Abstract
We investigated associations between early fearfulness and error-related negativity (ERN) in preschoolers. Children were classified as low, moderate, or high in fearfulness based on observations at age 2 years. ERN was measured at age 4.5 years. ERN was visible in preschoolers who were moderate or high in fear during toddlerhood, but was characterized differently in children based in association with fearfulness during toddlerhood. A non-localized ERN was visible across midline electrodes in high-fear children and an adult like ERN distribution was visible in moderately-fearful children. In contrast, low-fear children showed no evidence of ERN at 4.5 years of age.
Anxious individuals acutely monitor and reflect on their performance (Messer, 1970), suggesting heightened performance monitoring as a marker of and possible mechanism for anxiety problems (Olvet & Hajcak, 2008). Yet, previous work has focused on symptoms in clinically anxious adults. To examine performance monitoring as a marker of risk rather than correlate of symptoms, the current study tested links between early risk for anxiety problems preschool performance monitoring.
The error-related negativity (ERN) is an event-related potential (ERP) that, in adults, peaks at frontocentral midline scalp recording sites 50 to 100 ms following an incorrect behavioral response (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991). The ERN likely reflects a general process of performance monitoring, signaling a need to change behavior to improve performance (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000). A similar ERP follows correct responses (Correct Trial Negativity [CRN]), but at smaller amplitudes than ERN, suggesting specificity of the ERN to errors.
ERN has now been elicited in children as young as 4 years of age (Brooker, Buss, & Dennis, 2011). While largely replicating adult work, studies of young children have suggested a more broadly distributed ERN than in adults (Brooker et al., 2011; Torpey, Hajcak, & Klein, 2009), and more variability in younger relative to adults and to older children (Davies, Segalowitz, & Gavin, 2004).
Previous research has reported links between enhanced ERN and concurrent anxiety symptoms as early as adolescence (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006). Only one study has examined an early risk factor for anxiety problems, temperamental fearfulness, as a predictor of ERN. McDermott and colleagues (2009) showed that children who had high mean levels of temperamental fear between 14 months and 7 years of age had greater ERN amplitudes at age 15 than did less-fearful children. Yet, similar to work with anxious adults, a subset of adolescents had already developed clinically significant anxiety problems by the time ERN was assessed. Thus, it difficult to determine whether ERN marked anxiety risk or the presence of symptoms. A more optimal time for testing associations between ERN and early risk is during the preschool years, as cognitive control processes such as performance monitoring are rapidly developing (Rothbart, 2011) and most children have not yet developed disorders. To show that early fearfulness predicts ERN in early childhood would provide evidence that performance monitoring is a plausible mechanism by which early fearfulness transitions into symptoms of anxiety. To that end, we tested whether individual differences in temperamental fearfulness during toddlerhood were related to ERN during preschool. We hypothesized that ERN would be accentuated in highly fearful children.
Method
Participants
Sixty-six families who visited the laboratory at child age 2 years as part of a longitudinal study of emotional development were invited to participate in the current study. Inclusion criteria required children being 4.5 years of age and free of known developmental delays, neurological impairments, and psychostimulant medications. One invited family withdrew from the project, 7 families did not respond, 3 families had moved away from the area, 13 families declined participation, and 1 family failed to show for their laboratory visit. Thus, the final sample included 41 preschoolers (20 girls; M age = 4.59; SD = 0.13). Consistent with local demographics, participants were largely Caucasian (87.5%) and middle class.
Procedure and Measures
Fearfulness during toddlerhood
We focused on fear during a novel, 3-minute puppet show during the age 2 laboratory visit (Buss, 2011). Fearfulness was quantified as a standardized composite of latency to freeze (reverse scored) and durations of facial fear, bodily fear, freezing, and proximity to mother (agreement: 86 – 91% and κ = 0.73 – 0.81). Children whose fearfulness scores fell in the lowest third of the distribution (n = 13) were labeled low fear, children in the middle third of the distribution (n = 13) were labeled moderate fear, and children in the highest third of the distribution (n = 15) were labeled high fear. Sex of child was unrelated to group membership (χ2[2] = 1.35, p > .10). All three groups significantly differed in toddler fearfulness (F(2, 39) = 95.67, p < .01), with high-fear children showing the most fear and low-fear children showing the least fear.
Preschool ERN
At age 4.5 years, children returned to the laboratory and were fitted with a 128-channel Hydrocel Geodesic Sensor Net (Electrical Geodesics, Inc.) for EEG collection. ERN was elicited during a modified flanker task (Attention Network Test [ANT]; Rueda et al., 2004), completed on a Dell PC using E-Prime 1.1 (Psychology Software Tools, Inc: Pittsburg, PA). An ANT session consisted of two experimental blocks of 64 trials. Each trial began with the presentation of a fixation cross for 400 ms. On some trials, a warning cue was subsequently presented for 150 ms and represented one of three conditions: spatial cue, double cue, no cue. Because they were not relevant to study hypotheses, cue conditions were collapsed in the current study. Following a second fixation period of 450 ms, the target fish and four flanker fish appeared and remained on the screen until a response, indicating the direction faced by the target fish, occurred or 1700 ms had elapsed. Accuracy and response time were recorded.
EEG was recorded using NetStation (version 4.3.1) acquisition software (Electrical Geodesic, Inc.: Eugene, OR). Data were sampled at a rate of 500 Hz with a gain of 1,000. Prior to data collection, impedances were reduced to less than 80 kΩ. EEG data were filtered during acquisition using a 0.1 Hz highpass filter and a 100 Hz lowpass filter. All channels were referenced to Cz during data collection and rereferenced offline to an average of the two mastoid channels. Data from each participant were submitted to an Independent Components Analysis in EEGLab Version 8.0.3b (Delorme & Makeig, 2004) in order to extract eye blink and eye movement artifacts.
Data processing was performed in Brain Vision Analyzer (BVA; Brain Products: Gilching, Germany). EEG data were high-pass filtered at 0.10 Hz (12 dB rolloff) and low-pass filtered at 20 Hz (12 dB rolloff). Segments (1600 ms) were extracted beginning 600 ms prior to participant responses. Segments were baseline corrected by subtracting the average activity from −600 to 0 ms from each data point. Remaining trials were rejected when one of the following criteria were met: a step of more than 75 µV occurred between data points, a difference of 150 µV occurred in a single segment, amplitudes exceeded ±200 µV within a trial, or amplitudes were below 0.5µV during any 50 ms period. Trials were visually inspected for remaining artifacts. An average of 6.17 (SD = 3.46) trials per person were rejected across trial types. Grand averages for artifact-free segments were created for correct and error trials (Table 1). Trials were excluded if reaction times occurred outside of a 200–1600 ms time window.
Table 1.
Mean Reaction Times and Accuracy during the ANT
| Total | Low Fear | Moderate Fear | High Fear | |||||
|---|---|---|---|---|---|---|---|---|
| Number of errors | 23.22 | (15.93) | 24.77 | (13.95) | 15.69 | (14.53) | 28.40 | (17.13) |
| Number of correct responses | 75.83 | (24.54) | 74.00 | (23.58) | 75.23 | (34.27) | 77.93 | (15.14) |
| Accuracy (% correct) | 76.60 | (13.97) | 74.65 | (13.42) | 81.69 | (14.23) | 73.88 | (13.95) |
| Reaction Time for Error Trials | 904.52 | (199.41) | 842.57 | (173.53) | 927.23 | (220.18) | 940.03 | (203.75) |
| Reaction Time for Correct Trials | 936.10 | (141.43) | 897.55 | (102.80) | 935.68 | (133.43) | 969.88 | (173.86) |
| Reaction Time for Post-error Trials | 951.19 | (156.87) | 902.74 | (119.91) | 943.36 | (180.82) | 999.45 | (160.65) |
| Reaction Time for Post-correct Trials | 921.10 | (147.10) | 880.52 | (109.11) | 941.38 | (132.30) | 940.04 | (184.55) |
| Number of errors included in ERN | 21.25 | (19.79) | 18.58 | (10.83) | 22.70 | (31.88) | 22.50 | (15.54) |
| Number of errors included in correct trials average | 51.61 | (26.73) | 50.42 | (28.72) | 45.30 | (32.75) | 57.14 | (20.45) |
| Error Related Negativity at Fz (µV) | −9.65 | (14.73) | −3.42 | (12.74) | −17.95 | (14.55) | −9.06 | (14.52) |
| Error Related Negativity at Cz (µV) | −6.32 | (8.63) | −6.24 | (5.69) | −1.14 | (10.88) | −9.72 | (7.96) |
| Error Related Negativity at Pz (µV) | −8.85 | (9.37) | −7.01 | (16.16) | −6.65 | (8.94) | −12.10 | (9.67) |
| Correct Trial Negativity at Fz (µV) | −3.68 | (7.28) | −4.84 | (6.76) | −3.51 | (7.62) | −2.82 | (7.86) |
| Correct Trial Negativity at Cz (µV) | −2.52 | (7.97) | −1.94 | (7.25) | −1.71 | (9.56) | −3.54 | (7.96) |
| Correct Trial Negativity at Pz (µV) | −6.22 | (8.09) | −8.02 | (8.51) | −1.39 | (7.80) | −8.28 | (6.80) |
Note Numbers outside of parentheses are means; numbers inside of parentheses are standard deviations. Range for number of trials used in grand averages: 6–99.
An automated procedure was used to quantify CRN and ERN as the mean voltage surrounding the greatest negative deflection in the time window from −100 to +100 ms (+/−20 ms) around the response. ERN amplitudes were quantified at Fz, Cz, and Pz electrodes. Scored in this way, greater ERN corresponds to greater negative amplitudes during error trials. ANT performance was uncorrelated with ERN at Fz (r = .01, p > .10), Cz (r = .02, p > .10), or Pz (r = −.16, p > .10).
Missing Data
ERP averages were not created for participants with less than 6 usable trials (Pontifex et al., 2010), resulting in the exclusion of data from 5 children without a sufficient number of error trials. Thus final analyses are based on data from 36 children (14 high fear, 10 moderate fear, 12 low fear). Children with and without ERP data did not differ in fear group membership (χ2[2] = 2.11, p > .10).
Results
One child had an ERN at Pz greater than 3.5 SD away from the sample mean and was removed as an outlier. Analyses included an examination of response time data followed by a Repeated Measures ANOVA (Greenhouse-Geisser corrected probability values) to test whether fearfulness in toddlerhood was linked to ERN during preschool. We report the highest-ordered significant effect (Fquad or Flin) for each analysis below. For nonsignificant effects, main effects Fs (Fmain) are reported.
Response Time Data
Reaction time and accuracy data from the ANT are presented in Table 1. Although the pattern of means suggested that response times were significantly faster for error trials (M = 904.52, SD = 199.41) relative to correct trials (M = 937.96, SD = 142.72), as is typical, this difference was not significant (t(39) = −1.54, p > .10). Similarly, the difference in response times for correct and incorrect trials was similar across all three fear groups (F(1,37) = 1.05, p > .10).
There was a significant difference, however, between response times following correct (i.e., post-correct) and error (i.e., post-error) trials (t(39) = −2.59, p = .01, Cohen’s d = .20). This effect was driven by post-error slowing in the high-fear children (t(14) = −3.07, p < .01, d = .34). Significant post-error slowing was not observed in the low-fear (t(12) = −1.17, p > .10) or moderate-fear groups (t(11) = −0.10, p > .10). Fear group did not predict overall performance (% correct) on the task (F(2,40) = 1.29, p > .10).
Toddler Fearfulness Predicts Preschool ERN
A 3 (Electrode: Fz, Cz, and Pz) X 2 (Trial Type: Correct and Error) repeated measures ANOVA including fear status (high, moderate, low) as a between-subjects factor was conducted to determine whether toddler fearfulness was associated with preschool ERN. A significant main effect of trial type confirmed that errors were associated with greater negativity than correct trials (Flin(1, 30) = 10.55, p < .01, η2p = .26), consistent with the presence of ERN. Similarly, mean levels of negativity differed across electrode sites (Fquad(1, 30) = 10.78, p < .01, η2p = .26). A significant interaction between electrode site and trial type suggested that the difference between error negativity and correct trial negativity varied as a function of electrode site (Flin(1, 30) = 4.40, p < .05, η2p = .13). Of particular interest for the questions of the current study, main effects were subsumed under a significant three-way interaction among electrode site, trial type, and fear status (Fquad(2, 30) = 3.52, p < .05, η2p = .19).
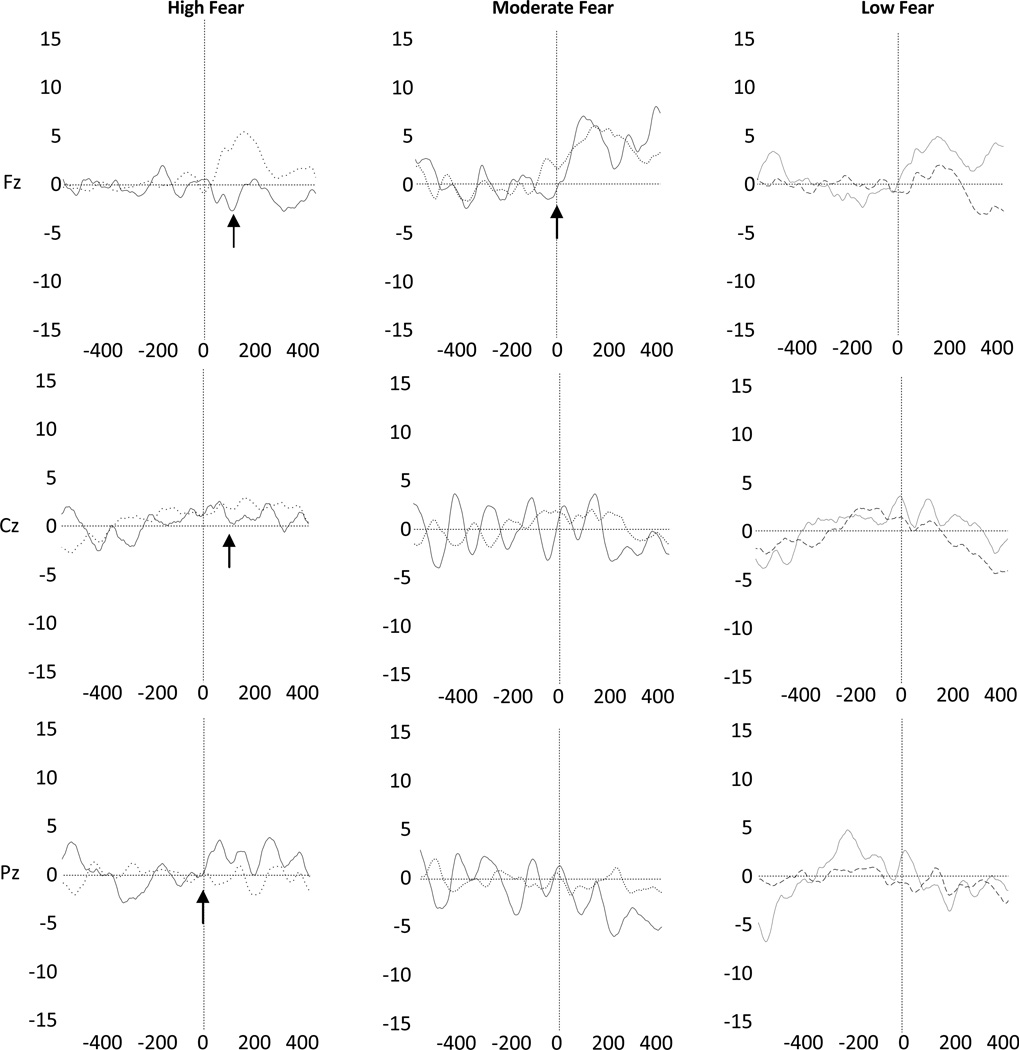
The three-way interaction was probed by rerunning the repeated measures ANOVA separately for each fear group. As shown in Figure 1, Low-fear children did not show greater negativity to error trials than to correct trials (Fmain(1, 10) = 0.10, p > .10), precluding the possibility of an ERN.
Figure 1.
Response-locked ERP waveforms at Fz, Cz, and Pz for high-fear, moderate-fear, and low-fear children.
Note: X-axis presents time in milliseconds (ms). Y-axis presents amplitude in microvolts (µV). For all groups, solid lines represent neural activity on error trials; broken lines represent neural activity on correct trials. Arrows are used to highlight the ERN effect.
In the moderate fear group, the difference between error negativity and correct trial negativity varied across electrodes (Flin(1, 8) = 4.84, p = .05, η2p = 0.38). Error trial negativity was greater than correct trial negativity at Fz (t(9) = −3.07, p < .05, d = 1.30), but not at Cz (t(8) = 0.10, p > .10) or Pz (t(9) = −1.62, p > .10).
In the high fear group, error-trial negativity was greater than correct-trial negativity (Flin(1, 12) = 10.41, p < .01, η2p = 0.46). This effect did not differ across electrodes (Fmain(2, 24) = 1.40, p > .10).
Discussion
We showed that fearfulness during toddlerhood predicted individual differences in ERN during preschool. Fear-related differences in ERN were visible as group-differences in distribution rather than amplitude. Like adults, moderately-fearful children showed ERN at Fz while highly-fearful children showed ERN across all electrode sites. Given that early-developing brain regions are overtaken by later-maturing structures during development (Bachevalier & Mishkin, 1984), a developmental shift in maxima from posterior to anterior regions has thus been interpreted as ERN maturation; thus, a more parietal ERN may reflect delayed maturation in more fearful children (e.g., Brooker et al., 2011; Torpey et al., 2009).
That anxious behaviors have been theoretically and empirically linked to inefficiencies in cognitive processing is also notable. In children, broadly distributed neural activity reflects cognitive inefficiency (Casey et al., 1997), as greater recruitment of neural resources is needed to maintain performance. While structural imaging data are necessary to distinguish inefficiency and immaturity, inefficient processing, similar to that seen in adults, may also be a plausible explanation for the presence of a non-localized ERN in high-fear children.
Unexpectedly, low-fear children did not show differences in neural processing during correct and incorrect trials. Risk for externalizing problems has been linked to temperamental surgency, one aspect of which is low fearfulness (Martin & Fox, 2006). If children at risk for anxiety problems are hyper-reactive to errors, children at risk for externalizing problems may be hypo-reactive to errors. Such reasoning has been used to explain reduced ERN in children with Attention Deficit-Hyperactivity Disorder relative to controls (Steiben et al., 2007). However inconsistent findings (e.g., Wiersema et al., 2005), suggest that additional work will be needed to clarify links between ERN and externalizing risk.
Limitations
Although our sample size and group ns are comparable to or larger than other samples from this domain of research (e.g., Ladouceur et al., 2006; Torpey et al., 2009; Wiersema et al., 2005), a small sample of such young participants may mean that residual artifacts still exist in ERP averages. Thus, replications of this work in larger, longitudinal samples will be necessary.
Additionally, levels of fear may have been restricted in high fear-children. Parents of highly-fearful children may have been less likely to enroll in the parent study. This possible bias is not uncommon in studies of temperamental fearfulness and should be considered when interpreting results.
Implications and Future Directions
The current work offers preliminary evidence for the role of ERN as marking mechanism of early risk for anxiety problems. Given an association between ERN and pre-clinical levels of fearfulness, our results suggest ERN is not simply a correlate of anxiety symptoms, but may denote an early risk for anxiety problems associated with heightened performance monitoring.
Acknowledgments
Data collection for this project was partially supported by R01 MH075750 from the National Institute of Mental Health (PI: Buss) and a RGSO dissertation support award from the Penn State College of the Liberal Arts to the first author. Training for the first author during the planning of this project was supported by R25 MH080794 (PI: Luck). The preparation of this manuscript was partially supported by the first author’s fellowship on T32 MH018931 (PI: Davidson) and by K01 MH100240 (PI: Brooker).
We thank the children and families who participated in this study and staff members of the Emotion Development Lab and the Social, Life, and Engineering Sciences Imaging Center who provided assistance and resources for recruitment, data collection, and data analysis.
Contributor Information
Rebecca J. Brooker, University of Wisconsin – Madison
Kristin A. Buss, The Pennsylvania State University
References
- Bachevalier J, Mishkin M. An early and a late developing system for learning retention in infant monkeys. Behavioral Neuroscience. 1984;98:770–778. doi: 10.1037//0735-7044.98.5.770. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Dennis TA. Error-monitoring brain activity is associated with affective behaviors in young children. Developmental Cognitive Neuroscience. 2011;1:141–152. doi: 10.1016/j.dcn.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA. Which fearful toddlers should we worry about? Effects of context on observed fear and risk for anxiety. Developmental Psychology. 2011;47:804–819. doi: 10.1037/a0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error-monitoring event-related potentials in adolescents. Annals of the New York Academy of Sciences. 2004;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Messer S. The effect of anxiety over intellectual performance on reflection-impulsivity in children. Child Development. 1970;41:723–735. [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox N. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopthology: Toward an endophenotype. Clinical Psychology Review. 2008;28:349–357. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu C, Themanson JR, Hillman CH. On the number of trials necessary for stabilization of error-related brain activity across the lifespan. Psychophysiology. 2010;47:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss B, Halparin J, Gruber D, Lercari L, Posner M. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Steiben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Klein DN. An examination of error-related brain activity and its modulation by error value in young children. Developmental Neuropsychology. 2009;34:749–761. doi: 10.1080/87565640903265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. ERP correlates of impaired error monitoring in children with ADHD. Journal of Neural Transmission. 2005;112:1417–1430. doi: 10.1007/s00702-005-0276-6. [DOI] [PubMed] [Google Scholar]