Abstract
With countless research papers using preclinical models and showing the superiority of nanoparticle design over current drug therapies used to treat cancers, it is surprising how deficient the translation of these nano-sized drug carriers into the clinical setting is. This review article seeks to compare the preclinical and clinical results for Doxil®, PK1, Abraxane®, Genexol-PM®, Xyotax™, NC-6004, Mylotarg®, PK2, and CALAA-01. While not comprehensive, it covers nano-sized drug carriers designed to improve the efficacy of common drugs used in chemotherapy. While not always available or comparable, effort was made to compare the pharmacokinetics, toxicity, and efficacy between the animal and human studies. Discussion is provided to suggest what might be causing the gap. Finally, suggestions and encouragement are dispensed for the potential that nano-sized drug carriers hold.
Keywords: Cancer Therapy, Drug Carrier, Nanomedicine, Drug Delivery, Clinical Translation
1. Introduction
Nanotechnology is a remarkable example of human achievement. In only a few decades of concerted effort, our knowledge of the laws of physics and chemistry has expanded to the point that we are able to manipulate matter at nearly the atomic scale to create complex structures with unique and potentially revolutionary functions. Nanotechnology describes the ability to manipulate matter at the scale of 1–100 nanometers in order to create and use structures with new, unique and useful properties [1]. This technology has far-reaching implications for improving the human condition, including more compact and powerful microchips and processors, more robust agriculture, cleaner, more efficient fuels, and better health. With this promise in mind, billions of dollars have been invested in nanotechnology research, and in some fields the return of that investment is starting to be realized [2]. However, as with most new technologies, progress has been uneven and the nature of future advancements uncertain.
Nanomedicine is the application of nanotechnology to improve the health of individuals through better diagnoses and treatments. However, nanomedicine is a very broad term, including applications in sensors, tissue engineering, imaging agents and other diagnostics, lab-on-a-chip devices, therapeutic agents, and drug carriers. Its usefulness has been diluted by a degree of irrational exuberance that has permeated the discussion of nanotechnology over the last few decades [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. Furthermore, certain technologies such as liposomes, polymer therapeutics, and protein therapeutics have existed long before “nanotechnology” was introduced. Thus, when considering drug delivery technology, particularly for anti-cancer therapeutics, it may be useful to abandon the term nanomedicine and instead adopt less loaded descriptors. This review will refer to these technologies as nano-sized drug carriers or simply drug carriers which are injectable into the blood stream, and focus on those drug carriers which were designed to provide better efficacy and lower toxicity for cancer therapeutics. The impact of drug carriers has in some ways been difficult to judge; they can be quite versatile, allowing researchers the flexibility to design delivery strategies specific to environmental challenges posed by the body. On the other hand, the more complex designs have thus far had little impact on clinical therapies beyond merely adding the rhetoric of targeted drug delivery to somewhat conventional therapies.
Cancer has been an area of particular interest for nano-sized drug carriers due to the enhanced permeability and retention (EPR) effect which is thought to provide them with significant therapeutic advantages over small molecule chemotherapy drugs [13], [14]. EPR refers to the tendency for nanoparticles and macromolecules to accumulate in tumors more, in comparison with the control solution formulations, due to the disorganized and ill-formed blood vessels that contain large fenestrae through which these large molecules can pass. Retention is increased by the dysfunctional lymph vessels which significantly hinder drainage from the tumor interstitial space. EPR was first discovered in the 1980s by Dr. Hiroshi Maeda and has subsequently become a key concept in the field of cancer drug delivery [15], [16]. According to the EPR hypothesis, nano-sized drug carriers should enjoy a natural advantage over traditional therapies as the increased drug concentration within a tumor should provide improved efficacy, and reduced toxicity due to the shielding of the drug from the rest of the body. This has generally been the case in the animal models used for preclinical studies [17], [18], [19], [20], but the improved efficacy promised by the EPR effect has often failed to materialize in clinical settings [21], [22].
The apparent gap between preclinical animal models and the clinical tumors encountered by clinicians is of great interest if drug carriers are to make a significant impact at the core of cancer therapy rather than just at the margins. Oncology drugs (including drug carrier technologies) suffer a 95% failure rate after entering human trials. Most of these failures occur in the efficacy phases and can cost hundreds of millions of dollars. A better understanding of the shortcomings of commonly used models could thus potentially save billions of dollars in wasted effort.
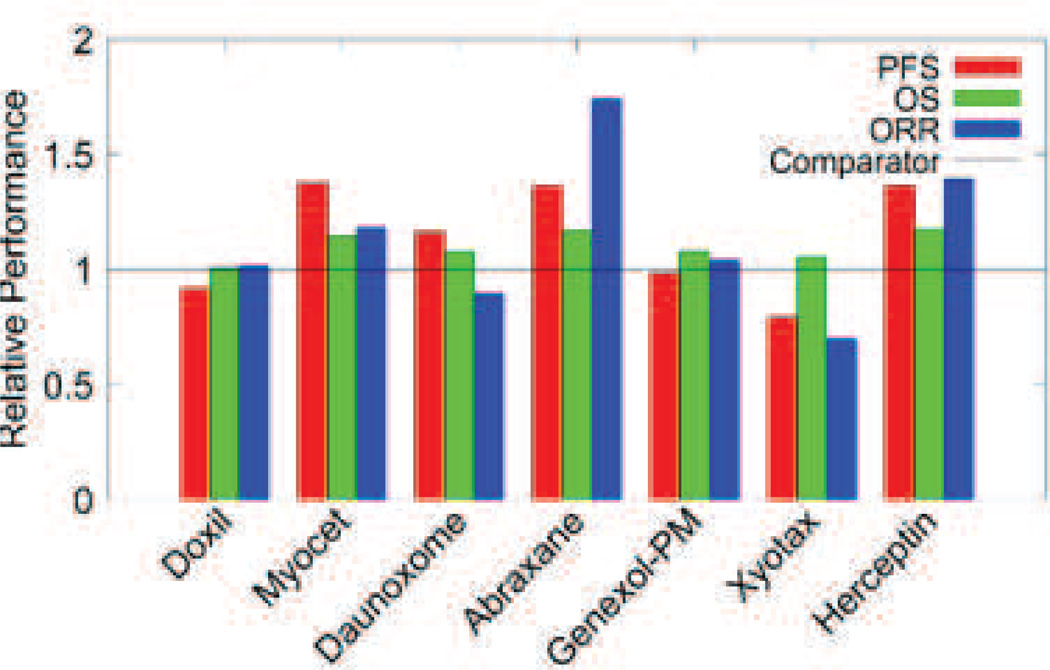
This review presents a cross section of some of the most important formulation strategies being pursued by researchers including liposomal formulations, micelles, linear polymers and protein carriers. Each of these formulations is unique in the way it changes the interactions between the drug and body. Some are designed with a half-life of several days, leaving a portion to circulate for weeks in the blood, with the intent to slowly accumulate in the tumor via EPR. Others simply attempt to improve the solubility of the drug cargo without harmful effects. Still others seek to actively target tumors by attaching groups that participate in ligand binding events with either cancer cell surface proteins or other targets of interest. Most of these formulations drastically impact how the drug and body interact, including starkly different pharmacokinetic parameters such as half-life, area under the curve, distribution, and clearance. Curiously, however, the impact of these formulations on efficacy is rarely significant outside the laboratory (Fig. 1).
Fig. 1.
Summary of phase III performance of selected drug carrier therapies compared to standard treatment. PFS: progression free survival; OS: overall survival; ORR: overall response rate. Data gathered from phase III trials [21], [22], [69], [106], [119], [122], [183], [184], [185].
Comparing human tumors to animal models can be a difficult game. In addition to the obvious differences in size, lifespan, physiology and metabolism between humans and mice which most commonly serve as models, the cancers themselves are significantly different from their naturally derived counterparts. Model cancers are grown artificially, from single cell lines, and in a matter of weeks, whereas human tumors emerge from complex tissues under immune surveillance over years. To make matters worse, preclinical and clinical rarely use the same benchmarks or endpoints. Preclinical studies often make use of measurements not available to the clinician, such as tumor diameter. Phase III efficacy studies generally use the best available therapy as a comparator, which often includes combinational therapies. Preclinical studies, on the other hand, do not always use the best available therapies as comparators but rather single compound formulations of the same drug contained in the carrier.
Given these differences, it may be reasonable to ask what can realistically be expected from human trials after successful animal trials, and are new drug formulations to treat human patients or mice being designed? Some of these discrepancies are beyond our control, but others can be ameliorated by concerted effort and greater global communication between the research and clinical communities as well as relevant regulatory agencies. Following the history of these drugs from the benchtop to the bedside provides some insight into where the shortcomings are, whether in the models used or in the discussions about them.
2. Doxorubicin
Doxorubicin is an anthracycline topoisomerase inhibitor. It was first isolated from culture medium of Streptomyces peucetius var. caesius by F. Arcamone from Farmitalia Research Laboratories in 1967 [23]. The anticancer activity of doxorubicin is given by multiple mechanisms [24], [25]. Doxorubicin intercalates between base pairs in the DNA helix, and inhibits the binding of DNA and RNA polymerase, resulting in the prevention of DNA replication and protein synthesis. Specifically, DNA intercalated with doxorubicin stabilizes the topoisomerase II-DNA complex during DNA replication, and prevents the ligation of the nucleotide strand after double-strand breakage. Since its approval, doxorubicin has been widely used to treat various malignancies (acute lymphoblastic leukemia, acute myelogenous leukemia, breast cancer, gastric cancer, Hodgkin’s lymphoma, neuroblastoma, non-Hodgkin’s lymphoma, ovarian cancer, small cell lung cancer, soft tissue and bone sarcomas, thyroid cancer, transitional cell bladder cancer, Wilms’ tumor and others) [26], [27], [28]. However, the use of doxorubicin has been limited by toxicities such as hematopoietic suppression, nausea, vomiting, apoplexy, alopecia and especially cardiotoxicity, which is a fatal adverse effect. Cardiotoxicity is characterized by a broad spectrum of symptoms ranging from asymptomatic electrocardiography (ECG)-changes, to pericarditis and decompensated cardiomyopathy. The probability of developing cardiotoxicity is largely dose-dependent. The usual dosage of doxorubicin is 60–75 mg/m2 every 3 weeks. Cardiomyopathy and congestive heart failure occur most frequently above a cumulative dose of 450–550 mg/m2 doxorubicin [29]. The most common cause of doxorubicin-related cardiotoxicity is oxidative stress. Doxorubicin produces oxygen free radical resulting in lipid peroxidation of cell membrane lipids [30], [31], [32], [33]. The specificity of the oxidative stress to the cardiac cells could be due to relatively low levels of antioxidant enzymes in heart [34].
2.1 Doxil®/Caelyx®
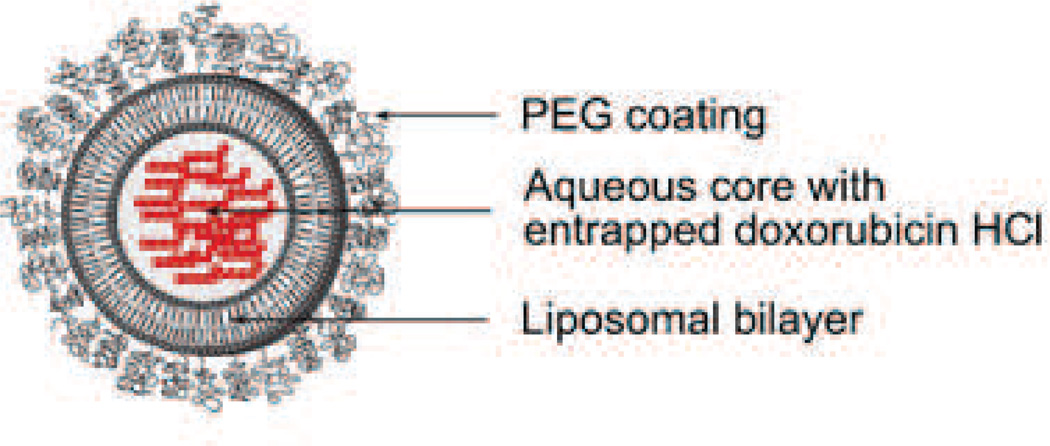
Doxil® [US], or Caelyx® [outside US], was the first FDA-approved nano-sized drug carrier formulation [35]. As seen in (Fig. 2), the doxorubicin molecules are encapsulated in a bilayer vesicle of lipids and is known as a liposome. This liposome is coated by a layer of poly(ethylene glycol) (PEG) to limit uptake by the mononuclear phagocyte system (MPS). Doxil® was developed as pegylated liposomal doxorubicin (PLD) to enhance efficacy and decrease toxicity of doxorubicin by virtue of its size (80–90 nm), pegylated surface, and stability in the blood, which allow prolonged blood circulation time considered necessary for high accumulation of doxorubicin in tumors [36].
Fig. 2.
Pegylated liposomal doxorubicin (Doxil® / Caelyx®), Particle size is 80–90 nm. Developed by Johnson and Johnson and approved in 1995 for treating AIDS-related Kaposi's sarcoma. So far, the indications have been extended to ovarian cancer and multiple myeloma. Illustration modified from FDA label.
2.1.1 Preclinical studies
Doxorubicin shows biphasic curves of plasma concentration after intravenous (i.v.) injection. First phase accounts for rapid distribution with a half-life of 5–10 min and second phase is an elimination with a half-life of 30 ± 8 h [37]. In contrast to the rapid elimination of free doxorubicin from blood circulation, Doxil maintains a significantly higher blood concentration than free doxorubicin for several days in various animals (rabbit, rat, and dog) [38], [39], [40], [41], [42]. The area under the plasma concentration-time curve (AUC) of doxorubicin in animals treated with Doxil is at least 60 times greater than that treated with free doxorubicin. The distribution volume of Doxil is close to the blood volume in the body. Clearance curves of total doxorubicin and doxorubicin entrapped in pegylated liposomes are superimposable [38], [43]. This indicates that the leakage of doxorubicin from pegylated liposomes while circulating is negligibly small.
A number of studies have investigated the tissue distribution of doxorubicin after i.v. injection of Doxil. Doxil treatment shows more accumulation of doxorubicin in various xenograft tumors (e.g., J6456 lymphoma [44], PC-3 human prostate adenocarcinoma [45], A375 human melanoma [46], N87 human gastric carcinoma [46], and C26 murine colon carcinoma [38]) than free doxorubicin treatment. Doxorubicin accumulation in the liver and spleen by Doxil® is also increased [45], [46].
The most important advantage of PLD is that doxorubicin accumulation in the heart was drastically reduced compared to free doxorubicin [47]. Comparative studies in rats, rabbits and dogs demonstrated the decrease in cardiotoxicity [37], [48].
Several efficacy studies using various tumor xenograft mice (e.g., C-26 murine colon carcinoma [49], PC-3 human prostate carcinoma [45], AsPC-1 human pancreatic carcinoma [50], HEY Human Ovarian Carcinoma [51], 3LL Human Lewis lung, carcinoma, [52], BT474 breast carcinoma [52] and MCF-7 breast adenocarcinoma [52]) revealed significant efficacy gains of Doxil over free doxorubicin [53], [54]. Studies using Lewis lung xenografts and breast cancer xenografts showed that the efficacy of Doxil at 2 mg/kg was approximately equivalent to that of doxorubicin at 4–9 mg/kg [52], indicating a 2 to 4.5-fold increase in potency of Doxil compared with free doxorubicin.
2.1.2 Clinical Studies
The first clinical trial of Doxil in patients with broad types of cancer (breast cancer, non-small cell lung cancer, ovarian cancer, mesothelioma, soft tissue sarcoma, and pancreatic cancer) was conducted from 1991 to 1994 in Jerusalem, and showed first proof of passive targeting based on the EPR effect in humans after intravenous administration [55]. Since then, a huge number of clinical studies have been conducted in various cancers: mainly AIDS-related Kaposi’s sarcoma (ARKS), ovarian cancer, breast cancer, multiple myeloma and soft tissue sarcoma.
Pharmacokinetic results are summarized in a review article by Gabizon et al.[39]. Doxil has significantly longer half-life than that of free doxorubicin. Surprisingly, the half-life found in clinical studies of ~45 h is even longer than that found in rodents and dogs of ~20 h and ~30 h, respectively.
Tissue distribution has also been studied from the first clinical trials. All results reveal Doxil had a higher accumulation in the tumor compared to the adjacent, normal tissues as well as the tumor treated with free doxorubicin [55], [56], [57], [58]. There is a tissue distribution study based on the use of 111In-DTPA labeled, drug free, pegylated liposomes of identical lipid composition to Doxil [36]. Tumors were visualized in fifteen of seventeen patients with breast, head and neck, bronchus, glioma and cervix cancer by gamma camera. Various normalized levels of accumulation (33% of injected dose/kg (ID/kg) in head and neck cancer, 18.3% ID/kg in lung cancer and 5.3% ID/kg in breast cancer) were detected. However, less than 5% ID was in a tumor as a whole due to small sizes of the solid tumors while a significant accumulation in other organs (liver, spleen and bone marrow) was detected.
Doxil shows a vastly different adverse-effect profile from that of free doxorubicin. These profiles are well summarized in review articles [59], [60]. While many toxicities induced by doxorubicin, as represented by cardiotoxicity, were dramatically decreased [61], [62], overall mucocutaneous toxicity was increased. Palmer-planter erythrodysesthesia (PPE) is particularly recognized as dose limiting toxicity.
The efficacy of Doxil has been extensively summarized in many articles and book chapters [63], [64]. Doxil showed better efficacy than conventional single or combinatory therapies in several cancers: bleomycin and vincristine [65], [66], paclitaxel [67] and highly active antiretroviral therapy [68] in ARKS, as well as topotecan in recurrent ovarian cancer [69], [70]. On the other hand, Doxil showed equivalent efficacy with reduced toxicity to free doxorubicin in metastatic breast cancer [22] and multiple myeloma [71], despite prolonged blood circulating time and improved accumulation in tumors (see Table 1).
Table 1.
Summary of clinical efficacy.
| Cancer type | ARKS | ARKS | ARKS | ARKS | Recurrent Ovarian Cancer |
Metastatic Breast Cancer |
Multiple Myeloma |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | PLD1 | BV | PLD1 | ABV | PLD1 | PTX | PLD1 | HAART | PLD2 | TPN | PLD2 | DOX | DVd | VAd |
| No. of patients | 121 | 120 | 133 | 125 | 37 | 36 | 13 | 15 | 239 | 235 | 254 | 255 | 97 | 95 |
| CR (%) | 6.0 | 1.0 | 1.0 | 0.0 | 5.0 | 8.0 | 31.0 | 13.0 | 3.8 | 4.7 | - | - | 3.1 | 0.0 |
| PR (%) | 53.0 | 22.0 | 45.0 | 25.0 | 41.0 | 47.0 | 46.0 | 7.0 | 15.9 | 12.3 | - | - | 41.3 | 41.1 |
| OR (CR + PR, %) | 59.0 | 23.0 | 46.0 | 25.0 | 46.0 | 55.0 | - | - | 19.7 | 17.0 | 33.0 | 38.0 | 44.4 | 41.1 |
| Stable (%) | 38.0 | 68.0 | 53.0 | 67.0 | 32.0 | 25.0 | - | - | 32.2 | 40.4 | 25.0 | 25.0 | 39.2 | 48.4 |
| Progressive (%) | 0.0 | 4.0 | 2.0 | 8.0 | 3.0 | 3.0 | - | - | 48.1 | 42.6 | 18.0 | 11.0 | 2.1 | 0.0 |
| Not assessable (%) | 3.0 | 5.0 | 0.0 | 0.0 | 24.0 | 17.0 | - | - | - | - | 18.0 | 21.0 | 14.4 | 10.5 |
| PFS (months) | - | - | - | - | 12.2 | 17.5 | - | - | 3.8 | 4.0 | 6.9 | 7.8 | 78%* | 76%* |
| OS (months) | 5.3 | 5.3 | - | - | - | 53.6 | - | - | 14.0 | 13.2 | 21.0 | 22.0 | 88%* | 85%* |
| Ref. | [64] | [63] | [65] | [66] | [67] | [20] | [69] | |||||||
CR: Complete response; PR: Partial response; OR: Overall response; PFS: Progression free survival; OS: Overall survival; ARKS: AIDS-related Kaposi’s sarcoma; PLD1: Pegylated liposomal doxorubicin (20 mg/m2); PLD2: Pegylated liposomal doxorubicin (50 mg/m2); BV: bleomycin (15 IU/m2) and vincristine (2 mg); ABV: doxorubicin (20 mg/m2), bleomycin (10 mg/m2) and vincristine (1 mg); PTX: Paclitaxel (100 mg/m2); HAART: highly active antiretroviral therapy; TPN: Topotecan (1.5 mg/m2) ; DOX: Doxorubicin (60 mg/m2); DVd: pegylated liposomal doxorubicin (40 mg/m2), vincristine (1.4 mg/m2; maximum, 2.0 mg) and reduced-dose dexamethasone (40 mg); VAd: vincristine (0.4 mg) doxorubicin (9 mg/m2) and reduced-dose dexamethasone (40 mg); ‘-’ not available; ‘*’ 1 year PFS or OS rate [22], [65], [66], [67], [68], [69], [71]
In summary, Doxil improved toxicity profile owing to its different pharmacokinetics and biodistribution from those of free doxorubicin; however, Doxil introduced other toxicities like PPE. Unlike preclinical results, Doxil has yet to show improvement in efficacy when compared with free doxorubicin. For the patient, Doxil absolutely has an advantage compared with conventional therapy due to reduced toxicity. Still, there seems to be an unknown gap in efficacy between preclinical and clinical studies.
2.2 PK1 / FCE 28068
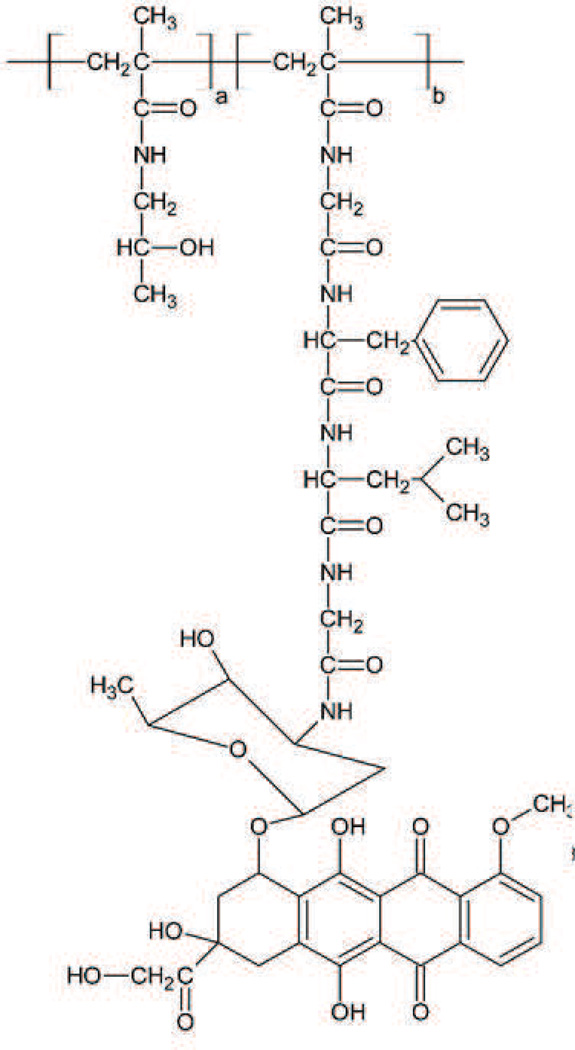
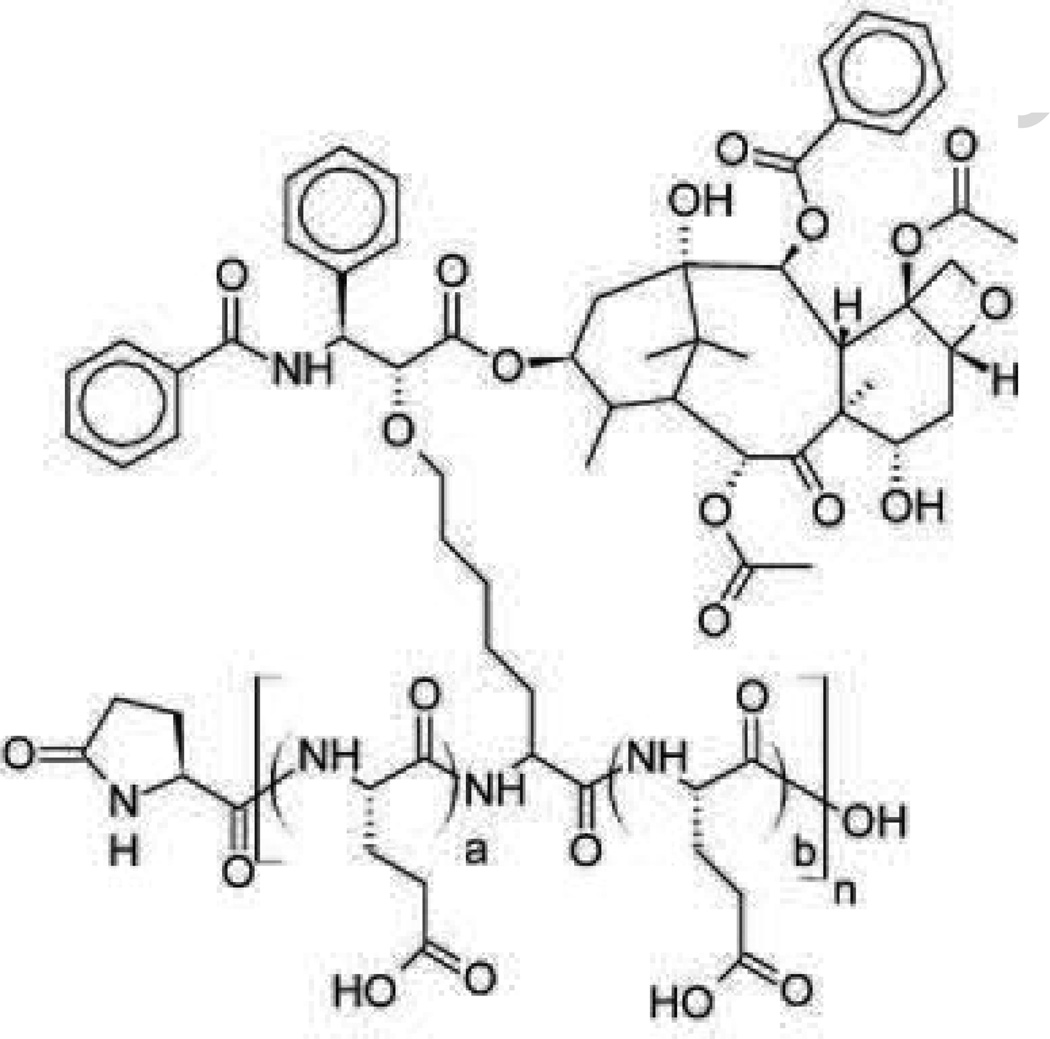
PK1 (FCE 28068) (Fig. 3) was the first drug-polymer conjugate to enter clinical trials. Doxorubicin is conjugated with a synthetic N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer via Gly-Phe-Leu-Gly peptidyl linker designed to degrade by thiol-dependent lysosomal protease [72], [73] and forms a unimolecular micelle in aqueous solution with a diameter of around 8 nm [74], [75]. HPMA is non-biodegradable and essentially nontoxic in the rat, even at doses of 30 g/kg [72]. Its hydrophobicity limits MPS sequestration and its relatively high molecular weight (~20–30 kDa) allows it to avoid renal elimination [74]. These features allow prolonged blood circulation time resulting in high accumulation in tumors. Furthermore, the high molecular weight prevents diffusion across the membrane, restricting cellular uptake to endocytic pathways and facilitates the cleavage of the linker and the release of doxorubicin in endosomes. Additionally, PK1 has been developed to overcome the resistance due to P-glycoprotein overexpression based on contributions of the endocytic (pinocytic) cellular uptake and the control release in the endosome. The in vitro results using A2780 resistant human ovarian carcinoma cell line showed no induction of MDR1 gene by PK1 and indicates that PK1 can avoid excretion by P-glycoprotein [76], [77], [78].
Fig. 3.
PK1/FCE 28068. a=96.1%, b= 3.9%, Particle size is around 8 nm. Developed by Pharmacia which merged with Pfizer [88].
2.2.1 Preclinical Studies
Since doxorubicin is conjugated with HPMA, free doxorubicin is not detected in plasma and tissues after administration of PK1. The pharmacokinetics study of PK1 using mice showed the half-life of PK1 was approximately 15 times longer than that of the free drug in mice [79].
Consistent with prolonged blood circulation time, several studies reveal relatively high accumulation of PK1 in xenograft tumors in mice [80], [81], [82], [83], [84], [85]. PK1 showed 17–77 times higher accumulation in B16FIO melanomas in mice compared with free doxorubicin [80]. The correlation between molecular weight of HPMA and tumor accumulation was studied as well [82], [83]. Higher molecular weight allows longer blood circulating time and higher accumulation in the tumor, liver and spleen. The molecular weight of PK1 is correlated with high tumor accumulation and low accumulation in other tissues. In a study using A2780 human ovarian carcinoma in mice, PK1 showed 45–250 times higher accumulation in the tumor than that in the other tissues (liver, kidney, lung, spleen, and heart) [81]. Remarkably, the accumulation of PK1 in normal tissues was 6–50 times lower than that of free doxorubicin over 30 days.
Due to decreased distribution of PK1 in normal tissues, PK1 significantly improves the doxorubicin toxicity profile [85], [86]. Regarding cardiotoxicity, rat models given free doxorubicin or the mixture of free doxorubicin and HPMA showed decreased cardiac output and heart rate, while rats given PK1 showed no significant change even in histological examination [85].
The efficacy studies of PK1 have been conducted using various ascites tumor models: (L1210 murine lymphocytic leukemia, B16F10 murine melanoma, Walker sarcoma, P388 murine leukemia, M5076 murine reticulum cell sarcoma, LS174T human colon adenocarcinoma, and A2780 multidrug resistant human ovarian carcinoma [19], [72], [81], [84]). In all models, PK1 demonstrated greater efficacy compared to free doxorubicin. Moreover, PK1 was effective in both the sensitive and drug resistant A2780 xenograft models, decreasing the tumor size to 3.5 and 5.6% of the initial size, whereas free doxorubicin was effective in only the sensitive model and decreased tumor size by about 33.3% [81], [84]. In addition to avoiding excretion by P-glycoprotein, the result was partially accounted for by decreasing the permeability of blood vessels and creating a more homogeneous drug distribution. Generally, doxorubicin increases the permeability of blood vessels by up-regulation of the VEGF gene and enhances the accumulation in the same location. This results in heterogeneous distribution of doxorubicin in a tumor. In contrast, PK1 decreases the permeability of blood vessels by down-regulation of VEGF gene and prevents excess accumulation in dead cells. This resulted in a more homogeneous distribution of nanoparticles in murine tumors [81], [84].
2.2.2 Clinical studies
Phase I and II clinical studies were conducted in thirty-six and sixty-two patients with non-small cell lung cancer (NSCLC), colorectal cancer, and breast cancer in the United Kingdom [87], [88].
Pharmacokinetic results showed a profile similar to that of preclinical studies. Blood circulating time was prolonged in PK1; the distribution half-life was 1.8–3.0 h while free doxorubicin was only several minutes.
Tissue distribution was also examined using the I-labeled analogue for gamma camera imaging. Minimal accumulation of PK1 in the liver, kidney, and tumor was observed after 24 h. Only six patients showed accumulation in the tumor in phase I studies [87] and there was no evidence of tumor accumulation in phase II studies [88].
PK1 showed no polymer-related toxicity and a significant decrease in dose-limiting toxicity caused by doxorubicin. Cardiotoxicity was not observed even when a cumulative dose of PK1 reached 1680 mg/m (doxorubicin equivalent), despite a cumulative dose of doxorubicin which most frequently causes cardiotoxicity is 450–550 mg/m2 [87].
Regarding efficacy, PK1 demonstrated only two partial and two minor responses in thirty-six patients with NSCLC, colorectal cancer and breast cancer in phase I studies [87]. However, it is notable that PK1 showed activity in the cancer considered resistant/refractory to conventional chemotherapy and can be interpreted as a proof of concept that polymer-drug conjugations may be advantageous in resistant cancers. In contrast, PK1 demonstrated 6 partial responses in forty patients with NSCLC or breast cancer in Phase II studies, but all patients that responded were anthracycline-naive or chemotherapy-naive patients [88]. Moreover, PK1 showed no activity in patients with colorectal cancer.
The pharmacokinetic results of PK1 of preclinical and clinical studies correlated. However, there seems to be a gap between preclinical and clinical studies on tissue distribution and efficacy.
3. Paclitaxel
Taxanes were discovered in the early 1960’s as part of an NCI initiative to screen synthetic and natural compounds for anti-cancer activity. It was initially collected from the bark of the rare and highly noxious Pacific yew tree [89]. The clinical utility of the drug was limited until the 1990’s due to both environmental concerns and the difficulty in formulating the highly insoluble compounds [90]. However, by the late 80’s clinical trials for paclitaxel and a slightly more soluble taxane, docetaxel, were being conducted in earnest, and new methods for synthesizing the drug from the more common varieties of the yew plant brought the drug into common use [91]. Once brought to market, taxanes had a significant impact on clinical therapies and were used as a first or second line therapy for numerous cancer types including lung, breast and ovarian cancers.
These drugs work by binding the beta-tubulin subunit and stabilizing the microtubules within the cell cytoplasm [92]. This prevents the microtubules from disassembling, even in the presence of signals normally causing catastrophe. The stabilized microtubules are unable to separate from the centromeres during mitosis which arrests the cell cycle and triggers apoptosis. Microtubules are capable of polymerizing stable microtubules even in the absence of guanosine triphosphate (GTP) [92].
Formulating paclitaxel and its counterpart for systemic administration proved to be a very difficult challenge. The drug contains a high number of fused rings and is very hydrophobic. To solubilize the drug, researchers turned to Cremophor® EL, an emulsifying compound closely related to Castor oil [93]. This formulation is marketed as Taxol®. Taxol sufficiently solubilized paclitaxel for systemic delivery and finally allowed paclitaxel to come to market and provide a new standard of care for many cancer patients. This solubilizing agent, however, could be highly toxic causing many patients to have acute hypersensitivity reactions and limiting the allowable dosage for the patient [94]. Acute toxicities associated with the Cremophor EL formulation are managed by premedication in current practice. The limiting toxicity for paclitaxel therapies is generally neutropenia, which is managed by giving the patient granulocyte stimulating factor before treatment in an effort to prevent crashing neutrophil levels [95]. Docetaxel is a slightly more soluble member of the taxane family and has been somewhat useful in reducing this toxicity [96], [97].
The current standard formulation of paclitaxel using Cremophor EL provides a low-hanging fruit for drug carrier technologies. Reformulating the drug in a less toxic carrier allows more of the drug to be used with less risk to the patient and improve outcomes without relying on EPR, targeted delivery or other characteristics of cancer drug delivery.
3.1 Abraxane®
Among the most successful second generation paclitaxel formulations is Abraxane®. Abraxane (Fig. 4) is indicated for metastatic breast cancer, non-small cell lung cancer, and has recently shown promise as a tool against pancreatic cancer [98], [99], [100]. It generated $385.9 million in 2011, which is expected to rise to $2 billion if Abraxane becomes standard care for pancreatic cancer [101]. Tens of thousands of patients have been treated with this formulation.
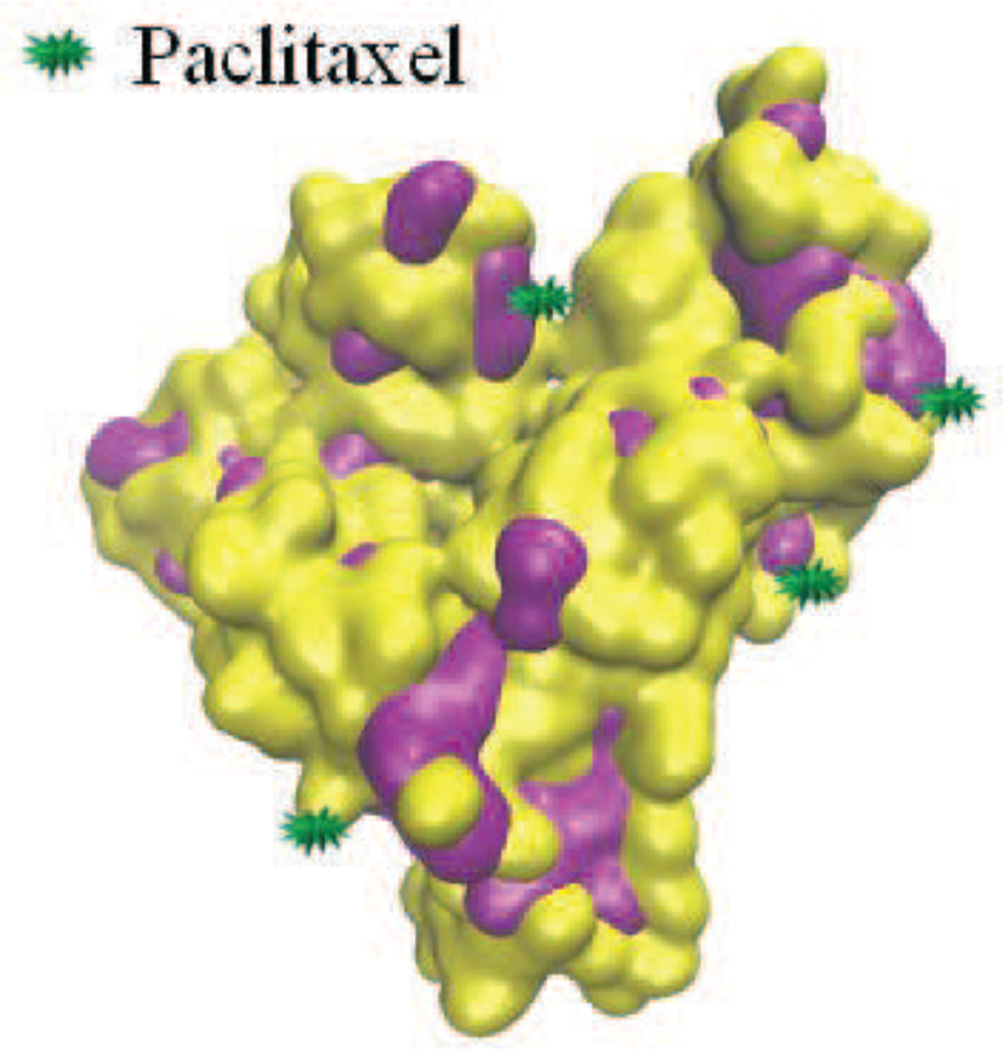
Fig. 4.
Albumin-bound paclitaxel (Abraxane). Purple represents hydrophobic regions. Particle size 7 nm or 67 kDa (size of albumin protein). FDA approval 2005 for treating advanced metastatic breast cancer. Recommended dose is 260 mg/m every 3 weeks while tolerated [186].
3.1.1 Preclinical efficacy
Preclinical evidence has suggested that albumin accumulates preferentially in tumors in comparison with surrounding tissue [102]. Multiple potential mechanisms have been invoked to explain the phenomena, including EPR and tumor protein targeting. However, the extent of albumin accumulation in clinical practice is still unknown and may or may not contribute to enhanced drug efficacy.
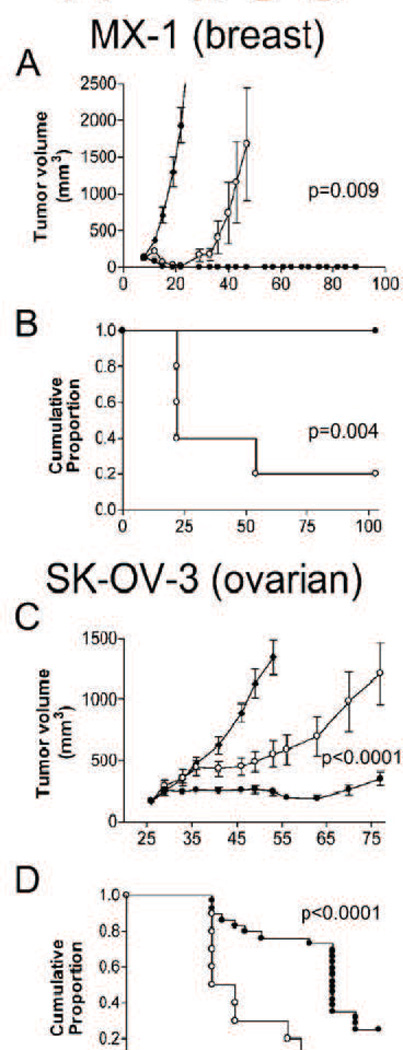
Fig. 5 shows the comparison of Abraxane and Taxol treatments in animal models [103]. For xenograft ovarian and breast tumors, the difference between the two is indeed remarkable, showing p-values of 0.004 and 0.0001, respectively, with impressive numbers of mice surviving tumor free for the duration of the study. The pharmacokinetic advantages were also significant though not dramatic compared with the pharmacokinetic changes in other drugs. The Abraxane formulation shows an approximately 50% reduction in AUC and a dramatic drop in maximum concentration (Cmax). Interestingly, the volume of distribution and clearance rate significantly increased, indicating that Abraxane behaves more like a small molecule drug than Taxol [104].
Fig. 5.
Tumor volume and Kaplan-Meier analysis of tumors in two xenograft mouse tumor models treated with Abraxane (closed circles), Taxol (open circles), and untreated controls (closed diamonds). A & B MX-1 (breast tumor xenograft); C & D SK-OV (ovarian tumor xenograft). [106]
3.1.2 Clinical Efficacy
Abraxane utilizes the serum protein albumin to solubilize and carry the drug in circulation, the formulation is Cremophor free and clinical trials indeed indicated a reduction in acute toxicity in patients (Table 2) [98], [105]. The reduction in toxicity allows Abraxane to be more aggressively dosed than Taxol. The recommended paclitaxel dosing for Taxol is 175 mg/m2, compared to 260 mg/m2 for Abraxane, though this leads to a total exposure AUC only 17% higher than Taxol [106]. Directly comparing these numbers can be difficult, however, because the ratio of free to bound paclitaxel will be different for each formulation. Lower toxicity also allows Abraxane to be administered in a fraction of the time that Taxol is (30 min vs. 3 h) and without premedication to mitigate acute side effects [105].
Table 2.
Summary of toxicities for various paclitaxel-based cancer treatments.
| Drug | Taxol [104] |
Abraxane [97] |
NK105 [105] |
Xyotax [106] |
Genexol-PM [107] |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Paclitaxel Dose | 175 mg/m2 | 260 mg/m2 | 150 mg/m2 | 210 mg/m2 | 300 mg/m2 | |||||
| All | Severe | All | Severe | All | Severe | All | Severe | All | Severe | |
| Neutropenia | 82 | 22 | 80 | 9 | 91 | 65 | 21 | 14 | 71 | 46 |
| Anemia | - | - | 33 | 1 | 54 | 12 | 17 | 5 | 19 | 3 |
| Platlet | 3 | 1 | 2 | 1 | 28 | 0 | 7 | 2 | 2 | 0 |
| Nausea | 22 | 1 | 30 | 3 | 28 | 0 | 33 | 4 | 70 | 3 |
| Vomiting | 10 | 1 | 18 | 4 | - | - | 19 | 2 | 52 | 3 |
| Diarrhea | 15 | 1 | 26 | 1 | 23 | 0 | 16 | 1 | 25 | 2 |
| Fatigue | 39 | 3 | 47 | 8 | 58 | 4 | 27 | 6 | 7 | 3 |
| Arthralgia | 49 | 4 | 44 | 8 | 46 | 2 | 12 | <1 | 29 | 7 |
| Myalgia | 49 | 4 | 44 | 8 | 60 | 0 | - | - | 54 | 6 |
| Neuropathy | 56 | 2 | 71 | 10 | 65 | 2 | 50 | 19 | 71 | 13 |
| Alopecia | 94 | N/A | 90 | N/A | 88 | N/A | 9 | N/A | 81 | N/A |
| AST | 32 | N/A | 36 | N/A | 32 | N/A | - | - | - | - |
| Hypersensitivity | 12 | 2 | 4 | 0 | - | - | - | - | 7 | 4 |
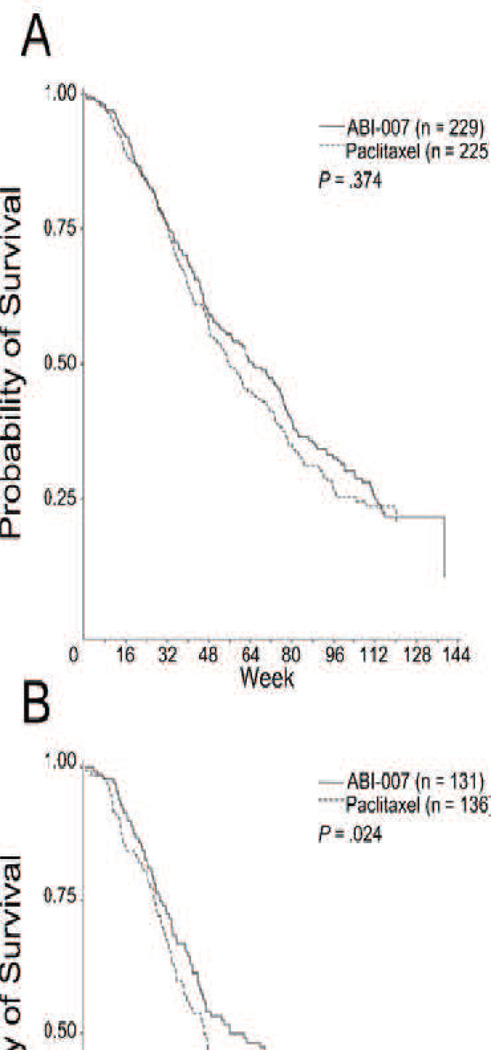
Abraxane does seem to have clear efficacy gains compared to Taxol. On average, the median time to disease progression was approximately five weeks longer for Abraxane treatment than Taxol (p=0.006), while quality of life (QOL) measurements were basically steady. Overall survival did not differ significantly between Taxol and Abraxane (Fig. 1) as a front line therapy, though as a second line therapy Abraxane appeared to extend life by nearly ten weeks (p=0.024) [106]. Toxicity also did not differ significantly (Table 2).
While extending life five to ten weeks may seem paltry in terms of the overall tragedy of cancer, seeing any improvement over standard care does provide hope. It cannot be construed, however, as anything more than an incremental gain or as a revolutionary technology. Especially in light of the tremendous promise Abraxane showed in preclinical studies.
Clinical and preclinical studies both indicate that albumin-conjugated paclitaxel enjoys an efficacy advantage over the Taxol formulation (see Figs. 5 and 6). This improvement is credited both to the higher dosage allowed by the elimination of the highly toxic Cremophor EL solubilizing agent and to tumor specific targeting via EPR and receptor-ligand targeting [107]. SPARC (secreted protein acidic and rich in cysteine) is a protein that appears to be up-regulated in many tumors. It is capable of interacting with albumin and potentially capable of increasing the retention of albumin bound drugs in the tumor [102]. Combined with EPR from a tumor’s pathological vasculature, SPARC binding may help explain some of the improved efficacy of Abraxane, especially in preclinical studies. However, the relatively minor efficacy improvement in human patients may not require any more explanation than the increased paclitaxel dosage and availability compared to Taxol. Still, Abraxane showed greater efficacy gains than any other drug carrier for which phase III were available. Interestingly, paclitaxel would likely be bound to albumin when administered as a free drug. The Cremophor EL emulsion that is used in Taxol, however, may act as a micelle nanocarrier that can significantly alter the drug concentration compared to a non-formulated state [108], [109]. Thus the improved efficacy of the Abraxane formulation over the Taxol in humans may be a result of Abraxane more closely approximating the free drug state than Taxol.
Fig. 6.
(A) Patient survival over time. (B) Patient survival over time in patients who received second-line or greater therapy. P values from log-rank test. Survival indicates time from first dose of study drug to date of death. [106]
3.2 NK105
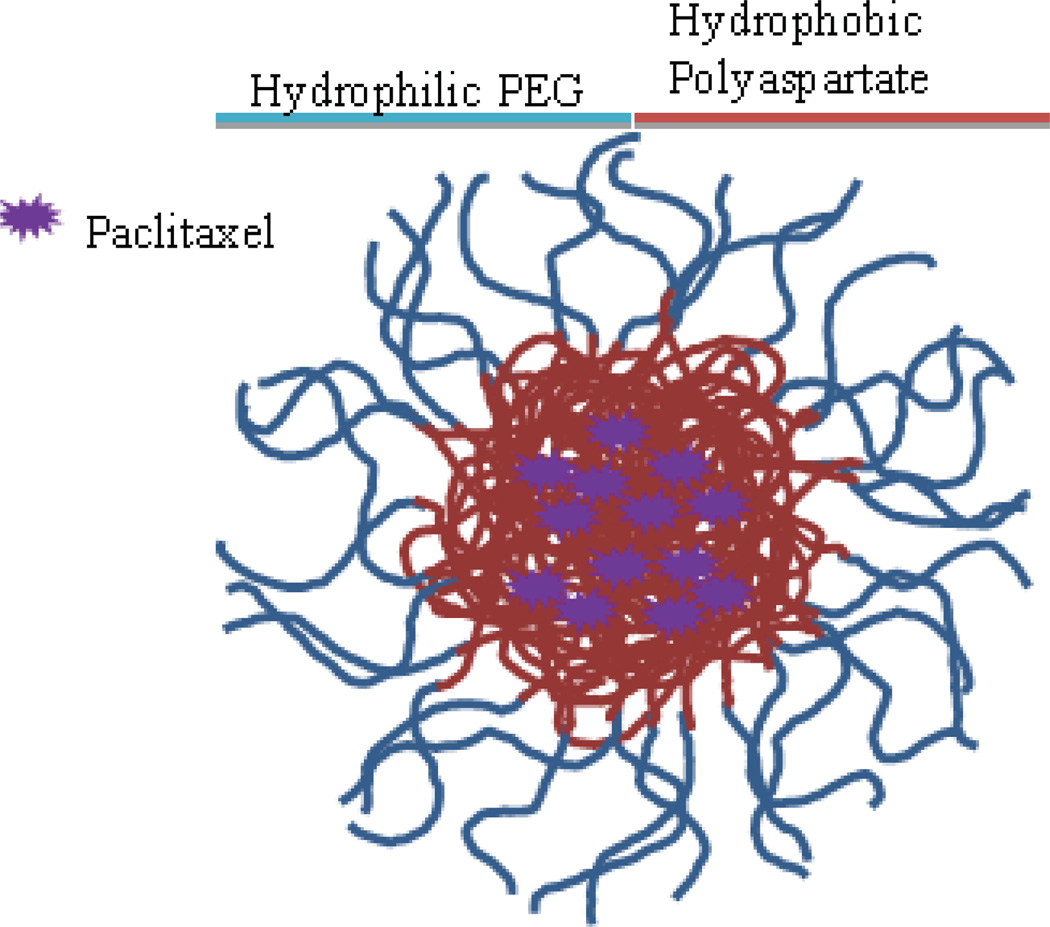
NK 105 (Fig. 7) is a micellar formulation of paclitaxel currently making its way through clinical trials in the United States. The micelles are formed using a diblock copolymer of PEG (hydrophilic) and polyaspartate (hydrophobic) modified to convert half the carboxylic groups to phenyl-1-butanol to create a more stable, hydrophobic core. Paclitaxel loads into the core by hydrophobic interactions. The process is well controlled and creates micelles with an average diameter of 85 nm [110].
Fig. 7.
NK105, micellar paclitaxel. Size range 20–430 nm, average size 85 nm. Not FDA approved but previously tested for use as second-line therapy for recurrent metastatic gastric cancer. Recommended dose is 150 mg/m2.
3.2.1 Preclinical efficacy
The PEG coating of the NK105 micelle radically alters the pharmacokinetics of the drug cargo. Preclinical studies indicated the paclitaxel AUC was 25 times higher for NK105 than for paclitaxel, with 4–6 times longer half-life and 3 times higher maximum concentration. Perhaps most impressive were the differences in intratumoral concentrations which were more than 100 times greater than paclitaxel after 72 h [110].
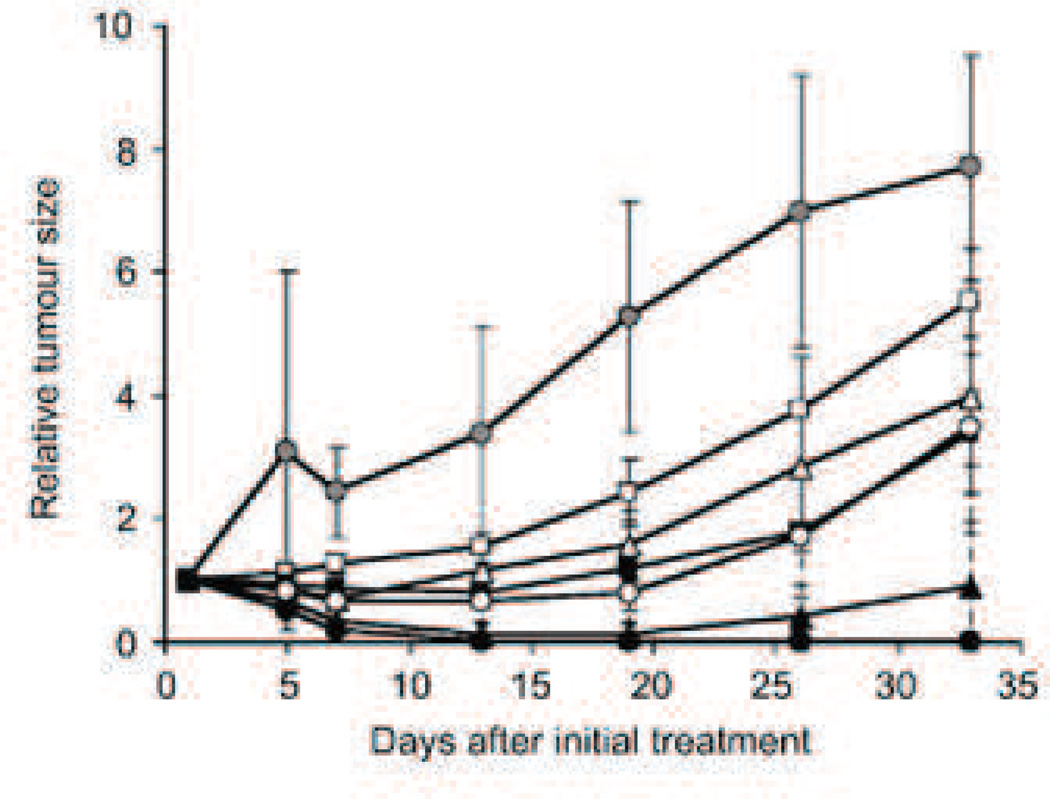
Preclinical trials showed remarkable promise for the NK105 paclitaxel formulation. In a one month study performed by Hamaguchi et al., escalating equivalent paclitaxel doses were administered using the NK105 and Taxol formulations. At every dose point tested, NK105 performed significantly better than free paclitaxel and at doses above 100 mg/kg, all mouse models receiving NK105 were completely cured (Fig. 8). Toxicity indicators did not appear significantly different between the two treatments, though neurotoxicity as measured by demyelination of nerve fibers was significantly less (p<0.001) [110].
Fig. 8.
Relative changes in HT-29 tumor growth rates in nude mice. (A) Effects of PTX (open symbols) and NK105 (closed symbols). PTX and NK105 were injected i.v. once weekly for 3 weeks at PTX-equivalent doses of 25 mg/kg (squares), 50 mg/kg (triangles), and 100 mg/kg (circles), respectively. Saline was injected to control animals (shaded circles) [110].
3.2.2 Clinical Efficacy
Clinical trials indicated a ninefold increase in the AUC though a lower paclitaxel dose was used (150 mg/kg vs. 210 mg/kg) [111]. In spite of the large increase in overall drug exposure as measured by the AUC, neutropenia, the primary toxicity of paclitaxel, was slightly changed from the Taxol formulation. Other hematological toxicities also changed little from Taxol. Hypersensitivity reactions were markedly reduced as well.
At the time of writing, NK105 was being recruited for phase III trials, leaving only limited clinical efficacy data from the phase II trial. In the phase II study for NK105, as a second-line therapy for gastric cancer, the overall response rate (ORR) was 25% with the 95% confidence intervals between 14.4% and 38.4%. Other indicators were progression-free survival (PFS) and overall survival (OS) which came in at 3.0 months and 14.4 months, respectively. Though the phase II study did not use a comparator, we may consider trials of other drugs and drug combinations to give a rough idea of how these values compare to existing treatments. Other trials using paclitaxel alone have yielded ORRs of 17–23% [112]. The recommended frontline treatment for gastric cancer is a combinational therapy using epirubicin, cisplatin and continuous infusion of 5-FU (ECF) [112]. Studies of previously untreated advanced gastric cancers have yielded ORRs as high as 71%, but generally above 40% [113], [114], [115]. If frontline treatment fails there are still numerous other combinational therapies from which to choose to treat the recurrent cancer [112], [116].
While upcoming phase III trials may show greater efficacy gains of NK105 over paclitaxel or currently available combinatory therapies against recurrent cancer, early evidence indicates that the ORR of the drug is unrelated to the orders-of-magnitude improvement in intratumoral drug accumulation seen in preclinical studies.
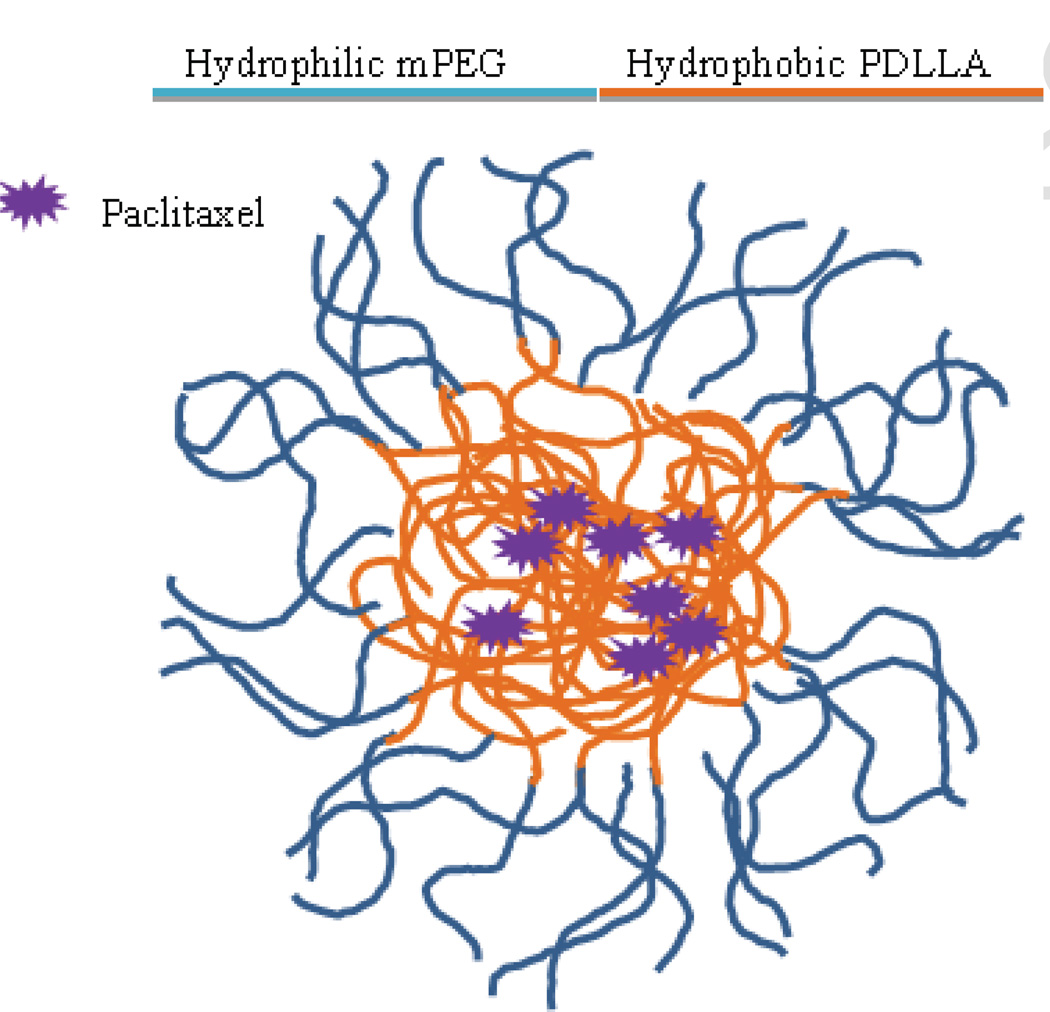
3.3 Genexol-PM®
Genexol-PM® is a polymeric micelle composed of a diblock copolymer of monomethoxy PEG-block-poly(D,L-lactide) (mPEG-PDLLA) (Fig. 9). The micelles are formed by the solid dispersion technique and form stable structures ranging from 20–50 nm in diameter [117]. The formulation is in numerous clinical trials including a phase IV trial for metastatic breast cancer.
Fig. 9.
Genexol-PM. Size range 20–50 nm. In phase III trials as second-line therapy for recurrent metastatic breast cancer. Recommended dose 300 mg/m2 paclitaxel equivalent.
3.3.1 Preclinical Efficacy
Preclinical pharmacokinetic studies showed a unique drug distribution pattern. In a preclinical study, the paclitaxel dosage for the Cremophor EL formulation was 20 mg/kg and the micelle formulation was dosed at 50 mg/kg. In spite of the lower dosage of the Taxol and the pegylation of the micelle, the AUC of the Genexol-PM formulation was nearly 30% lower than Taxol. In spite of the lower AUC, the drug accumulation was higher in all sampled tissues including a nearly 100% increase in tumor accumulation. At equivalent doses the AUC could be an order of magnitude lower yet still more than double tissue concentrations [117]. The unique pharmacokinetics of the Genexol PM formulation may simply be a result of the relatively unstable nature of the mPEG-b-PDLLA copolymer micelle, which appears to substantially release the drug into the bloodstream very soon after administration. This allows the drug to be cleared much more rapidly, but the higher overall dose allowed for greater tissue accumulation. When equivalent paclitaxel doses were administered, the tissue exposure remained the same in spite of the fact that the AUC of the Genexol-PM formulation was an order of magnitude lower.
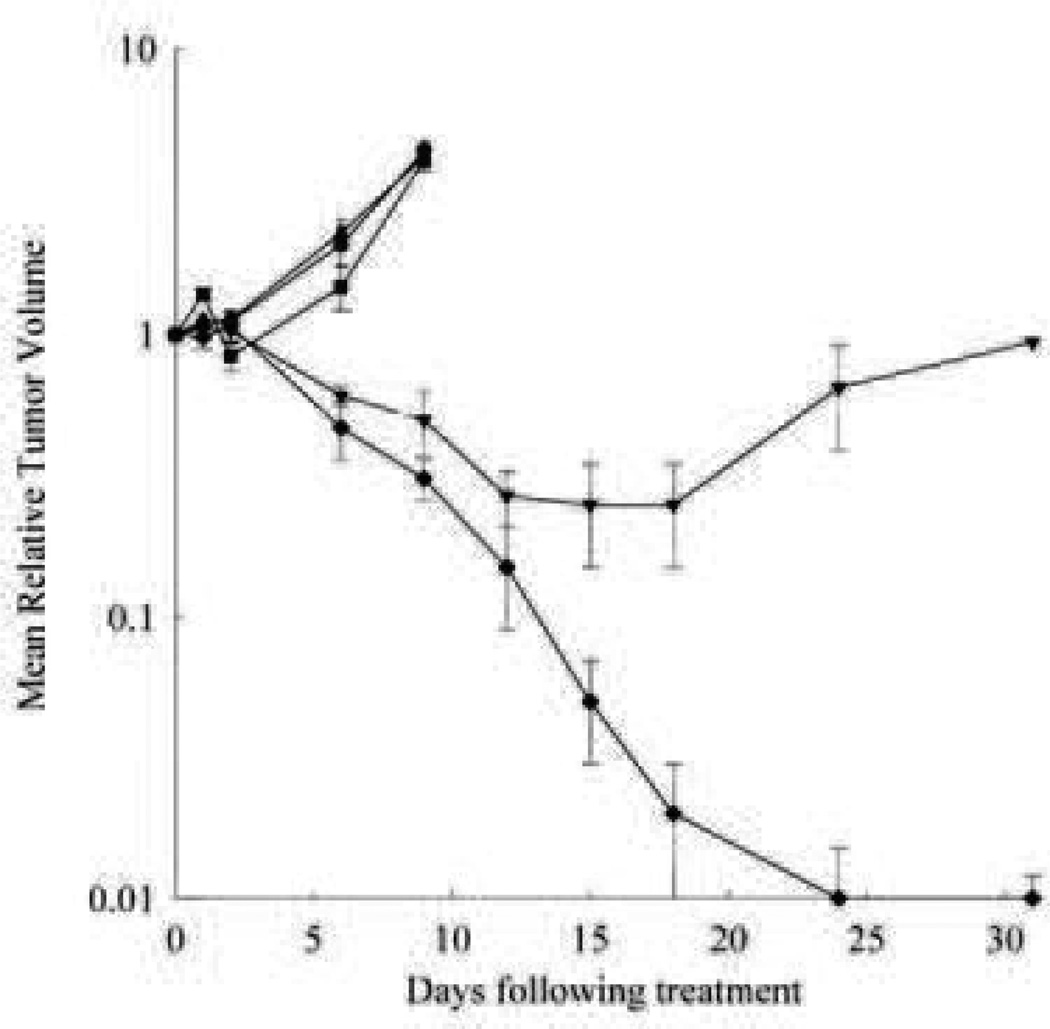
Preclinical studies proved Genexol-PM to be remarkably capable at producing complete tumor remission in mouse models. One study done on athymic mice bearing MX-1 human breast tumor xenograft showed Genexol-PM administered at the maximum tolerated dose (MTD) of 60 mg/kg was able to produce complete remission in all tumors while Taxol at 20 mg/kg was only able to delay tumor growth for approximately 14 days, after which growth resumed as quickly as without treatment. The authors indicated that the increased efficacy is likely due to the significantly lower toxicity, allowing a dose three times higher than could be achieved with Taxol. A similar protocol with a SKOV-3 human ovarian cell line xenograft tumor also showed a marked improvement over Taxol. While Taxol was only able to slow tumor growth, Genexol-PM significantly reduced the tumor mass and caused complete remission in some mice, giving an overall delay in tumor progression of nearly six weeks (Fig. 10) [117].
Fig. 10.
Antitumor efficacy of Genexol®-PM and Taxol® on Tac:Cr:(NCr)-nu athymic mice bearing MX-1 human breast tumor xenograft. Tumors were allowed to establish and mice were treated on 3 consecutive days with saline (•), Taxol® vehicle (■), Genexol®-PM vehicle (▴), Taxol® 20 mg/kg (▾) or Genexol®-PM 60 mg/kg (♦). Each point represents a mean ± S.D. [117]
3.3.2 Clinical trials
As in the preclinical trials, early clinical studies showed that the AUC of Genexol-PM was consistently lower than Taxol. Reduced toxicity allowed a much higher dose of paclitaxel to be administered than was possible for Taxol (300 mg/m2). In clinical studies the AUC and plasma half-life of Genexol PM remained well below that of Taxol [118]. This can be explained by the instability of the PEG-PDLLA micelles, which can break up soon after administration, allowing the paclitaxel to be carried in the blood as a small molecule drug.
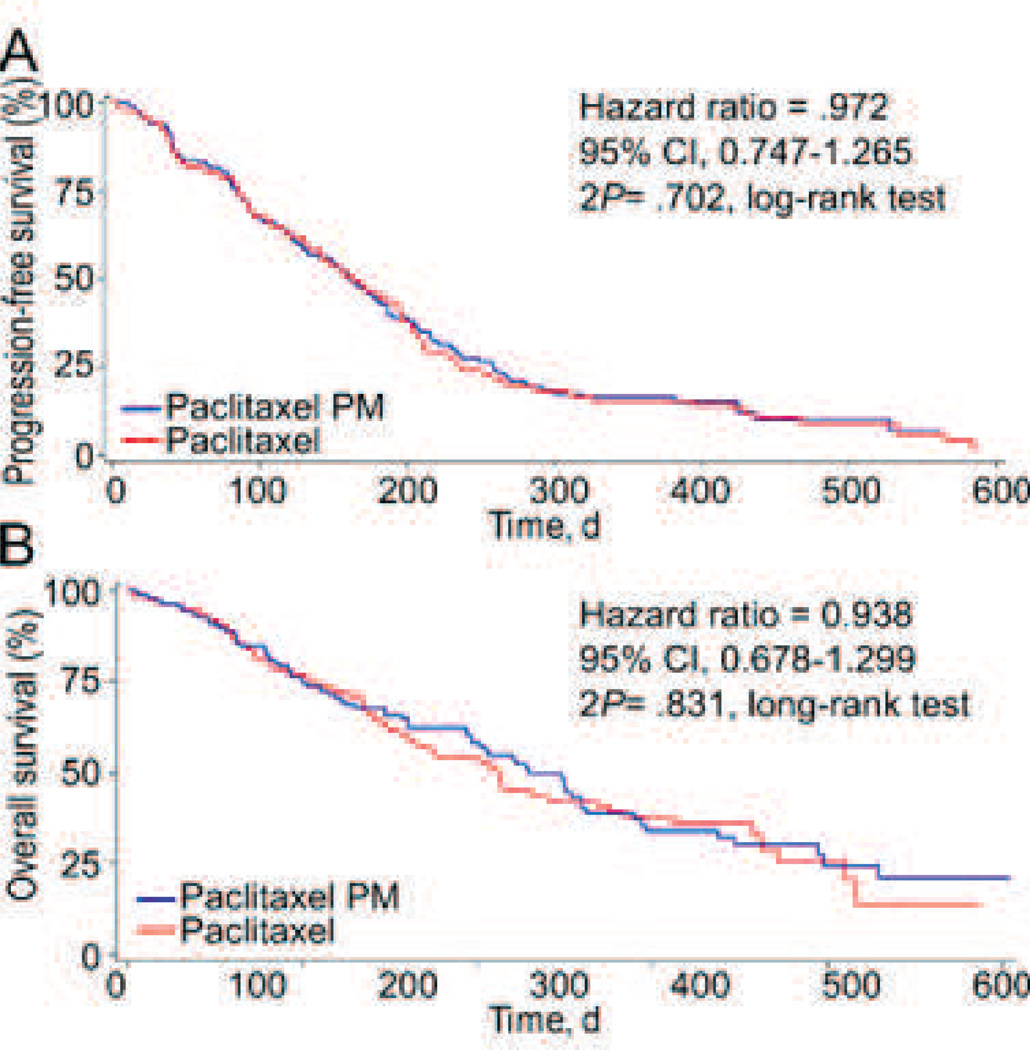
Samyang has conducted or is currently conducting numerous clinical trials for Genexol-PM covering multiple indications and often in combination with small molecule drugs. The toxicity profile of the drug from phase II studies is generally in line with the paclitaxel formulations previously discussed, though at a much higher dose (Table 2). A phase IIb study of Genexol-PM in combination with cisplatin to treat non-small-cell lung cancer was able to show only non-inferiority providing progression free survival and overall survival curves that were nearly identical to the paclitaxel + cisplatin controls (Fig. 11). Toxicity was also substantially similar for both treatments, though neutropenia was greater in the Genexol-PM + paclitaxel formulation. Progression free survival for the Genexol PM + cisplatin formulation was 5.4 months compared to 5.5 months for free paclitaxel + cisplatin and overall survival was one month longer for the former [119].
Fig. 11.
PFS and OS among the study patients. (A) Kaplan-Meier Curves for PFS in the Intent-to-Treat (ITT) Population (2P = .70). (B) OS in the ITT Population (2P = .83). Blue represents Genexol-PM + cisplatin and red represents paclitaxel + cisplatin. Figure from [119].
Genexol-PM seems to provide another case of a seemingly revolutionary drug becoming rather ordinary during clinical trials. The formulation allows a much higher paclitaxel dose to be administered without increasing the toxicity, but the higher dose apparently fails to provide greater efficacy. Samyang is still pursuing FDA approval for the drug, which may be granted on the same basis as Doxil, based on an altered toxicity profile making it more suitable for a subset of patients.
3.4 Xyotax™
Xyotax™ (Fig. 12), paclitaxel poliglumex (PPX) was among most promising of the second generation paclitaxel formulations. The formulation grafts paclitaxel in a comb-like pattern to a linear polymer of L-glutamic acid using a degradable linker. The link between the paclitaxel and the polymer backbone effectively disables the active site of the taxane drug rendering it inert until it is released from the conjugate. Because the link is relatively stable, drug release tends to happen slowly. The total size of the conjugated system is approximately 48 kDa, of which more than a third is the paclitaxel drug.
Fig. 12.
Paclitaxel poliglumex (PPX; Xyotax). Size range 40–50 kDa. Not FDA approved. Under investigation to treat NSCLC. Recommended dose 210 mg/m paclitaxel equivalent.
3.4.1 Preclinical efficacy
As with other drug carriers, the poliglumex formulation can drastically alter the pharmacokinetic profile of the drug. Due to the stable binding of paclitaxel to the polymer backbone, the patient exposure to the bioactive drug can be quite low even when the total paclitaxel dose becomes very high. Preclinical pharmacokinetic studies using equivalent paclitaxel doses in mice showed that even with a twelvefold increase in total AUC for poliglumex in comparison with Taxol (due to slower clearance), the plasma exposure to active taxane was more than sixteenfold less. Intratumoral exposure to active drug, on the other hand, was nearly double that of the Taxol formulation. On the surface, this result shows great potential for improving efficacy while drastically reducing systemic drug exposure and toxicity, but the slow release of the drug into surrounding tissue could drive high drug concentrations in tissues other than the tumor by a similar mechanism.
Early preclinical studies showed great promise for the PPX formulation to improve anti-tumor efficacy of paclitaxel for some cell lines. In one study by Li et al., PPX at a dose of 40 mg/kg effected complete regression in OCA-1 ovarian and 13762F breast cancer tumors in mice, whereas free paclitaxel was only able to suppress growth for approximately ten days after which growth resumed [120]. The formulation did not appear to be so successful in all cell lines. HCa-1 hepatocarcinoma tumors were only marginally slowed by PPX at a dose of 80 mg/kg, though a statistically significant gain was seen over free paclitaxel administered at 800 mg/kg. Efficacy gains against FSa-II fibrosarcoma tumors were similarly modest [121].
3.4.2 Clinical efficacy
Phase I clinical studies determined the MTD for PPX to be 233 mg/m2 given in three week doses. At that level, the AUC of bound taxane was 1583 mg.h/L with a half-life of approximately 120 h. Exposure to free paclitaxel was significantly less, only 27.8 mg.h/L, a nearly sixtyfold decrease between total dose and available paclitaxel. This result proved similar to the preclinical results which also showed a large increase in total AUC, even while exposure to the active drug declined.
The success of PPX in eradicating some types of tumors in preclinical studies helped fuel a large number of clinical trials, many of which progressed to phase III [21], [122], [123], [124], [125]. Xyotax generally shows less toxicity than other paclitaxel formulations; neutropenia and alopecia, in particular, showed marked improvement with the poliglumex formulation. Neuropathy on the other hand, was increased in comparison with the docetaxel standard used in the study [21].
At least six phase III clinical trials were launched with Xyotax, based on enthusiasm from preclinical and early clinical results. A few trials were based on administering PPX alone, and several others tested PPX in combination with other drugs. A large portion of these studies were completed in 2008 leaving a wealth of efficacy data. Almost all of this data indicates that PPX is no more efficacious than the comparative treatment. A phase III study comparing either PPX or paclitaxel in combination with carboplatin against NSCLC. This study failed to show any benefit of PPX over paclitaxel; overall median survival for the PPX group was 7.9 months and 8 months for the paclitaxel group, similarly the time to progression was 3.9 months and 4.6 months, respectively. Neither of these differences was statistically significant. Toxicities differed somewhat between the two treatments, with patients receiving PPX experiencing less cardiac toxicity, alopecia and muscle toxicity, but more nausea and vomiting [124].
Another trial for treating NSCLC using PPX alone and comparing with single agent docetaxel yielded similar results, the docetaxel treatment is ostensibly better than the PPX. Overall median survival for both was 6.9 months, though median time to progression was 2.6 months for docetaxel and 2.0 months for PPX. Again, toxicities differed only slightly with neutropenia being more common in patients receiving docetaxel and neuropathy being more common in patients receiving PPX [21]. A third trial published in 2008 comparing single agent PPX with single agent control (gemcitabine or vinorelbine) for treating NSCLC yet again tells the same story. The study showed similar overall median survival times between the experimental and control groups (7.3 months vs. 6.6 months), similar time to progression (2.9 months vs. 3.6 months), and slightly different toxicities (improved hematological and gastrointestinal effects but worsened neuropathy) [122].
Xyotax is a fairly simple drug carrier that packages paclitaxel in a slow releasing formulation. The goal is to allow the nanoparticle form of the drug to accumulate in a tumor via EPR then slowly release the drug in the tumor while sparing the rest of the body drug exposure. The approach was largely successful in preclinical trials, causing complete remission of some tumors in mice, though success was limited in other cell lines. As with other drug formulations, clinical trials did not bear out the promise of preclinical studies. All three clinical trials reported show no significant difference in efficacy, in spite of the radically different pharmacokinetic profile of the formulation.
4. Cisplatin
Cisplatin, cis-diamminedichloroplatinum (II), cis-[PtCl2(NH3)2] (CDDP) is the first anti-cancer drug containing inorganic platinum and one of the most widely used anti-cancer drugs. In the 1960s, Barnett Rosenberg unexpectedly discovered the inhibition of cell division by platinum salts from “inert” platinum electrodes [126], [127], [128] and its potent anti-cancer activity was revealed in sarcoma 180 and leukemia L1210 in mice [129], [130].
The anti-cancer activity of cisplatin is given by crosslinking DNA [131]. Cisplatin forms highly reactive and charged platinum complexes which bind to nucleophilic groups, such as guanine and cytosine in DNA and form intrastrand and interstrand DNA cross-links, as well as DNA-protein crosslinks. These cross-links result in apoptosis and cell growth inhibition. Since approved for use in testicular and ovarian cancers by the FDA in 1978, cisplatin is widely used to treat various malignancies (bladder cancer, cervical cancer, malignant mesothelioma, non-small cell lung cancer, ovarian cancer, squamous cell carcinoma of the head and neck, and testicular cancer) [132]. However, the major toxicity of cisplatin therapy is irreversible nephrotoxicity, which has been recognized in rats as early as 1971 [133]. Cisplatin accumulates in proximal tubular epithelial cells by both active and passive uptake. The active uptake is mediated by organic cation transporter (OCT2) and is especially recognized as a critical pathway for accumulation in proximal tubules [134]. Nephrotoxicity was a serious obstacle during clinical trials and remains a major dose-limiting toxicity. To reduce nephrotoxicity, patients are hydrated excessively pre- and post-treatment and associated inconveniences can decreases their quality of life (QOL) [135]. Cisplatin therapy also causes neurotoxicity, gastrointestinal toxicity (nausea and vomiting), hematological toxicity and ototoxicity [136]. Ototoxicity is usually bilateral, irreversible, and cumulative. Audiological studies have indicated that up to 90% of the patients treated with cisplatin experience significant hearing loss, especially at high frequencies [137], [138]. Various types of cisplatin analogues (carboplatin, oxaliplatin, satraplatin, and picoplatin) have been developed to reduce these toxicities [139], [140], [141]. Furthermore, its anti-tumor activity is limited by various types of resistance such as increased efflux, inactivation by sulfhydryl molecules (e.g., glutathione), and increased DNA repair [142].
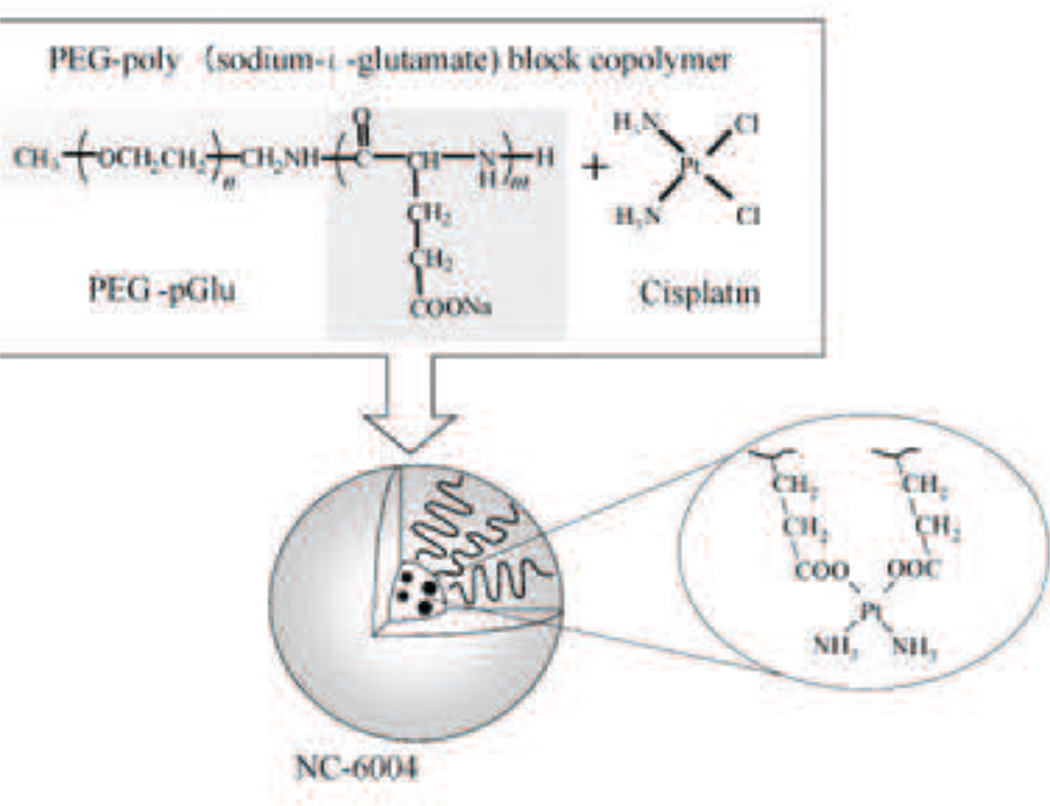
4.1 NC-6004
As early as 1996, cisplatin-incorporated polymeric micelles using PEG-poly amino acid block copolymer has been developed to decrease cisplatin’s toxicity [143], [144], [145], [146]. NC-6004 (Fig. 13) was first introduced in 2003 [147] and is currently undergoing phase II clinical trials. NC-6004 consists of the block copolymer, PEG which constitutes the outer shell and poly(glutamic acid) which constitutes the inner core. The cisplatin drug forms a complex with the carboxyl group of poly(glutamic acid) in this formulation. NC-6004 is clearly distinguished from typical micelles consisting of an amphiphilic polymer. The micelle formation is driven by the ligand substitution of platinum (II) from chloride to carboxyl groups of glutamine. The molar ratio of cisplatin to the carboxyl groups in the copolymers was 0.71. The size is around 30 nm with a narrow distribution. NC-6004 is remarkably stable in distilled water and it dissociates into unimers accompanied by cisplatin release in chloride-rich solutions. The high stability is assumed to be allowed by the rigid structure stabilized by interpolymer cross-linking by Pt(II) atom.
Fig. 13.
Cisplatin incorporated polymeric micelle (NC-6004). Particle size is around 50 nm. Developed by Nanocarrier. [187]
4.1.1 Preclinical studies
NC-6004 shows significantly prolonged blood circulating time compared with cisplatin in rats [148], [149]. Using frameless atomic absorption spectrophotometer (FAAS) to detect elemental platinum, it was found that free cisplatin was detected in plasma only up to 4 h after i.v. injection while cisplatin delivered by NC-6004 was detected up to 48 h after i.v. injection. The AUC and Cmax of elemental platinum in the rat treated with NC-6004 are 65 times and 8 times greater, respectively, compared with cisplatin treatment. Furthermore, total body clearance and volume at steady state of NC-6004 are significantly lower than that of cisplatin. These findings are accounted for by the “stealth effect” seen in pegylated nanoparticles.
As mentioned in the introduction of cisplatin, it accumulates in the kidney within 1 h after i.v. injection in the rat. In contrast, NC-6004 shows relatively high accumulation in the liver and the spleen instead of the kidney, and highest accumulation is seen at 48 h after i.v. injection [148], [149]. The maximum concentration of platinum in the kidney is 3.8 fold lower for NC-6004 than for cisplatin. Tumor accumulation was examined using a xenograft model of MKN-45 human gastric carcinoma in mice. Although Cmax is observed 10 min after i.v. injection of cisplatin, NC-6004 shows 3.6 fold higher Cmax than cisplatin at 48 h after i.v. injection. These results are consistent with the prolonged blood circulating time in pharmacokinetics results.
Nephrotoxicity (dose limiting toxicity) and neurotoxicity were mainly studied using rats [148], [149], [150]. Seven days after injection of cisplatin or NC-6004, the concentration of blood urea nitrogen (BUN) and creatinine, assessors of kidney function, was increased only in rats treated with cisplatin. Moreover, 4 of 12 rats treated with cisplatin died while no deaths were observed in the rats treated with NC-6004. Histological studies using TUNEL assay also revealed the significant decrease of nephrotoxicity of NC-6004 compared with cisplatin [150].
Neurotoxicity was examined by electrophysiological methods. There was no delay of sensory nerve conduction velocities observed in the rats treated with NC-6004 although a significant delay was observed in the rats treated with cisplatin. Additionally, NC-6004 shows significantly lower accumulation of platinum in the sciatic nerve compared with cisplatin in histological examination. These results clearly revealed NC-6004’s improved toxicity compared to cisplatin.
Recently another serious toxicity, ototoxicity, was studied in guinea pigs [151]. Auditory brainstem responses to sound stimulation of various frequencies were examined before and after treatment with NC-6004 or cisplatin. Although cisplatin induced a threshold shift of auditory brainstem response, particularly at high frequency, NC-6004 showed no apparent threshold shifts.
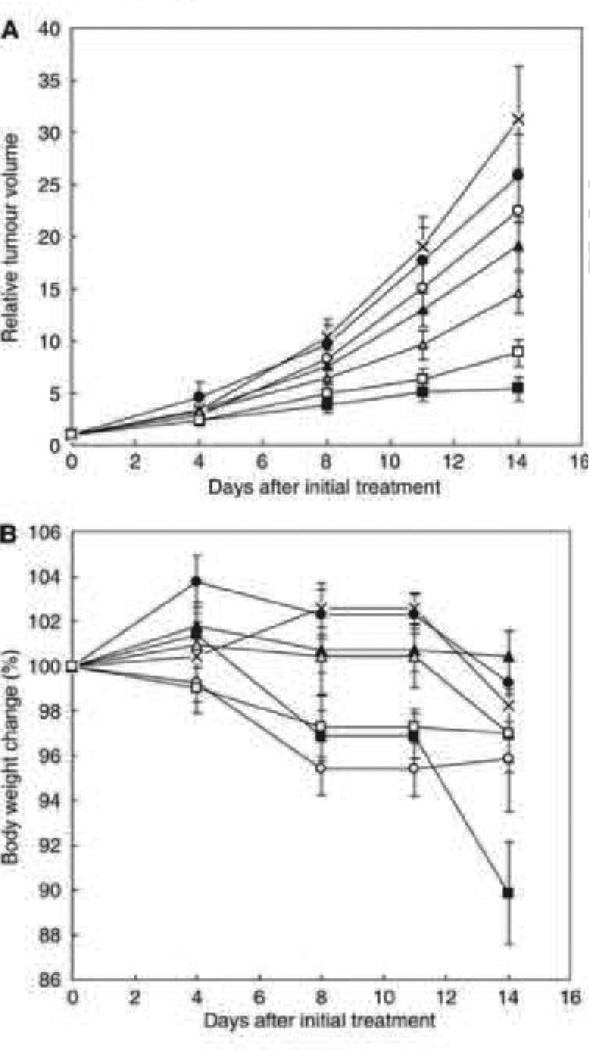
NC-6004 significantly inhibits the xenograft tumor growth of MKN-45 human gastric carcinoma in mice [148] and OSC-19 oral squamous cell carcinoma in mice [150], but there was no significant difference from the cisplatin at the same dosage. Additionally, significant weight loss was observed in the mice treated with cisplatin. On the other hand, the mice treated with NC-6004 did not show as much weight loss as the mice treated with cisplatin which is accounted for by the difference of toxicity profile between the two formulations (Fig. 14).
Fig. 14.
Relative changes in MKN-45 tumor growth rates in nude mice. (A) Cisplatin and NC-6004 were injected i.v. every 3 days, three administrations in total, at CDDP-equivalent doses of 0.5 mg/kg(●, ○), 2.5 mg/kg (▲, Δ), and 5 mg/kg (■, □), respectively. Glucose (5%) was injected in the control mice (×). (B) Changes in relative body weight. Data were derived from the same mice as those used in the present study. Values are expressed as the mean ± S.E. [148].
4.1.2 Clinical Studies
A phase I study was conducted with seventeen patients that had various advanced solid tumors (lung, colon, hepatic cell, mesothelioma, esophagus, pancreas, melanoma, and renal cell) in the UK starting in the year 2000 [152]. NC-6004 was administered intravenously every 3 weeks. The dose escalation started at 10 mg/m2 and reached 120 mg/m2.
The pharmacokinetics was examined at 120 mg/m2. The Cmax and AUC of total platinum in plasma were 11-fold higher than those of cisplatin, and 88% of those were due to intact micelle form. Therefore, Cmax and AUC of active species of platinum in plasma is 35-fold smaller and 8.5-fold larger compared with the values of cisplatin from other clinical trials [153]. Consistent with these results, NC-6004 generally shows less severe and less frequent toxicities compared with cisplatin. No significant myelosuppression, ototoxicity, emesis, or neurotoxicity was observed. Although nephrotoxicity was mitigated, it was still observed in patients who received 120 mg/m2 even with a pre- and post-treatment hydration regimen. Cisplatin therapy generally requires 8 h of hydration both before and after the cisplatin treatment to reduce nephrotoxicity. Therefore, low nephrotoxicity of NC-6004 is clearly advantageous over cisplatin due to increased QOL. On the other hand, unanticipated hypersensitivity was frequently observed and was related to all dose interruptions. The mechanism of hypersensitivity has not been determined yet. As for efficacy, seven out of seventeen patients showed stable disease status and the median progression free survival time was 49 days. However, neither complete nor partial responses were seen.
Remarkably, these clinical results generally correspond to preclinical results despite unpredicted toxicities such as hypersensitivity. It could be accounted for by the sustained release of platinum from NC-6004 which is promoted by chloride replacement unlike the designs of other drug carriers. It might prevent a significant increase of plasma concentration of active platinum that in turn decreases the toxicity. Still, released free active platinum in plasma could be functioning in the same manner as cisplatin.
5. Targeting moieties
One of the ways that drug carriers may improve cancer therapy is by targeted therapy. While many drug carriers claim targeting based on improved distribution profiles, this section will focus on drug carriers that have specific groups added to provide receptor-specific targeting functionality. Obviously, these targeted therapies cannot sense their target from a distance nor do they have any control over their own trajectory. Drug carriers that are designed to be targeted must rely on circulation in the blood stream and must rely on access to the cancer cells. Given access to the target, interactions between the drug and the cancer cell can be increased with targeting moieties. Targeting moieties present on the drug carrier can be antibodies that bind to a specific antigen or they can be various biological molecules that have been shown to interact with receptors on certain cell types. Essentially, enabling receptor-ligand binding between the drug and the target increases the likelihood of the drug being in the presence of the desired target. The most common and most successful (in terms of FDA approval) method of receptor-ligand binding involves the use of antibodies. Some of these antibodies act as a drug when binding their ligand (antigen), others are linked to a drug such as with antibody-drug conjugates. Antibodies are excellent tools for providing mechanisms of binding. They can be selected to have high specificity and affinity for their target ligand. However, care must be taken to select the correct ligand target. It is difficult to find binding targets that are unique to cancer cells. Often, therapies have to rely on overexpression of certain proteins by cancer cells. Care must also be taken in the selection of an appropriate linker to conjugate the drug to the drug carrier/targeting moiety. Beyond antibodies, there are numerous preclinical studies showing how adding targeting moieties to drug carriers such as polymers, liposomes, micelles, etc. will improve efficacy - many have even made it to clinical trials. However, they have yet to make a presence in the field of clinically approved drug carriers for targeted cancer treatment.
5.1 Mylotarg®
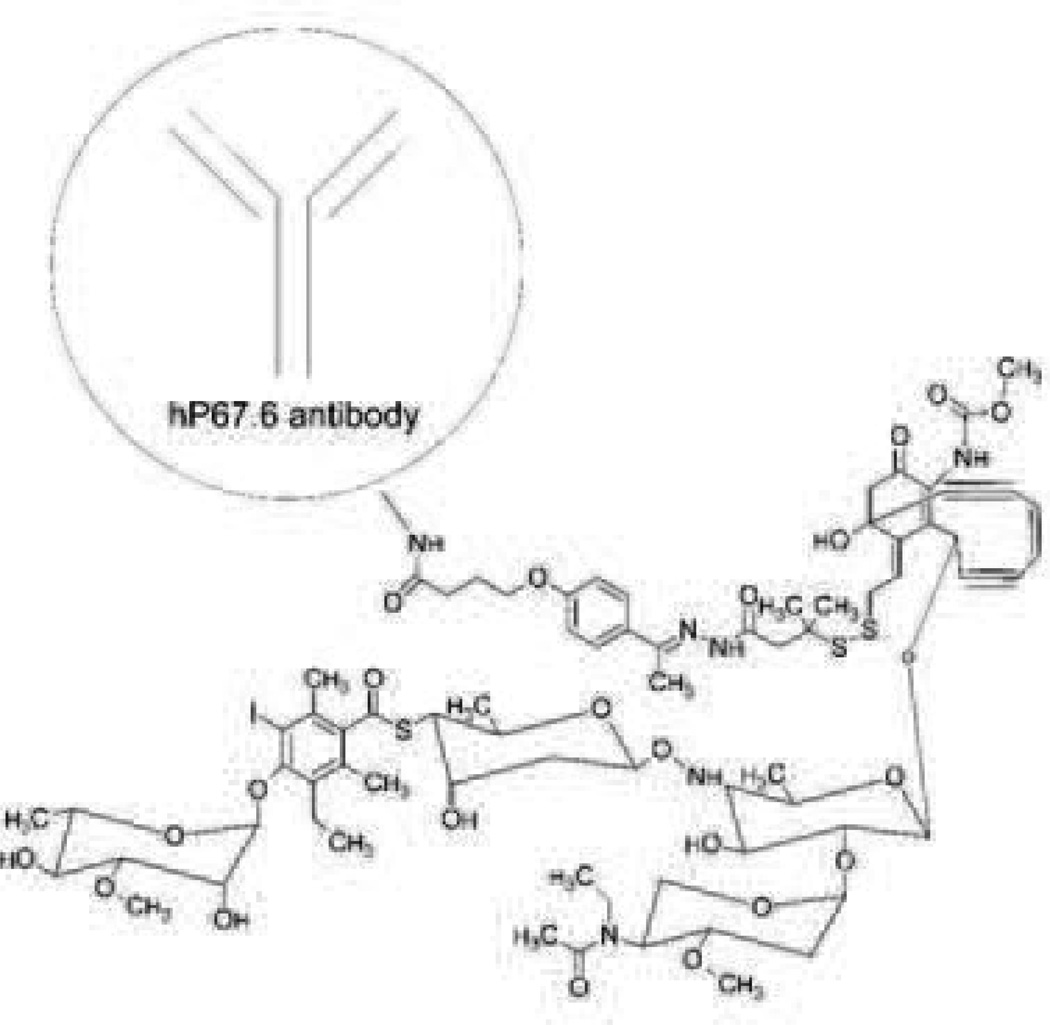
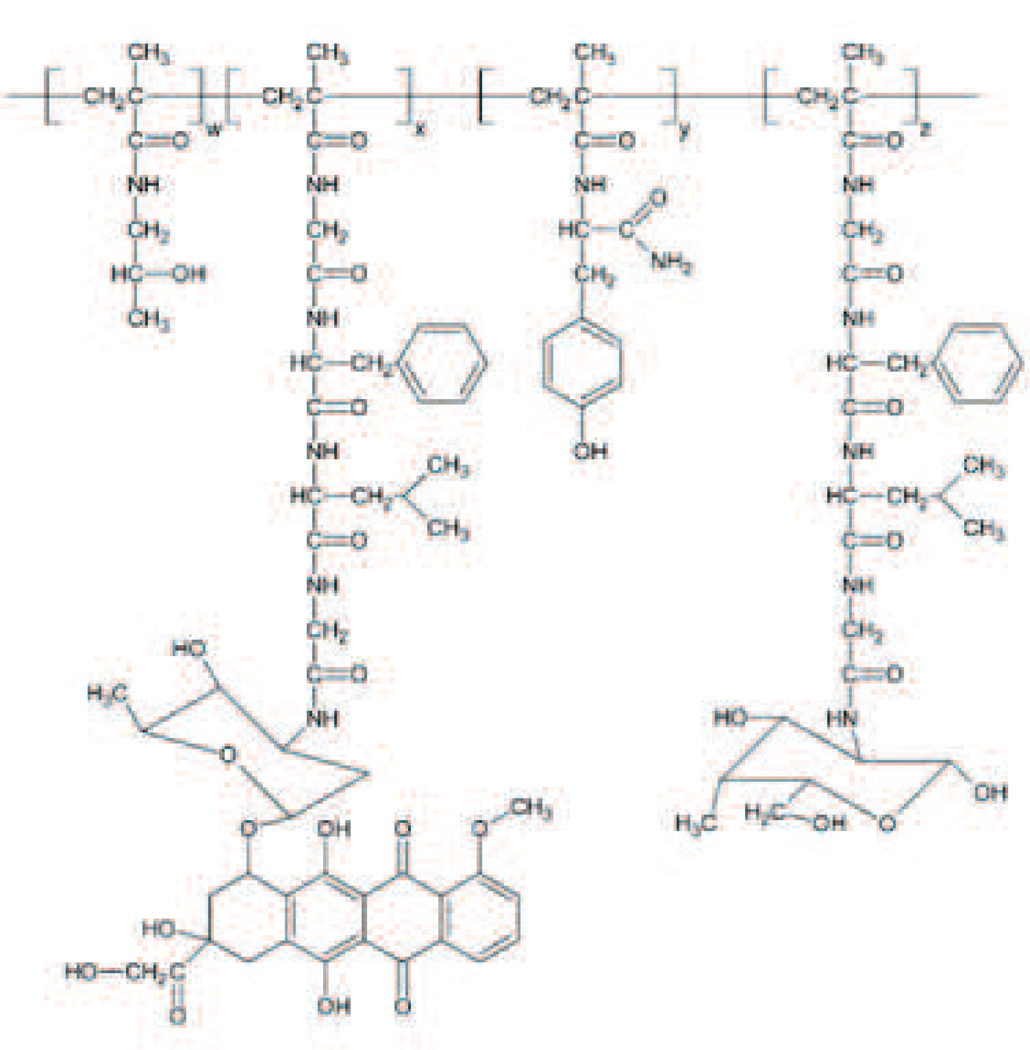
Mylotarg (Fig. 15) is the product name for gemtuzumab ozogamicin which is a humanized monoclonal antibody (hP67.6) against the CD33 antigen that is linked to a derivative of calicheamicin, an antibiotic that is toxic due to its ability to damage DNA [154], [155]. Approximately 50% of the antibodies have about four to six drug molecules per antibody (the remainder of the antibodies are unconjugated). CD33 was chosen as a target because approximately 90% of AML patients have CD33+ leukemia cells and because it is internalized upon binding [156], [157]. Following encouraging phase II trials, it gained accelerated approval to address an unmet medical need for relapsed AML [154], [155].
Fig. 15.
Mylotarg/gemtuzumab ozogamicin. Size range: 5–20 nm or 151–153 kDa. Binding Site: CD33. FDA approved in 2000 and withdrawn in 2010. Indicated for CD33 positive acute myeloid leukemia. Recommended dose is 9 mg/m i.v. over 4 h and repeated in 14 days. Company: Pfizer.
5.1.1 Preclinical
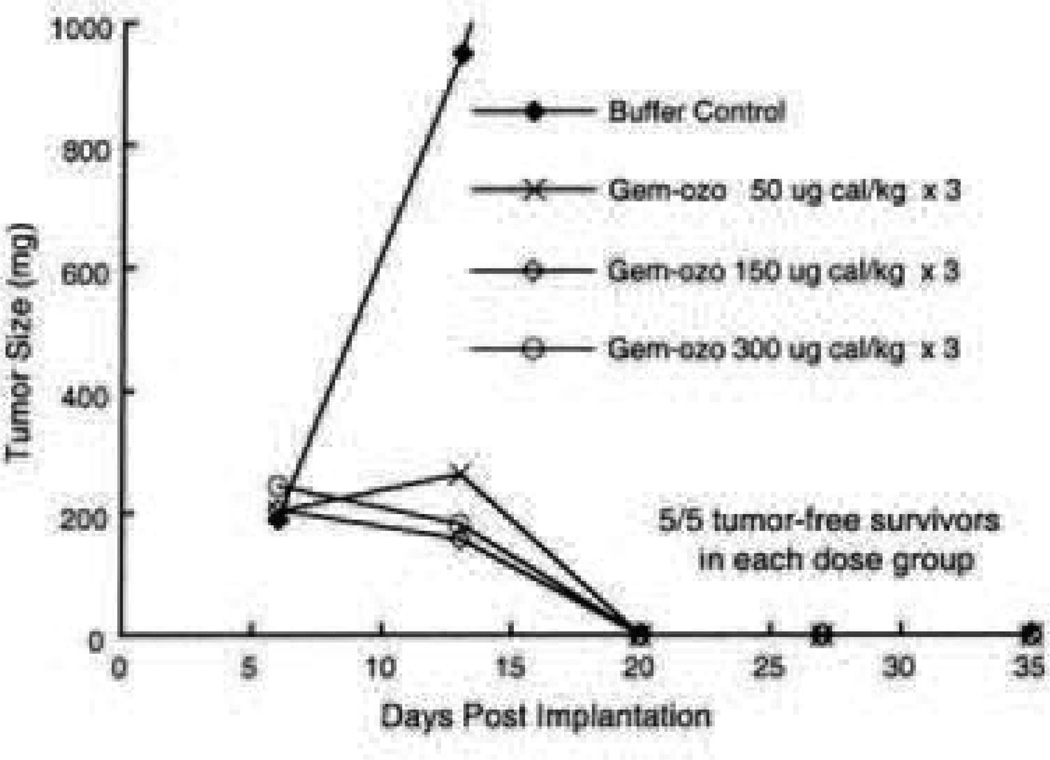
In vitro and in vivo studies tested various linkers joining the P67.6 antibody (not yet humanized for preclinical studies) with the calicheamicin drug derivative. The in vivo studies used HL-60 human promyelocytic leukemia cells implanted subcutaneously into athymic mice and found a formulation that showed a complete inhibition of tumor growth but was only compared to a buffer control. Unfortunately, that formulation was abandoned due to an unexpected problem with sensitivity to periodate oxidation after humanizing the P67.6 antibody [158]. A new formulation with a bifunctional linker was chosen and named gemtuzumab ozogamicin. In vivo studies found the formulation to be effective with complete inhibition of HL-60 xenografts in mice when tested at various doses (Fig. 16) [159].
Fig. 16.
Complete cure rates in mice for various dose strengths of Mylotarg [159].
A summary of preclinical trials boasts selective in vitro activity against a CD33+ cell line when treatment was compared in cells not expressing the CD33 antigen. When administering 6 weekly doses up to 7.2 mg/m2 in mice and 22 mg/m2 in monkeys, myelotoxicity and toxicity in the liver, kidney, and testes were present, though not lethal. Preclinical studies also showed that Mylotarg distributed preferentially to the liver and the major excretion pathway was biliary [154].
5.1.2 Clinical
Phase I trials used a dosing interval of 2 weeks, as the half-life of the drug is over 3 days. It was found that three biweekly doses caused myelosuppression, thus, most received only two doses. The major toxicity found in phase I trials was myelosuppression (neutropenia and thrombocytopenia) because CD33 is expressed on myeloid progenitor cells [154]. The dose limiting toxicity was stated to be neutropenia. Other complications were fever, nausea, chills, leukopenia, vomiting, rash, and pain. Furthermore, some patients developed antibodies against Mylotarg after 2–3 doses. While testing the safety of various doses in the phase I trial, two out of forty-one patients (one dosed at 1 mg/m2 and the other at 4 mg/m2) achieved a complete response (CR) defined by transfusion independence and clearance of leukemic blasts as well as a recovery of hemoglobin, neutrophils, and platelets. Seven patients (dosed at 5, 6, and 9 mg/m2) had clearance of leukemic blasts but not a recovery of their platelet count and were designated as a partial response (PR). The approval summary for phase I trials states that the hepatobiliary excretion pathway was not included in the pharmacokinetic studies and no mention was made of liver toxicity, despite evidence in preclinical studies. As only 50% of the antibodies are actually linked to the calicheamicin, the methods used to characterize the pharmacokinetics of Mylotarg may be questionable. The data simply do not provide much information when the half-life of a 9 mg/m2 dose of antibody varied greatly from 67 ± 37 h to 88 ± 58 h for the first and second doses, respectively. The AUCs for the antibody were also quite varied-ranging from 132 ± 136 to 243 ± 198 mg.h/L for the first and second doses. For comparison, the total calicheamicin half-life was found to be 39 ± 25 h for the first dose and 63 ± 63 h for the second dose. Finally, the AUC of total calicheamicin was 2.1 ± 1.8 mg.h/L for the first dose and 4.7 ± 4.1 mg.h/L for the second dose. Following the Phase I trial, the recommended phase II dose was 9 mg/m2 i.v. over 2 h for two doses [154].
The phase II trials consisted of 142 patients between three study groups labeled 201, 202, and 203. They used the recommended dose of 9 mg/m2 and all 142 patients received at least one dose while 109 received two doses and 5 received 3 doses. The phase II studies saw 16% with a CR and 13% with a PR. The CR and PR were summed to 30% and termed as the overall response (OR) rate. Among these responders, the CR group had a relapse-free survival (RFS) of 7.2 months and the PR group had a RFS of 4.4 months, thus averaging 6.8 months for the OR group. Treatment was generally well tolerated except for cases of infusion related symptoms, fever, chills, hypotension, hypoxia, difficulty breathing, and bleeding. However, treatment caused tumor lysis syndrome in four cases with one resulting in death. Furthermore, in the phase II clinical trials the hepatotoxicity was evident with hepatic function abnormalities, bilirubin elevations, and transaminase elevations. Most cases were treatable, but one patient died of hepatic failure. However, by combining the CR and PR to form an impressive OR rate, there was enough evidence for efficacy and to gain a fast track approval [154].
5.1.3 Postmarketing data
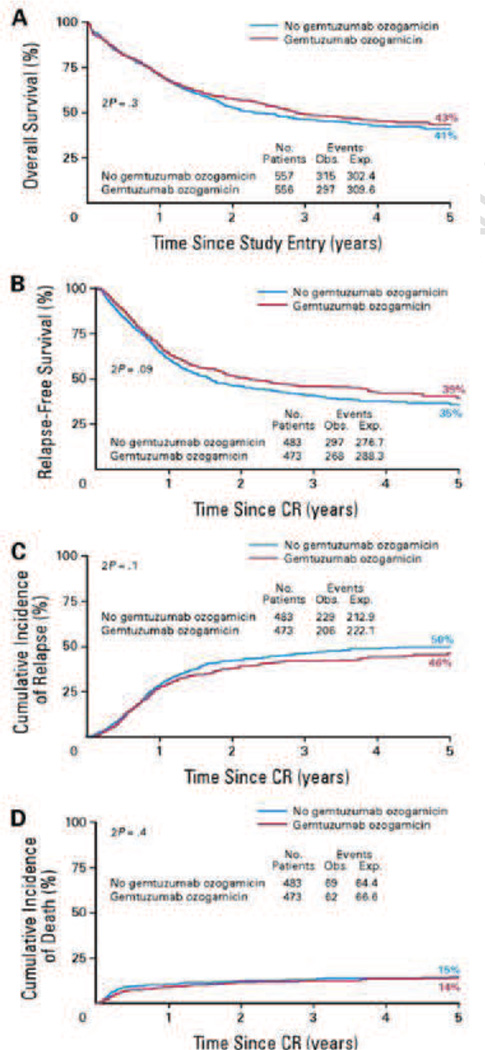
The postmarketing data revealed some severe adverse events such as hypersensitivity reactions, pulmonary toxicity, and hepatotoxicity-especially veno-occlusive disease (VOD). A post-market phase III trial from 2004 to 2009 at the Southwest Oncology Group showed a poor response rate and adverse effects of Mylotarg plus various chemotherapies compared to traditional chemotherapies alone [160]. Not long after, on June 21, 2010, there was a FDA news release announcing that Pfizer voluntarily withdrew Mylotarg from the U.S. market [161]. A recent study completed in Germany showed a similar lack of improved efficacy. The CR rate for gemtuzumab ozogamicin therapy was 31% while standard care had a CR rate of 32%. While there may be some slight advantage in the relapse free survival, due to the improvements in preventing relapse, there is very little difference between the overall survival percentages. Unfortunately, this study also showed serious toxicities associated with the gemtuzumab ozogamicin therapy. There were eight induction deaths in the gemtuzumab ozogamicin group and three in the standard care and two of the induction deaths from the GO group had severe VOD as an underlying complication [162]. Another large randomized open-label trial in the UK produced results of lack of superiority in efficacy for those patients receiving gemtuzumab ozogamicin therapy. The survival percentage and relapse free survival percentages are shown in Fig. 17. It was only by separating patients with favorable cytogenics or prognostic index that benefits could be seen [163]. The gap present could be a matter of choice for the target. Inconsistent expression is much more prevalent in clinical cancers with a varied human population. Prior results from ex vivo studies on inhibition of colony formation were less than impressive compared to the in vivo studies on tumor growth suppression in mice (Table 3) [159]. Furthermore, liver toxicity was an issue and it is likely that Mylotarg was passively targeting the liver by targeting CD33+ cells of myeloid lineage (leukemia and others) in the liver sinusoids [164].
Fig. 17.
An open-label trial involving 1,113 patients that were randomly assigned whether or not to include gemtuzumab ozogamicin as part of their induction therapy. Broadly, there were only slight differences in overall survival (A), relapse-free survival (B), cumulative incidence of relapse (C), and cumulative incidence of death (D) [163].
Table 3.
Colony formation of patient-derived leukemic marrow cells in the presence of gemtuzumab ozogamicin [159].
| concentration (ng/mL)a |
total no samples |
0–25% inhib |
25–60% inhib |
60–90% inhib |
>90% inhib |
|
|---|---|---|---|---|---|---|
| adult samples | 2 | 27 | 17 | 6 | 3 | 1 |
| 10 | 27 | 12 | 3 | 6 | 6 | |
| pediatric samples | 2 | 15 | 2 | 5 | 3 | 5 |
| 10 | 5 | 0 | 1 | 1 | 3 |
Concentration of N-acetyl-gamma calicheamicin dimethyl hydrazide. Antibody concentration is 40-fold greater.
5.2 PK2
PK2 is an HPMA polymer drug conjugate very similar to PK1 except that it has a galactosamine moiety added to target the liver (Fig. 18). The galactosamine moiety is a biomimetic ligand for the asialoglycoprotein receptor found on differentiated hepatocytes; thus, this receptor is not uniquely expressed by hepatomas. Furthermore, depending on the state of differentiation of the cancer cells, expression can even exist at lower levels than healthy liver tissue [165]. Still, the hope was to induce more accumulation of the drug into the liver to treat hepatomas which is aided by the fact that liver physiology is conducive to accumulation of nanoparticles that rely on the EPR effect.
Fig. 18.
PK2/FCE 28069. Size: about 27,100 Da. Binding Site: Asialoglycoprotein receptor. Review articles state phase I/II FDA trials, though not currently found on clinicaltrials.gov. Indicated for hepatocellular carcinoma. The recommended dose is a 4 mg/kg loading dose with 2 mg/kg weekly doses or an 8 mg/kg loading dose with a 6 mg/kg dose every 3 weeks. Developed by Pharmacia which merged with Pfizer.
Preclinical studies in mice have shown drastically faster blood clearance and higher liver uptake in formulations containing higher galactosamine content (4 mol% compared to 1 mol%). These studies showed formulations with greater than 80% of the 125I-labeled dose accumulated in the liver [166]. Another preclinical study grew human colon carcinomas in the livers of nude mice. As the colon carcinoma does not have asialoglycoprotein receptors, radio labeled HPMA copolymer-doxorubicin-galactosamine conjugates were absent in tumor areas in the liver as shown by Fig. 19 [167].
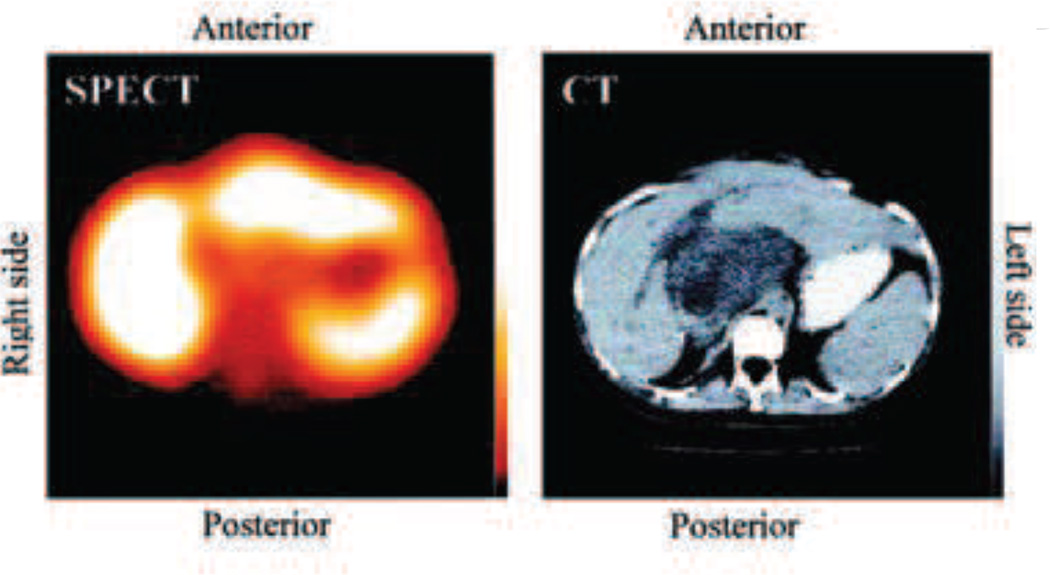
Fig. 19.
SPECT and CT imaging of a patient treated with PK2 showing decreased accumulation of targeted nanoparticles in the tumor region. The SPECT shows accumulation of PK2 in the liver except in the central region. The tumor is visible in the CT image as the dark mass in the central region. [167]
In the preliminary clinical study where a formulation of 1.0 mol% of galactosamine for PK2 was chosen, only 30% of the dose accumulated in the liver when scanned at 24 hours following administration [168]. In the phase I clinical study they chose 1.5 mol% galactosamine for the PK2 formulation. However, this same study compared PK2 with PK1 (almost identical, save it has no galactosamine moiety) and planar gamma-camera imaging showed PK2 preferentially accumulating in the liver while PK1 was not [165]. Unfortunately, while it was accumulating in the liver, SPECT imaging showed it accumulating to a lesser degree in the cancerous regions of the liver likely lacking asialoglycoprotein receptors—where the treatment needs to go—and possibly explains why no efficacy gains were reported in clinical studies.
The AUC (from 0–192 h) at 120 mg/m2 reached as high as 296 mg.h/L when administered by a 24 h infusion [165], [168]. The MTD was found to be 160 mg/m2 due to doxorubicin associated toxicity of myelosuppression, mucositis, and fatigue. The recommended dose for phase II trials was 120 mg/m2. For comparison, the MTD of PK1 was higher at 320 mg/m2 [87], [165].
Some dated review articles state that PK1 is in phase I/II clinical trials; however, it is notably absent in lists of more recent review articles and government databases of clinical trials [13], [169], [170], [171]. It may be safe to assume it has “quietly disappeared” after not performing as well in clinical studies.
5.3 CALAA-01
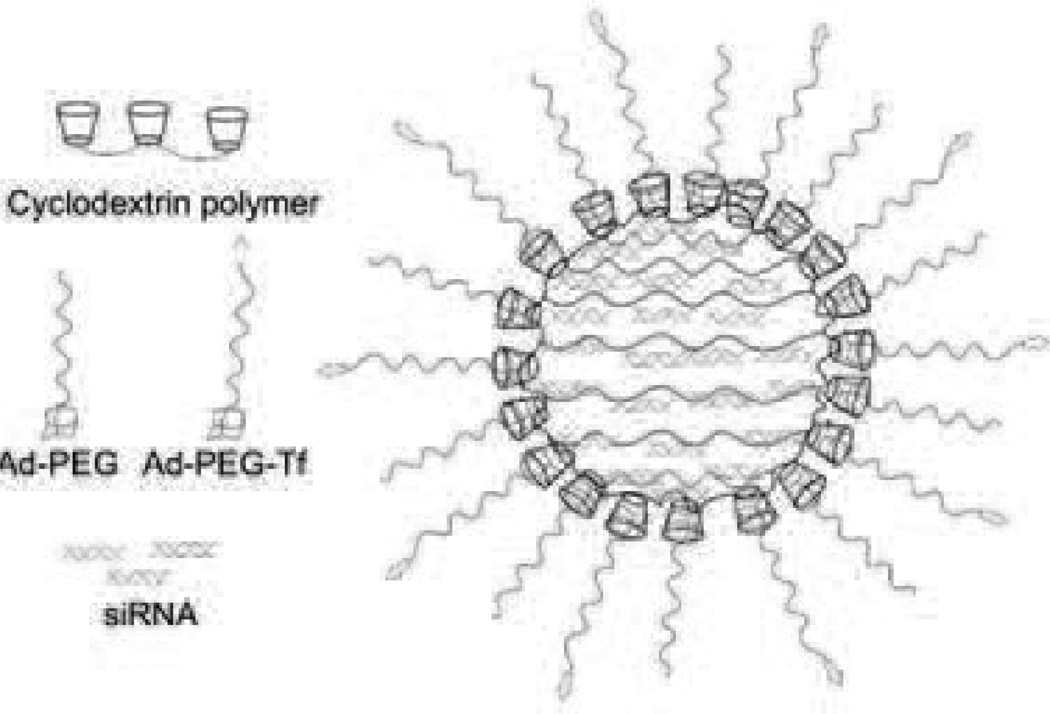
Another example of a targeted drug carrier uses siRNA as the delivered drug. The product name is CALAA-01 (Fig. 20) and the nanoparticle is the delivery vehicle Rondel™. It is composed of a linear polycationic polymer containing cyclodextrin and imidazole groups [172]. The imidazole groups are added to enhance unpacking and delivery of siRNA [173]. The cyclodextrin groups form a complex with hydrophobic adamantane (Ad) groups where each are conjugated to PEG alone or with a human transferrin (Tf) protein for targeting. The PEG is to help stabilize and minimize unwanted interactions (such as aggregation, protein binding, or macrophage uptake). They conjecture that these nanoparticles are able to take advantage of the tumor physiology to aid in intended tissue targeting [172]. Additionally, the Tf groups bind to Tf receptors that are found in a wide variety of malignant tissues [174], [175]. Using siRNA to inhibit the expression of RRM2 results in an antiproliferative effect that could potentially treat a wide range of tumors [176].
Fig. 20.
CALAA-01. Size: about 70 nm. Binding site: Transferrin receptor. Currently in phase I of FDA clinical trials. Seeking indication for solid tumors. The recommended dose is 18–30 mg siRNA/m2. Developed by Calando Pharmaceuticals, Inc.
5.3.1 Preclinical
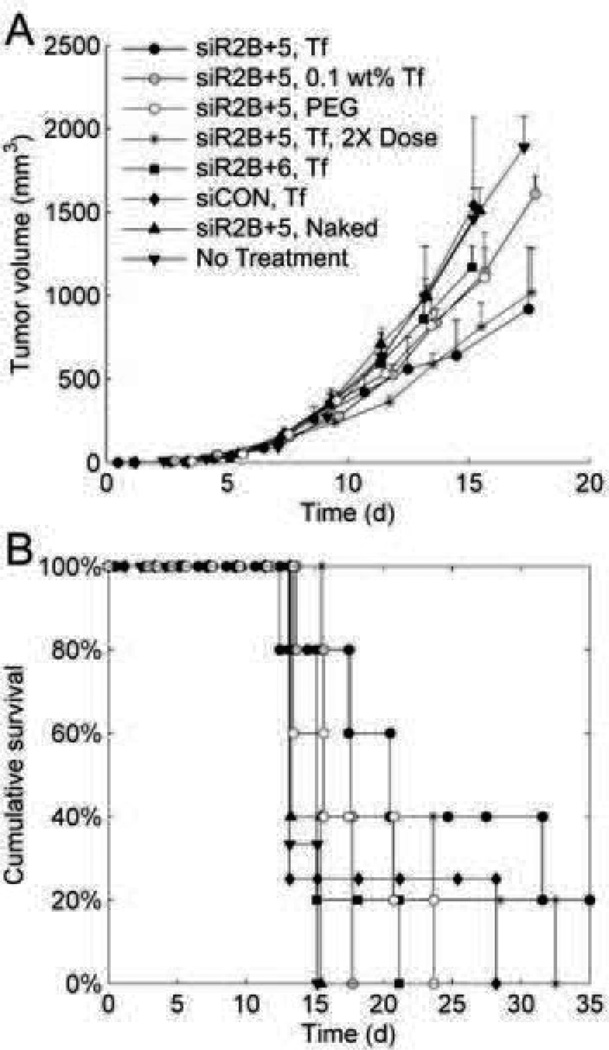
In non-human primate studies, they tested the safety of the nanoparticles at doses of 3, 9, and 27 mg siRNA/kg. There was concern about the possibility of the PEG component causing hypersensitivity reactions, activating complement, or accelerating clearance from the blood. The results showed that there was some antibody formation only at the highest dosage, but that it was formed against the Tf protein on the drug carrier. Regardless of the dose, the majority of nanoparticles and siRNA had been cleared from plasma within 2 hours. While ELISA studies showed an increase in cytokine levels for IL-6, IL-12 and IFN-gamma, they saw no salient physiological signs of toxicity. However, the highest dose saw an increase in blood urea nitrogen and creatinine significant enough to indicate kidney toxicity. Researchers doubted the toxicity was due to siRNA; unfortunately, they did not test a formulation without siRNA as a control. Still, they suggested there was a large therapeutic index by reasoning that the equivalent effective dose found in mice was near 0.5–1.0 mg/kg and well below the 27 mg/kg that had some indicated toxicities [177].
In vivo studies in mice using 64Cu-DOTA-siRNA delivered by Tf-targeted and non-targeted nanoparticles showed similar tissue distribution, blood clearance, and tumor accumulation. The authors state that their methodology does not differentiate between the different states of 64Cu-DOTA-siRNA [178].
The majority of the preclinical efficacy data focuses on protein expression levels and cell growth inhibition. A/J mice using siR2B+5 had the best results in blocking protein expression in vivo. Also, Fig. 21 shows tumor volume progressed slower when treated with the targeted and pegylated nanoparticle formulation containing siR2B+5 (which was eventually chosen for the CALAA-01 formulations) as well as an improvement in cumulative survival [179], [180]. Unfortunately, an improvement of 20 days for cumulative survival in mice may not be predictive of how CALAA-01 will prolong life for humans. On the other hand, mathematical models were employed to elucidate the mechanisms affecting the threshold and duration of the knockdown in order to improve treatment design [180].
Fig. 21.
The targeted and pegylated formulation containing the RRM2 sequence denoted as siR2B+5 performed the best in tumor growth inhibition. (A) tumor growth rates and (B) Kaplan-Meier survival curves. [180]
5.3.2 Clinical
CALAA-01 is only in phase I of clinical trials. Thus, clinical data is limited for comparison against preclinical data. As the best performer in preclinical trials, siR2B+5 was selected for the siRNA component of CALAA-01 to be used for clinical trials and is termed C05C. Research is ongoing, but a review article states that there is fast clearance and an acute elevation of cytokines as seen in preclinical trials [172].
Fifteen patients were enrolled where the average age was 62. Dosing was tested at escalating doses of 3, 9, 18, 24, and 30 mg/m2 for 30 min iv on days 1, 3, 8, and 10 of 21 day cycles. There were no dosage limiting toxicity issues and the most common adverse events were fatigue, fever/chills, allergic reactions, constipation, and nausea/vomiting. One patient had anemia and two patients had thrombocytopenia. One patient had sinus bradycardia [181]. While the purpose of the phase I trial was safety, they reported on some aspects of efficacy. One patient at the highest dose saw their metastatic melanoma change course and become stable for 4 months. Furthermore, a total of four patients with melanoma had biopsies that showed nanoparticles internalized in cells. At the highest dose, RRM2 knockdown had occurred at the mRNA and protein level [179], [181].
Still, it remains to be seen what kind of response will be seen in the clinical setting. In the clinical trial report, the researchers saw no tumor responses in any of the patients. While the improvements of CALAA-01 in tumor growth deceleration and cumulative survival rates in preclinical studies are promising, early reports from phase I trials indicate that it may not have as much efficacy in clinical tumors. The targeted binding site of CALAA-01, transferrin receptors, is also found on some normal tissues and could be a potential pitfall in its clinical progression [174]. However, delivery of a less toxic siRNA that is antiproliferative-rather than a toxic small molecule drug-could abrogate some of the dangers from lack of specificity in their chosen target.
5.4 Discussion—Targeted formulations
There are general factors that make some of the products from this section unfit for market approval. Some of these targeted drug carriers suffered from similar problems as non-targeted drug carriers: unacceptable toxicity and insufficient efficacy in the clinical setting (e.g., Mylotarg). Sometimes, targeted drug carriers appear to have issues due to their implementation of targeting and they may have performed better without targeting (e.g., PK2). At times, the issues become apparent only after conducting clinical trials. Essentially, the selection of appropriate receptor-ligand binding is a key to its success in practice. There were two examples of how poorly chosen targeting groups likely caused problems in clinical trials and the third targets receptors known to be found on some normal tissues. Unfortunately, one of the difficulties with cancer in the clinical setting is its inherent heterogeneity and similarity to ‘self’, thus making it difficult to choose a ligand that will be universally expressed by cancer cells yet not expressed by healthy tissue. This is not as much of an issue in preclinical studies that use more homogeneous cancer cell lines and could explain the disparity in results when translating to the clinical setting.
The limited market success in drug carriers with targeting groups has occurred mostly with antibody therapies. A simple search on the FDA drug database, Drugs@FDA, for the suffix “mab” will return numerous FDA approved antibodies (a considerable fraction is for cancer treatment). Some are antibody-drug conjugates such as Adcetris® and Kadcyla®, while others act as a drug simply by binding to the ligand, such as Herceptin® and Erbitux®. Antibodies are on the lower range of drug carrier sizes at approximately 5–20 nm in diameter (depending on the axis). They are made by cells resulting in uniform and biocompatible products. However, variation does exist due to metabolism/degradation, immune responses, organism of origin, drugs, and linkers. Still, there are more and more antibody therapies being approved by the FDA when compared to other targeted drug carriers such as polymers, colloids, or microemulsions. Preclinical studies are replete with polymers, micelles, liposomes, etc., that contain multifunctional aspects, beyond just targeting moieties, that provide preferential treatment to the target tissue; however, it seems relatively few of these enter clinical trials and they have yet to successfully navigate through clinical trials to market approval. Apparently, the simplest designs have the best hope for causing positive effects in the complex clinical cancer setting.
6. Conclusion
This survey is meant to be a broad, though not comprehensive, review of intravenously injectable cancer drug carriers that have undergone some clinical testing. The formulation strategies used vary widely from compound to compound. Drugs can be covalently bonded to proteins or polymers, loaded into micellar or liposomal structures by hydrophobic interactions, bound to the nanoparticle surface by ionic bonds, or sterically trapped within a structure. The drug carriers have a drastic effect on the dosing of the drug either by frequency or MTD. Comparable doses ranged from slightly lower to many-fold higher drug equivalents than their free-drug counterparts. Pharmacokinetic properties were also substantially altered with circulation half-life in some cases extended from hours to days or even weeks; drug exposure as measured by the AUC often increased as well. In some of these cases, these increases in half-life and AUC compared to the free drug occurred in both preclinical and clinical trials. Unfortunately, these improvements in dosing or pharmacokinetics and this focus on comparing against a free drug do not coincide with the gap between preclinical and clinical results. Comparing the half-life, AUC, Cmax, and other pharmacokinetic values between preclinical and clinical data is often not possible for the drug carriers as they are either not comparable or missing from the literature.
The efficacy in preclinical studies can be incredible; an HPMA-drug conjugate [81], Abraxane [103], NK105 [110], Genexol-PM [117], Xyotax [120], and gemtuzumab ozogamicin [159] were able to completely cure all mice of specific tumors (i.e., show tumor regression and complete growth inhibition for the remainder of the study). In spite of the tremendous advantages in efficacy the drug carriers seem to enjoy in preclinical studies, the difference seems to collapse to nearly zero in human tumors. The question of why the nanoparticle should perform in clinical trials, essentially the same as the free drug, may be an important one for understanding how to best proceed with new drug carrier therapies. Furthermore, one must understand the differences that are present in the preclinical and clinical studies. Some of the differences are obvious and the known shortcomings in using animal models for human cancer therapy testing are accepted because the murine model is the most widely used model in preclinical studies and has been invaluable in providing information and saving human lives. Still we must acknowledge the differences with regard to immunocompetency, metabolism, biological rhythms, hormone cycles, and heterogeneity-among others-and how these can affect the rate and nature of tumor growth.
The clinical environment also differs in attitudes, goals, training, protocols, and sample population characteristics. Due to an increased level of complexity, successful clinical trials are highly dependent on the proper design of the experiments. There is heterogeneity not only from patient to patient, but clinical tumors are more heterogeneous in terms of environmental and cellular composition. Depending on the nature of the cancer and the patient, the results can be drastically different. It is not suggested that chaos theory rules supreme in clinical trials, but it does help to explain the difficulty in reproducing results. The butterfly effect suggests the importance of consistency in initial conditions. For example, a ball may come to rest in entirely different locations at the bottom of a hill based on where it is placed at the crest. Imagine now, the topography of that hill can change as cancer is different from patient from patient and the resulting path of treatment can be different. In reality, heterogeneity in clinical cancers (including genetic, epigenetic, and environmental differences) is considered to play a major role in failed treatments.
There are some promising strategies to navigate and bridge the gap between research and clinical practice. These include advancements in personalized medicine and theranostics which would both address the issue of adapting to the unique circumstances of an individual patient. There are also increased efforts in modifying the tumor environment, which could perhaps serve as a way to make the disease population more uniform and treatable. As a means of stimulating discussion and creativity in bridging the gap, a short list of suggestions is being submitted that might help. Initially, consistent protocols for specific experiments could be developed that would serve as a prerequisite for clinical trials. Similar experiments would then be required, and must be reported on, in clinical trials. This would, hopefully, improve reproducibility and allow for comparisons to be made between preclinical and clinical trials. This could be an opportunity to provide more transparent reporting of pharmacokinetic and efficacy results, even if the results are negative. As reporting negative results is perceivably not as rewarding, they are a lot more difficult to find in publications and sometimes get summarized in conference meetings. However, the Declaration of Helsinki states that authors have an ethical duty to publish all results from clinical trials-whether positive, negative, or inconclusive [182]. While there are provisions in section 801 of the Food and Drug Administration Amendments Act that require a summary of results, there seem to be loopholes in the system that allow for some negative results from clinical trials to avoid being published. If made publicly available, the negative data could be extremely useful to understand the faults of the drug, model, or protocols and to learn from such mistakes. A journal, forum, or collection could be created where all results will be made publicly available as a requirement in taking part in clinical trials. Finally, a simple suggestion for a continued effort of scientific criticism and skepticism is recommended. In more than one of the drugs presented in this paper, negative results were found in clinical studies that were also present (and should have been addressed) in the preclinical studies. It would be wise to trust the bad news and be skeptical of the good. Still, one must acknowledge that great progress has indeed occurred in the fight against cancer and that many drug carriers have been approved with good reasons and have saved or improved the quality of countless lives.
Acknowledgment
This work was supported by NIH CA101850 and 140348.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roco MC. Nanotechnology: convergence with modern biology and medicine. Current Opinion in Biotechnology. 2003;14:337–346. doi: 10.1016/s0958-1669(03)00068-5. [DOI] [PubMed] [Google Scholar]
- 2.Wagner V, Dullaart A, Bock A-K, Zweck A. The emerging nanomedicine landscape. Nature Biotechnology. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 3.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, et al. Multifunctional Polymeric Micelles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems. Nano Letters. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 4.Pickup JC, Zhi Z, Khan F, Saxl T, Birch DJS. Nanomedicine and its potential in diabetes research and practice. Diabetes/Metabolism Research and Reviews. 2008;24:604–610. doi: 10.1002/dmrr.893. [DOI] [PubMed] [Google Scholar]
- 5.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. American Journal of Respiratory and Critical Care Medicine. 2005;172:1487–1490. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pison U, Welte T, Giersig M, a Groneberg D. Nanomedicine for respiratory diseases. European Journal of Pharmacology. 2006;533:341–350. doi: 10.1016/j.ejphar.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 7.V Kabanov A, Gendelman HE. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Progress in Polymer Science. 2007;32:1054–1082. doi: 10.1016/j.progpolymsci.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yatuv R, Robinson M, Dayan-Tarshish I, Baru M. The use of PEGylated liposomes in the development of drug delivery applications for the treatment of hemophilia. International Journal of Nanomedicine. 2010;5:581–591. doi: 10.2147/ijn.s8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallet-Regí M. Nanostructured mesoporous silica matrices in nanomedicine. Journal of Internal Medicine. 2010;267:22–43. doi: 10.1111/j.1365-2796.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B-BS, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumourinitiating cells: challenges and opportunities for anticancer drug discovery. Nature Reviews. Drug Discovery. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 11.Kim BYS, Rutka JT, Chan WCW. Nanomedicine. New England Journal of Medicine. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 12.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine--challenge and perspectives. Angewandte Chemie (International Ed. in English) 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. Journal of Controlled Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 14.Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009;2:99–112. doi: 10.1002/wnan.66. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 16.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advanced Drug Delivery Reviews. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Science. 2009;100:572–579. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2005;11:4136–4143. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 19.Duncan R, Seymour LW, O’Hare KB, Flanagan PA, Wedge S, Hume IC, et al. Preclinical evaluation of polymer-bound doxorubicin. Journal of Controlled Release. 1992;19:331–346. [Google Scholar]
- 20.Xu Z, Gu W, Huang J, Sui H, Zhou Z, Yang Y, et al. In vitro and in vivo evaluation of actively targetable nanoparticles for paclitaxel delivery. International Journal of Pharmaceutics. 2005;288:361–368. doi: 10.1016/j.ijpharm.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Paz-Ares L, Ross H, O’Brien M, Riviere A, Gatzemeier U, Von Pawel J, et al. Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. British Journal of Cancer. 2008;98:1608–1613. doi: 10.1038/sj.bjc.6604372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien MER, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Annals of Oncology. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 23.Carter SK, Di Marco A, Krakoff IH. International Symposium on Adriamycin: Milan, 9th-10th September, 1971. Softcover, Springer London, Limited; 2012. [Google Scholar]
- 24.Di Marco A. Adriamycin (NSC-123127): Mode and Mechanism of Action. Cancer Chemotherapy Reports. 1975;6:91–106. Part 3. [Google Scholar]
- 25.Potmesil M, Hsiang YH, Liu LF, Bank B, Grossberg H, Kirschenbaum S, et al. Resistance of human leukemic and normal lymphocytes to drug-induced DNA cleavage and low levels of DNA topoisomerase II. Cancer Research. 1988;48:3537–3543. [PubMed] [Google Scholar]
- 26.Weiss RB. The anthracyclines: will we ever find a better doxorubicin. Seminars in Oncology. 1992;19:670–686. [PubMed] [Google Scholar]
- 27.Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Current Medicinal Chemistry. Anti-cancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- 28.Cancer Drug Information - Doxorubicin Hydrochloride. [Accessed: 08-Aug-2013];National Cancer Institute. [Online]. Available at: http://www.cancer.gov/cancertopics/druginfo/doxorubicinhydrochloride.
- 29.Von Hoff DD, Layard MW, Basa P, Davis HL, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Annals of Internal Medicine. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 30.Alderton PM, Gross J, Green MD. Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Research. 1992;52:194–201. [PubMed] [Google Scholar]
- 31.Rossi F, Filippelli W, Russo S, Filippelli A, Berrino L. Cardiotoxicity of doxorubicin: effects of drugs inhibiting the release of vasoactive substances. Pharmacology & Toxicology. 1994;75:99–107. doi: 10.1111/j.1600-0773.1994.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 32.Vásquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA, Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36:11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopalan S, Politi PM, Sinha BK, Myers CE. Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Research. 1988;48:4766–4769. [PubMed] [Google Scholar]
- 34.Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. The Journal of Clinical Investigation. 1980;65:128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. Journal of Controlled Release : Official Journal of the Controlled Release Society. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, et al. Effective Targeting of Solid Tumors in Patients With Locally Advanced Cancers by Radiolabeled Pegylated Liposomes. Clinical Cancer Research. 2001;7:243–254. [PubMed] [Google Scholar]
- 37.Working PK, Dayan AD. Pharmacological-toxicological expert report. CAELYX. (Stealth liposomal doxorubicin HCl) Human Experimental Toxicology. 1996;15:751–785. [PubMed] [Google Scholar]
- 38.Vail DM, Amantea MA, Colbern GT, Martin FJ, Hilger RA, Working PK. Pegylated liposomal doxorubicin: Proof of principle using preclinical animal models and pharmacokinetic studies. Seminars in Oncology. 2004;31:16–35. doi: 10.1053/j.seminoncol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clinical Pharmacokinetics. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 40.Siegal T, Horowitz A, Gabizon A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: biodistribution and therapeutic efficacy. Journal of Neurosurgery. 1995;83:1029–1037. doi: 10.3171/jns.1995.83.6.1029. [DOI] [PubMed] [Google Scholar]
- 41.Gabizon AA, Barenholz Y, Bialer M. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: pharmacokinetic studies in rodents and dogs. Pharmaceutical Research. 1993;10:703–708. doi: 10.1023/a:1018907715905. [DOI] [PubMed] [Google Scholar]
- 42.Amantea M, Newman MS, Sullivan TM, Forrest A, Working PK. Relationship of dose intensity to the induction of palmar-plantar erythrodysesthia by pegylated liposomal doxorubicin in dogs. Human & Experimental Toxicology. 1999;18:17–26. doi: 10.1177/096032719901800103. [DOI] [PubMed] [Google Scholar]
- 43.Gabizon AA, Pappo O, Goren D, Chemla M, Tzemach D, Horowitz AT. Preclinical Studies with Doxorubicin Encapsulated in Polyethyleneglycol-Coated Liposomes. 2008 [Google Scholar]
- 44.Gabizon AA. Selective tumor localization and improved therapeutic index of anthracyclines encapsulated in long-circulating liposomes. Cancer Research. 1992;52:891–896. [PubMed] [Google Scholar]
- 45.Vaage J, Barberá-Guillem E, Abra R, a Huang, Working P. Tissue distribution and therapeutic effect of intravenous free or encapsulated liposomal doxorubicin on human prostate carcinoma xenografts. Cancer. 1994;73:1478–1484. doi: 10.1002/1097-0142(19940301)73:5<1478::aid-cncr2820730526>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Gabizon A, Goren D, Horowitz AT, Tzemach D, Lossos A, Siegal T. Long-circulating liposomes for drug delivery in cancer therapy: a review of biodistribution studies in tumor-bearing animals. Advanced Drug Delivery Reviews. 1997;24:337–344. [Google Scholar]
- 47.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Working PK, Newman MS, Sullivan T, Yarrington J. Reduction of the cardiotoxicity of doxorubicin in rabbits and dogs by encapsulation in long-circulating, pegylated liposomes. The Journal of Pharmacology and Experimental Therapeutics. 1999;289:1128–1133. [PubMed] [Google Scholar]
- 49.Huang SK, Mayhew E, Gilani S, Lasic DD, Martin FJ, Papahadjopoulos D. Pharmacokinetics and Therapeutics of Sterically Stabilized Liposomes in Mice Bearing C-26 Colon Carcinoma. Cancer Res. 1992;52:6774–6781. [PubMed] [Google Scholar]
- 50.Vaage J, Donovan D, Uster P, Working P. Tumour uptake of doxorubicin in polyethylene glycol-coated liposomes and therapeutic effect against a xenografted human pancreatic carcinoma. British Journal of Cancer. 1997;75:482–486. doi: 10.1038/bjc.1997.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaage J, Donovan D, Mayhew E, Abra R, Huang A. Therapy of human ovarian carcinoma xenografts using doxorubicin encapsulated in sterically stabilized liposomes. Cancer. 1993;72:3671–3675. doi: 10.1002/1097-0142(19931215)72:12<3671::aid-cncr2820721219>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 52.Colbern GT, Hiller AJ, Musterer RS, Pegg E, Henderson IC, Working PK. Significant Increase in Antitumor Potency of Doxorubicin Hc1 by its Encapsulation in Pegylated Liposomes. Journal of Liposome Research. 1999;9:523–538. [Google Scholar]
- 53.Gabizon A, Martin F. Polyethylene Glycol-Coated (Pegylated) Liposomal Doxorubicin. Drugs. 1997;54:15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 54.Working PK, Newman MS, Huang SK, Mayhew E, Vaage J, Lasic DD. Pharmacokinetics, Biodistribution and Therapeutic Efficacy of Doxorubicin Encapsulated in Stealth® Liposomes (Doxil®) Journal of Liposome Research. 1994;4:667–687. [Google Scholar]
- 55.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Research. 1994;54:987–992. [PubMed] [Google Scholar]
- 56.Northfelt DW, Martin FJ, Working P, Volberding PA, Russell J, Newman M, et al. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi’s sarcoma. Journal of Clinical Pharmacology. 1996;36:55–63. doi: 10.1002/j.1552-4604.1996.tb04152.x. [DOI] [PubMed] [Google Scholar]
- 57.Northfelt DW. Liposomal Anthracycline Chemotherapy in the Treatment of AIDS-Related Kaposi’s Sarcoma. Oncology. 1997;11:21–32. [Google Scholar]
- 58.Symon Z, Peyser A, Tzemach D, Lyass O, Sucher E, Shezen E, et al. Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes. Cancer. 1999;86:72–78. [PubMed] [Google Scholar]
- 59.Alberts DS, Muggia FM, Carmichael J, Winer EP, Jahanzeb M, Venook AP, et al. Efficacy and safety of liposomal anthracyclines in phase I/II clinical trials. Seminars in Oncology. 2004;31:53–90. doi: 10.1053/j.seminoncol.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 60.Soloman R, Gabizon AA. Clinical pharmacology of liposomal anthracyclines: focus on pegylated liposomal Doxorubicin. Clinical Lymphoma & Myeloma. 2008;8:21–32. doi: 10.3816/clm.2008.n.001. [DOI] [PubMed] [Google Scholar]
- 61.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2000;11:1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 62.Ewer MS, Martin FJ, Henderson C, Shapiro CL, Benjamin RS, Gabizon AA. Cardiac safety of liposomal anthracyclines. Seminars in Oncology. 2004;31:161–181. doi: 10.1053/j.seminoncol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Gabizon A, Shmeeda H, Grenader T. Pharmacological basis of pegylated liposomal doxorubicin: impact on cancer therapy. European Journal of Pharmaceutical Sciences : Official Journal of the European Federation for Pharmaceutical Sciences. 2012;45:388–398. doi: 10.1016/j.ejps.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Udhrain A, Skubitz KM, Northfelt DW. Pegylated liposomal doxorubicin in the treatment of AIDS-related Kaposi’s sarcoma. International Journal of Nanomedicine. 2007;2:345–352. [PMC free article] [PubMed] [Google Scholar]
- 65.Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 1998;16:2445–2451. doi: 10.1200/JCO.1998.16.7.2445. [DOI] [PubMed] [Google Scholar]
- 66.Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 1998;16:683–691. doi: 10.1200/JCO.1998.16.2.683. [DOI] [PubMed] [Google Scholar]
- 67.Cianfrocca M, Lee S, Von Roenn J, Tulpule A, Dezube BJ, Aboulafia DM, et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer. 2010;116:3969–3977. doi: 10.1002/cncr.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin-Carbonero L, Barrios A, Saballs P, Sirera G, Santos J, Palacios R, et al. Pegylated liposomal doxorubicin plus highly active antiretroviral therapy versus highly active antiretroviral therapy alone in HIV patients with Kaposi’s sarcoma. AIDS (London, England) 2004;18:1737–1740. doi: 10.1097/01.aids.0000131385.60974.b9. [DOI] [PubMed] [Google Scholar]
- 69.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 70.Gordon AN, Tonda M, Sun S, Rackoff W. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecologic Oncology. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Rifkin RM, Gregory SA, Mohrbacher A, Hussein MA. Pegylated liposomal doxorubicin, vincristine, and dexamethasone provide significant reduction in toxicity compared with doxorubicin, vincristine, and dexamethasone in patients with newly diagnosed multiple myeloma: a Phase III multicenter randomized trial. Cancer. 2006;106:848–858. doi: 10.1002/cncr.21662. [DOI] [PubMed] [Google Scholar]
- 72.Duncan R. Drug-polymer conjugates: potential for improved chemotherapy. Anti-cancer Drugs. 1992;3:175–210. doi: 10.1097/00001813-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Muggia FM. Doxorubicin-polymer conjugates: further demonstration of the concept of enhanced permeability and retention. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 1999;5:7–8. [PubMed] [Google Scholar]
- 74.Duncan R. Development of HPMA copolymer-anticancer conjugates: clinical experience and lessons learnt. Advanced Drug Delivery Reviews. 2009;61:1131–1148. doi: 10.1016/j.addr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Paul A, Vicent MJ, Duncan R. Using small-angle neutron scattering to study the solution conformation of N-(2-hydroxypropyl)methacrylamide copolymer-doxorubicin conjugates. Biomacromolecules. 2007;8:1573–1579. doi: 10.1021/bm060925s. [DOI] [PubMed] [Google Scholar]
- 76.Minko T, Kopeckovà P, Pozharov V, Kopecek J. HPMA copolymer bound adriamycin overcomes MDR1 gene encoded resistance in a human ovarian carcinoma cell line. Journal of Controlled Release : Official Journal of the Controlled Release Society. 1998;54:223–233. doi: 10.1016/s0168-3659(98)00009-1. [DOI] [PubMed] [Google Scholar]
- 77.Minko T, Kopeckovà P, Kopecek J. Comparison of the anticancer effect of free and HPMA copolymer-bound adriamycin in human ovarian carcinoma cells. Pharmaceutical Research. 1999;16:986–996. doi: 10.1023/a:1018959029186. [DOI] [PubMed] [Google Scholar]
- 78.Minko T, Kopečkovà P, Kopeček J. Chronic exposure to HPMA copolymer-bound adriamycin does not induce multidrug resistance in a human ovarian carcinoma cell line. Journal of Controlled Release. 1999;59:133–148. doi: 10.1016/s0168-3659(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 79.Seymour LW, Ulbrich K, Strohalm J, Kopecek J, Duncan R. The pharmacokinetics of polymer-bound adriamycin. Biochemical Pharmacology. 1990;39:1125–1131. doi: 10.1016/0006-2952(90)90293-t. [DOI] [PubMed] [Google Scholar]
- 80.Seymour LW, Ulbrich K, Steyger PS, Brereton M, Subr V, Strohalm J, et al. Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma. British Journal of Cancer. 1994;70:636–641. doi: 10.1038/bjc.1994.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minko T, Kopeckovà P, Kopecek J. Efficacy of the chemotherapeutic action of HPMA copolymer-bound doxorubicin in a solid tumor model of ovarian carcinoma. International Journal of Cancer. Journal International Du Cancer. 2000;86:108–117. doi: 10.1002/(sici)1097-0215(20000401)86:1<108::aid-ijc17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 82.Seymour LW, Miyamoto Y, Maeda H, Brereton M, Strohalm J, Ulbrich K, et al. Influence of molecular weight on passive tumour accumulation of a soluble macromolecular drug carrier. European Journal of Cancer (Oxford, England : 1990) 1995;31A:766–770. doi: 10.1016/0959-8049(94)00514-6. [DOI] [PubMed] [Google Scholar]
- 83.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, et al. Early Phase Tumor Accumulation of Macromolecules: A Great Difference in Clearance Rate between Tumor and Normal Tissues. Cancer Science. 1998;89:307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Minko T, Kopeckova P, Pozharov V, Jensen KD, Kopecek J. The influence of cytotoxicity of macromolecules and of VEGF gene modulated vascular permeability on the enhanced permeability and retention effect in resistant solid tumors. Pharmaceutical Research. 2000;17:505–514. doi: 10.1023/a:1007500412442. [DOI] [PubMed] [Google Scholar]
- 85.Yeung TK, Hopewell JW, Simmonds RH, Seymour LW, Duncan R, Bellini O, et al. Reduced cardiotoxicity of doxorubicin given in the form of N-(2-hydroxypropyl)methacrylamide conjugates: and experimental study in the rat. Cancer Chemotherapy and Pharmacology. 1991;29:105–111. doi: 10.1007/BF00687318. [DOI] [PubMed] [Google Scholar]
- 86.Rihova B, Bilej M, Vetvicka V, Ulbrich K, Strohalm J, Kopecek J, et al. Biocompatibility of N-(2-hydroxypropyl) methacrylamide copolymers containing adriamycin. Immunogenicity, and effect on haematopoietic stem cells in bone marrow in vivo and mouse splenocytes and human peripheral blood lymphocytes in vitro. Biomaterials. 1989;10:335–342. doi: 10.1016/0142-9612(89)90075-6. [DOI] [PubMed] [Google Scholar]
- 87.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, et al. Phase I Clinical and Pharmacokinetic Study of PK1 [N-(2-Hydroxypropyl)methacrylamide Copolymer Doxorubicin]: First Member of a New Class of Chemotherapeutic Agents--Drug-Polymer Conjugates. Clin. Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 88.Seymour LW, Ferry DR, Kerr DJ, Rea D, Whitlock M, Poyner R, et al. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. International Journal of Oncology. 2009;34:1629–1636. doi: 10.3892/ijo_00000293. [DOI] [PubMed] [Google Scholar]
- 89.Guenard D, Gueritte-Voegelein F, Potier P. Taxol and taxotere: discovery, chemistry, and structure-activity relationships. Accounts of Chemical Research. 1993;26:160–167. [Google Scholar]
- 90.Stierle A, Strobel G, Stierle D, Grothaus P, Bignami G. The Search for a Taxol-Producing Microorganism Among the Endophytic Fungi of the Pacific Yew, Taxus brevifolia. Journal of Natural Products. 1995;58:1315–1324. doi: 10.1021/np50123a002. [DOI] [PubMed] [Google Scholar]
- 91.Ganem B, Franke RR. Paclitaxel from primary taxanes: a perspective on creative invention in organozirconium chemistry. The Journal of Organic Chemistry. 2007;72:3981–3987. doi: 10.1021/jo070129s. [DOI] [PubMed] [Google Scholar]
- 92.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 1994;5(Suppl 6):S3–S6. [PubMed] [Google Scholar]
- 93.Dosio F, Brusa P, Crosasso P, Arpicco S, Cattel L. Preparation, characterization and properties in vitro and in vivo of a paclitaxel-albumin conjugate. Journal of Controlled Release. 1997;47:293–304. [Google Scholar]
- 94.Szebeni J, Muggia FM, Alving CR. Complement Activation by Cremophor EL as a Possible Contributor to Hypersensitivity to Paclitaxel: an In Vitro Study. Journal of the National Cancer Institute. 1998;90:300–306. doi: 10.1093/jnci/90.4.300. [DOI] [PubMed] [Google Scholar]
- 95.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Seminars in Oncology. 1993;20:1–15. [PubMed] [Google Scholar]
- 96.Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Seminars in Oncology. 1992;19:646–662. [PubMed] [Google Scholar]
- 97.Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L, Lower E, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2005;23:5542–5551. doi: 10.1200/JCO.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 98.Moreno-Aspitia A, Perez EA. Nanoparticle albumin-bound paclitaxel (ABI-007): a newer taxane alternative in breast cancer. Future Oncology (London, England) 2005;1:755–762. doi: 10.2217/14796694.1.6.755. [DOI] [PubMed] [Google Scholar]
- 99.Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2006;17:1263–1268. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 100.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tirrell M. Celgene Rises as Abraxane Meets Pancreatic Cancer Goals. Bloomberg [Google Scholar]
- 102.Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC Expression Correlates with Tumor Response to Albumin-Bound Paclitaxel in Head and Neck Cancer Patients. Translational Oncology. 2009;2:59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 104.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clinical Cancer Research. 2005;11:4136–4143. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 105.Stinchcombe TE, Socinski MA, Walko CM, O’Neil BH, Collichio FA, Ivanova A, et al. Phase I and pharmacokinetic trial of carboplatin and albumin-bound paclitaxel, ABI-007 (Abraxane) on three treatment schedules in patients with solid tumors. Cancer Chemotherapy and Pharmacology. 2007;60:759–766. doi: 10.1007/s00280-007-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. Journal of Clinical Oncology. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 107.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. International Journal of Nanomedicine. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, et al. Cremophor EL-mediated Alteration of Paclitaxel Distribution in Human Blood: Clinical Pharmacokinetic Implications. Cancer Res. 1999;59:1454–1457. [PubMed] [Google Scholar]
- 109.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological Effects of Formulation Vehicles. Clinical Pharmacokinetics. 2003;42:665–685. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 110.Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. British Journal of Cancer. 2005;92:1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kato K, Chin K, Yoshikawa T, Yamaguchi K, Tsuji Y, Esaki T, et al. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Investigational New Drugs. 2012;30:1621–1627. doi: 10.1007/s10637-011-9709-2. [DOI] [PubMed] [Google Scholar]
- 112.Wöhrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2004;15:1585–1595. doi: 10.1093/annonc/mdh422. [DOI] [PubMed] [Google Scholar]
- 113.Findlay M, Cunningham D, Norman A, Mansi J, Nicolson M, Hickish T, et al. A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF) Ann. Onc. 1994;5:609–616. doi: 10.1093/oxfordjournals.annonc.a058932. [DOI] [PubMed] [Google Scholar]
- 114.Ross P. Prospective Randomized Trial Comparing Mitomycin, Cisplatin, and Protracted Venous-Infusion Fluorouracil (PVI 5-FU) With Epirubicin, Cisplatin, and PVI 5-FU in Advanced Esophagogastric Cancer. Journal of Clinical Oncology. 2002;20:1996–2004. doi: 10.1200/JCO.2002.08.105. [DOI] [PubMed] [Google Scholar]
- 115.Webb A, Cunningham D, Scarffe J, Harper P, Norman A, Joffe J, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J. Clin. Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 116.Eguchi T, Fujii M, Wakabayashi K, Aisaki K, Tsuneda Y, Kochi M, et al. Docetaxel plus 5-fluorouracil for terminal gastric cancer patients with peritoneal dissemination. Hepato-gastroenterology. 2003;50:1735–1738. [PubMed] [Google Scholar]
- 117.Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. Journal of Controlled Release. 2001;72:191–202. doi: 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 118.Kim T-Y, Kim D-W, Chung J-Y, Shin SG, Kim S-C, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 119.Lee SY, Park HS, Lee KY, Kim HJ, Jeon YJ, Jang TW, et al. Paclitaxel-loaded polymeric micelle (230 mg/m(2)) and cisplatin (60 mg/m(2)) vs. paclitaxel (175 mg/m(2)) and cisplatin (60 mg/m(2)) in advanced non-small-cell lung cancer: a multicenter randomized phase IIB trial. Clinical Lung Cancer. 2013;14:275–282. doi: 10.1016/j.cllc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 120.Li C, Yu D-F, Newman RA, Cabral F, Stephens LC, Hunter N, et al. Complete Regression of Well-established Tumors Using a Novel Water-soluble Poly(L-Glutamic Acid)-Paclitaxel Conjugate. Cancer Res. 1998;58:2404–2409. [PubMed] [Google Scholar]
- 121.Li C, Price JE, Milas L, Hunter NR, Ke S, Yu D-F, et al. Antitumor Activity of Poly(L-glutamic acid)-Paclitaxel on Syngeneic and Xenografted Tumors. Clin. Cancer Res. 1999;5:891–897. [PubMed] [Google Scholar]
- 122.O’Brien MER, Socinski MA, Popovich IN, Bondarenko Alexander Y, Tomova A, Bilynsky BT, Hotko YS, et al. Randomized Phase III Trial Comparing Single-Agent Gemcitabine or Vinorelbine for the Treatment of PS 2 Patients with Chemotherapy-Naıve Advanced Non-small Cell Lung Cancer. Journal of Thoracic Oncology. 2008;3:728–734. doi: 10.1097/JTO.0b013e31817c6b68. [DOI] [PubMed] [Google Scholar]
- 123.Sabbatini P, Sill MW, O’Malley D, Adler L, Secord AA. A phase II trial of paclitaxel poliglumex in recurrent or persistent ovarian or primary peritoneal cancer (EOC): a Gynecologic Oncology Group Study. Gynecologic Oncology. 2008;111:455–460. doi: 10.1016/j.ygyno.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 124.Langer CJ, O’Byrne KJ, a Socinski M, Mikhailov SM, Leśniewski-Kmak K, Smakal M, et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naïve advanced non-small cell lung cancer. Journal of Thoracic Oncology : Official Publication of the International Association for the Study of Lung Cancer. 2008;3:623–630. doi: 10.1097/JTO.0b013e3181753b4b. [DOI] [PubMed] [Google Scholar]
- 125.Richards DA, Richards P, Bodkin D, Neubauer MA, Oldham F. Efficacy and safety of paclitaxel poliglumex as first-line chemotherapy in patients at high risk with advanced-stage non-small-cell lung cancer: results of a phase II study. Clinical Lung Cancer. 2005;7:215–220. doi: 10.3816/CLC.2005.n.039. [DOI] [PubMed] [Google Scholar]
- 126.Rosenberg B, VanCamp L, Krigas T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 127.Rosenberg B, VanCamp L, Grimley EB, Thomson AJ. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes. The Journal of Biological Chemistry. 1967;242:1347–1352. [PubMed] [Google Scholar]
- 128.Rosenberg B, Renshaw E, Vancamp L, Hartwick J, Drobnik J. Platinum-induced filamentous growth in Escherichia coli. Journal of Bacteriology. 1967;93:716–721. doi: 10.1128/jb.93.2.716-721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum Compounds: a New Class of Potent Antitumour Agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 130.Rosenberg B, VanCamp L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Research. 1970;30:1799–1802. [PubMed] [Google Scholar]
- 131.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Pérez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anti-cancer Agents in Medicinal Chemistry. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 132.Cancer Drug Information - Cisplatin. [Accessed: 08-Aug-2013];National Cancer Institute. [Online]. Available at: http://www.cancer.gov/cancertopics/druginfo/cisplatin.
- 133.Kociba RJ, Sleight SD. Acute toxicologic and pathologic effects of cis-diamminedichloroplatinum (NSC-119875) in the male rat. Cancer Chemotherapy Reports. 1971;55:1–8. Part 1. [PubMed] [Google Scholar]
- 134.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. The American Journal of the Medical Sciences. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 135.Pinzani V, Bressolle F, Haug IJ, Galtier M, Blayac JP, Balmès P. Cisplatin-induced renal toxicity and toxicity-modulating strategies: a review. Cancer Chemotherapy and Pharmacology. 1994;35:1–9. doi: 10.1007/BF00686277. [DOI] [PubMed] [Google Scholar]
- 136.Hartmann JT, Lipp H-P. Toxicity of platinum compounds. Expert Opinion on Pharmacotherapy. 2003;4:889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- 137.Helson L, Okonkwo E, Anton L, Cvitkovic E. cis-Platinum ototoxicity. Clinical Toxicology. 1978;13:469–478. doi: 10.3109/15563657808988252. [DOI] [PubMed] [Google Scholar]
- 138.Ding D, Allman BL, Salvi R. Review: ototoxic characteristics of platinum antitumor drugs. Anatomical Record (Hoboken, N.J. : 2007) 2012;295:1851–1867. doi: 10.1002/ar.22577. [DOI] [PubMed] [Google Scholar]
- 139.Kelland LR, Sharp SY. Platinum compounds in cancer therapy, Current Opinion in Oncologic. Endocrine & Metabolic Investigational Drugs. 1999;1:380–385. [Google Scholar]
- 140.Judson I, Kelland LR. New developments and approaches in the platinum arena. Drugs. 2000;59(Suppl 4):29–36. doi: 10.2165/00003495-200059004-00004. discussion 37–8. [DOI] [PubMed] [Google Scholar]
- 141.Sharp SY, O’Neill CF, Rogers P, Boxall FE, Kelland LR. Retention of activity by the new generation platinum agent AMD0473 in four human tumour cell lines possessing acquired resistance to oxaliplatin. European Journal of Cancer (Oxford, England : 1990) 2002;38:2309–2315. doi: 10.1016/s0959-8049(02)00244-7. [DOI] [PubMed] [Google Scholar]
- 142.Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutation Research. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 143.Yokoyama M, Okano T, Sakurai Y, Suwa S, Kataoka K. Introduction of cisplatin into polymeric micelle. Journal of Controlled Release. 1996;39:351–356. [Google Scholar]
- 144.Nishiyama N, Yokoyama M, Aoyagi T, Okano T, Sakurai Y, Kataoka K. Preparation and Characterization of Self-Assembled Polymer-Metal Complex Micelle from cis-Dichlorodiammineplatinum(II) and Poly(ethylene glycol)-Poly(α, β-aspartic acid) Block Copolymer in an Aqueous Medium. Langmuir. 1999;15:377–383. [Google Scholar]
- 145.Nishiyama N, Kataoka K. Preparation and characterization of size-controlled polymeric micelle containing cis-dichlorodiammineplatinum(II) in the core. Journal of Controlled Release : Official Journal of the Controlled Release Society. 2001;74:83–94. doi: 10.1016/s0168-3659(01)00314-5. [DOI] [PubMed] [Google Scholar]
- 146.Nishiyama N, Kato Y, Sugiyama Y, Kataoka K. Cisplatin-loaded polymer-metal complex micelle with time-modulated decaying property as a novel drug delivery system. Pharmaceutical Research. 2001;18:1035–1041. doi: 10.1023/a:1010908916184. [DOI] [PubMed] [Google Scholar]
- 147.Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Research. 2003;63:8977–8983. [PubMed] [Google Scholar]
- 148.Uchino H, Matsumura Y, Negishi T, Koizumi F, Hayashi T, Honda T, et al. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. British Journal of Cancer. 2005;93:678–687. doi: 10.1038/sj.bjc.6602772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matsumura Y. Poly (amino acid) micelle nanocarriers in preclinical and clinical studies. Advanced Drug Delivery Reviews. 2008;60:899–914. doi: 10.1016/j.addr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 150.Endo K, Ueno T, Kondo S, Wakisaka N, Murono S, Ito M, et al. Tumor-targeted chemotherapy with the nanopolymer-based drug NC-6004 for oral squamous cell carcinoma. Cancer Science. 2013;104:369–374. doi: 10.1111/cas.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baba M, Matsumoto Y, Kashio A, Cabral H, Nishiyama N, Kataoka K, et al. Micellization of cisplatin (NC-6004) reduces its ototoxicity in guinea pigs. Journal of Controlled Release : Official Journal of the Controlled Release Society. 2012;157:112–117. doi: 10.1016/j.jconrel.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 152.Plummer R, Wilson RH, Calvert H, V Boddy A, Griffin M, Sludden J, et al. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. British Journal of Cancer. 2011;104:593–598. doi: 10.1038/bjc.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kitajima K, Fukuoka M, Kobayashi S, Kusunoki Y, Takada M, Negoro S, et al. [Studies on the appropriate administration of cisplatin based on pharmacokinetics and toxicity]., Gan to Kagaku Ryoho. Cancer & Chemotherapy. 1987;14:2517–2523. [PubMed] [Google Scholar]
- 154.Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval Summary: Gemtuzumab Ozogamicin in Relapsed Acute Myeloid Leukemia. Clin. Cancer Res. 2001;7:1490–1496. [PubMed] [Google Scholar]
- 155.Larson RA, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL, et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin) Leukemia. 2002;16:1627–1636. doi: 10.1038/sj.leu.2402677. [DOI] [PubMed] [Google Scholar]
- 156.van der Jagt RHC, Badger CC, Appelbaum FR, Press OW, Matthews DC, Eary JF, et al. Localization of Radiolabeled Antimyeloid Antibodies in a Human Acute Leukemia Xenograft Tumor Model. Cancer Res. 1992;52:89–94. [PubMed] [Google Scholar]
- 157.Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leukemia Research. 1984;8:521–534. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- 158.Hamann PR, Hinman LM, Beyer CF, Lindh D, Upeslacis J, Flowers DA, et al. An Anti-CD33 Antibody-Calicheamicin Conjugate for Treatment of Acute Myeloid Leukemia. Choice of Linker. Bioconjugate Chemistry. 2002;13:40–46. doi: 10.1021/bc0100206. [DOI] [PubMed] [Google Scholar]
- 159.Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. Gemtuzumab Ozogamicin, A Potent and Selective Anti-CD33 Antibody-Calicheamicin Conjugate for Treatment of Acute Myeloid Leukemia. Bioconjugate Chemistry. 2002;13:47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 160.Petersdorf S, Kopecky K, Stuart RK, Larson RA, Nevill TJ, Stenke L, et al. Preliminary Results of Southwest Oncology Group Study S0106: An International Intergroup Phase 3 Randomized Trial Comparing the Addition of Gemtuzumab Ozogamicin to Standard Induction Therapy Versus Standard Induction Therapy Followed by a Second Randomiz. ASH Annual Meeting Abstracts. 2009;114:790. [Google Scholar]
- 161.FDA-News-Release. [Accessed: 09-May-2013];2010 - FDA: Pfizer Voluntarily Withdraws Cancer Treatment Mylotarg from U.S. Market. [Online]. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm216448.htm.
- 162.Brunnberg U, Mohr M, Noppeney R, Dürk HA, Sauerland MC, Müller-Tidow C, et al. Induction therapy of AML with ara-C plus daunorubicin versus ara-C plus gemtuzumab ozogamicin: a randomized phase II trial in elderly patients. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2012;23:990–996. doi: 10.1093/annonc/mdr346. [DOI] [PubMed] [Google Scholar]
- 163.Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. Journal of Clinical Oncology. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 164.Rajvanshi P. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002;99:2310–2314. doi: 10.1182/blood.v99.7.2310. [DOI] [PubMed] [Google Scholar]
- 165.Seymour LW. Hepatic Drug Targeting: Phase I Evaluation of Polymer-Bound Doxorubicin. Journal of Clinical Oncology. 2002;20:1668–1676. doi: 10.1200/JCO.2002.20.6.1668. [DOI] [PubMed] [Google Scholar]
- 166.Duncan R, Seymour LCW, Scarlett L, Lloyd JB, Rejmanovà P, Kopeček J. Fate of N-(2-hydroxypropyl)methacrylamide copolymers with pendent galactosamine residues after intravenous administration to rats. BBA - General Subjects. 1986;880:62–71. doi: 10.1016/0304-4165(86)90120-0. [DOI] [PubMed] [Google Scholar]
- 167.V Pimm M, Perkins AC, Strohalm J, Ulbrich K, Duncan R. Gamma scintigraphy of a 123I–labelled N-(2-hydroxypropyl)methacrylamide copolymer-doxorubicin conjugate containing galactosamine following intravenous administration to nude mice bearing hepatic human colon carcinoma. Journal of Drug Targeting. 1996;3:385–390. doi: 10.3109/10611869608996829. [DOI] [PubMed] [Google Scholar]
- 168.Julyan P. Preliminary clinical study of the distribution of HPMA copolymers bearing doxorubicin and galactosamine. Journal of Controlled Release. 1999;57:281–290. doi: 10.1016/s0168-3659(98)00124-2. [DOI] [PubMed] [Google Scholar]
- 169.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular Pharmaceutics. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. [Accessed: 06-Aug-2013];ClinicalTrials.gov. [Online]. Available at: http://www.clinicaltrials.gov/.
- 171. [Accessed: 06-Aug-2013];UK Clinical Trials Gateway. [Online]. Available at: http://www.ukctg.nihr.ac.uk/.
- 172.Heidel JD, Schluep T. Cyclodextrin-containing polymers: versatile platforms of drug delivery materials. Journal of Drug Delivery. 2012;2012:262731. doi: 10.1155/2012/262731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mishra S, Heidel JD, Webster P, Davis ME. Imidazole groups on a linear, cyclodextrin-containing polycation produce enhanced gene delivery via multiple processes. Journal of Controlled Release : Official Journal of the Controlled Release Society. 2006;116:179–191. doi: 10.1016/j.jconrel.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 174.Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. Transferrin receptors in human tissues: their distribution and possible clinical relevance. Journal of Clinical Pathology. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Molecular Pharmaceutics. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 176.Heidel JD, Liu JY-C, Yen Y, Zhou B, Heale BSE, Rossi JJ, et al. Potent siRNA inhibitors of ribonucleotide reductase subunit RRM2 reduce cell proliferation in vitro and in vivo. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2007;13:2207–2215. doi: 10.1158/1078-0432.CCR-06-2218. [DOI] [PubMed] [Google Scholar]
- 177.Heidel JD, Yu Z, Liu JY-C, Rele SM, Liang Y, Zeidan RK, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bartlett DW, Davis ME. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnology and Bioengineering. 2008;99:975–985. doi: 10.1002/bit.21668. [DOI] [PubMed] [Google Scholar]
- 181.Ribas A, Kalinoski L, Heidel JD, Peterkin J, Seligson DB, Zuckerman JE, et al. Systemic delivery of siRNA via targeted nanoparticles in patients with cancer: Results from a first-in-class phase I clinical trial. J Clin Oncol (Meeting Abstracts) 2010;28 abstract 3022. [Google Scholar]
- 182. [Accessed: 01-Aug-2013];W.M. Association, World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. [Online]. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html.
- 183.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 184.Chan S, Davidson N, Juozaityte E, Erdkamp F, Pluzanska A, Azarnia N, et al. Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2004;15:1527–1534. doi: 10.1093/annonc/mdh393. [DOI] [PubMed] [Google Scholar]
- 185.Gill P, Wernz J, Scadden D, Cohen P, Mukwaya G, von Roenn J, et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcoma. J. Clin. Oncol. 1996;14:2353–2364. doi: 10.1200/JCO.1996.14.8.2353. [DOI] [PubMed] [Google Scholar]
- 186.Wang Z-M, Ho JX, Ruble JR, Rose J, Rüker F, Ellenburg M, et al. Structural studies of several clinically important oncology drugs in complex with human serum albumin. Biochimica et Biophysica Acta. 2013 doi: 10.1016/j.bbagen.2013.06.032. In Press. [DOI] [PubMed] [Google Scholar]
- 187.Matsumura Y. Polymeric micellar delivery systems in oncology. Japanese Journal of Clinical Oncology. 2008;38:793–802. doi: 10.1093/jjco/hyn116. [DOI] [PubMed] [Google Scholar]