amino acid at the X position displayed a profound effect in melanoma targeting and clearance properties. First of all, we found that the switch from RGD to RAD dramatically improved the MC1 receptor binding affinity of RAD-Lys-(Arg11)CCMSH as compared to RGD-Lys-(Arg11)CCMSH in M21 and B16/F1 melanoma cells. 5,6 The stronger MC1 receptor binding yielded higher melanoma uptake for 99mTc-RAD-Lys-(Arg11)CCMSH than 99mTc-RGD-Lys-(Arg11)CCMSH. 6 Moreover, the residues of Ser, Val and Thr resulted in more favorable melanoma targeting and clearance properties than the residues of Nle, Phe or dPhe.7,8 However, all 99mTc-RXD-Lys-(Arg11)CCMSH peptides exhibited high non-specific renal uptake. Thus, it is desirable to reduce the renal uptake to facilitate their potential applications.
Despite the profound effect of single amino acid (Gly, Ala, Ser, Val and Thr) at the X position in 99mTc-RXD-Lys-(Arg11)CCMSH peptides, the positively-charged amino acid residues were same among the 99mTc-RXD-Lys-(Arg11)CCMSH peptides. Specifically, each 99mTc-RXD-Lys-(Arg11)CCMSH peptide has three arginine residues and one lysine linker. In our previous reports, L-lysine co-injection successfully reduced the renal uptake of 99mTc-RXD-Lys-(Arg11)CCMSH peptides by 37–55% at 2 h post-injection.6–8 Meanwhile, it was reported that the replacement of Lys with an Arg dramatically decreased the renal uptake of 99mTc-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH by 41–64% at 2 h post-injection.9–11 Therefore, we hypothesized that the replacement of the Lys linker with an Arg linker would decrease the renal uptake of 99mTc-RXD-Lys-(Arg11)CCMSH peptides. Thus, as a proof-of-principal study, we prepared and evaluated the biodistribution property of 99mTc-RAD-Arg-(Arg11)CCMSH to examine our hypothesis in this study.
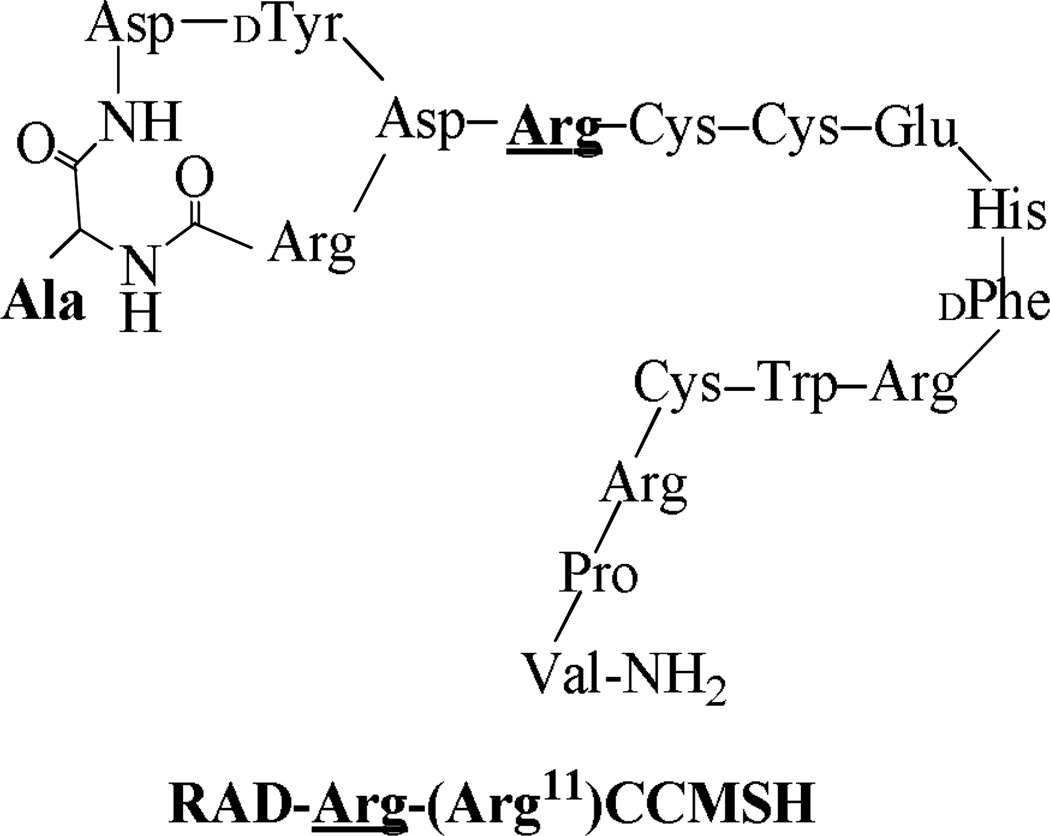
Firstly, RAD-Arg-(Arg11)CCMSH was synthesized using fluorenylmethyloxycarbonyl (Fmoc) chemistry, purified by reverse phase-high performance liquid chromatography (RP-HPLC) and characterized by electrospray ionization mass spectrometry according to our published procedure.12 The schematic structure of RAD-Arg-(Arg11)CCMSH is presented in Figure 1. The competitive binding study of RAD-Arg-(Arg11)CCMSH was determined in B16/F1 melanoma cells. The competitive binding curve of RAD-Arg-(Arg11)CCMSH is presented in Figure 2. The MC1 receptor binding affinity of RAD-Arg-(Arg11)CCMSH was 0.22 nM which was comparable to that of RAD-Lys-(Arg11)CCMSH (0.26 nM) in B16/F1 cells. The receptor binding result indicated that the replacement of the Lys linker with an Arg linker retained its nanomolar MC1 receptor binding affinity. 99mTc-RAD-Lys-(Arg11)CCMSH was readily prepared with greater than 95% radiolabeling yield and was separated from its excess nonlabeled peptide by high performance liquid chromatography (HPLC). The radiochemical purity of 99mTc-RAD-Lys-(Arg11)CCMSH was greater than 98%. The specific activity of 99mTc-RAD-Lys-(Arg11)CCMSH was 8.406 × 109 MBq/g.
Figure 1.
Schematic structure of RAD-Arg-(Arg11)CCMSH.
Figure 2.
The competitive binding curve of RAD-Arg-(Arg11)CCMSH in B16/F1 melanoma cells. The IC50 value of RAD-Arg-(Arg11)CCMSH was 0.22 nM.
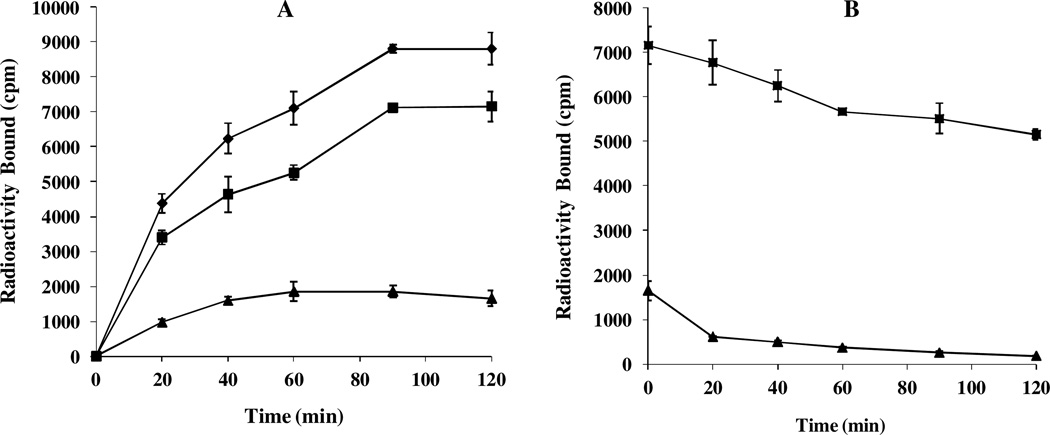
Secondly, cellular internalization and efflux properties of 99mTc-RAD-Arg-(Arg11)CCMSH were examined in B16/F1 melanoma cells. The cellular results are presented in Figure 3. 99mTc-RAD-Arg-(Arg11)CCMSH exhibited rapid cellular internalization and extended cellular retention. Approximately 77.81 ± 4.46% of the 99mTc-RAD-Arg-(Arg11)CCMSH activity internalized at 20 min post incubation, whereas 81.26 ± 4.81% of the 99mTc-RAD-Arg-(Arg11)CCMSH activity internalized after 2 h incubation. The efflux results demonstrated that 72.07 ± 1.61% of the 99mTc-RGD-Arg-(Arg11)CCMSH activity remained inside the cells 2 h after incubating cells in culture medium. 99mTc-RAD-Arg-(Arg11)CCMSH displayed similar rapid internalization and extended retention pattern as 99mTc-RAD-Lys-(Arg11)CCMSH.11
Figure 3.
Cellular internalization (A) and efflux (B) of 99mTc-RAD-Arg-(Arg11)CCMSH in B16/F1 melanoma cells. Total bound radioactivity (♦), internalized radioactivity (■) and cell membrane radioactivity (▲) were presented as counts per minute (cpm).
Thirdly, the melanoma targeting and pharmacokinetic properties of 99mTc-RAD-Arg-(Arg11)CCMSH were determined in B16/F1 melanoma-bearing C57 mice. The biodistribution results are presented in Table 1. 99mTc-RAD-Arg-(Arg11)CCMSH exhibited rapid and high tumor uptake in melanoma-bearing mice. The tumor uptake was 17.98 ± 4.96 and 14.07 ± 2.90% ID/g at 2 and 4 h post-injection. In peptide blocking study, approximately 87% of the tumor uptake of 99mTc-RAD-Arg-(Arg11)CCMSH was blocked by 10 µg (6.1 nmol) of non-radiolabeled NDP-MSH at 2 h post-injection (p<0.05), demonstrating that the tumor uptake was specific and MC1 receptor-mediated. Whole-body clearance of 99mTc-RGD-Arg-(Arg11)CCMSH was rapid, with approximately 75% of the injected radioactivity cleared through the urinary system by 2 h post-injection (Table 1). Normal organ uptake of 99mTc-RGD-Arg-(Arg11)CCMSH was generally lower than 1.9% ID/g except for kidneys after 2 h post-injection. High tumor/blood and tumor/muscle uptake ratios were demonstrated as early as 0.5 h post-injection (Table 1). The renal uptake of 99mTc-RAD-Arg-(Arg11)CCMSH reached its highest value of 41.24 ± 4.48% ID/g at 0.5 h post-injection and decreased to 11.99 ± 2.29% ID/g at 24 h post-injection. In peptide blocking study at 2 h post-injection, the renal uptake was not significantly (p = 0.063) different with or without the peptide blockade, suggesting that the renal uptake was not receptor-mediated.
Table 1.
Biodistribution of 99mTc-RAD-Arg-(Arg11)CCMSH in B16/F1 melanoma-bearing C57 mice. The data was presented as percent injected dose/gram or as percent injected dose (mean±SD, n=5)
| Tissue | 0.5 h | 2 h | 4 h | 24 h | 2 h NDP blockade |
|---|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | |||||
| Tumor | 8.46 ± 3.70 | 17.98 ± 4.96 | 14.07 ± 2.90 | 10.16 ± 2.66 | 2.39 ± 0.66* |
| Brain | 0.16 ± 0.04 | 0.04 ± 0.02 | 0.02 ± 0.02 | 0.02 ± 0.00 | 0.06 ± 0.02 |
| Blood | 3.43 ± 0.37 | 0.59 ± 0.17 | 0.10 ± 0.06 | 0.04 ± 0.01 | 0.99 ± 0.05 |
| Heart | 1.71 ± 0.58 | 0.50 ± 0.14 | 0.21 ± 0.03 | 0.10 ± 0.02 | 0.66 ± 0.12 |
| Lung | 5.42 ± 0.70 | 1.15 ± 0.22 | 0.40 ± 0.16 | 0.16 ± 0.03 | 1.38 ± 0.49 |
| Liver | 3.07 ± 0.08 | 1.88 ± 0.35 | 1.47 ± 0.11 | 1.18 ± 0.28 | 2.13 ± 0.27 |
| Skin | 5.43 ± 0.41 | 1.10 ± 0.39 | 0.53 ± 0.09 | 0.24 ± 0.10 | 1.15 ± 0.20 |
| Spleen | 2.12 ± 0.57 | 0.61 ± 0.31 | 0.25 ± 0.12 | 0.41 ± 0.14 | 0.84 ± 0.36 |
| Stomach | 3.13 ± 0.62 | 1.63 ± 0.43 | 1.41 ± 0.74 | 0.28 ± 0.09 | 2.35 ± 0.61 |
| Kidneys | 41.24 ± 4.48 | 35.64 ± 9.61 | 26.51 ± 0.62 | 11.99 ± 2.29 | 27.16 ± 5.58 |
| Muscle | 0.80 ± 0.46 | 0.20 ± 0.15 | 0.15 ± 0.10 | 0.03 ± 0.02 | 0.23 ± 0.07 |
| Pancreas | 1.06 ± 0.33 | 0.20 ± 0.11 | 0.10 ± 0.05 | 0.10 ± 0.05 | 0.29 ± 0.10 |
| Bone | 1.97 ± 0.22 | 0.64 ± 0.25 | 0.34 ± 0.13 | 0.14 ± 0.04 | 0.92 ± 0.26 |
| Percent injected dose (%ID) | |||||
| Intestines | 2.53 ± 0.18 | 1.38 ± 0.41 | 2.24 ± 1.08 | 0.41 ± 0.11 | 2.50 ± 0.16 |
| Bladder | 46.28 ± 2.59 | 74.50 ± 7.30 | 80.16 ± 2.77 | 91.45 ± 1.86 | 77.85 ± 2.34 |
| Uptake Ratio of Tumor/Normal Tissue | |||||
| Tumor/Blood | 2.47 | 30.47 | 140.70 | 254.00 | 2.41 |
| Tumor/Kidneys | 0.21 | 0.50 | 0.53 | 0.85 | 0.09 |
| Tumor/Lung | 1.56 | 15.63 | 35.18 | 63.50 | 1.73 |
| Tumor/Liver | 2.76 | 9.56 | 9.57 | 8.61 | 1.12 |
| Tumor/Muscle | 10.58 | 89.90 | 93.80 | 338.67 | 10.39 |
p<0.05 (p=0.00006), significance comparison in tumor and kidney between 99mTc-RAD-Arg-(Arg11)CCMSH with/without peptide blockade at 2 h post-injection.
As compared to 99mTc-RAD-Lys-(Arg11)CCMSH,11 99mTc-RAD-Arg-(Arg11)CCMSH exhibited comparable melanoma uptake at 2, 4 and 24 h post-injection. The comparable melanoma uptake between 99mTc-RAD-Arg-(Arg11)CCMSH and 99mTc-RAD-Lys-(Arg11)CCMSH was attributed to the similar receptor binding affinities between RAD-Arg-(Arg11)CCMSH and RAD-Lys-(Arg11)CCMSH (0.22 vs. 0.26 nM). Despite that 99mTc-RAD-Arg-(Arg11)CCMSH and 99mTc-RAD-Lys-(Arg11)CCMSH displayed similar distribution pattern in normal organs, the renal uptake of 99mTc-RAD-Arg-(Arg11)CCMSH was dramatically lower than that of 99mTc-RAD-Lys-(Arg11)CCMSH. The renal uptake of 99mTc-RAD-Lys-(Arg11)CCMSH was 3.1, 2.6, 3.7 and 2.8 times the renal uptake of 99mTc-RAD-Arg-(Arg11)CCMSH at 0.5, 2 ,4 and 24 h post-injection, respectively. The comparable melanoma uptake and dramatically decreased renal uptake of 99mTc-RAD-Arg-(Arg11)CCMSH resulted in higher tumor to kidney uptake ratios as compared to 99mTc-RAD-Lys-(Arg11)CCMSH.
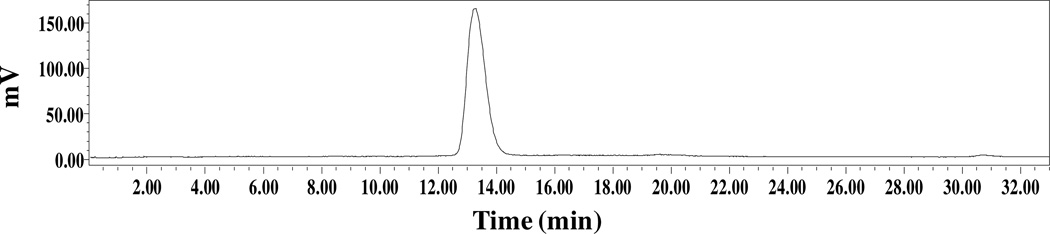
Finally, the melanoma imaging property of 99mTc-RAD-Arg-(Arg11)CCMSH was examined in a B16/F1 melanoma-bearing C57 mouse in this study. The representative whole-body single photon emission computed tomography (SPECT)/CT image is presented in Figure 4. Flank melanoma tumors were visualized clearly by 99mTc-RAD-Arg-(Arg11)CCMSH at 2 h post-injection. 99mTc-RAD-Arg-(Arg11)CCMSH exhibited high tumor to normal organ uptake ratios except for kidney, which was consistent with the biodistribution results (Table 1). The urine collected from the imaging mouse was analyzed for the metabolites by HPLC. The urinary HPLC profile of 99mTc-RAD-Arg-(Arg11)CCMSH is shown in Figure 6. 99mTc-RAD-Arg-(Arg11)CCMSH remained intact at 2 h post-injection.
Figure 4.
Representative whole-body SPECT/CT image of 99mTc-RAD-Arg-(Arg11)CCMSH in a B16/F1 melanoma-bearing C57 mouse at 2 h post-injection. Flank melanoma lesions (T) are highlighted with an arrow on the images.
In conclusion, the replacement of the Lys linker with an Arg linker dramatically decreased the renal uptake of 99mTc-RAD-Arg-(Arg11)CCMSH while retaining its high melanoma uptake. B16/F1 melanoma lesions were clearly visualized by SPECT/CT using 99mTc-RAD-Arg-(Arg11)CCMSH as an imaging probe. High melanoma uptake and decreased renal uptake of 99mTc-RAD-Arg-(Arg11)CCMSH suggests that the replacement of the Lys linker with an Arg linker may be useful in reducing the renal uptake of other 99mTc-RXD-Lys-(Arg11)CCMSH peptides in future studies.
The experimental details are presented in References and notes. 13–16
Figure 5.
Radioactive HPLC profile of urine sample of a B16/F1 melanoma-bearing C57 mouse at 2 h post-injection of 99mTc-RAD-Arg-(Arg11)CCMSH. The retention time (13.3 min) of the original compound of 99mTc-RAD-Arg-(Arg11)CCMSH prior to the tail vein injection is identical with that of the urine sample of 99mTc-RAD-Arg-(Arg11)CCMSH.
Acknowledgments
We appreciate Dr. Fabio Gallazzi for his technical assistance. This work was supported in part by the NIH grant NM-INBRE P20RR016480 and the University of New Mexico RAC Award. The image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, Eberle AN. Cancer Res. 1989;49:6352. [PubMed] [Google Scholar]
- 2.Tatro JB, Reichlin S. Endocrinology. 1987;121:1900. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- 3.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Bioconjug. Chem. 2003;14:1177. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Shenoy N, Gershman BM, Yang J, Sklar LA, Miao Y. Nucl. Med. Biol. 2009;36:267. doi: 10.1016/j.nucmedbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Guo H, Miao Y. Nucl. Med. Biol. 2010;37:873. doi: 10.1016/j.nucmedbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Miao Y. Bioorg. Med. Chem. Lett. 2012;22:1541. doi: 10.1016/j.bmcl.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flook AM, Yang J, Miao Y. Mol. Pharm. 2013;10:3417. doi: 10.1021/mp400248f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flook AM, Yang J, Miao Y. J. Med. Chem. 2013 in press. [Google Scholar]
- 9.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Cancer Res. 2000;60:5649. [PubMed] [Google Scholar]
- 10.Miao Y, Benwell K, Quinn TP. J. Nucl. Med. 2007;48:73. [PubMed] [Google Scholar]
- 11.Yang J, Miao Y. Bioorg. Med. Chem. 2010;18:6695. doi: 10.1016/j.bmc.2010.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Guo H, Gallazzi F, Berwick M, Padilla RS, Miao Y. Bioconjug. Chem. 2009;20:1634. doi: 10.1021/bc9001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MC1 receptor binding affinity: Amino acid and resin were purchased from Advanced ChemTech Inc. (Louisville, KY) and Novabiochem (San Diego, CA). 125I-Tyr2-[Nle4, dPhe7]-α-MSH {125I-(Tyr2)-NDP-MSH} was obtained from PerkinElmer, Inc. (Shelton, CT) for MC1 receptor binding assay. B16/F1 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA). The RAD-Arg-(Arg11)CCMSH was synthesized, purified by RP-HPLC and characterized by LC-mass spectroscopy according to our published procedure.11 The IC50 value of RAD-Arg-(Arg11)CCMSH for the MC1 receptor was determined in B16/F1 melanoma cells according to our published procedure11 Briefly, the B16/F1 cells (0.2×106 cells/well, n=3) were incubated at 25 °C for 2 h with approximately 30,000 counts per minute (cpm) of 125I-(Tyr2)-NDP-MSH in the presence of 10−13 to 10−6 M of RAD-Arg-(Arg11)CCMSH in 0.3 mL of binding medium. The IC50 value of RAD-Arg-(Arg11)CCMSH was calculated using the Prism software (GraphPad Software, La Jolla, CA).
- 14.Cellular internalization and efflux of 99mTc-RAD-Arg-(Arg11)CCMSH: 99mTcO4− was purchased from Cardinal Health (Albuquerque, NM) for peptide radiolabeling. All other chemicals used in this study were purchased from Thermo Fisher Scientific (Waltham, MA) and used without further purification. RAD-Arg-(Arg11)CCMSH was radiolabeled with 99mTc using the method described previously.11 The radiolabeled peptide was purified to single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytic column (Deerfield, IL) using a 20 min gradient of 16–26% acetonitrile in 20 mM HCl aqueous solution at a flow rate of 1 mL/min. Cellular internalization and efflux of 99mTc-RAD-Arg-(Arg11)CCMSH were evaluated in B16/F1 melanoma cells according to our published procedure.11
- 15.Biodistribution studies: All the animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The biodistribution of 99mTc-RAD-Arg-(Arg11)CCMSH was determined in B16/F1 melanoma-bearing C57 mice (Harlan, Indianapolis, IN). C57 mice were subcutaneously inoculated on the right flank with 1×106 B16/F1 cells. Tumor weights reached approximately 0.2 g at 10 days post cell inoculation. Each melanoma-bearing mouse was injected with 0.037 MBq of 99mTc-RAD-Arg-(Arg11)CCMSH via the tail vein. Groups of 5 mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight. The specificity of tumor uptake was determined at 2 h post-injection by co-injecting 99mTc-RAD-Arg-(Arg11)CCMSH with 10 µg (6.1 nmol) of unlabeled NDP-MSH. Statistical analysis was performed using the Student’s t-test for unpaired data to determine the significance of differences in tumor and kidney uptake between the groups in the biodistribution studies with/without peptide blockade. Differences at the 95% confidence level (p<0.05) were considered significant.
- 16.Melanoma imaging and urinary metabolites of 99mTc-RAD-Arg-(Arg11)CCMSH: Approximately 7.4 MBq of 99mTc-RAD-Arg-(Arg11)CCMSH was injected in a B16/F1 melanoma-bearing C57 mouse for imaging and urine analysis. The mouse was euthanized at 2 h post-injection for small animal SPECT/CT (Nano-SPECT/CT®, Bioscan) imaging, as well as to collect urine for analyzing the metabolites. The 9-min CT imaging was immediately followed by the whole-body SPECT scan. The SPECT scans of 24 projections were acquired. Reconstructed SPECT and CT data were visualized and co-registered using InVivoScope (Bioscan, Washington DC). The collected urine sample was centrifuged at 16,000 g for 5 min before the HPLC analysis. Thereafter, aliquots of the urine were injected into the HPLC. A 20-minute gradient of 16–26% acetonitrile / 20 mM HCl with a flow rate of 1 mL/min was used for urine analysis.