Abstract
estD encodes a carboxylic ester hydrolase and is part of the NmlR regulon in Neisseria gonorrhoeae. An estD mutant was found to be susceptible to nitrite and to S-nitrosoglutathione. This mutant was also unable to infect and survive within human cervical epithelial cells, and it showed reduced ability to form a biofilm on these cells. We conclude that esterase D is an integral part of the nitrosative stress defense system of N. gonorrhoeae and that it has potential importance in pathogenesis.
Neisseria gonorrhoeae is an obligate human pathogen. Gonococcal infection is associated with inflamed mucosa of the urethra in males but often results in asymptomatic infection of the cervix in females [1]. The innate immune response produces reactive oxygen species and reactive nitrogen species (RNS), and some of the strategies used by N. gonorrhoeae against these host defense molecules have been reviewed elsewhere [2]. Defense against RNS may be of particular importance; gonococci possess a nitrite reductase (AniA) and a nitric oxide reductase (NorB) that catalyze the conversion of nitrite to nitrous oxide via an energy-conserving respiratory process [3]. Although respiration can confer protection against nitric oxide (NO), it has been established that cytoplasmic enzymes in enteric bacteria are also involved in protection against RNS. Such enzymes include flavorubredoxin (NorV) and its reductase partner (NorW), which exhibit anaerobic NorB activity [4], and flavohemoglobin (Hmp), which converts NO to nitrate and nitrous oxide under aerobic and anaerobic conditions, respectively [5]. However, these enzymes are not present in N. gonorrhoeae.
We recently described a novel transcriptional regulator, NmlR, that belongs to the MerR family of regulators and binds to 2 sites on the N. gonorrhoeae chromosome [6]. One site exhibits dyad symmetry between the divergent and overlapping promoters between trxB (NGO0580), which encodes thioredoxin reductase, and the divergently transcribed gene copA (NGO0579). In a recent study, we showed that trxB mutants were susceptible to killing by NO and had reduced ability to survive during interactions with cervical epithelial cells, compared with wild-type (WT) gonococci [7]. These phenotypic properties were correlated with a low level of expression of aniA and norB in the mutant strains. The other site of NmlR binding contains divergent and overlapping promoters between nmlR (NGO0602) and adhC-estD (NGO0601–NGO0600). It has been established in bacterial and mammalian systems that glutathione (GSH)–dependent alcohol dehydrogenase (AdhC) and esterase D (EstD) can protect cells against the toxicity of formaldehyde; S-(hydroxymethyl)glutathione, formed from the reaction of formaldehyde with GSH, is oxidized by NAD+-dependent AdhC to generate S-formylglutathione, which is then hydrolyzed to formate and GSH by EstD, a carboxylic ester hydrolase [8]. It has also been suggested that AdhC might also provide protection against nitrosative stress by catalyzing the NADH-dependent reduction of S-nitrosoglutathione (GSNO) [9]. Surprisingly, we have found that the adhC gene of N. gonorrhoeae is a pseudogene and, thus, has no functional role in GSH-based stress defenses [10]. In contrast, estDappears to be functional in N. gonorrhoeae, and we report herein an analysis of the role of estD in gonococcal stress defense and virulence.
Materials and methods
N. gonorrhoeae strain 1291 was routinely cultured on brain-heart infusion (BHI; Oxoid) supplemented with 10% (vol/vol) Levinthal base and 1% (vol/vol) IsoVitaleX (Beckton Dickinson). GC agar (Oxoid) was used for disk diffusion susceptibility assays. Growth on solid media was performed at 37°C with 5% carbon dioxide, and liquid cultures were incubated at 37°C. Kanamycin was used at a concentration of 100 μg/mL.
The N. gonorrhoeae adhC mutant has already been described elsewhere [10]. estD was inactivated in N. gonorrhoeae 1291 by means of marker exchange mutatgenesis. estD was polymerase chain reaction (PCR) amplified using primers estD-F1/R1 (table 1, which appears only in the electronic version of the Journal), cloned into pGEM-T Easy (Promega), and interrupted by insertion of the kanamycin resistance cassette from pUC4kan (HincII digest) into the unique BseR1 restriction site. The resulting plasmid, pGEM-T::estD::kan, was linearized with EaeI and used to transform N. gonorrhoeae 1291. Correct insertion into the chromosome was verified by PCR performed using primers external to the construct used for mutagenesis; estD-F-check/R-check (table 1, which appears only in the electronic version of the Journal), and primers complementary to the kanamycin resistance cassette (kan-F/R) (table 1, which appears only in the electronic version of the Journal).
Table 1.
Primers used in the present study.
| Primer name | Nucleotide sequence (5′-3′) |
|---|---|
| nthn-F | GACGCGTTTTTCTTATCAAGG |
| nthn-R | AAAATGCTCCTCAATCAGACG |
| estD-Fl | CGGCAATGTAAACGTAATGCGTC |
| estD-Rl | CACAAAGGGGGTATAAGCAAAGG |
| estD-F-check | CCTGCAAAATTCGAGTTGG |
| estD-R-check | CGGGGCTTTAAAAAGTTGG |
| kan-F | CGAGGCCGCGATTAAATTCC |
| kan-R | ATCGGTCTGCGATTCCGACT |
Potter A J et al. J Infect Dis. 2009;200:273-278
Formaldehyde susceptibility was assessed using the disk diffusion susceptibility assay. Triplicate disks were saturated with a 0.37% formaldehyde solution (Sigma) and placed onto a lawn of cells, and a zone of clearing was measured after incubation at 37°C for 15 h. The effect of nitrite on cell growth was measured by preculturing gonococcus microaerobically in BHI broth to midexponential phase before diluting cells to a 0.1 optical density measured at 600 nm (OD600) in fresh BHI broth. A total of 100 μL of this suspension was then added to 100 μL of BHI broth in a microtiter plate that contained 2-fold dilutions of sodium nitrite. Plates were incubated at 37°C in a candle jar for 20 h, and the end point OD600 value was recorded. The effect of nitrite on the microaerobic growth rate of N. gonorrhoeae was determined by adding 5 mmol/L sodium nitrite to cultures after incubation at 37°C for 4 h. Microaerobic cultures were performed as described elsewhere [7].
The effect of GSNO on the growth of N. gonorrhoeae was determined in chemically defined growth medium [11]. GSNO was prepared as described elsewhere [10]. The assay was performed by growing cells to midexponential phase and then diluting cells 10-fold into fresh medium that contained 0, 0.1, 0.5, and 1 mmol/L GSNO before incubation at 37°C overnight. Growth was determined by recording the end point OD600 value of cultures.
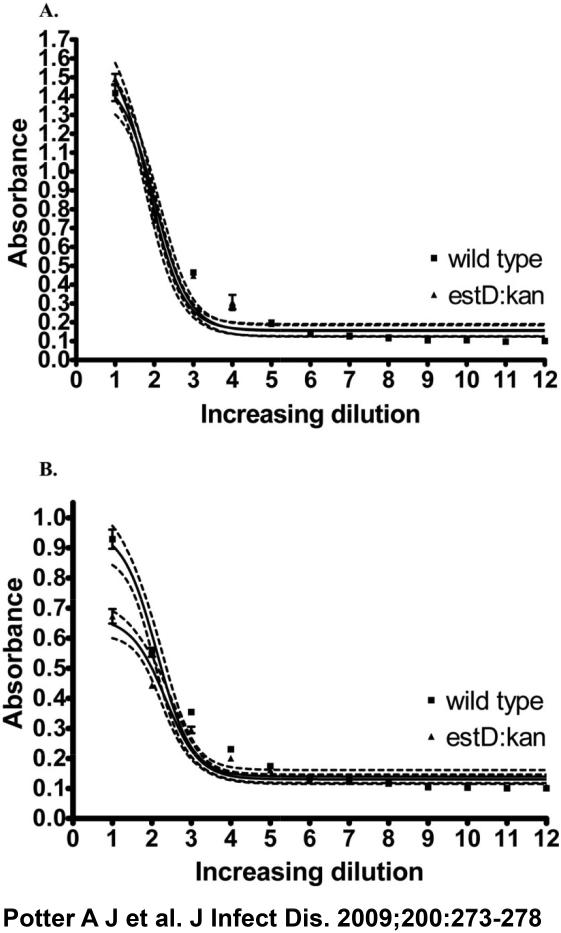
RNA was extracted from bacterial cells by use of the RNeasy Mini kit (Qiagen) and was treated with RQ1 RNase-Free DNase (Promega) at 37°C for 30 min to remove any genomic DNA contamination. The treated RNA was reverse transcribed to complementary DNA by use of the Omniscript RT Kit (Invitrogen). N. gonorrhoeae biofilm growth and cervical cell survival assays were performed as described elsewhere [7]. As a standard procedure, only piliated bacteria are used in cell interaction/ biofilm studies. Levels of pili and lipid oligosaccharide in the cells used in biofilm growth and cervical cell survival assays were confirmed using an enzyme-linked immunosorbent assay (figure 1, which appears only in the electronic version of the Journal).
Figure 1.
Enzyme-linked immunosorbent assay of expression of pilus and lipid oligosaccharide in Neisseria gonorrhoeae.
Results
Although adhC is a pseudogene in gonococci, we have shown that it is transcribed [6]. It seemed likely that estD is expressed on the same transcript and, hence, coregulated by NmlR. This possibility was investigated using reverse-transcriptase (RT)–PCR performed with a primer (nthn-R) (table 1, which appears only in the electronic version of the Journal) complementary to part of the estD coding sequence and a primer (nthn-F) containing part of the adhC coding sequence (table 1, which appears only in the electronic version of the Journal). A PCR product of ~1.2 kb was generated (data not shown), indicating that adhC and estD are cotranscribed and constitute an operon. To examine the role of EstD in N. gonorrhoeae, estD was inactivated in strain 1291 by marker exchange mutagenesis. We tested the susceptibility to formalde-hyde of a WT gonococcus, as well as that of estD and adhC mutants, using disk diffusion susceptibility assays. There was an identical zone of clearing for all 3 strains (data not shown), suggesting that estD does not confer resistance to formaldehyde in N. gonorrhoeae.
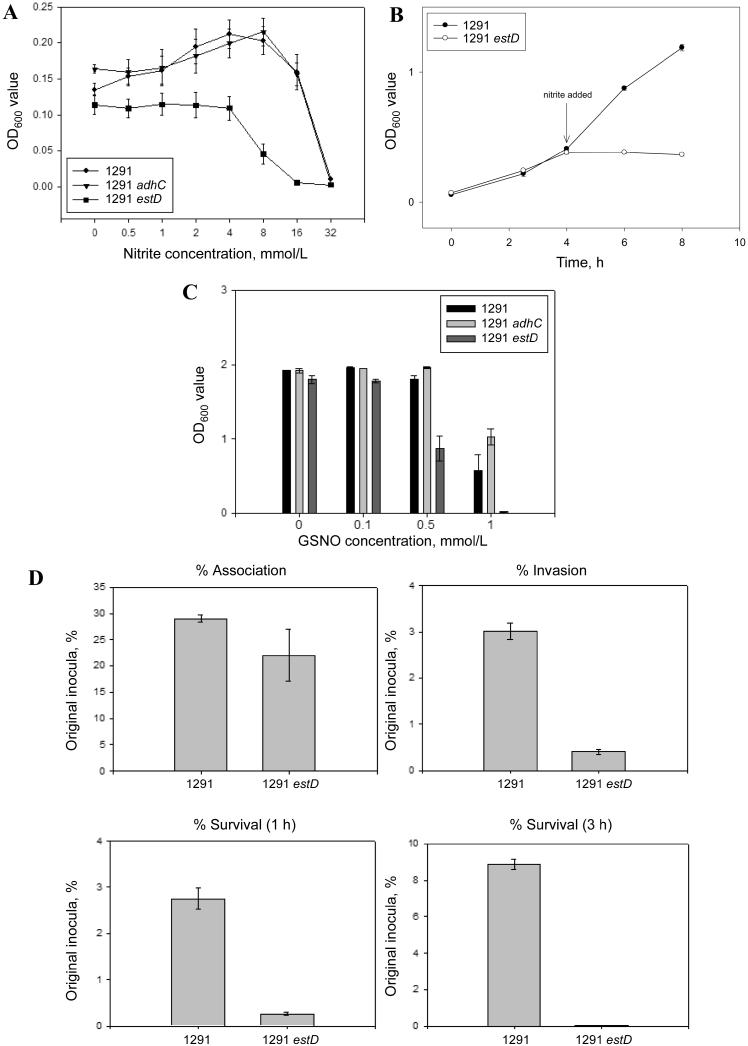
In view of the association between the NmlR regulon and nitrosative stress, as was established during our analysis of the trxB mutant [7], we tested the ability of N. gonorrhoeae 1291, adhC, and estD strains to grow microaerobically in the presence of sodium nitrite. Figure 2A shows that nitrite stimulated growth of 1291 and adhC strains but that no such stimulation was observed for the estD mutant; furthermore, this strain exhibited decreased growth in the presence of nitrite concentrations >4 mmol/L. We further examined the effect of nitrite on the estD mutant by adding this anion to a growing culture. Figure 2B shows that nitrite caused immediate cessation of growth of the estD mutant. In view of the aforementioned results, we tested whether estD was required for protection of N. gonorrhoeae against killing by GSNO. Figure 2C shows that the growth of the estD mutant was inhibited in the presence of GSNO, compared with the WT strain and the adhC strain.
Figure 2.
A, Microaerobic growth of Neisseria gonorrhoeae 1291 wild-type (WT), adhC, and estD strains in the presence of sodium nitrite. Points denote the mean end point absorbance reading of triplicate cultures, and Y-error bars denote ±1 standard deviation (SD) from the mean. B, The effect of the addition of nitrite on the microaerobic growth rate of N. gonorrhoeae 1291 WT and estD strains. A total of 5 mmol/L sodium nitrite was added to cultures after incubation at 37°C for 4 h. Points denote the mean absorbance readings of triplicate cultures, and Y-error bars denote ±1 SD from the mean. C, Growth of N. gonorrhoeae WT, adhC, and estD strains in chemically defined media in the presence of different concentrations of S-nitrosoglutathione (GSNO). Bars denote the mean end point absorbance readings of duplicate cultures, and Y-error bars denote ±1 SD from the mean. D, Gonococcal association with and intracellular survival within primary human cervical epithelial (pex) cells. Bars depicted in each graph denote the mean percentage of total association, invasion, or survival calculated from 3 experiments performed in triplicate; error bars denote the variance of the mean. Percentages were determined as a function of the original inoculum and the no. of colony-forming units evident after plating the cervical cell lysates. A Kruskal-Wallis nonparametric analysis of variance was used to determine the statistical significance of the data obtained for the estD mutant after comparison with assays performed using wild-type gonococci. P< .001, for the estD mutant under each condition assayed.
To determine whether EstD activity might be physiologically relevant for successful gonococcal infection, the ability of N. gonorrhoeae 1291 and 1291 estD to associate with, invade, and survive within primary human ectocervical epithelial (pex) cells was tested. Pex cells were challenged with either the WT or mutant strain, and infection was allowed to progress for 3 h. There was a small but significant difference observed in the ability of the estD strain to associate with pex cells, in comparison with the WT strain (figure 2D). However, the estD strain was severely attenuated in its ability to survive and replicate within pex cells, compared with the WT strain (figure 2D).
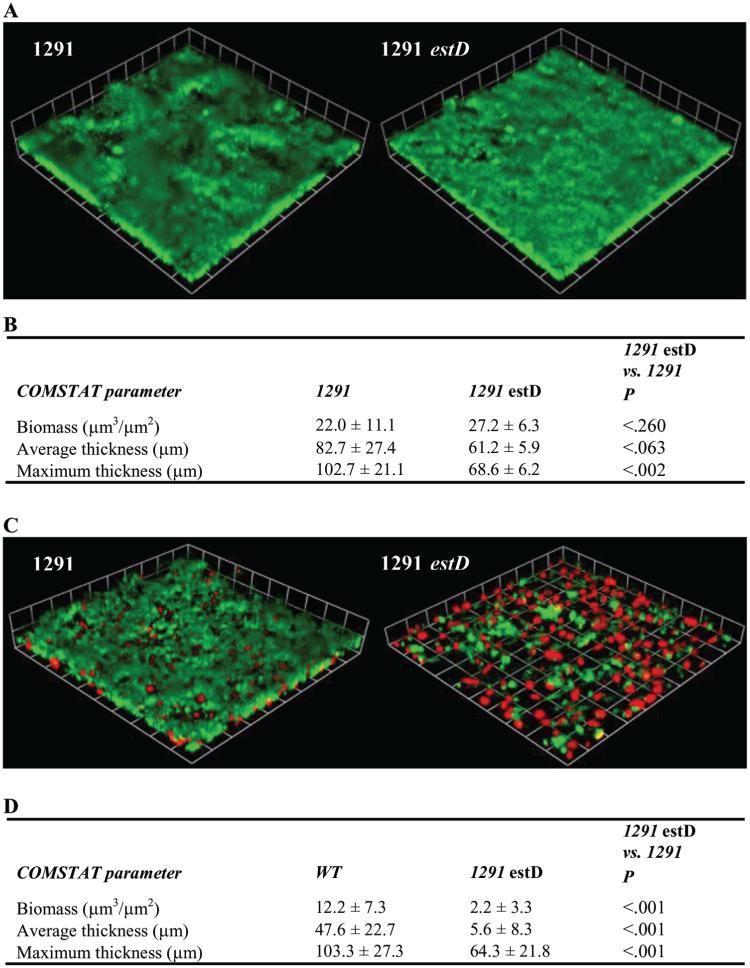
The comparative ability of the N. gonorrhoeae 1291 and 1291 estD strains to form a biofilm was evaluated after 2 days of growth under continuous-flow conditions. COMSTAT analyses [12] showed that the estD mutant was markedly deficient in biofilm formation, compared with the WT strain, when grown over transformed human cervical epithelial cells (THCECs) (figure 3D), but it formed a biofilm on glass equally as well as did the WT strain (figure 3B), with only a minor but statistically significant difference in maximum thickness. Three-dimensional images of these biofilms showed that the estD mutant formed a sparse biofilm over THCECs, with large gaps between biofilm clusters. This differs from the WT biofilm, which showed confluent growth with fewer and smaller gaps between biofilm clusters (figure 3C and 3D). The differences between the WT strain and the estD mutant strain mentioned above were not a result of the differences in the variable surface factors pili and lipid oligosaccharide (figure 1, which appears only in the electronic version of the Journal).
Figure 3.
Biofilm formation by Neisseria gonorrhoeae 1291 wild-type (WT) and estD mutant. A, Three-dimensional reconstructions of stacked z-series of biofilms (green) grown over glass, which were taken at 200× magnification and rendered by Volocity high-performance 3-dimensional imaging software (Improvisation). These experiments were performed on 3 different occasions, and representative images are shown. B, Summary of COMSTAT [12] analysis of biomass, average thickness, and maximum thickness of WT and estD mutant biofilms grown over glass, for all replicates. Statistical significance was determined using a Student’s t test. C, Three-dimensional reconstructions of stacked z-series of biofilms (green) grown over transformed human cervical epithelial cells (THCECs) (red), which were taken at 200× magnification and rendered by Volocity. These experiments were performed on 4 different occasions, and representative images are shown. D, Summary of COMSTAT analysis for biofilms grown over THCECs.
Discussion
The NmlR regulon was initially identified as having a role in the defense of N. gonorrhoeae against oxidative and disulfide stress [6]. In a recent study, we showed that NmlR-regulated trxB, which encodes thioredoxin reductase, is required to confer protection of N. gonorrhoeae against killing by NO, and our data indicate that TrxB is required to maintain a high level of expression of aniA and norB [7]. NmlR itself is under the control of Fnr [13]; therefore, a role for the NmlR regulon in the anaerobic physiology of the gonococcus is not unexpected. The data presented in this report indicate that another NmlR-regulated gene, estD, is also required for defense of N. gonorrhoeae against nitrosative stress. However, unlike the trxB mutant, which is susceptible to killing by NO, the estD mutant is susceptible to killing by GSNO and nitrite but is not susceptible to killing by the NO generator DEA-NONOATE than is the WT strain (A.J.P. and A.G.M., unpublished observation).
EstD is a carboxylic ester hydrolase that is known to catalyze the hydrolysis of S-formylglutathione [8]. This enzyme usually works in tandem with AdhC, which catalyzes the oxidation of S-(hydoxymethyl)glutathione, which is formed from the reaction of formaldehyde with GSH [8]. However, our data do not provide any evidence that EstD has a role in defense against formaldehyde in N. gonorrhoeae. The inhibition of growth of the N. gonorrhoeae estD strain by GSNO and nitrite may reflect susceptibility to a common compound within the cell that is produced during respiration with nitrite and NO. GSNO is formed from the reaction of nitrosonium cation (NO+), formed by the metal ion-dependent oxidation of NO, with GSH. AdhC can catalyze the reduction of GSNO to N-hydroxysulfenamide (GSNHOH) [9], but this enzyme is not functional in N. gonorrhoeae [11]. However, GSNHOH can also be formed from the reaction of GSH with nitroxyl [14], which can be formed by the reductive resolution of S-nitrosothiols [15]. GSNHOH is unstable, and it can form a variety of oxidized GSH derivatives [14]. Such reactions would cause oxidation of the GSH pool with deleterious effects on defense against oxidative and nitrosative stress. Although a specific substrate for EstD has not yet been identified, it is possible that EstD catalyzes the hydrolysis of a toxic intermediate produced from GSNHOH.
Our observation that mutation of estD severely impaired the ability of the gonococcus to survive within human cervical epithelial cells provides strong evidence that this enzyme has an important protective role during infection of cervical epithelial cells. Furthermore, the importance of EstD in enabling the gonococcus to form a biofilm on these same cells has also been demonstrated. Oxidative/nitrosative killing mechanisms of cervical epithelial cells have not yet been fully explored; however, recent data indicate that pex cells produce NO in response to gonococcal infection and, furthermore, that NO actually promotes the intracellular survival of these bacteria during ex vivo infection (J.L.E., unpublished observation). In view of this, it appears likely that GSNO would be a physiologically relevant compound formed during gonococcal infection, and the presence of a functional EstD may be required for metabolism of GSNO derivatives.
Acknowledgment
We thank the Cooperative Human Tissue Network (Columbus, Ohio) for providing cervical tissue specimens.
Footnotes
Potential conflicts of interest: none reported.
Financial support: National Health and Medical Research Council of Australia (program grant 284214 to M.P.J. and A.G.M.); National Institutes of Health (grant AI045728 to M.A.A.); Research Institute at Nationwide Children’s Hospital (funding to J.L.E.).
References
- 1.Sparling PF. Biology of Neisseria gonorrhoeae. In: Holmes KK, Mardh PA, Sparling PF, et al., editors. Sexually transmitted diseases. 3rd McGraw-Hill; New York: 1999. pp. 433–49. [Google Scholar]
- 2.Seib KL, Wu HJ, Kidd SP, Apicella MA, Jennings MP, McEwan AG. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol Mol Biol Rev. 2006;70:344–61. doi: 10.1128/MMBR.00044-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Householder TC, Fozo EM, Cardinale JA, Clark VL. Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect Immun. 2000;68:5241–6. doi: 10.1128/iai.68.9.5241-5246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner AM, Helmick RA, Gardner PR. Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J Biol Chem. 2002;277:8172–7. doi: 10.1074/jbc.M110471200. [DOI] [PubMed] [Google Scholar]
- 5.Poole RK, Hughes MN. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol Microbiol. 2000;36:775–83. doi: 10.1046/j.1365-2958.2000.01889.x. [DOI] [PubMed] [Google Scholar]
- 6.Kidd SP, Potter AJ, Apicella MA, Jennings MP, McEwan AG. NmlR of Neisseria gonorrhoeae: a novel redox responsive transcription factor from the MerR family. Mol Microbiol. 2005;57:1676–89. doi: 10.1111/j.1365-2958.2005.04773.x. [DOI] [PubMed] [Google Scholar]
- 7.Potter AJ, Kidd SP, Falsetta M, Apicella MA, Jennings MP, McEwan AG. Thioredoxin reductase is essential for protection of Neisseria gonorrhoeae against killing by nitric oxide and for bacterial growth during interaction with cervical epithelial cells. J Infect Dis. 2009;199:227–35. doi: 10.1086/595737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harms N, Ras J, Reijnders WNM, van Spanning RJM, Stouthamer AH. S-formylglutathione hydrolase of Paracoccus denitrificans is homologous to human esterase D: a universal pathway for formaldehyde detoxification? J Bacteriol. 1996;178:6296–9. doi: 10.1128/jb.178.21.6296-6299.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedberg JJ, Griffiths WJ, Nilsson SJF, Höög JO. Reduction of S-nitrosoglutathione by human alcohol dehydrogenase 3 is an irreversible reaction as analysed by electrospray mass spectrometry. Eur J Biochem. 2003;270:1249–56. doi: 10.1046/j.1432-1033.2003.03486.x. [DOI] [PubMed] [Google Scholar]
- 10.Potter AJ, Kidd SP, Jennings MP, McEwan AG. Evidence for distinctive mechanisms of S-nitrosoglutathione metabolism by AdhC in two closely related species, Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 2007;75:1534–6. doi: 10.1128/IAI.01634-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse SA, Bartenstein L. Purine metabolism in Neisseria gonorrhoeae—requirement for hypoxanthine. Can J Microbiol. 1980;26:13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- 12.Heydorn A, Nielsen AT, Hentzer M, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiol. 2000;146:2395–407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 13.Whitehead RN, Overton TW, Snyder LAS, et al. The small FNR regulon of Neisseria gonorrhoeae: comparison with the larger Escherichia coli FNR regulon and interaction with the NarQ-NarP regulon. BMC Genomics. 2007;8:35. doi: 10.1186/1471-2164-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong PSY, Hyun J, Fukuto JM, et al. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–71. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta R, Ryter SW, Zuckerbraun BS, Tzeng E, Billiar TR, Stoyanovsky DA. Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry. 2007;46:8472–83. doi: 10.1021/bi700449x. [DOI] [PubMed] [Google Scholar]