SUMMARY
We have translated a powerful genetic tool, designer receptors exclusively activated by designer drugs (DREADDs), from mammalian systems to Drosophila melanogaster to selectively, rapidly, reversibly, and dose-dependently control behaviors and physiological processes in the fly. DREADDs are muscarinic acetylcholine G protein-coupled receptors evolved for loss of affinity to acetylcholine and for the ability to be fully activated by an otherwise biologically inert chemical, clozapine-N-oxide. We demonstrate its ability to control a variety of behaviors and processes in larvae and adults, including heart rate, sensory processing, diurnal behavior, learning and memory, and courtship. The advantages of this particular technology include the dose-responsive control of behaviors, the lack of a need for specialized equipment, and the capacity to remotely control signaling in essentially all neuronal and nonneuronal fly tissues.
INTRODUCTION
Pharmacological tools have traditionally been a primary method used to modify neural signal transduction and function in mammalian systems. Although these methods are generally quite successful, certain limitations associated with pharmacological strategies include frequent deleterious off-target effects (Keiser et al., 2009). In the model organism Drosophila melanogaster, approaches taking advantage of the extensive genetic toolkit to elucidate the neuronal basis of behavior have been primarily utilized instead of small-molecule-based pharmacological tools. The bipartite GAL4/UAS expression system (Brand and Perrimon, 1993) and its modifications to optimize both spatial and temporal control of gene expression have been the primary workhorse. More recent methods of control of transgene expression used in combination with the GAL4/UAS system include the use of FLP recombinase (Keller et al., 2002; Struhl and Basler, 1993) and the use of hormones or drugs to induce expression of UAS transgenes (e.g., GeneSwitch) (Osterwalder et al., 2001; Roman et al., 2001). Advantages of these newer modifications include increased control of spatial and/or temporal expression and reversibility of expression through the addition or removal of a stimulus like the hormone RU486. Although greater control of targeted expression of transgenes has allowed for an enhanced understanding of the neuronal circuits in an intact organism, disadvantages to these systems include developmental and physiological “off-target” effects associated with constitutively expressed transgenes and slow on/off rates for hormonal induction systems (e.g., several days).
Recently developed methods target expression of proteins that, when activated, inhibit or enhance neuronal activity and circuitry function. Some strategies include using constitutively active sodium channels (NaChBac) or potassium channels (Kir2.1) to excite or hyperpolarize neurons, respectively (Nitabach et al., 2002, 2006). Conditional activation of neurons can also be achieved with dTRPA1, a temperature- and voltage-gated cation channel that regulates thermotactic behavior (Hamada et al., 2008; Parisky et al., 2008; Shang et al., 2008). The most common conditional method to silence neurons in the fly is to inhibit synaptic release using a temperature-sensitive dominant-negative allele of dynamin (shibirets). Using this system, shifting the fly to the nonpermissive temperature (>29°C) leads to loss of neurotransmitter vesicular recycling and a rapid inhibition of neurotransmitter release (Kitamoto, 2001). Another valuable tool for inducing activity in neuronal tissue is a light-sensitive cation channel, Channelrhodopsin-2 (ChR2) (Nagel et al., 2003). Exposure to intense blue light activates these channels and leads to depolarization and neuronal firing where expressed; therefore, activity can be precisely controlled at the temporal level (Boyden et al., 2005; Li et al., 2005; Nagel et al., 2003, 2005). Altering the duration or intensity of the light pulse can vary the response of the channels (Zhang et al., 2007). Although ChR2 is used successfully to manipulate neural circuit activity in Drosophila embryos and adults (Schroll et al., 2006; Zhang et al., 2007), limitations of the “optogenetic” approach include poor penetration of blue light into whole organisms (Eichler et al., 1977), sensitization issues, and a narrow dose-response control. A significant disadvantage of optogenetics is the need for specialized and expensive equipment like custom blue light sources with fiber optics or two-photon illumination. These limitations, taken together, contribute to the inadequacy of optogenetics for types of studies that require long-term or chronic modulation of neuronal activity in Drosophila. A significant limitation of each of these channel-based methods, as well as that of shibirets for neuronal control, is that they are essentially unidirectional switches and primarily either activate or inactivate neurons in an “all on” or “all off” manner. Additionally, such channel-based methods do not regulate G protein signaling.
Powerful new tools combining genetics and pharmacology that examine physiological functions and behaviors associated with specific neuronal circuits have recently been developed for mammalian systems that overcome many of the limitations of other methods. These systems make use of modified G protein coupled receptors (GPCRs) that respond to synthetic ligands that the wild-type receptor does not respond to and have been termed receptors activated solely by synthetic ligands (RASSLs) and designer receptors exclusively activated by a designer drug (DREADDs) (Armbruster et al., 2007; Coward et al., 1998; Nichols and Roth, 2009). While the RASSLs represent GPCRs that have been rationally designed or found in nature, and often still respond to the native ligand, the DREADDs were created through directed evolution to respond to a synthetic ligand but to have no affinity for its native ligand. The current set of DREADDs largely consist of mammalian muscarinic acetylcholine receptors that no longer have affinity for, and therefore have no response to their natural ligand, acetylcholine, and are fully and potently activated by the synthetic ligand clozapine-N-oxide (CNO), which is inert at all known mammalian GPCRs (Armbruster et al., 2007). In mammalian systems, DREADDs have produced conditional control of neuronal function through manipulation of effector pathways that is rapid, reversible, and does not require specialized equipment (Alexander et al., 2009; Krashes et al., 2011; Ray et al., 2011; Sasaki et al., 2011). Activating ligand can be easily and conveniently administered by simply feeding or injecting the drug to the animal. Importantly, and unlike all channel-based methods, DREADDs reliably control neuronal and effector pathway function in a true dose-dependent fashion.
We have adapted and translated the DREADD technology to Drosophila and show that the mammalian DREADD receptors effectively couple to Drosophila G protein effector pathways and alter behaviors and physiological function in both larvae and adults when activated by CNO. These effects are rapid, reversible, and dose responsive. We thereby demonstrate the utility of the DREADD technology for conditional control of signaling, behaviors, and physiological processes in the fly.
RESULTS
CNO Is Biologically Inert in the Fly
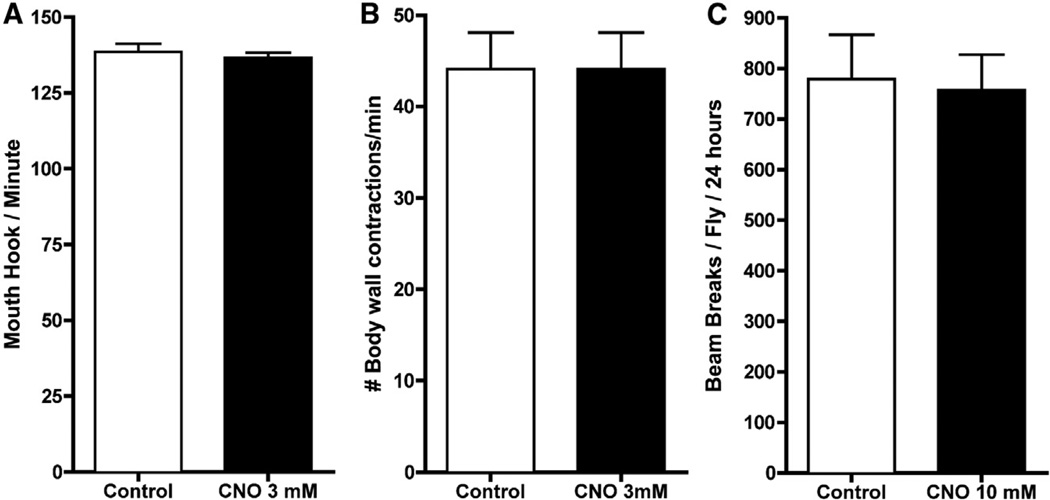
To determine if CNO had any effects on normal flies, we grew wild-type Canton-S (CS) flies on food containing 3 mM CNO and observed no gross overt adverse developmental consequences, developmental delays, or effects on lifespan (Figure S1). To test the effects of CNO on overt fly behavior, we tested larval activity and feeding, as well as adult activity. No significant differences in either the number of mouth hook contractions (a measure of feeding behavior) or body wall contractions were observed between the control and larvae fed either 3.0 mM CNO (Figure 1). To examine overt activity levels in adult flies, we used the Drosophila activity monitoring system (DAMS) photobeam break counting system. Adult CS flies fed with food containing 10 mM CNO for 5 days did not exhibit a significant change in activity when compared to flies not fed CNO (Figure 1C).
Figure 1. CNO Has No Effect on Wild-Type Larvae or Adults.
(A and B) Mouth hood contractions (A; a measure of feeding), and body wall contractions (B; a measure of locomotion) were counted for 1 min after Canton S (CS) larvae were fed 10% sucrose with no CNO (white bars) or 3.0 mM CNO (black bars). There were no statistical differences in flies fed CNO in either the mouth hook or body wall contractions when compared to control flies (N = 20).
(C) Sixteen CS adult males were loaded into the DAMS system with 10% sucrose (white bars) or 10% sucrose with CNO up to 10.0 mM (black bars), and their activity was measured. The activity levels of CNO-fed flies were comparable to control levels. Error bars indicate SEM.
DREADDs Couple to Drosophila Effector Pathways and Can Alter Neuronal Properties
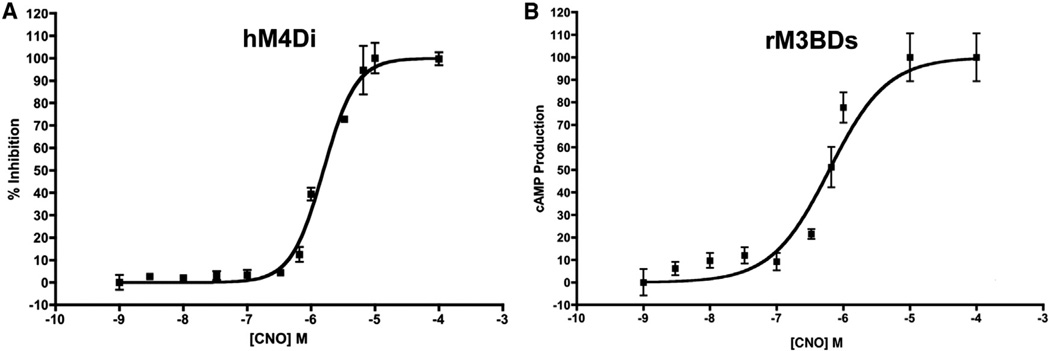
To verify that the mammalian DREADDs effectively couple to insect G proteins, we measured the abilities of the Gαi-coupled and Gαs-coupled DREADDs to modulate cyclic AMP (cAMP) production in Drosophila S2 cells when stimulated with CNO. We observed that stimulation of the Gαi-coupled hM4Di receptor with CNO negatively modulates cAMP levels in a dose-dependent manner and that the CNO stimulated Gαs-coupled rM3BDs receptor dose-dependently increases cAMP (Figures 2A and 2B).
Figure 2. Mammalian DREADD Receptors Couple to Insect G Proteins.
(A and B) The hM4Di and rM3BDs receptors were expressed in Drosophila S2 cells in culture, stimulated with CNO, and cAMP levels measured.
(A) The percent inhibition of forskolin-stimulated cAMP in S2 cells transfected with the hM4Di receptor. The hM4Di receptor negatively couples to adenylate cyclase, and the hM3BDs receptor positively couples to adenylate cyclase. N = 3 for each data point.
(B) Adenylate cyclase accumulation in S2 cells transfected with the rM3BDs receptor.
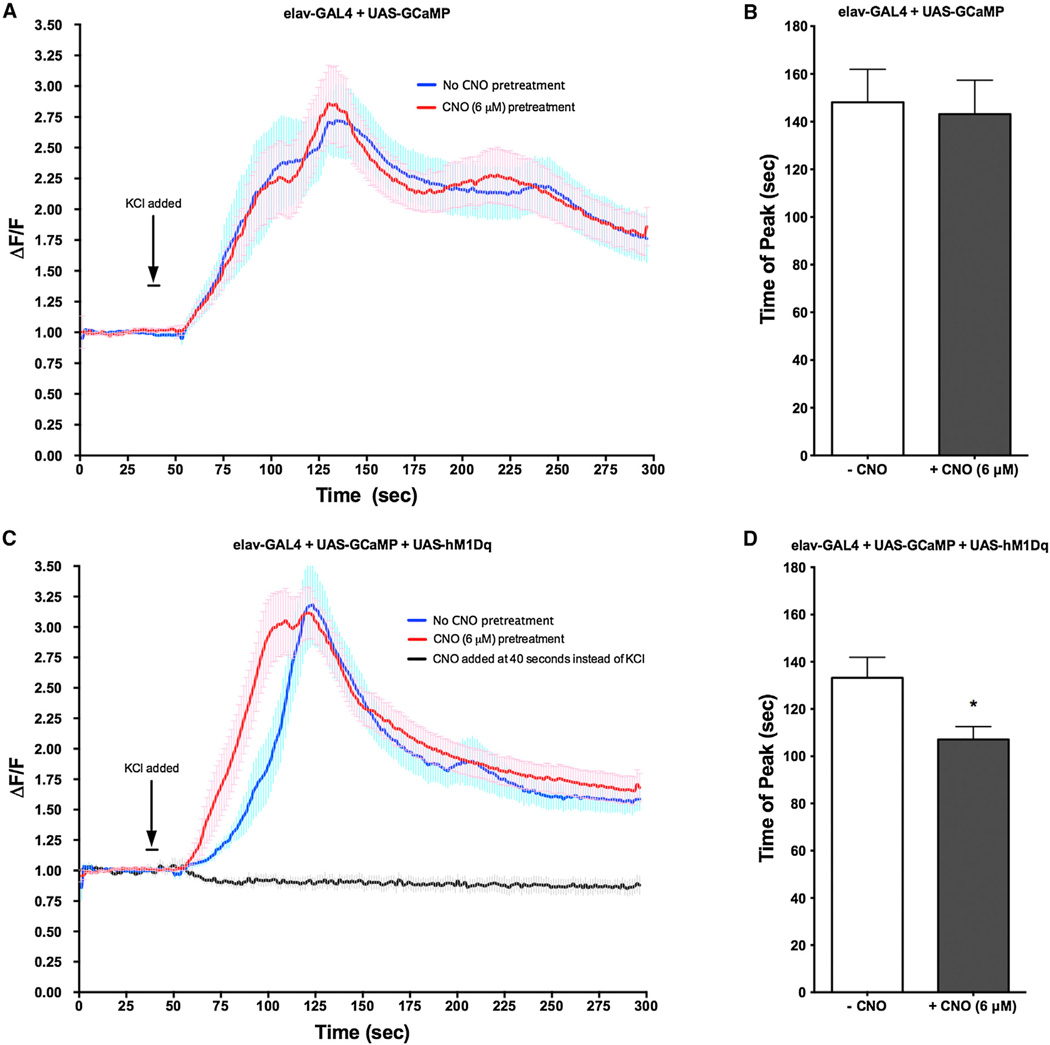
To examine the effects of activation of the hM1Dq receptor on neuronal properties in vivo, we coexpressed hM1Dq receptor along with a live cell fluorescent calcium sensor, GCaMP (Tian et al., 2009), in neurons of the third-instar larval brain using the pan-neuronal elavC155-GAL4 driver. The response to a depolarizing amount of KCl (75 mM) applied to the brain was measured in neurons of the ventral ganglion that were pretreated for 5 min with either ringers or ringers + CNO (6 uM). In fly brains carrying one copy of all three transgenes (elavC155-GAL4 + UAS-hM1Dq + UAS-GCaMP3), we observed that pretreatment with CNO resulted in a more rapid increase in the fluorescence intensity of neurons and in a reduced latency to the maximum peak intensity (Figures 3B and 3D). CNO had no effect on neuronal firing or maximum peak latency in control flies expressing only GCaMP by the elavc155-GAL4 driver (Figures 3A and 3B) or when added directly to the brain instead of KCl (Figure 3C). Together, these data are consistent with activation of the hM1Dq receptor facilitating neuronal depolarization, as has been previously observed in mammalian systems.
Figure 3. Activation of Mammalian hM1Dq Receptor Facilitates Neuronal Depolarization.
(A–D) The hM1Dq receptor was expressed in flies carrying the elavc155-GAL4 driver and the UAS-GcAMP. Brains from third-instar larvae were stimulated with KCl (75 mM), either in the presence or absence of CNO (6 µM). GCaMP fluorescence response was measured and the maximum peak latency was determined.
(A) In neurons only expressing GCaMP (elavc155-GAL4 + UAS-GCaMP), pretreatment with CNO had no effect on KCl-induced depolarization.
(B) Maximum peak latency was unaffected by pretreatment of CNO in larvae only expressing GCaMP (elavc155-GAL4 + UAS-GCaMP).
(C) In neurons expressing both GCaMP and hM1Dq (elavc155-GAL4 + UAS-GCaMP + hM1Dq), pretreatment with CNO resulted in a decreased latency to firing.
(D) Maximum peak latency was significantly reduced in CNO-pretreated brains expressing hM1Dq (elavc155-GAL4 + UAS-GCaMP + hM1Dq). *p < 0.005, Student’s t test. N = 15 brains per treatment, ten neurons per brain. Errors bars indicate SEM.
DREADD Activation Dose-Dependently Modulates Olfactory Response in Larva
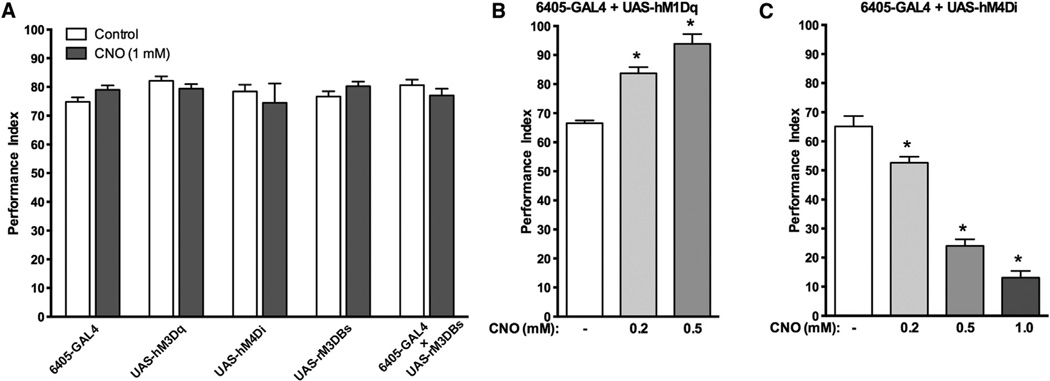
Each DREADD receptor was expressed in larval sensory neurons under the control of the SG18.1-GAL4 (6405-GAL4) driver (Sarpal et al., 2003). Next, their response to the attractant odor, ethyl acetate, was measured. In adults and larvae, attraction to ethyl acetate has been shown to involve Gαq signaling (Kain et al., 2009). Activation of hM1Dq expressed in sensory neurons with CNO produced a dose-dependent increase in the performance index (Figure 4B). CNO activation of sensory-neuron-expressed hM4Di receptor produced a dose-dependent decrease in the preference for ethyl acetate (Figure 4C). There was no effect of CNO on any of the parental lines or for the rM3BDs 3 6405-GAL4 F1 larva for the preference to ethyl acetate (Figure 4A). The maximal effective concentration in the food was approximately 1.0 mM CNO. Our previous studies with CNS active drugs at GPCRs have demonstrated robust behavioral activity in the low-millimolar range. Therefore, we chose to perform our subsequent experiments with low-millimolar CNO concentrations in the food.
Figure 4. CNO Activation of DREADDs in Chemosensory Neurons Dose-Dependently Controls Behavior.
(A–C) Flies carrying either the UAS-rM3BDs, UAS-hM1Dq, or UAS-hM4Di transgene were crossed to flies carrying the SB18.1-GAL4 (6405-GAL4) driver, which drives expression in sensory neurons, and the attraction to ethyl acetate measured.
(A) Performance index of flies carrying either the 6405-GAL4 driver, UAS- hM4Di, UAS-hM1Dq, UAS-rM3BDs, or both the 6405-GAL4 driver and the UAS-rM3BDs construct either fed food without CNO (white) or fed food with 1 mM CNO (gray bars). CNO had no effect on any of the parental lines or on flies carrying both 6406-GAL4 and UAS-rM3BDs.
(B) CNO activation of hM1Dq in sensory neurons (6405-GAL4 + UAS-hM1Dq) produces a dose-dependent increase in the performance index.
(C) Activation of hM4Di in the same neurons (6405-GAL4 + UAS-hM4Di) produces a dose-dependent decrease in the performance index (right). There was no observable difference in the number of immotile animals between all groups. *p < 0.005, ANOVA with Tukey post hoc test for multiple comparison. N = 3, 50 larva per trial. Error bars indicate SEM.
Gαi Activation in Larval Heart Disrupts Heart Rate
To express hM4Di in the heart, the 24B-GAL4 driver was used. This driver is expressed in all larval somatic muscle, including cardiac (Schuster et al., 1996). In nearly all larvae preparations where hM4Di was expressed in heart muscle, the heart dramatically slowed immediately after exposure to 500 nM CNO (Table 1; Movie S1). Each time the heart was revitalized when the CNO was washed out with fresh HL3 saline (pH 7.1). Flies that did not express hM4Di (but containing the UAS-hM4Di element) from the same cross (control sibling progeny that lacked the 24B-GAL4 element) showed no effect upon exposure to CNO (Table 1). Although 24B-GAL4 expresses in skeletal muscles, they have no impact on heart rate in dissected preparations because the heart is directly visualized contracting and the segmental roots to skeletal muscles from the CNS have been transected.
Table 1.
Activation of hM4D in Larval Heart Disrupts Heart Rate
| Treatment | hM4D | Control | ||
|---|---|---|---|---|
| Saline | CNO | Saline | CNO | |
| 31 | 0 | 39 | 68 | |
| 80 | 0 | 65 | 86 | |
| 108 | 122 | 96 | 60 | |
| 136 | 55 | 84 | 105 | |
| 59 | 0 | 58 | 30 | |
| 54 | 0 | 169 | 171 | |
| 38 | 0 | 19 | 0 | |
| 25 | 0 | 138 | 146 | |
| 70 | 0 | 81 | 47 | |
| 102 | 0 | 70 | 17 | |
| 144 | 0 | 58 | 63 | |
| 43 | 0 | 87 | 81 | |
| 93 | 127 | |||
| 107 | 85 | |||
| Mean ± SEM | 77.8 ± 10.2 | 27.8 ± 12.9a | 80.3 ± 11.8 | 72.8 ± 14.4 |
The UAS-hM4Di was crossed to the 24B-GAL4 driver line. “24B” is heterozygous for the 24B-Gal4 transgene and homozygous for the UAS-GFP transgene. CNO (500 nM) was applied to the hearts of partially dissected live third-instar larvae and the effects directly visualized. The application of CNO rapidly and dramatically slowed the heart in nearly all larvae. Values represent heartbeats per minute from the same larvae before (saline) and after CNO application (CNO). hM4Di = 24B-GAL4 +/−; UAS-mCD8:GFP +/−; UAS-hM4D +/−. Control (siblings from the same F1 cross not carrying the 24B-GAL4) = UAS-mCD8:GFP +/−; UAS-hM4D +/−.
p < 0.01 saline versus CNO; ANOVA with Bonferroni post hoc test for multiple comparison.
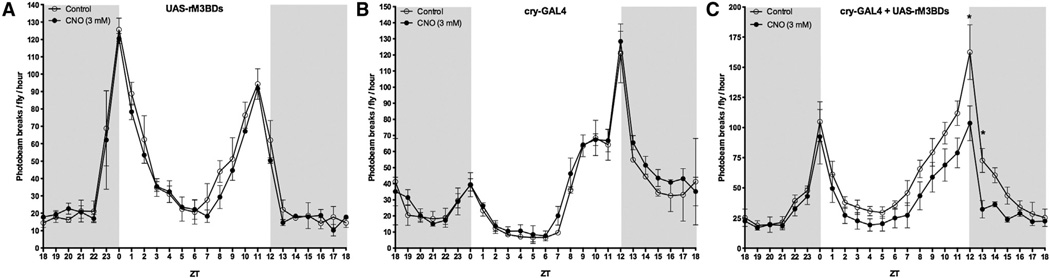
Gαs Activation in Cry-GAL4 Neurons Affects Diurnal Behavior in Adults
The neurons defined by the cryptochrome (cry) GAL4 driver define many cells of the circadian clock (Im et al., 2011). An important component of the cry circuitry is the PDF receptor, which is a Gas-coupled receptor (Im and Taghert, 2010). Therefore, manipulating cAMP levels through Gαs activation will likely influence certain circadian behaviors. UAS-rM3BDs 3 cry-GAL4 F1 flies fed 3.0 mM CNO had decreased early night activity levels compared to the same genotype flies not fed CNO (Figure 5C). There were no observable differences in activity levels in either parental strains fed CNO (Figures 5A and 5B).
Figure 5. Activation of hM3BDs in Cry Neurons Affects Diurnal Behavior in Adults.
(A–C) Males carrying transgenes for the cry-GAL4 driver, the UAS-rM3BDs, or both were fed either 10% sucrose (open circles) or 10% sucrose + 3 mM CNO closed circles) and their activity levels were monitored using the DAMS system for 5 days.
(A and B) Flies carrying either the UAS-rM3BDs (A) or the cry-GAL4 (B) exhibited no significant alterations in diurnal behaviors when fed CNO as compared to the flies not fed CNO.
(C) F1 flies expressing rM3BDs in cry-GAL4 neurons (cry-GAL4 + UAS-rM3BDs) fed CNO exhibited decreased late day activity levels compared to non-CNO-fed F1 flies. Gray shading indicates dark conditions (ZT 0 = lights on). Results represent the average of two (A and B) and three (C) separate experiments, with each separate experiment consisting of 16 male adult flies each for each treatment condition. *p < 0.05, two-way ANOVA with Bonferroni post hoc test for multiple comparison. Error bars indicate SEM. There appears to be an unknown mutation/background effect affecting normal diurnal behavior in the parental cry-GAL4 driver. Nevertheless, the F1 progeny demonstrate normal diurnal behavior in the absence of CNO, which is affected by CNO, whereas the parental behavior is not.
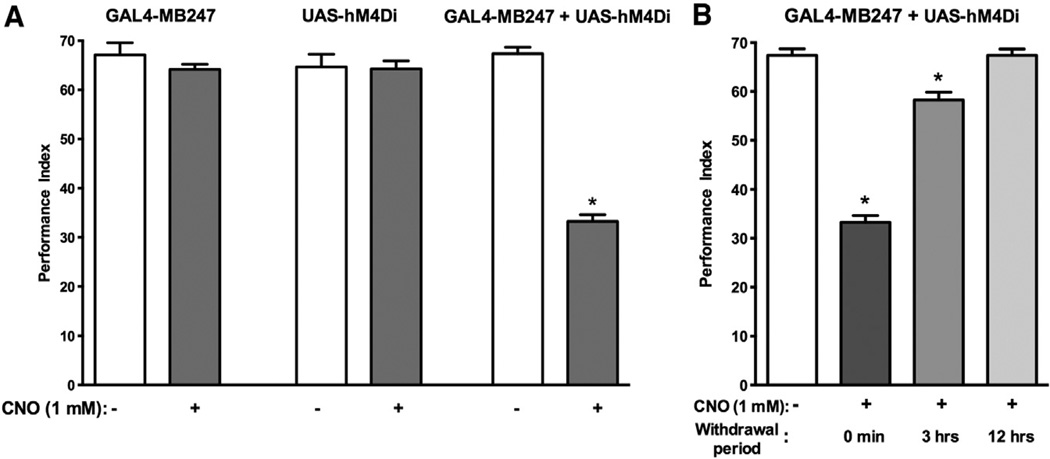
Stimulation of hM4Di in the Mushroom Body Disrupts Short-Term Learning and Memory
The mushroom body (MB) has been shown to be necessary for proper short-term memory (STM) function (Zars et al., 2000). Furthermore, cAMP levels in the MBs are crucial for olfactory learning and memory (Davis et al., 1995). Disruption of cAMP levels in the MBs with DREADDs is therefore predicted to negatively influence learning and memory. To determine if we could use the DREADD system to probe MB circuitry function, we expressed the hM4Di receptor in a subset of MB lobes using the MB247-GAL4 driver, activated them with CNO, and measured the resulting performance. The UAS-hM4Di 3 MB247-GAL4 F1 flies fed CNO (1.0 mM) prior to training exhibited a 50% decrease in the performance index compared to the non-CNO-fed F1 flies (Figure 6A). We observed no difference in the performance of any of the parental strains fed CNO (Figure 6A).
Figure 6. Activation of hM4Di in the Mushroom Bodies Disrupts Short-Term Learning and Memory.
(A) Flies carrying either the UAS-hM4Di transgene (left), the MB247-GAL4 driver (center), or both in combination (right) were fed CNO and the STM performance measured [PI = (# of flies avoiding the paired odor) – (# of flies avoiding the unpaired odor)/total flies]. The parental strain flies carrying only one or the other of the transgenes exhibited no difference in the performance index after being fed CNO. The F1 flies carrying both transgenes exhibited a 50% decrease in the performance index when the expressed hM4Di receptor (MB247-GAL4 + UAS-hM4Di) was activated with 1 mM CNO. *p < 0.05, Student’s t test. N = 8 trials. Error bars indicate SEM.
(B) The effects of DREADD activation are reversible. Flies expressing hM4Di in the mushroom bodies (MB247-GAL4 + UAS-hM4Di) were again monitored for short-term memory, this time after being removed from the CNO. Flies immediately trained and tested exhibited the expected 50% decrease in performance index (dark gray). When flies were removed from the CNO for 3 hr, the decrease in PI was only 12% (gray). Flies that were removed from the CNO for 12 hr prior to training and testing did not exhibit a decrease in PI (light gray) when compared to F1 flies that were not fed CNO (white). *p < 0.05, ANOVA with Bonferroni post hoc test for multiple comparison. N = 8. Error bars indicate SEM.
The Behavioral Effects of DREADD Activation Are Reversible
To determine if DREADD activation is reversible, we used the UAS-hM4Di × MB247-GAL4 F1 flies in additional short-term learning and memory assays. The flies were removed from the CNO at T = 0, 3, and 12 hr prior to training. When the flies were immediately trained (T = 0) and tested, we observed the expected 50% decrease in the PI (Figure 6B). When flies were removed from the drug and placed on food without CNO for 3 hr prior to training, the PI was decreased only by 12% (Figure 6B). Flies removed from the CNO and placed on food without drug for 12 hr prior to training exhibited no statistically significant difference in short-term olfactory learning and memory performance compared to flies not fed CNO (Figure 6B). Based upon these results, the half-life for the reversibility of the CNO/ DREADD activation, at least for this behavior, is approximately 90 min.
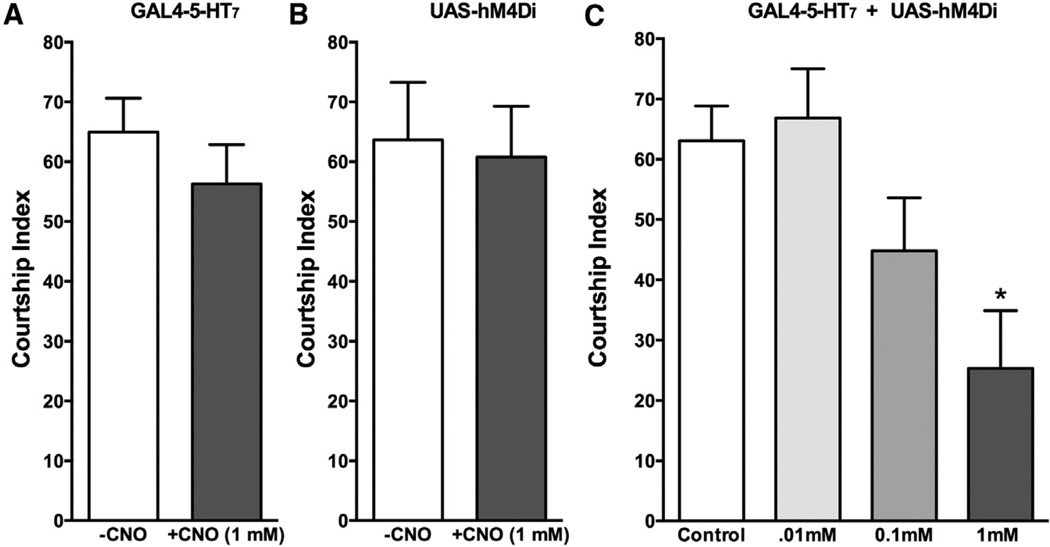
hM4Di Activation of 5-HT7Dro-GAL4 Neurons Modulates Behaviors
Our previous studies have indicated that the 5-HT7Dro receptor, as well as the circuitry defined by this driver, is involved in courtship and mating (Becnel et al., 2011). To further validate the role of 5-HT7Dro receptor circuitry in this behavior, we expressed the hM4Di receptor under the control of the 5-HT7Dro-GAL4 driver and assessed the effects of CNO activation on courtship. When fed CNO, the UAS-hM4Di × 5-HT7Dro-GAL4 F1 flies exhibited a decrease in the courtship index (Figure 7). These results are consistent with our previous methods utilizing receptor antagonists and RNAi methods (Becnel et al., 2011).
Figure 7. hM4Di Activation in 5-HT7-Dro Circuitry Dose-Dependently Disrupts Courtship and Mating.
(A–C) Flies expressing the hM4Di receptor under the control of the 5-HT7Dro-GAL4 driver were fed CNO and the effects on courtship index tested. (A and B) CNO (1.0 mM in the food for the 48 hr prior to testing) had no effect on either parental strain.
(C) Flies expressing the hM4Di receptor (5-HT7Dro-GAL4 + UAS-hM4Di) and fed CNO in the food at the indicated concentrations for 48 hr prior to testing had their courtship index dose-dependently disrupted, consistent with our previous results demonstrating a role of this neuronal circuit in courtship and mating behaviors. Courtship index was calculated by the amount of time a pair of flies spent engaged in courtship rituals divided by the total amount of time of the assay (10 min), expressed as a percentage. *p < 0.05, ANOVA with Bonferroni post hoc test. N = 10–20 mating pairs per treatment. Error bars indicate SEM.
DISCUSSION
The major finding of this paper is that the DREADD system allows for reversible, spatiotemporal control of signaling and behavior as well as physiological processes in Drosophila melanogaster. DREADD technology overcomes many limitations of other methods, including the need for expensive specialized equipment, dedicated temperature environments, and the inability to easily titrate signaling and induced behaviors in a dose-responsive manner. As demonstrated, flies of the appropriate genotype can be simply fed CNO, either acutely or chronically, to modulate signaling and downstream behaviors. Significantly, this system not only examines the role of signal transduction effector pathways in cells, but could conceivably silence and facilitate the electrical activity of neurons in the traditional sense. For example, we observed that activation of the Gαq-coupled hM1Dq DREADD facilitates depolarization of neurons. This is possibly due to PIP2 gating of inwardly rectifying potassium channels (Alexander et al., 2009). In developing the DREADD technology, clozapine-N-oxide was chosen as the ligand to evolve the receptors around because it demonstrates no appreciable affinity to any known mammalian GPCR or enzyme (Armbruster et al., 2007). We found no overt behavioral or developmental effects of CNO in flies indicating that, as in mammals, CNO is likely biologically inert in the fly.
Another issue to address in developing this system was the coupling of mammalian GPCRs to insect effector pathways. Evidence in the literature examining this question is sparse and mixed with respect to heterologous coupling. A number of reports, however, demonstrate positive coupling (Han et al., 1996; Kemp and Manahan-Vaughan, 2005; Perret et al., 2003; Saudou et al., 1992; Wei et al., 2000). Mammalian muscarinic acetylcholine receptors expressed in insect cells have been previously shown to fully couple to appropriate insect heterotrimeric G proteins to activate canonical effector pathways (Knight et al., 2003). We cloned the DREADD receptors into insect expression vectors and tested them in a Drosophila S2 cell culture expression system and found that they correctly couple to adenylate cyclase with the rM3BDs increasing, and the hM4Di decreasing, cAMP production in a dose-dependent manner upon activation with CNO. The EC50 values of receptor activation were, however, slightly less than in a purely mammalian system; nevertheless, this is still within an effective range for GPCR activation. Some neurotransmitters, including acetylcholine at muscarinic receptors, have micromolar receptor affinities (PDSP database; http://pdsp.med.unc.edu).
To validate DREADD activity, we chose a variety of basic behavioral assays to test. In the larvae, we examined chemosensation and locomotor activity. For the chemosensory assay, we expressed the receptors under the control of a sensory neuron driver and tested their response to the attractant odor, ethyl acetate. Although activation of the rM3BDs receptor with CNO had no effect, activation of the hM4Di receptor produced a dose-responsive decrease in the performance index, and activation of the hM1Dq receptor produced a dose-responsive increase in the performance index. Significantly, this experiment demonstrates the dose-responsiveness of the system to silence/activate neurons to influence behaviors. These results likely represent both increased sensitivity (more larvae chose to move toward the odorant disc) and maximal response (larvae appeared to travel more rapidly toward the odorant disc).
To explore the ability of DREADDs to control physiological processes, in addition to behaviors, we expressed the silencing hM4Di receptor in larval heart muscle. The effects were rapid and profound for completely or nearly completely abolishing heart activity. This effect recapitulates the bradycardia induced by activation of Gαi-coupled GPCRs in mammalian heart. Dramatically, upon washout of CNO, most of the hearts began to beat again. We anticipate that other physiological processes regulated by intracellular signaling pathways in nonneuronal tissues can also be manipulated with the DREADD system.
In the adult, we examined diurnal behavior, and olfactory learning and memory. For the diurnal tests, we used a crypto-chrome (cry)-GAL4 driver to drive DREADD expression. There is significant overlap with cry expression and the Gαs-coupled PDF receptor (PDFR) (Im et al., 2011), which is a key regulatory component of the circadian clock (Im and Taghert, 2010). Flies mutant for the PDFR primarily have defects in the evening peak, with a slight forward shift and increased general activity in the afternoon (Im and Taghert, 2010). We observed that activation of rM3BDs under expression control of a cry-GAL4 driver led to a decrease in evening peak activity, without affecting the morning peak. Because PDFR mutants demonstrate a general increase in afternoon activity, an increase in PDFR activity in cry circuitry may be predicted to decrease afternoon activity, which is consistent with our results. Our system may therefore prove useful in experiments to further elucidate the role of specific neurons and circuits in the circadian clock and the role of effectors including cAMP within these neurons in experiments utilizing circadian relevant GAL4 drivers and doses of CNO.
For adult olfactory learning and memory performance, we tested if we could negatively control the performance index by expressing and activating the hM4Di receptor under the control of the MB247-GAL4 driver. The MB247-GAL4 driver expresses in a subset of the mushroom body lobes (Aso et al., 2009), and is a commonly used GAL4 driver in the study of olfactory learning and memory. When we fed flies expressing hM4Di in the MBs CNO prior to training and testing, we significantly reduced the performance index for short-term learning and memory. Neither of the parental strains exhibited an effect of CNO. It is not possible at this point to determine if the effect was due to the silencing properties of receptor activation through Gβγ-coupled opening of inwardly rectifying potassium channels and hyperpolarization of the neurons or a Gai-mediated decrease in cAMP, which is a key molecule involved in learning and memory (Davis et al., 1995). We hypothesize that the inhibition is likely a combination of the two mechanisms. Regardless of the exact mechanism, we demonstrate here the ability of the hM4Di receptor to disrupt behavior in the adult. In any conditional activation system, it is crucial to determine if the effects are reversible or not, as well as the kinetics of the reversibility. We examined this utilizing the MB247-GAL4 + UAS-hM4Di fly in olfactory short-term learning and memory experiments. We observed a time-dependent recovery to a normal performance index with a predicted half-time of ~90 min after removal from food containing CNO prior to training. Although not as rapid as methods involving channels, this system is inducible on the scale of minutes and reversible on the scale of minutes to hours for modulation of behaviors. For many types of studies, these kinetics are more than adequate.
Based upon our results with these particular DREADD strains, the practical effective dose appears to be between 1 and 3 mM CNO in the food. Flies can be fed either acutely to produce immediate behaviors for observation or chronically to observe behaviors over an extended length of time. We routinely maintain adult flies on food containing CNO for 48 hr prior to a particular acute behavioral test in order to allow for steady-state accumulation of drug levels. Our results indicate a half-life of ~90 min after removal from food. This treatment regimen may result in some degree of receptor desensitization and downregulation; nevertheless, they effectively alter behaviors when activated by ligand. Flies can also be starved overnight and fed a large bolus dose and tested individually for acute behaviors. In our previous experiments, we have observed that CNS active drugs in acute administration experiments begin to have effects ~15 min, with a maximal effect ~20 min that persists at full strength until ~60 min (Nichols et al., 2002).
The elucidation of the function of unknown neural circuits and tissues defined by GAL4 drivers is a major utility we envision for this system. Although other methods employing channels are used for this purpose, they are more of a “switch” approach and maximally activate/inactivate neuronal circuits. Our system allows for a more graded analysis by gradually altering neuronal signaling through dose response experiments, which may uncover and distinguish more subtle, but distinct, behaviors mediated by the same neurons and circuits. To address this, we used our system to investigate the circuitry defined by the 5-HT7Dro-GAL4 driver, which demonstrates high expression in large field R-neurons innervating the ellipsoid body (EB) (Becnel et al., 2011). We have previously reported that the neurons defined by this driver, and the 5-HT7Dro receptor itself, are involved in courtship and mating behaviors (Becnel et al., 2011), as well as in olfactory learning and memory (Johnson et al., 2011). Previous studies by others utilizing shibirets expressed in a subset of large-field R-neurons have shown that when the neurons innervating the ellipsoid body are completely inactivated, severe locomotor and coordination deficits are produced (Krashes and Waddell, 2008), precluding an accurate analysis of the role of the EB in other behaviors dependent on normal activity and coordination. In agreement with our previous studies, activation of hM4Di expressed in 5-HT7Dro neurons dose-dependently inhibited courtship and mating, with no observable overt deficits in locomotor or coordination abilities. These results indicate that the DREADD system can indeed be used to examine more subtle behaviors otherwise masked by all-on or all-off approaches.
In summary, we have successfully translated a method for inducible and reversible remote control of behaviors and physiological processes to the fly utilizing a combination of genetics and pharmacology (e.g., “pharmacogenetics”). Perhaps the most important attribute of this system is its ability control behaviors and physiology in a dose-dependent manner, which allows for more precise and subtle examination of neuronal function and behaviors as well as physiological processes. Further, no specialized equipment is necessary; one simply feeds CNO to the fly. Due to the ubiquitous nature of GPCRs, we anticipate that this system will also be useful in the examination of the role of signal transduction pathway effectors in almost every tissue of the fly for which there is an available GAL4 driver.
EXPERIMENTAL PROCEDURES
Chemicals
General chemicals were obtained from Sigma. The odors 3-octonal and 4-methylcyclohexanol were obtained from Sigma. Ethyl acetate was from Fisher Scientific. Clozapine-N-oxide (CNO) was generously synthesized and provided by Dr. David E. Nichols at Purdue University from clozapine purchased from Enzo Life Sciences.
Drosophila Strains and Rearing
Canton-S (CS), white1118, the cry-GAL4 and the 6405-GAL4 drivers, and the UAS-GCaMP (chr3) strain were obtained from the Bloomington Stock Center. The 24B-GAL4 driver and Balancer lines [+/+; Adv1/CyO; +/+ ] and [ +/+; +/+; Sco/CyO] were provided by Dr. Udai Pandey (LSU Health Sciences Center, New Orleans, LA). The MB247-GAL4 strain was generously provided by Dr. Kyung-An Han (UTEP, El Paso, TX). The elavc155-GAL4 strain was provided by Dr. Chunlai Wu (LSU Health Sciences Center, New Orleans, LA). The creation of the 5-HT7Dro-GAL4 transgenic line was previously described (Becnel et al., 2011). For routine maintenance, flies were reared on standard corn-meal-molasses food at 25°C under 12 hr light/dark conditions, unless otherwise stated.
Creation of UAS-DREADDs
The UAS-hM1Dq, UAS-hM4Di, and UAS-M3BDs fly strains were created, and inducibility of expression tested, as described in the Extended Experimental Procedures and as shown in Figure S2.
Generation of the pMT/V5-DREADDs
The hM4Di and rM3BDs complementary DNAs (cDNAs) were excised from the UAS-DREADD plasmids using KpnI and XhoI restriction enzymes (Promega). The pMT-V5-His vector from the DES-Inducible Kit (Invitrogen) was digested using the same enzymes. The hM4Di cDNA was ligated into the pMT-V5-His vector using the Fast Link DNA ligation kit following the manufacturer’s instructions. To generate the rM3BDs expression vector, both the excised rM3BDs fragment and the vector were blunt-ended using the End-IT DNA End Repair Kit (Epicenter) following the manufacturer’s directions and subsequently ligated together using the Fast Link DNA ligation kit following the manufacturer’s instructions. The final constructs were verified using a panel of restriction enzymes.
Culture of S2 Cells
Drosophila S2 Cells (Invitrogen) were cultured in Schneider’s Drosophila medium (Invitrogen) at 28°C in a nonhumidified, ambient-air-regulated incubator.
Transfection of Drosophila S2 Cells
Drosophila S2 cells were transfected using Fugene HD Transfection Reagent (Roche) following the manufacturer’s directions using a 3:1 ratio of Fugene HD:DNA in six-well culture dishes.
cAMP assay
To determine the activity of the DREADD receptors, a single 10 cm plate of S2 cells was transfected with plasmid as described above. Then 24 hr later, transfected cells were split into 96-well plates. After an additional 24 hr, cells were stimulated with CNO. Increases in cAMP, as well as percent inhibition of forskolin-induced cAMP, were determined using the cAMP-Glo Kit (Promega) and measured using luminescence. The following modification was performed: 96-well plates containing the S2 cells were placed on ice for the addition of the induction buffer, the CNO, and forskolin (where appropriate). Luminescence was measured using a Tristar LB941 luminometer (Berthold).
GCaMP Assay
Assays were performed in 29 mM glass bottom dishes (In Vitro Scientific). To prepare the dishes, a drop of 0.01% (w/v) poly-L-lysine (Sigma) was placed on the glass and allowed to dry for 30 min at 42°C using a slide warmer.
Crosses were performed to generate flies expressing either GCaMP alone or GCaMP and hM1Dq together under the control of the pan-neuronal elavc155-GAL4 driver. Brains were dissected from third-instar larvae in Ringer’s solution (130 mM NaCl, 5 mM KCl, 2 mM MgCl2, 36 mM sucrose, 5 mM HEPES) without calcium. The brains were then adhered to the bottom of the 29 mM glass-bottom cell culture dishes coated with poly-L-lysine. Brains were then incubated for 30–45 min in 3 ml Ringer’s solution with 2 mM CaCl2 at room temperature.
Real-time GCaMP fluorescence was measured using a Leica SP2-TCS confocal microscope with the following settings: XYT mode, 1,000 Hz, one frame captured every 1.3 s for a total of 5 min. At frame 40 (52 s), 200 mL of KCL (75 mM) in Ringer’s solution was pipetted onto the brain. For brains pretreated with CNO, brains were incubated in Ringer’s + calcium containing CNO (6 mM) at room temperature for 5 min prior to imaging and/or KCl addition. A custom MATLAB program was written and used to analyze the movie files for changes in fluorescence intensity (see Extended Experimental Procedures). In brief, ΔF/F over time was measured and averaged for ten neurons per ventral ganglia per brain and the results for 15 brains per treatment group averaged to generate the final response curves and determine the mean maximum peak latencies.
Larval Behavior Assays
Larvae Collection
To obtain larva, 8 oz bottles were set up with 10–15 virgin females of one parental strain and 10–15 males of the other parental line and incubated at 25°C. Flies were allowed to mate and lay eggs for 24 hr before being removed from the bottle. When the majority of the larvae in the bottle had reached the third-instar stage (day 6), the bottle was flooded with 50 ml of a 20% sucrose solution in water (w/v), causing the larvae to float to the surface. Larvae were collected, transferred to a small mesh basket, and washed twice with deionized water before further use (Nichols et al., 2012).
Chemosensory Assay
Fifty larvae were collected as described above and transferred to a small beaker containing 1.2 ml of 5% sucrose (either alone or with the appropriate concentration of CNO) for 15 min. The larvae were transferred to a Petri dish containing a 1% agarose gel with two 1 cm discs of Whatman filter paper directly opposite one another and evenly spaced from the center. One disc was spotted with 20 µl of deionized water as a control, and the other was spotted with 20 µl of ethyl acetate (1:104 dilution). The larvae were placed in the center of the plate using an overturned lid of a 35 mM dish. Once larvae were transferred to the plate, the lid was removed, the Petri dish covered, and the larvae were allowed to roam freely for 3 min, at which time the number of larvae on either side of the dish were recorded. Larvae on the half of the dish with the odorant disc were counted as positive, those on the opposite side as negative, and those in the middle were not counted. The performance index (PI) was calculated as the number of larvae in the half of the dish containing the odorant disc subtracted by the number or larvae in the half of the dish containing the water disc, divided by the total number of larvae.
Heart Rate
The UAS-hM4Di transgenic line was crossed to a heterozygous 24B-GAL4 (24B-GAL4 +/−, UAS-mCD8:GFP +/+) transgenic line. Half of the resulting progeny will carry all three of the transgenes (24B-GAL4 +/−; UAS-mCD8:GFP +/−; UAS-hM4D +/−). Experimental controls were siblings from the same cross that did not carry the 24B-GAL4 (UAS-mCD8:GFP +/−; UAS-hM4D +/−). Heart rate measures in larval Drosophila are readily obtained within the whole animal or in an in situ preparation. Detailed procedures are described and illustrated in Cooper et al. (2009). In this study, we used early third-instar larvae and exposed the larval heart tube by dissecting the larvae on the ventral longitudinal axis. Dissection time takes about 3–6 min. During the dissection and for observations the preparation is bathed in HL3 saline (pH 7.1). This saline was a good media for maintaining synaptic transmission at neuromuscular junctions (Stewart et al., 1994). For heart measures, pH was carefully controlled (pH 7.1) in the HL3 saline and for the HL3 saline containing CNO. Each larva was recorded by video microscopy for the duration of an experiment. Heart rate was first observed under baseline conditions in HL3 saline alone for 5 min. Next, media was replaced with HL3 saline + 500 nM CNO and the heart observed again for 5 min. Finally, media was then replaced with HL3 saline alone (pH 7.1) (washout), and the heart observed for 5 min.
Adult Fly Behavior Assays
Courtship and Mating Assay
For the courtship and mating assays, bottles of wild-type CS flies were cleared and newly eclosed virgin females and males were collected and matured in 10 ml glass tubes containing ~300 ml of food (10% sucrose, 1 % agarose, and drug where appropriate) for 5 days. Between five and six virgin females were housed together during this process, while sexually naive males were individually housed. During the isolation period, all flies were maintained at 25° Cundera 12 hr light/dark cycle until testing. Following the isolation period, one male and one female were transferred to a single chamber of a mating wheel, and each mating pair was closely monitored for 10 min (Nichols et al., 2012). The courtship index was calculated as the amount of time a male spent performing courtship behaviors out of 10 min or until copulation occurred. All testing was performed at 25°C at 70%–80% relative humidity, and between the hours of 11 am and 4 pm.
Adult Locomotor Assay and Diurnal Activity
Crosses were made between the different UAS-DREADD responder strains and the appropriate GAL4 driver strain. Then 2- to 3-day eclosed adult male flies were individually placed into 5 mm diameter glass capillary tubes with an agar plug at one end consisting of 1% agarose, 10% sucrose, and CNO (where appropriate), and then plugged with cotton at the other end. Tubes were then placed into Trikinetics activity monitor arrays, which were subsequently placed into a humidified incubator at 25°C with a 12 hr light-dark cycle (unless stated otherwise) and infrared beam breaks counted with the Trikinetics Drosophila Activity Monitor System (DAMS) at 5 min intervals. Sixteen male flies were used in each experimental trial for each treatment. Only activity data for days 3 and greater were used for analysis, omitting the first 2 days to allow for acclimation to the environment and to build up steady state drug levels.
Learning and Memory
Flies were grown in 8 oz polypropylene bottles on standard cornmeal-molasses food at 25°C under a 12 hr light/dark cycle. Both male and female adult flies were used for all conditioning procedures. For selection of flies for assays, bottles were cleared, and 48 hr later the recently emerged adult flies were transferred to large 64 oz plastic commercial juice bottle for 48 hr with the large end cut off and replaced with fine plastic mesh containing ~2 ml of food in the cap, with or without CNO where appropriate, and without anesthetization. The larger environment prevents over exposure to the food source, and allows flies to properly groom and feed, resulting in clean and dry flies for optimal performance in the conditioning apparatus.
STM was performed as previously described (Johnson et al., 2011). Essentially, ~100 flies were transferred to the training chamber containing an electrifiable grid where they received 4-methylcyclohexanol (MCH) in the presence of shock, followed by a brief period of rest in the absence of shock and odor, and finally benzaldehyde (BA) in the absence of shock. The flies were then transferred to the T-maze (choice point) for testing where they were presented with MCH and BA from either side and allowed to choose for 120 s. The PI was calculated as the number of flies avoiding the shock paired odor minus the number of flies avoiding the unpaired odor divided by the total number of flies assayed. The entire procedure was then repeated using BA as the shock-paired odor and MCH as the unpaired odor, with both calculated performance indices combined to give the overall PI for the trial. For the reversibility studies, flies were maintained on food + CNO (1 mM) for 48 hr and then either tested immediately or placed on food without CNO for 3 and 12 and hours before training and testing.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Dan Mangiavellano for comments on the manuscript and the Higher Committee for Education Development in Iraq (Z.R.M.). This work was funded by R01MH083689 (C.D.N).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, two figures, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.08.003.
REFERENCES
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Grübel K, Busch S, Friedrich AB, Siwanowicz I, Tanimoto H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- Becnel J, Johnson O, Luo J, Nässel DR, Nichols CD. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS ONE. 2011;6:e20800. doi: 10.1371/journal.pone.0020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cooper AS, Rymond KE, Ward MA, Bocook EL, Cooper RL. Monitoring heart function in larval Drosophila melanogaster for physiological studies. J. Vis. Exp. 2009;33:e1596. doi: 10.3791/1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H, Conklin BR. Controlling signaling with a specifically designed Gi-coupled receptor. Proc. Natl. Acad. Sci. USA. 1998;95:352–357. doi: 10.1073/pnas.95.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Cherry J, Dauwalder B, Han PL, Skoulakis E. The cyclic AMP system and Drosophila learning. Mol. Cell. Biochem. 1995;149–150:271–278. doi: 10.1007/978-1-4615-2015-3_31. [DOI] [PubMed] [Google Scholar]
- Eichler J, Knof J, Lenz H. Measurements on the depth of penetration of light (0.35–1.0 microgram) in tissue. Radiat. Environ. Biophys. 1977;14:239–242. doi: 10.1007/BF01323942. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J. Comp. Neurol. 2010;518:1925–1945. doi: 10.1002/cne.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Li W, Taghert PH. PDFR and CRY signaling converge in a subset of clock neurons to modulate the amplitude and phase of circadian behavior in Drosophila. PLoS ONE. 2011;6:e18974. doi: 10.1371/journal.pone.0018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson O, Becnel J, Nichols CD. Serotonin receptor activity is necessary for olfactory learning and memory in Drosophila melanogaster. Neuroscience. 2011;192:372–381. doi: 10.1016/j.neuroscience.2011.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain P, Chandrashekaran S, Rodrigues V, Hasan G. Drosophila mutants in phospholipid signaling have reduced olfactory responses as adults and larvae. J. Neurogenet. 2009;23:303–312. doi: 10.1080/01677060802372494. [DOI] [PubMed] [Google Scholar]
- Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Sweeney ST, Zars T, O’Kane CJ, Heisenberg M. Targeted expression of tetanus neurotoxin interferes with behavioral responses to sensory input in Drosophila. J. Neurobiol. 2002;50:221–233. doi: 10.1002/neu.10029. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The 5-hydroxytryptamine4 receptor exhibits frequency-dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region in vivo. Cereb. Cortex. 2005;15:1037–1043. doi: 10.1093/cercor/bhh204. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Knight PJ, Pfeifer TA, Grigliatti TA. A functional assay for G-protein-coupled receptors using stably transformed insect tissue culture cell lines. Anal. Biochem. 2003;320:88–103. doi: 10.1016/s0003-2697(03)00354-3. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J. Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Mar-atos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Roth BL. Engineered G-protein Coupled Receptors are Powerful Tools to Investigate Biological Processes and Behaviors. Front. Mol. Neurosci. 2009;2:16. doi: 10.3389/neuro.02.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CD, Ronesi J, Pratt W, Sanders-Bush E. Hallucinogens and Drosophila: linking serotonin receptor activation to behavior. Neuroscience. 2002;115:979–984. doi: 10.1016/s0306-4522(02)00354-8. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Becnel J, Pandey UB. Methods to assay Drosophila behavior. J. Vis. Exp. 2012;61:e3795. doi: 10.3791/3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J. Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret BG, Wagner R, Lecat S, Brillet K, Rabut G, Bucher B, Pattus F. Expression of EGFP-amino-tagged human mu opioid receptor in Drosophila Schneider 2 cells: a potential expression system for large-scale production of G-protein coupled receptors. Protein Expr. Purif. 2003;31:123–132. doi: 10.1016/s1046-5928(03)00140-2. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K, Eberl DF. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr. Biol. 2003;13:1687–1696. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS ONE. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Völler T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc. Natl. Acad. Sci. USA. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemo-lymph-like physiological solutions. J. Comp. Physiol. A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Zhou DH, Shen QX, Chen J, Chen LW, Wang TL, Pei G, Chi ZQ. Human mu-opioid receptor overexpressed in Sf9 insect cells functionally coupled to endogenous Gi/o proteins. Cell Res. 2000;10:93–102. doi: 10.1038/sj.cr.7290039. [DOI] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge W, Wang Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur. J. Neurosci. 2007;26:2405–2416. doi: 10.1111/j.1460-9568.2007.05862.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.