Abstract
Background
Accumulating evidence suggests that opioid overdose and HIV infection are burgeoning intertwined epidemics among people who inject drugs (PWID) in Central Asia. To date, however, research on overdose and its associations with HIV risks among PWID in Central Asia remains virtually absent. This paper aims to provide a regional overview of the hidden epidemic of overdose and how it is linked to HIV among PWID in Central Asia, using a syndemic framework that is guided by risk environment research.
Methods
We conducted a comprehensive literature search of peer-reviewed publications and grey literature on opioid overdose and its associations with HIV in five countries of Central Asia (Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan and Uzbekistan) as well as on policies and programs that address these co-occurring epidemics.
Results
Regional data indicate high rates of fatal and non-fatal overdose among PWID. Evidence suggests mortality rates from overdose exceed HIV/AIDS as the leading cause of death among PWID. The syndemic framework suggests multiple macro-level and micro-level environmental risk factors that drive the co-occurring epidemics of HIV and overdose. This framework identifies several interacting biological and behavioral risks that result in additive effects for HIV and overdose.
Conclusion
The high rates of overdose and its associations with HIV underscore the need for a syndemic approach that considers overdose on parity with HIV. Such an approach should focus on the biological, behavioral and structural interactions between these epidemics to reduce social suffering, morbidity and mortality among PWID in Central Asia.
Keywords: Opioid Overdose, HIV, Naloxone, Injection Drug Use, Central Asia, syndemic
1. THE NEGLECTED EPIDEMIC OF OPIOID OVERDOSE IN CENTRAL ASIA
The steep rise in HIV among PWID in Central Asia over the past decade has coincided with a dramatic increase in the rates of opioid overdose. Opioid overdose has reached epidemic proportions both in terms of its scope and severity. Recent research suggests that overdose is a leading, if not primary, cause of morbidity and mortality among PWID in the region (see Table 1). To date, however, opioid overdose remains a largely hidden epidemic that does not appear to be on the radar screen of public health and government officials in Central Asia.
Table 1.
The prevalence of HIV and Overdose among PWID in Central Asia and Status of Naloxone
| Country | Estimated # of IDUS1 |
HIV Prevalence1 |
Non-Fatal Opioid OD Prevalence |
Mortality Rate from Overdose Deaths |
Naloxone Status |
Naloxone Distribution Data |
|---|---|---|---|---|---|---|
| Kazakhstan | 186,000 | 3.98% – 28%2 |
20% of PWID in past year3,4 |
2.1% (11 deaths) found over a 1- year period among 480 PWID3 |
Registered, but not included in MOH purchase list since 2011 |
Pilot naloxone distribution projects in NGOs in Almaty and Karaganda have distributed naloxone to a total of 388 PWID and 97 PWID have reported successfully reversing an opioid overdose.3, 8 These pilots were interrupted from September 2011 until January 2013 when naloxone was not included on the MOH purchase list. |
| Kyrgyzstan | 44,000 | 11% | 40% life time prevalence 5i |
Not Available (N/A) |
Registered and purchased by global fund |
Narcology dispensaries around the country have distributed naloxone kits to 2200 PWID since November 2012. To date, NGOs have reported 22 cases where naloxone was successfully used8. |
| Tajikistan | 34,000 | 23.6% | 23.6% of PWID in past year6 |
N/A | Registered and purchased by global fund |
Tajikistan NGOs distributed a total of 1,836 ampoules of naloxone to PWID clients since 2011. To date, 108 cases were reported where naloxone was used to successfully reverse an OD8 |
| Turkmenistan | 11,148 | N/A | N/A | N/A | N/A | -- |
| Uzbekistan | 80,000 | 15.3% | N/A | 0.13 per 100,0007 |
Registered but not included in purchase list |
-- |
| Total | 247,0009 – 355,14810 |
11% | N/A | N/A | -- | -- |
UNODC. (2010). Accessibility of HIV Prevention, Treatment and Care Services for People who use Drugs and Incarcerated People in Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan and Uzbekistan.. Ashgabat, Turkmenistan.
El-Bassel, N., Gilbert, L., Terlikbayeva, A., Wu, E., Beyrer, C., Shaw, S., et al. (in press). HIV among Injection Drug Users and their Intimate Partners in Almaty, Kazakhstan. AIDS & Behavior.
Gilbert, L., El-Bassel, N., Primbetova, S., Hunt, T., Terlikbayeva, A., & Chang, M. (2012). Co-occurring Opioid Overdose and HIV risks among PWID in Kazakhstan: Findings from Project Renaissance. Paper presented at the National Treatment as Prevention Conference.
Kazakhstan RAC. (2010). Results of a Behavioral Surveillance Survey. Republican AIDS Center of Kazakhstan. Almaty, Kazakhstan.
Republican Narcological Center. (2012). Results of a Behavioral Surveillance Survey. Republican Narcological Center. Bishkek, Kyrgyzstan.
Tajikistan RAC. (2011). Results of Behavioral Surveillance Survey. Tajikstan Republican AIDS Center. Dushanbe, Tajikstan.
The National Centre on Drug Control. (2012). 2012 National Report on the Drug Situation in the Republic of Uzbekistan. CADAP. Tashkent, Uzbekistan.
Primbetova, S., El-Bassel, N., Gilbert, L., Terlikbayeva, A., & Hunt, T. (2012). Use of DatStat sofware for data collection, monitoring, and evaluation of naloxone peer distribution programs to respond to opioid overdoses among people living with HIV and injecting drug users in Central Asia. Paper presented at the XIX International AIDS Conference.
Degenhardt, l. 2012. Extent of Illicit Drug Use and Dependence, and their Contribution to the Global Burden of Disease. Lancet, 379, 55–70.
The estimate of 355,148 represents the sum of the estimates of PWID of all five countries listed above.
The total estimated number of PWID in the five countries of Central Asia is similar or greater in size to the estimated population of PWID in the U.S. (see Table 1). About one-tenth (29,000) are estimated to be infected with HIV (Degenhardt, 2012). Although there remains a dearth of surveillance data on overdose, some evidence suggests alarming rates of overdose in the region. Data reported in Table 1 indicate that between 21–24% of the PWID in the region reported experiencing a non-fatal overdose in the past year (Tajikistan Republican AIDS Center (RAC), 2011; Kazakhstan RAC, 2010). A study of 480 PWID in Almaty, Kazakhstan found that overdose was the leading cause of death among this sample over a 12-month follow-up period (2.1%,N=11 deaths) compared to only one AIDS-related death (0.002%; Gilbert et al., 2012). Another study conducted among PWID in Tajikistan, Kyrgyzstan and Kazakhstan found that one-quarter (25.1%) reported witnessing a death of a PWID in the past year. These data are consistent with a 2013 systematic global review of mortality (Mathers et al., 2013) and other recent research which suggests that overdose is the leading cause of mortality among PWID, exceeding AIDS mortality among HIV-positive PWID (Baggett et al., 2013, Degenhardt, 2012; UNODC/WHO, 2013).
The public health response to opioid overdose in Central Asia remains woefully inadequate. Mounting research worldwide indicates that lay administration of naloxone (an opioid antagonist) to reverse overdose is safe, highly effective, and cost-effective (Compton et al., 2013; Coffin and Sullivan, 2013; CDC, 2012). Some small pilot peer-administered naloxone overdose prevention projects in Kazakhstan, Kyrgyzstan and Uzbekistan are under way with promising results, as shown in Table 1 (Primbetova et al., 2012, EHRN, 2012). These pilot projects, however, have yet to be scaled up. Major structural barriers continue to thwart these efforts including interruptions in the supply of naloxone due to low pharmaceutical profits and limited shelf life, failure to register or include naloxone on government purchase lists and lack of knowledge or training on naloxone use by medical professionals (Coffin, 2008; Gilbert et al., 2012).
2. DISENTANGLING OVERDOSE AND HIV EPIDEMICS: BEHAVIORAL AND BIOLOGICAL LINKAGES
Emerging research suggests multiple biological and behavioral links between overdose and HIV infection. PWID who experience non-fatal overdose are more likely to engage in drug-related and sexual risk behaviors and to test positive for HIV and hepatitis C infections (Coffin et al., July 2012; Gilbert et al., 2012; Green et al., 2012). A recent meta-analysis conducted by Green et al., (2012) found that HIV positive serological status carried a 74% greater risk of mortality from overdose among PWID, most likely as a result of abnormal liver or pulmonary problems and lowered CD4 counts. It is well documented that PWID with lower CD4 counts are at higher risk of transmitting HIV. To date, only one study has examined the associations between HIV and overdose among PWID in Central Asia. In this study of 480 PWID in Almaty, Kazakhstan, experiencing a non-fatal overdose in the past 6 months was associated with sharing syringes, injecting with multiple partners, having sex under the influence of drugs, and engaging in unprotected sex (Gilbert et al., 2012). Although the behavioral mechanisms linking overdose and HIV risks have yet to be researched, being under the heavy addictive influence of heroin impairs judgment and cognitive capacity of PWID to recognize risks and enact protective behaviors to prevent HIV transmission and overdose (e.g., using condoms, refusing to share drug equipment, testing purity of drugs before injection and avoiding excessive use or mixed use of drugs that increases the likelihood of overdose). Research suggests that integrating naloxone overdose prevention with HIV prevention interventions and harm reduction programs may not only reduce overdose rates (Walley et al., 2013), but also lead to better HIV outcomes, including a reduction in syringe sharing and an increased likelihood of initiating ARV treatment (Green et al., 2012; Coffin et al., 2012). By addressing the primary life-threatening concern of overdose among PWID, HIV services are more likely to build trust with PWID, link PWID to drug treatment, and retain PWID in harm reduction services and HIV care (Curtis and Dasgupta, 2010; Gilbert et al., 2012).
3. RETHINKING THE LINKS BETWEEN HIV AND OVERDOSE USING A SYNDEMIC FRAMEWORK
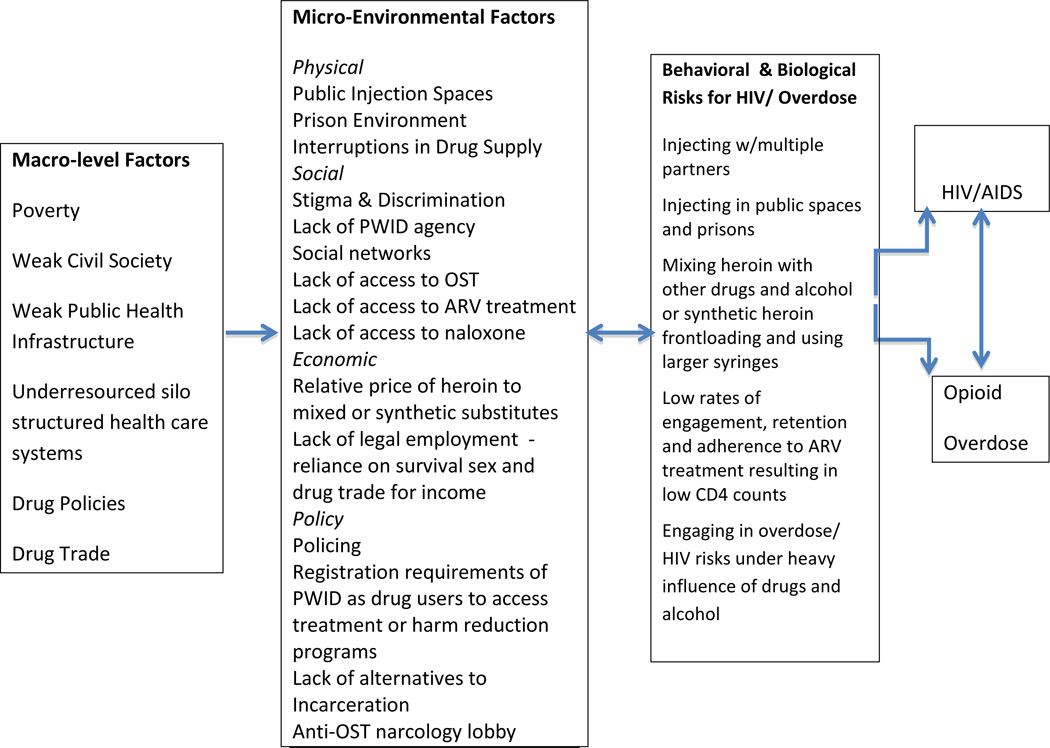
Syndemic theory provides a useful framework for understanding the social determinants of HIV and other co-occurring problems in vulnerable populations (Talman et al., 2013; Singer et al., 2006, Stall et al., 2003). A syndemic occurs when a concentration of co-occurring epidemics that are linked to common social conditions interact and reinforce each other. Syndemic interactions create additive risks that ultimately give rise to other health and social problems in specific populations (Singer et al., 2006). The conceptual framework shown in Figure 1 identifies the most salient macro-level and micro-level environmental risk factors associated with both HIV and overdose among PWID in Central Asia (Jolley et al., 2012; Coffin, 2008). This syndemic framework draws on previous risk environment research on HIV (Rhodes et al., 2006; Strathdee et al., 2010; Jolley et al., 2012) and overdose (Green et al., 2009). As shown in Figure 1, the co-occurring epidemics of HIV and overdose meet criteria of a syndemic, both in terms of their interacting and additive behavioral and biological risks and in terms of their common environmental risk factors.
Figure 1.
Conceptual Framework of Macro-level and Micro-level Environmental Risk factors Associated with the Syndemic Production of HIV and Opiate Overdose in Central Asia
4. THE INTERPLAY OF ENVIRONMENTAL RISK FACTORS FUELING THE HIV AND OVERDOSE SYNDEMIC
Dramatic social, political and economic transitions, which have transformed Central Asian countries over the past two decades following the Soviet Union collapse, have fueled the syndemic of HIV and overdose (Jolley et al., 2012; Beyrer et al., 2011). These transitions have resulted in pervasive poverty and widespread unemployment. The lack of employment and educational opportunities has contributed to widespread heroin use among youth. Lack of legal employment options have also pushed PWID into the drug trade and survival sex work, which increase their risks for HIV transmission and overdose (Strathdee et al., 2010). These transitions have also weakened civil society, which has impeded the capacity of NGOs and PWID to advocate for their needs and contributed to the lack of government transparency and accountability in their response to HIV and overdose as well as to the excessive punishment and corrupt policing of PWID. Furthermore, these transitions have led to a collapse of the public health infrastructure and brain drain of medical and scientific expertise needed to counter these epidemics (Jolley et al., 2012). The legacy of Russian style silo-structured health care systems that currently exists in these countries require PWID to go to separate service structures for HIV treatment, drug treatment, harm reduction services, and psychiatric care. The lack of coordination among these services and the lack of integration of these services into primary care continue to impede the delivery of essential services to PWID.
These macro-level risk factors along with the expansion of drug trafficking routes throughout Central Asia and punitive drug policies (e.g., mandatory long-term sentences for small amounts of drug use for personal use; lack of alternatives-to-incarceration), have created a “perfect storm” of environmental conditions for the syndemic production of HIV and overdose (Jolley et al., 2012, Beyrer, 2011). These macro-level factors shape and are reinforced by the interplay of micro-level environmental factors in which HIV and overdose co-occur as shown in Figure 1.
Key physical micro-environmental factors include injecting with multiple partners in public injection locations, interruptions in heroin supply and prison risk environments. These physical risk environments interact with social, economic and policy environmental factors. Research suggests that injecting with multiple partners in public spaces in Kazakhstan increases the risk of both overdose and HIV among PWID in Kazakhstan (El-Bassel et al., in press; Gilbert et al., 2012), consistent with previous research (Strathdee et al., 2010; Rhodes et al., 2005). Injecting in public spaces heightens fear of police arrest, creating a risk environment for hurried social injection practices, including sharing injection equipment (Rhodes et al., 2005) and not testing the purity of the drug prior to injection, increasing the risk of overdose. Fear of arrest also prevents PWID from calling emergency services to respond to an overdose, as police often accompany ambulances in Central Asia (Coffin 2008; Gilbert et al., 2012). PWID are reluctant to access syringe exchange programs and drug treatment as these sites are heavily policed. The requirement to register PWID as drug users by harm reduction or drug treatment programs in all five countries also prevents PWID from accessing these essential services, as these registration lists are often shared with police (Grund et al., 2013).
Interruptions in the heroin supply resulting from increased interdiction in the region reduces the purity and increases the cost of heroin, which in turn, increases the likelihood of HIV transmission and overdose (UNODC, 2010; Grund et al., 2013). As heroin becomes unavailable or unaffordable, PWID will often mix heroin with benzodiazepines, inject liquid forms of heroin or turn to heroin substitute drugs like “krokodil”, which has become increasingly prevalent in Central Asia (Grund et al., 2013). Krokodil and other synthetic substitute drugs are often frontloaded with shared cookers and require larger size needles for injection, increasing the likelihood of HIV transmission (UNODC, 2010; Grund et al., 2013). The use of these synthetic opioid drugs and mixing of heroin with other drugs or alcohol also increases the risk of overdose among PWID (Coffin, 2008; Grund et al., 2013).
The risk environment of prison and experience of incarceration is associated with HIV transmission and overdose (Green et al., 2009; Strathdee et al., 2010; UNODC/WHO, 2013). In all Central Asian countries, except for Kyrgyzstan, PWID in prison do not have access to new syringes, despite evidence of injection drug use in prison throughout Central Asia (UNODC, 2010). Injecting in prison also creates a “hurried” environment where syringes are more likely to be shared and drugs are not first tested for their purity. Furthermore, the high rates of arrest, pre-detention and incarceration of PWID in all countries contribute to a “revolving door” risk environment, which fuels the spread of HIV, as PWID transition between prison and home environments, mixing their drug and sexual networks (El-Bassel et al., in press). Upon release from prison, PWID are also at elevated risk for overdose as a result of lower tolerance along with excessive use associated with “coming home from prison parties” (Green et al., 2009; UNODC, 2013).
Micro-level social risk factors interact with physical, policy and economic factors to shape the risk environment in which HIV and overdose co-occur. Shared group norms and the size and density of networks influence patterns of syringe sharing and risky drug use for overdose (Strathdee et al., 2010; Green et al., 2009). The coverage rates for opioid substitution therapy (OST), anti-retroviral therapy (ARV), and SEPs among PWID remain low in all five countries (El-Bassel et al., in press; UNAIDS, 2012). Stigma and widespread discrimination against drug users marginalize and isolate PWID, deterring them from seeking essential harm reduction services, HIV care and opioid substitution therapy (OST; Needle and Zhao, 2010; UNODC, 2010). The Russian-influenced narcology lobby continues to block efforts to initiate or scale up OST in all five countries (Jolley et al., 2012).
5. CONCLUSION: CALL FOR POLICIES, PROGRAMS AND RESEARCH TO CURB THIS SYNDEMIC
Given that opioid overdose remains a major, if not the leading, cause of mortality and morbidity among PWID in Central Asia, there is a critical need to prioritize the public health response to overdose on parity with HIV. Coffin’s (2013) cost-effectiveness analyses found that lay naloxone administration for heroin users is highly cost effective under multiple assumptions. This includes the unthinkable “elephant in the room” assumption of projected medical cost savings associated with greater mortality rates of PWID from overdose that some government officials and policy makers may hold. The multiple biological, behavioral and structural linkages between HIV and overdose epidemics underscore the need to scale up combined naloxone overdose and HIV prevention interventions that will most efficiently address this deadly syndemic.
However, scaling up such programs alone is likely to have a minimal impact without a more comprehensive approach that addresses the macro-level and micro-level risk factors driving this syndemic. This syndemic framework points to several key first steps to redress these intertwined epidemics. First, the lack of surveillance of on overdose continues to preempt a strategic response to tackle the nexus of overdose and HIV risks in all five countries. Generating an accurate up-to-date surveillance of opioid overdose is not only necessary for targeting overdose prevention efforts, but may also illuminate “hot spots” where PWID social networks at risk for HIV infection exist. Second, reform of drug policies is needed, including decriminalizing possession of opiates for personal use, creating alternatives-to-incarceration, and removing policy and legislative barriers that prevent PWID from accessing harm reduction services (UNODC, 2010). Pilot programs are underway in the region to deliver harm reduction services in alternative venues, including pharmacies and mobile vans, which may be less subject to policing, and thus, enhance engagement of PWID. At the same time, police, prosecutors, and judges also need to be trained on the importance of drug policy reform, held accountable for excessive or corrupt policing or prosecution and engaged as key stakeholders in prevention efforts. Public health education efforts to raise awareness of harm reduction legislation among police in Kyrgyzstan show promise as a model that may be used to redress excessive policing (Beletsky et al., in press). Third, removing policy and programmatic barriers to scaling up OST in the region is critical for reducing the risks of HIV and overdose. Fourth, raising public awareness about the widespread problem of overdose and the use of naloxone as an antidote is needed to alert PWID and their family and friends how to identify and respond to an overdose. Finally, to echo a recent call to expand overdose prevention research by NIDA (Compton et al., 2013), future research is urgently needed to advance knowledge on what multi-level interventions and policies may effectively counter this syndemic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of Funding Source:
There was no funding source for this commentary.
Contributors:
Louisa Gilbert took a lead role in conceptualizing the paper and wrote the first draft of the paper. Sholpan Primbetova managed the literature review and collection of secondary data from the region as well as reviewed and provided feedback on drafts. Danil Nikitin collected secondary data from the region and assisted with the literature review as well as reviewing and providing feedback on drafts. Timothy Hunt, Assel Terlikbayeva and Azzi Momenghalibaf assisted in the systematic review of macro-level and micro-level conceptual factors and provided feedback on drafts. Murodali Ruziev collected secondary data from the region and assisted in the literature review. Nabila El-Bassel provided feedback on the outline, concept and draft of the paper.
- Louisa Gilbert has no conflict of interest.
- Shopan Primbetova has no conflict of interest.
- Danil Nikitin has no conflict of interest.
- Timothy Hunt has no conflict of interest.
- Assel Terlikbayeva has no conflict of interest.
- Azzi Momenghalibaf has no conflict of interest.
- Murodali Ruziev has no conflict of interest.
- Nabila El-Bassel has no conflict of interest.
REFERENCES
- Baggett TP, Hwang SW, J OCJ, Porneala BC, Stringfellow EJ, Orav EJ, Singer DE, Rigotti NA. Mortality among homeless adults in Boston: shifts in causes of death over a 15-year period. JAMA Intern. Med. 2013;173:189–195. doi: 10.1001/jamainternmed.2013.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletsky L, Thomas R, Shumskaya N, Artamonova I, Smelyanskaya M. Police Education as a Component of National HIV Response: Lessons from Kyrgyzstan. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2013.06.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrer C. The Golden Crescent and HIV/AIDS in Central Asia: deadly interactions. Global Public Health. 2011;6:570–576. doi: 10.1080/17441692.2011.572080. [DOI] [PubMed] [Google Scholar]
- CDC. Community-based opioid overdose prevention programs providing naloxone -- United States, 2010. MMWR. 2012;61:101–105. [PMC free article] [PubMed] [Google Scholar]
- Coffin P. Overdose: A Major Cause of Preventable Death in Central and Eastern Europe and Central Asia. Vilnius, Lithuania: Eurasian Harm Reduction Network; 2008. [Google Scholar]
- Coffin P, Coffin L, Fitzpatrick T, Murphy S. Drug Overdose, Lay Naloxone and HIV Risk Behaviors among Persons who Inject Drugs. XIX International AIDS Conference; Washington, D.C.. 2012. Jul, [Google Scholar]
- Coffin P, Sullivan SD. Cost effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann. Intern. Med. 2013;158:1–9. doi: 10.7326/0003-4819-158-1-201301010-00003. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND, Throckmorton DC, Lurie P. Expanded access to opioid overdose intervention: research, and policy needs. Ann. Intern. Med. 2013;158:65–66. doi: 10.7326/0003-4819-158-1-201301010-00013. [DOI] [PubMed] [Google Scholar]
- Curtis M, Dasgupta N. Why Overdose Matters for HIV. New York: Open Society Foundations; 2010. [Google Scholar]
- Degenhardt L. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- EHRN. The Voice Harm Reduction: Overdose Edition. Vilinius, Lithuania: Eurasian Harm Reduction Network; 2012. [Google Scholar]
- El-Bassel N, Gilbert L, Terlikbayeva A, Wu E, Beyrer C, Shaw S, Hunt T, Ma X, Chang M, Isamyilova L, Tukeyev M, Zhussupov B, Rozental E. HIV among injection drug users and their intimate partners in Almaty, Kazakhstan. AIDS Behav. doi: 10.1007/s10461-013-0484-2. in press. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L, El-Bassel N, Primbetova S, Hunt T, Terlikbayeva A, Chang M. Cooccurring Opiate Overdose and HIV risks among PWID in Kazakhstan: Findings from Project Renaissance. Kazakhstan National Treatment as Prevention Conference; Almaty, Kazakhstan. 2012. [Google Scholar]
- Green TC, Grau LE, Blinnikova KN, Tobin M, Krupitsky E, Ilyuk R, Kozlov A, Heimer R. Social and structural aspects of the overdose risk environment in St. Petersburg, Russia. Intl. J. Drug Policy. 2009;20 doi: 10.1016/j.drugpo.2008.07.002. 270-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Mcgowan SK, Yokell MA, Pouget ER, Rich JD. HIV Infection and risk of overdose: a systematic review and meta-analysis. AIDS. 2012;26:403–417. doi: 10.1097/QAD.0b013e32834f19b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund J-PC, Latypov A, Harris M. Breaking worse: the emergence of krokodil and excessive injuries among people who inject drugs in Eurasia. Intl. J. Drug Policy. 2013 doi: 10.1016/j.drugpo.2013.04.007. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jolley E, Rhodes T, Platt L, Hope V, Latypov A, Donghoe M, Wilson D. HIV among people who inject drugs in Central And Eastern Euorpe and Central Asia. BMJ Open. 2012;2:1–20. doi: 10.1136/bmjopen-2012-001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakhstan RAC. Results of a Behavioral Surveillance Survey. Almaty, Kazakhstan: 2010. [Google Scholar]
- Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull. WHO. 2013;91:102–123. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needle RH, Zhao L. HIV Prevention among Injection Drug Users: Strengthening U.S. Support for Core Interventions. Washington, D.C.: CSIS; 2010. [Google Scholar]
- Primbetova S, El-Bassel N, Gilbert L, Terlikbayeva A, Hunt T. Use of DatStat Sofware for Data Collection, Monitoring, and Evaluation of Naloxone Peer Distribution Programs to Respond to Opiate Overdoses Among People Living with HIV and Injecting Drug Users in Central Asia. XIX International AIDS Conference; Washington, D.C.. 2012. [Google Scholar]
- Rhodes T, Singer MC, Bourgouis P, Friedman S, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc. Sci. Med. 2005;61:1026–1044. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Singer MC, Erickson PL, Badiane L, Diaz R, Ortiz D, Abraham T, Nicolaysen AM. Syndemics, sex and the city: understanding sexually transmitted diseases in social and cultural context. Soc. Sci. Med. 2006;63:2010–2021. doi: 10.1016/j.socscimed.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall RD, Mills TC, Williamson J, Hart T, Greenwood G, Paul J. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am. J. Public Health. 2003;93:939–942. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Hallett T, Bobrova N, Rhodes T, Booth R, Abdool R, Hankins CA. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376:263–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajikistan RAC. Results of Behavioral Surveillance Survey. Dushanbe, Tajikistan: 2011. [Google Scholar]
- Talman A, Bolton S, Watson JL. Interactions between HIV/AIDS and the environment: towards a syndemic framework. Am. J. Public Health. 2013;103:253–261. doi: 10.2105/AJPH.2012.300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Global report: UNAIDS Report on the Global AIDS Epidemic 2011. New York: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2012. [Google Scholar]
- UNODC. Accessibility of HIV Prevention, Treatment and Care Services for People who use Drugs and Incarcerated People in Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Turkemenistan and Uzbekistan: Legislative and Policy Analysis. Ashgabat, Turkemenistan: 2010. [Google Scholar]
- UNODC/WHO. Opioid overdose: preventing and reducing opioid overdose mortality. Vienna: 2013. [Google Scholar]
- Walley AY, Xuan Z, Hackman H, Quinn E, Do-Simkins M, Ruiz S, Ozonoff A. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]