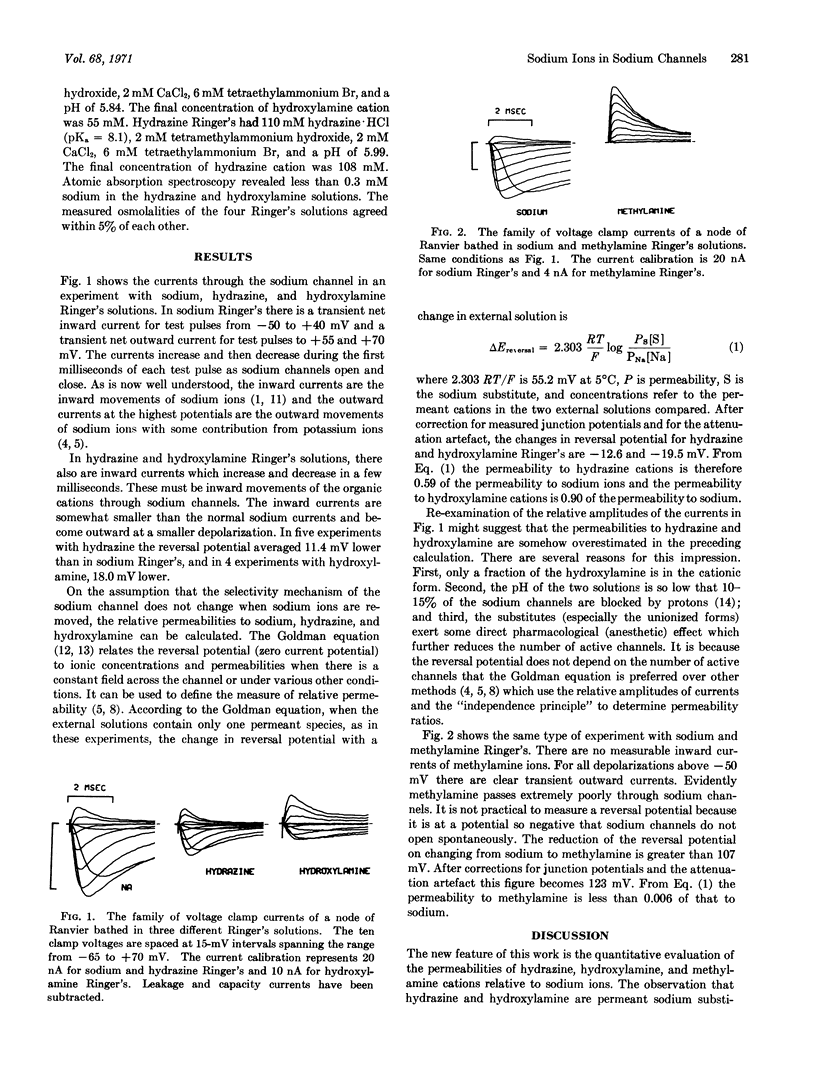
Abstract
The sodium channel of the excitability mechanism in nerve membranes is about as permeable to hydroxylamine and hydrazine cations as it is to sodium ions. It is impermeable to methylamine cations. This selectivity is explained by supposing that an oxygen group in the channel must receive a hydrogen bond from the permeating cation at the same time as the cation lies against another negatively charged oxygen acid. If these conditions are not satisfied the cation cannot permeate. Sodium ions can satisfy this hydrogen-bonding requirement if they have a water of hydration. The H2O·Na complex also has almost the same dimensions as the hydroxylamine and hydrazine cations. This hydrated ion is probably part of the critical complex between sodium ions and the selectivity mechanism of the sodium channel.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binstock L., Lecar H. Ammonium ion currents in the squid giant axon. J Gen Physiol. 1969 Mar;53(3):342–361. doi: 10.1085/jgp.53.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., MOORE L. E. THE SPECIFICITY OF THE INITIAL CURRENT IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. VOLTAGE CLAMP EXPERIMENTS. J Physiol. 1963 Nov;169:438–444. doi: 10.1113/jphysiol.1963.sp007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORENTE DE NO R., VIDAL F., LARRAMENDI L. M. Restoration of sodium-deficient frog nerve fibres by onium ions. Nature. 1957 Apr 6;179(4562):737–738. doi: 10.1038/179737b0. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Singer I., Watanabe A. Excitation of internally perfused squid giant axons in sodium-free media. Proc Natl Acad Sci U S A. 1965 Sep;54(3):763–769. doi: 10.1073/pnas.54.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]



