Abstract
We recently demonstrated tumor-selective iodide uptake and therapeutic efficacy of combined radiovirotherapy after systemic delivery of the theranostic sodium iodide symporter (NIS) gene using a dendrimer-coated adenovirus. To further improve shielding and targeting we physically coated replication-selective adenoviruses carrying the hNIS gene with a conjugate consisting of cationic poly(amidoamine) (PAMAM) dendrimer linked to the peptidic, epidermal growth factor receptor (EGFR)-specific ligand GE11. In vitro experiments demonstrated coxsackie-adenovirus receptor-independent but EGFR-specific transduction efficiency. Systemic injection of the uncoated adenovirus in a liver cancer xenograft mouse model led to high levels of NIS expression in the liver due to hepatic sequestration, which were significantly reduced after coating as demonstrated by 123I-scintigraphy. Reduction of adenovirus liver pooling resulted in decreased hepatotoxicity and increased transduction efficiency in peripheral xenograft tumors. 124I-PET-imaging confirmed EGFR-specificity by significantly lower tumoral radioiodine accumulation after pretreatment with the EGFR-specific antibody cetuximab. A significantly enhanced oncolytic effect was observed following systemic application of dendrimer-coated adenovirus that was further increased by additional treatment with a therapeutic dose of 131I. These results demonstrate restricted virus tropism and tumor-selective retargeting after systemic application of coated, EGFR-targeted adenoviruses therefore representing a promising strategy for improved systemic adenoviral NIS gene therapy.
Introduction
We recently reported on the feasibility of noncovalent adenovirus surface modification using synthetic polycationic dendrimers resulting in partial protection from neutralizing antibodies, coxsackie-adenovirus receptor (CAR)-independent infectivity and efficient liver detargeting after systemic vector administration, leading to reduced toxicity as well as enhanced tumoral transduction and therapeutic efficacy.1,2
Once a viral gene transfer vehicle has been developed that allows for systemic application and provides sufficiently high transgene expression in the target tissue, a key task is to further increase levels of oncolysis and tumoral transgene expression with optimal specificity and lowest possible toxicity in nontarget organs.3,4 A variety of different methods have been tested in recent times to make viral gene transfer even more secure and successful in terms of development of targeted and shielded vectors for future clinical applications in humans.5,6 Among others, targeting ligands that have been tested recently to optimize tumor-selectivity of viral vectors include ligands of the epidermal growth factor receptor (EGFR), the fibroblast growth factor receptor 2, CGKRK motifs, and α-v integrins on the cell surface.7,8,9 Targeting the EGFR is of particular interest since it has been shown that EGFR triggers tumor growth and progression and is significantly upregulated in a large number of epithelial tumors.10 Therefore, the EGFR has been evaluated as a promising target structure for viral and nonviral gene transfer.11,12,13,14 In a recent study, we reported on systemic nonviral sodium iodide symporter (NIS) gene transfer using polyplexes coupled to the synthetic peptide GE11 as an EGFR-targeting ligand with high receptor affinity that does not activate the receptor tyrosine kinase,15 capable of inducing high levels of tumor-specific transgene expression.14 NIS represents one of the oldest targets for molecular imaging and therapy. Due to its ability to concentrate iodine in the thyroid gland it provides the molecular basis for thyroid scintigraphy and radioiodine whole body scanning as well as therapeutic application of radioiodine in thyroid cancer—the most effective form of systemic anticancer radiotherapy available today.16 Transduction of cancer cells with the theranostic NIS gene therefore gives us the possibility of noninvasive monitoring of NIS biodistribution before application of a therapeutic dose of radioiodine, which is of particular importance after systemic vector application.17,18
In a further study, we have previously reported on the feasibility of systemic NIS gene transfer using a dendrimer-coated replication-selective adenovirus. To further improve safety, tumor selectivity, and therapeutic efficacy of the dendrimer-coated adenovirus vector, in the current study we added another level of tumor specificity by combining the two approaches through attachment of the EGFR-specific peptide GE11 to the virus coating polymer. Thereby NIS transgene expression is not only detargeted from the liver after systemic virus administration and passively accumulated in the tumor by the enhanced permeability and retention effect,19 but also actively targeted to the EGFR-expressing tumor cells.
Based on the dual function of the NIS gene encoded by our adenovirus as reporter and therapy gene, at first we investigated its potential for noninvasive imaging of vector biodistribution and transgene expression of our targeted and shielded adenovirus by 2D 123I-scintigraphy as well as 3D high resolution 124I-PET-imaging. Furthermore, the potential of further stimulation of therapeutic efficacy of adenovirus-mediated oncolysis was investigated by subsequent combination with systemic NIS-mediated radiotherapy (radiovirotherapy).
Results
Influence of EGFR-targeted adenoviral surface modification in vitro
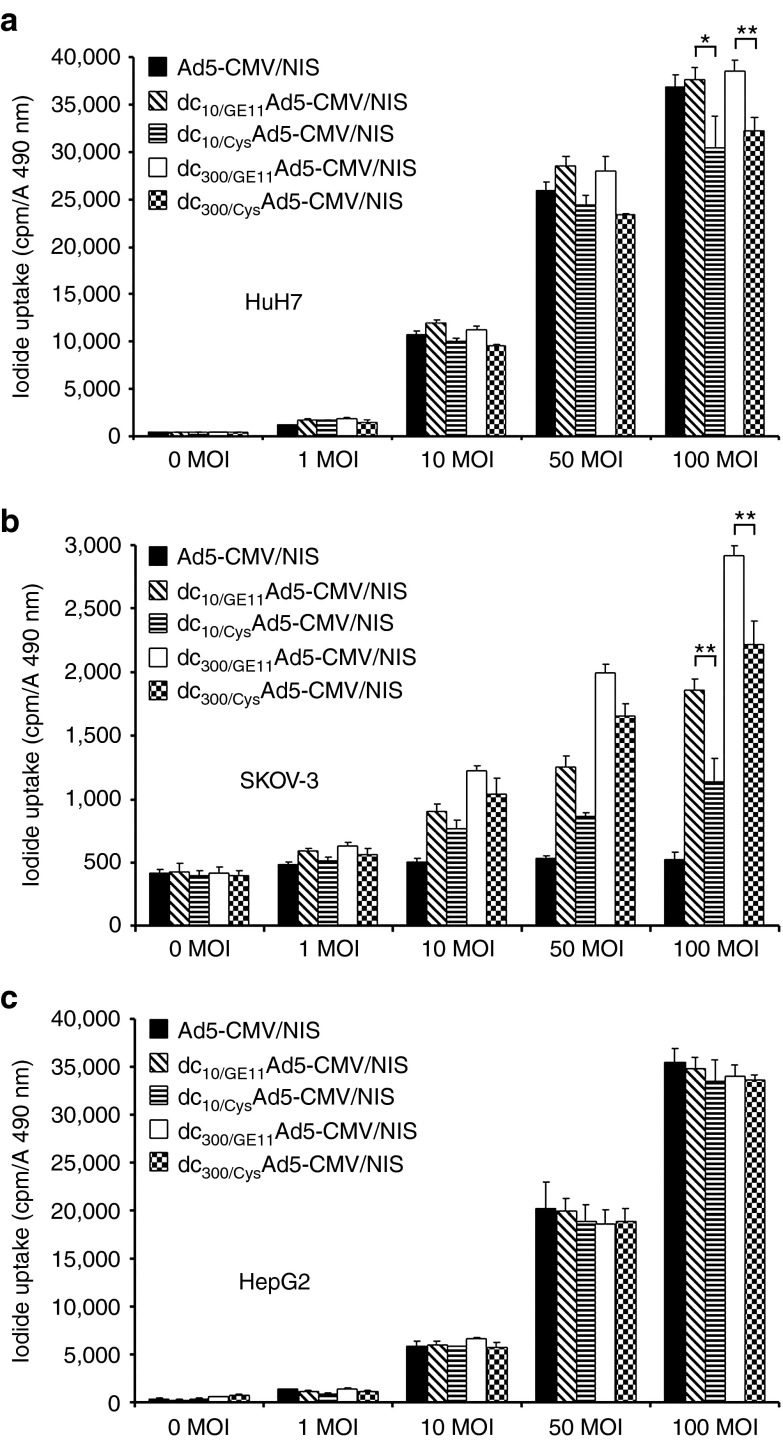
Three cell lines with different levels of CAR and EGFR expression (HuH7: high CAR, high EGFR; SKOV-3: CAR-negative, high EGFR; HepG2: High CAR, low EGFR) as determined by flow cytometry (data not shown) were used. After infection with uncoated Ad5-CMV/NIS the CAR-positive cell lines HuH7 and HepG2 showed a dose-dependent increase in perchlorate-sensitive 125I uptake activity of up to 80-fold, which was fully retained after EGFR-targeted coating of the adenovirus with increasing amounts of dendrimer (Figure 1a,c). The CAR-negative cell line SKOV-3 showed no iodide accumulation above background level, even when incubated with high multiplicity of infection of the uncoated Ad5-CMV/NIS. Adenovirus coating with increasing amounts of EGFR-targeted dendrimer led to an increase in perchlorate-sensitive iodide uptake activity of up to six orders of magnitude (Figure 1b), thereby indicating CAR-independent uptake mechanisms of dendrimer-coated adenovirus. Replacement of the targeting ligand GE11 by a cysteine residue (Cys) significantly lowered transduction efficiency in EGFR-positive HuH7 and SKOV-3 cells (Figure 1a,b) whereas transduction efficiency in the low EGFR-expressing HepG2 cells remained unchanged (Figure 1c), thereby demonstrating targeting specificity and increased transduction efficiency by the use of the EGFR-specific targeting ligand. Viral NIS gene transfer using uncoated or dendrimer-coated Ad5-CMV/NIS did not alter cell viability as measured by 3-(4,5-dimethylthiazol)-2-yl-5-(3-carboxymeth-oxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay (data not shown).
Figure 1.
In vitro iodide uptake studies of EGFR-targeted adenovirus. In vitro transduction experiments with uncoated Ad5-CMV/NIS showed dose-dependent transduction efficiency in CAR-positive cells (HuH7, HepG2), which was fully retained after EGFR-targeted coating of the adenovirus with increasing amounts of dendrimer (a, c). The CAR-negative cell line SKOV-3 showed no iodide accumulation above background level, even when incubated with high MOI of the uncoated Ad5-CMV/NIS but adenoviral coating with increasing amounts of EGFR-targeted dendrimer caused a significant increase in perchlorate-sensitive iodide uptake activity (b; **P < 0.01). Replacement of the targeting ligand GE11 by a cysteine residue (Cys) lowered transduction efficiency in EGFR-positive HuH7 and SKOV-3 cells (a: *P < 0.05, **P < 0.01; b: **P < 0.01) whereas transfection efficiency in the low EGFR expressing HepG2 cells remained unchanged (c). CAR, coxsackie-adenovirus receptor; EGFR, epidermal growth factor receptor; MOI, multiplicity of infection; NIS, sodium iodide symporter.
Noninvasive imaging of EGFR-targeted NIS gene delivery
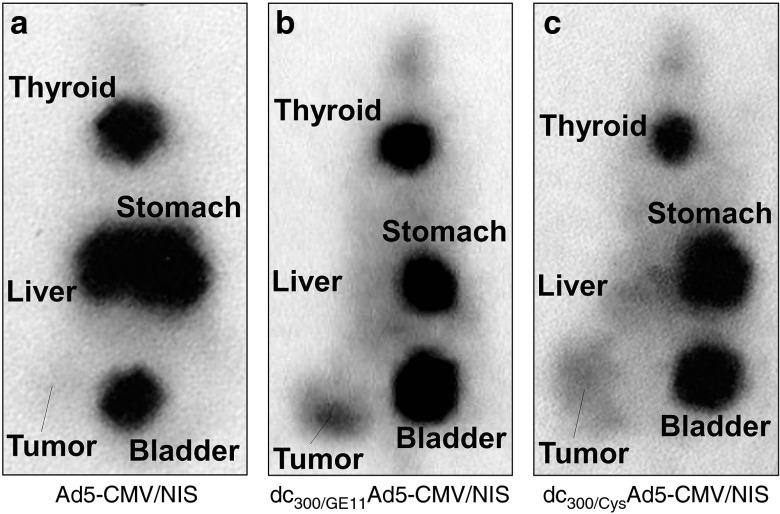
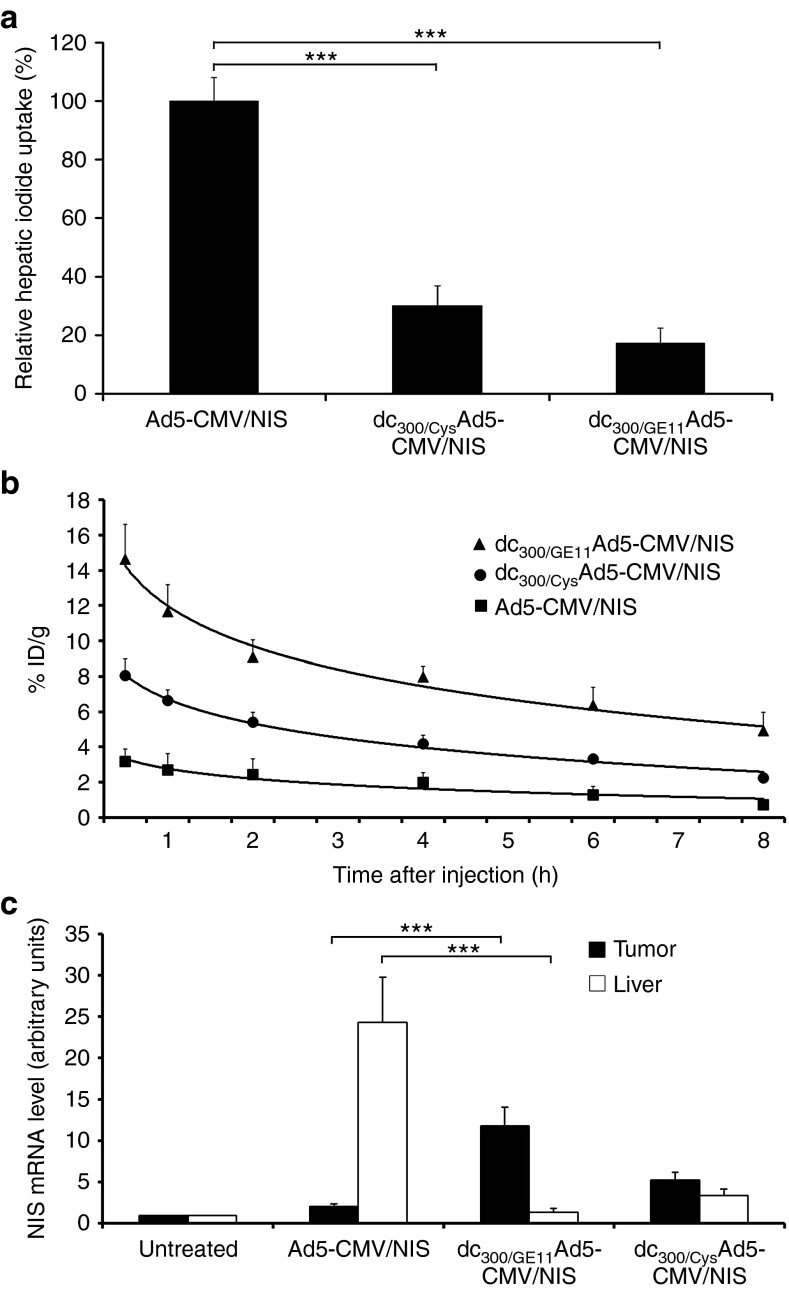
Mice bearing high EGFR-expressing HuH7 xenograft tumors were imaged after intravenous vector administration for functional NIS expression via whole body 123I-scintigraphy. In vivo imaging of vector biodistribution demonstrated high levels of NIS-mediated radionuclide accumulation in the livers of mice after systemic injection of uncoated Ad5-CMV/NIS due to hepatic sequestration of the vector. Because of hepatic vector trapping only very low 123I accumulation above background level was observed in peripheral xenograft tumors (Figure 2a). By coating of Ad5-CMV/NIS with EGFR-targeted PAMAM-G2-PEG-GE11 (dc300/GE11Ad5-CMV/NIS) before systemic administration liver transduction was strongly reduced by over 80% (Figures 2b,3a) resulting in significantly increased transduction efficiency of xenograft tumors. Serial scanning of mice revealed an accumulated dose of ~15% of the injected dose per gram tumor tissue (%ID/g) with an average biological half-life of 4.5 hours (Figures 2b,3b), resulting in a calculated tumor-absorbed dose of 103 mGy/MBq for 131I. Replacement of the dendrimer-coupled targeting ligand by a cysteine residue (dc300/CysAd5-CMV/NIS) still prevented liver pooling of the vector but significantly reduced tumor-specific radionuclide accumulation nearly by half (Figures 2c, 3a,b). In addition to 123I uptake in liver and tumor, radioiodine accumulation was also observed in stomach and thyroid that physiologically express NIS and in the urinary bladder due to radionuclide elimination but in no case in other nontarget organs (Figure 2a–c). This physiologic uptake due to endogenous NIS expression or renal radionuclide elimination was confirmed by imaging of untreated control mice that showed the same uptake in thyroid, stomach, and bladder, as mice treated with NIS-expressing adenovirus vectors (Supplementary Figure 1a, online). Furthermore, pretreatment with the competitive NIS-inhibitor perchlorate (NaClO4) demonstrated that the uptake observed in thyroid and stomach was indeed NIS-mediated (Supplementary Figure 1b, online). Ex vivo analysis of NIS mRNA expression in livers, tumors, and other organs (kidney, spleen, lungs) correlated well with the 123I scintigraphy data and showed no NIS mRNA expression above background level in nontarget organs (Figure 3c, Supplementary Figure 2, online).
Figure 2.
In vivo iodide uptake studies of EGFR-targeted adenovirus. 123I-scintigraphy of mice bearing high EGFR-expressing HuH7 xenografts demonstrated high hepatic and low tumoral NIS-mediated radionuclide accumulation after systemic injection of uncoated Ad5-CMV/NIS (a). Coating of Ad5-CMV/NIS with EGFR-targeted PAMAM-G2-PEG-GE11 (dc300/GE11Ad5-CMV/NIS) before systemic administration strongly reduced liver transduction resulting in significantly increased transduction efficiency of xenograft tumors (b). Replacement of the dendrimer-coupled targeting ligand by a cysteine residue (dc300/CysAd5-CMV/NIS) still prevented liver pooling of the vector but significantly reduced tumor-specific radionuclide accumulation (c). EGFR, epidermal growth factor receptor; NIS, sodium iodide symporter.
Figure 3.
Biodistribution of NIS transgene expression. Quantification of hepatic transgene expression revealed over 80% reduction after intravenous injection of dendrimer-coated dc300/GE11Ad5-CMV/NIS as compared with injection of uncoated Ad5-CMV/NIS (a: ***P < 0.001). Detargeting of hepatic transgene expression resulted in significantly increased transduction efficiency of xenograft tumors (b). Replacement of the dendrimer-coupled targeting ligand by a cysteine residue (dc300/CysAd5-CMV/NIS) still prevented liver pooling of the vector but reduced tumor-specific radionuclide accumulation nearly by half (a: ***P < 0.001, b). Ex vivo analysis of NIS mRNA expression in livers and tumors correlated well with the observed radionuclide biodistribution and confirmed the findings of 123I scintigraphy (c: ***P < 0.001). NIS, sodium iodide symporter.
Influence of vector modification on hepatotoxicity
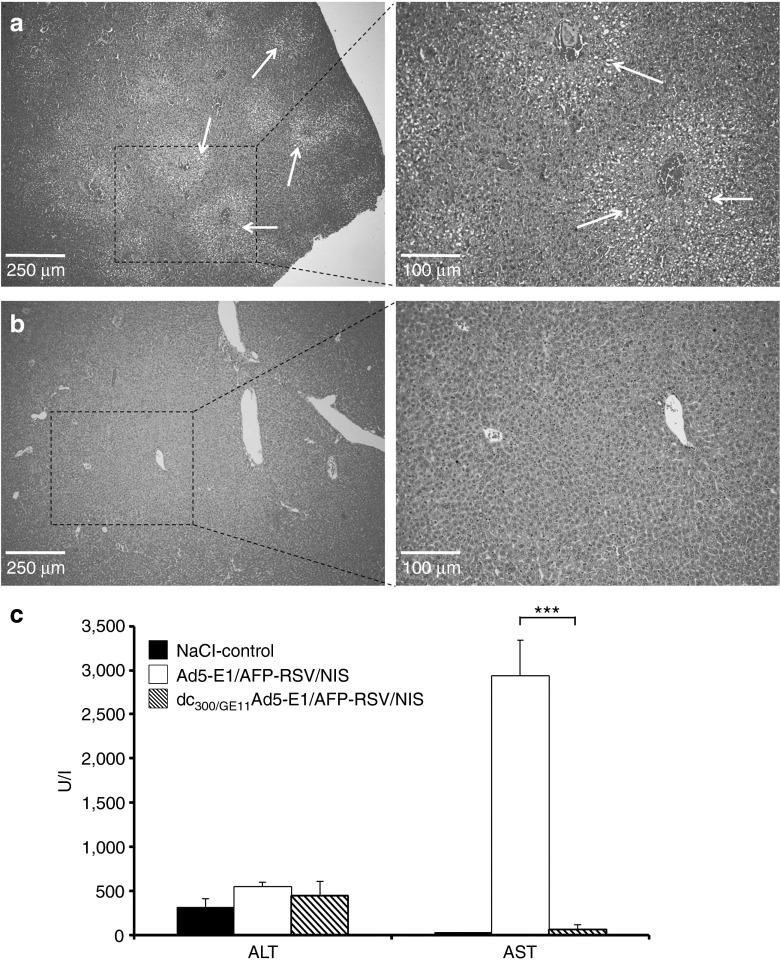
Assessment of hepatotoxicity after intravenous injection of uncoated Ad5-E1/AFP-RSV/NIS demonstrated a small 1.75-fold increase in alanine aminotransferase and a strong 128-fold increase in aspartate aminotransferase (Figure 4c) as well as a significant increase in fatty degeneration of liver tissue (Figure 4a). Coating of the adenovirus before intravenous injection abrogated hepatotoxic effects almost completely as seen by a reduction of increase in alanine aminotransferase by half (45.6%) and in aspartate aminotransferase by 98.6 % (Figure 4c) as well as liver histology without pathological findings (Figure 4b).
Figure 4.
Analysis of liver toxicity. H/E staining of liver sections of mice injected intravenously with Ad5-E1/AFP-RSV/NIS showed fatty degeneration of liver tissue (a), which was not observed in livers of mice treated with dc300/GE11Ad5-E1/AFP-RSV/NIS (b). Injection of Ad5-E1/AFP-RSV/NIS without surface modification led to a minor increase in ALT level and a strong increase in AST level as compared with mice treated with saline only, which was mostly avoided by coating of the adenovirus before systemic administration (c: ***P < 0.01). AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NIS, sodium iodide symporter.
EGFR-specificity of vector targeting
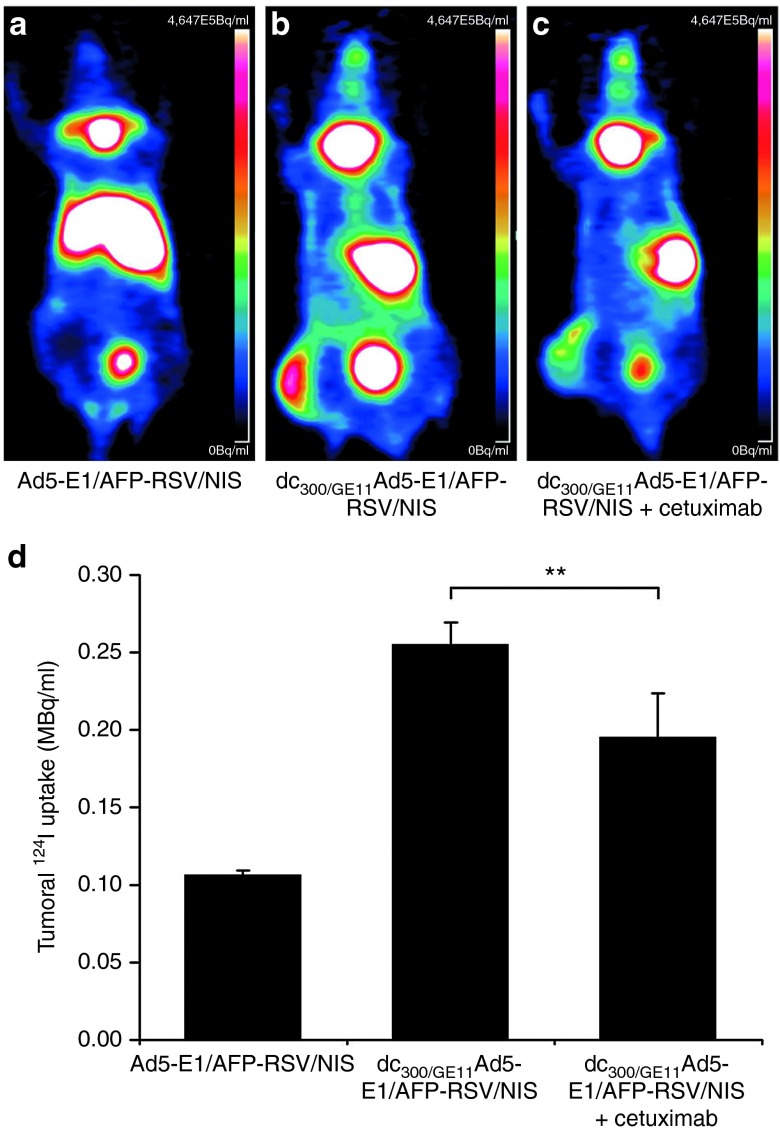
3-dimensional high resolution 124I-PET-imaging was used to investigate EGFR-specificity of NIS gene delivery after intravenous injection of the targeted replication-selective dc300/GE11Ad5-E1/AFP-RSV/NIS. As shown before by 123I-scintigraphy, i.v. injection of the uncoated vector (Ad5-E1/AFP-RSV/NIS) resulted in strong transduction of liver tissue that resulted in poor tumoral transduction (Figure 5a,d). In contrast, coating of the adenovirus with PAMAM-G2-PEG-GE11 resulted in prevention of hepatic radioiodine accumulation and significantly enhanced transduction of tumor xenografts (Figure 5b,d). By pretreatment of mice with the monoclonal anti-EGFR antibody cetuximab before systemic dc300/GE11Ad5-E1/AFP-RSV/NIS administration tumoral radioiodine accumulation was significantly reduced while liver detargeting of NIS expression was not affected, thereby confirming EGFR-specificity of the targeted vector (Figure 5c,d).
Figure 5.
In vivo analysis of EFGR-specificity. 124I-PET-imaging demonstrated strong hepatic transduction after i.v. injection of the uncoated vector (Ad5-E1/AFP-RSV/NIS) (a) and quantification of radioiodine accumulation revealed only poor tumoral transduction (a, d). In contrast, coating of the adenovirus with PAMAM-G2-PEG-GE11 (dc300/GE11Ad5-E1/AFP-RSV/NIS) before systemic injection resulted in prevention of hepatic radioiodine accumulation and distinct transduction of tumor xenografts (b, d). By pretreatment of mice with the monoclonal anti-EGFR antibody cetuximab before systemic dc300/GE11Ad5-E1/AFP-RSV/NIS administration tumoral radioiodine accumulation was significantly reduced while liver detargeting of NIS expression was still effective (c, d; **P < 0.01). AFP, α-fetoprotein; EGFR, epidermal growth factor receptor; NIS, sodium iodide symporter.
Radionuclide therapy study in vivo
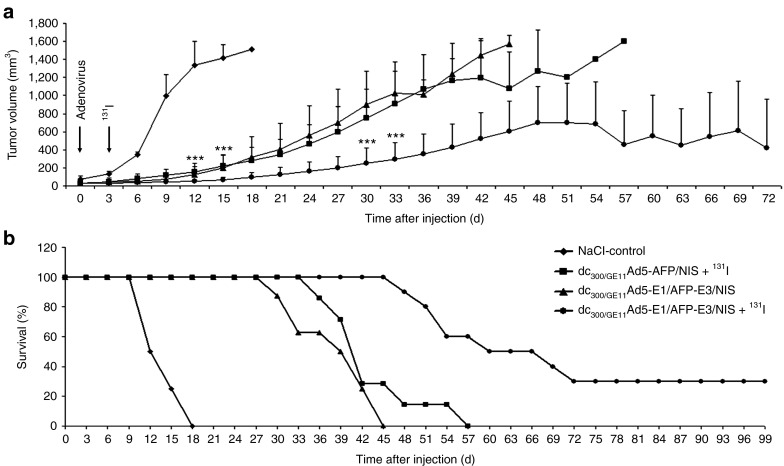
A single i.v. injection of the replication-deficient Ad5-AFP/NIS coated with PAMAM-G2-PEG-GE11 followed by a therapeutic dose of 131I (dc300/GE11Ad5-AFP/NIS + 131I, radiotherapy) showed a significant delay in tumor growth and improved survival (Figure 6a,b) as compared with mice treated with saline only (NaCl-control, Figure 6a,b). I.v. application of the oncolytic replication-selective Ad5-E1/AFP-E3/NIS with EGFR-targeted surface modification (dc300/GE11Ad5-E1/AFP-E3/NIS, virotherapy) revealed a comparable delay in tumor growth and enhancement of survival due to the oncolytic activity of the adenovirus (Figure 6a,b). Combined radiovirotherapy by i.v. injection of PAMAM-G2-PEG-GE11-coated replication-selective Ad5-E1/AFP-E3/NIS followed by application of 131I (dc300/GE11Ad5-E1/AFP-E3/NIS + 131I, radiovirotherapy) resulted in a strongly enhanced therapeutic effect, as seen by significantly delayed tumor growth and further improvement of survival (Figure 6a,b). While mice treated with saline only (NaCl-control) had to be killed within 2–3 weeks after onset of the experiment due to excessive tumor growth, 50% of mice treated with combined radiovirotherapy survived at least 9 weeks and 30% were even still alive at day 100, the endpoint of the observation period (Figure 6b). None of the treated mice, even with combined radiovirotherapy, showed major adverse effects in terms of lethargy or respiratory failure due to oncolytic virotherapy or radionuclide treatment.
Figure 6.
In vivo therapeutic efficacy. A single i.v. injection of the replication-deficient Ad5-AFP/NIS coated with PAMAM-G2-PEG-GE11 followed by a therapeutic dose of 131I (dc300/GE11Ad5-AFP/NIS + 131I, radiotherapy) showed a significant delay in tumor growth and improved survival (a, b; ***P < 0.01) as compared with mice treated with saline only (NaCl-control, a, b). I.v. application of the oncolytic replication-selective Ad5-E1/AFP-E3/NIS with EGFR-targeted surface modification (dc300/GE11Ad5-E1/AFP-E3/NIS, virotherapy) revealed a comparable delay in tumor growth and enhancement of survival (a, b). Combined radiovirotherapy treatment (dc300/GE11Ad5-E1/AFP-E3/NIS + 131I, radiovirotherapy) resulted in a strongly enhanced therapeutic effect, as seen by significantly delayed tumor growth and further improved survival (a, b; ***P < 0.01). AFP, α-fetoprotein; EGFR, epidermal growth factor receptor; NIS, sodium iodide symporter.
Discussion
The tropism of adenoviruses is greatly influenced by interaction with several blood components and the widespread expression of the CAR.6,20,21,22 Aside from the inherent hepatic tropism after intravenous injection,23 the CAR-dependent way of infection results in a broad cellular tropism with no intrinsic cancer specificity of wild-type adenovirus.24 On the other hand, therapeutic efficacy of an adenovirus can be diminished by the lack of CAR on the tumor cell surface.25 Hence, a variety of methods have been developed, to alter the natural virus tropism and to detarget the vector away from its natural receptors. Through surface modification by chemical or genetical engineering, the adenovirus can be retargeted to cancer cell-specific targets in order to achieve sufficient transgene expression in cancerous tissues while expression in nontarget organs and toxic side effects are minimized.26 In recent years, several studies provided evidence that adenoviruses can enter cells via cell surface molecules that are not natural viral receptors, e.g., the EGFR.27 Cancer specificity of the ligand chosen for retargeting purposes is of great importance since unspecific infection of nontarget cells can significantly reduce the availability of the therapeutic vector24 as well as infection of nontarget tissues may result in increased toxicity,28 in particular regarding NIS-mediated radiotherapy. Moreover, targeted delivery of the NIS gene potentially allows for direct radiation treatment of tumors on-site and owns the advantage of achieving high radiation doses in the tumor while minimizing side effects to normal tissue. Although NIS as normal human protein is also endogenously expressed, in particular in the thyroid, the patient's thyroid gland can be protected by pretreatment with thyroid hormone L-T4 (levothyroxine), which effectively downregulates thyroidal NIS expression. In fact, NIS-mediated radiotherapy is well known to be remarkably safe in humans and has been routinely used as standard therapy in the management of thyroid cancer patients for over 70 years.17 Moreover, NIS as normal human gene and protein causes no toxicity or diminished efficacy by immune responses as it is often observed after the use of other protein and gene therapeutics.6
As a consequence of our recent characterization of a high-affinity, EGFR-selective peptide (GE11) coupled to synthetic nanoparticles for systemic nonviral NIS gene delivery,14 in this study we combined dendrimeric adenovirus surface modification with the EGFR-targeting strategy in order to generate a shielded, targeted, and armed adenovirus for systemic radiovirotherapy of high EGFR-expressing hepatocellular carcinoma. In this way a triple cancer-specific adenovirus was developed that is transcriptionally targeted to hepatocellular carcinoma by the use of the α-fetoprotein (AFP) promoter29 to control replication and NIS expression, that is actively targeted to the EGFR through attachment of the GE11 peptide14 and passively targeted to leaky tumor vasculature through the enhanced permeability and retention effect.30 Here, we show the biodistribution and the retargeting capacity of adenovirus vectors coated with EGFR-specific dendrimer in vitro and in vivo using NIS in its dual function as reporter and therapy gene for noninvasive imaging of transgene expression and calculated radiotherapeutic treatment of hepatocellular carcinoma.
In vitro experiments using the EGFR-targeted adenovirus proved that transduction efficiency in CAR-positive cancer cells is barely hampered after dendrimer coating with mild but nonsignificant improvement if the cells additionally express the EGFR. In contrast, the CAR-negative ovarian cancer cells SKOV-3, that were shown to be refractory to infection with uncoated adenovirus, can be efficiently infected by dendrimer-coated adenoviral vectors with a significant additional increase in transduction efficiency by attachment of the targeting ligand GE11. These experiments suggest that adenovirus vectors coated with targeted dendrimer can transduce cells CAR-independently by employing a different receptor for cell entry and may be of great potential for therapy of EGFR-expressing neoplasms lacking CAR.
In the current study, 123I scintigraphy after intravenous administration of the dendrimer-coated adenovirus revealed strong detargeting of hepatic transgene expression that is usually caused by i.v. administration of the uncoated vector. The reduction in hepatic NIS-mediated iodine accumulation is even stronger than observed in our former study1 (80 vs. 70 %), which might be due to improved covering of the adenoviral surface epitopes by the smaller dendrimer used for surface modification (molecular weight PAMAM-G2 3,284 Da vs. PAMAM-G5 28,854 Da). By improved liver detargeting the vector was able to infect peripheral hepatoma xenografts of mice upon systemic delivery even more efficiently than the previous vector (15 vs. 13 %ID/g) with an increased average biological half-life and tumor absorbed dose calculated for therapeutic 131I (103 vs. 91 mGy/MBq). This may be explained by active GE11-mediated tumor targeting combined with an extended blood circulation time of the vector enforcing the passive tumor targeting through the enhanced permeability and retention effect as it was shown before by Yao et al.8 Substitution of the targeting ligand by a cysteine residue led to a significant decrease of tumoral transgene expression, thereby confirming the targeting benefit. These results were further corroborated by ex vivo analysis of NIS mRNA expression that confirmed in vivo biodistribution imaging data and did not show NIS expression in nontarget organs. To ensure that the targeting ligand was indeed targeting the EGFR, we pretreated mice bearing high EGFR-expressing HuH7 xenografts with the high affinity anti-EGFR antibody cetuximab before infection with the targeted adenovirus. Pretreatment with cetuximab lowered the tumoral transduction efficiency of dc300/GE11Ad5-E1/AFP-RSV/NIS significantly as shown by high resolution 124I-PET-imaging, while hepatic detargeting was not affected and remained stable.
Despite these impressive data convincingly demonstrating effective liver detargeting and tumor retargeting of adenovirus-mediated NIS transgene expression by EGFR-targeted dendrimer coating, we have to be aware of the limitations of mice as assay system for safety evaluations due to restricted CAR expression in murine tissues31,32,33 and severely restricted viral replication of adenovirus serotype 5-based vectors in mice.34 As outlined above, our dendrimer-coated adenovirus is still able to use CAR for cell entry and therefore bears the potential to infect tissues other than EGFR-positive tumors, which cannot be fully evaluated in a human xenograft mouse model, which, however, serves well as proof-of-principle model to evaluate the feasibility of systemic application of dendrimer-coated adenovirus.
For reliable quantitative analysis of such experiments, highly sensitive imaging modalities are needed to display even small changes in biodistribution that, however, may have great biological impact. Recently, [18F]-tetrafluoroborate, a known alternative substrate of NIS, was evaluated as new PET-imaging agent in preclinical models, demonstrating high sensitivity and significantly improved resolution as compared with 124I, which will improve NIS biodistribution analysis in orthotopic tumor models with overlap in radioiodine accumulation with organs endogenously expressing NIS.35,36
Wild-type adenovirus is initially recognized by the scavenger receptors of Kupffer cells due to its negative surface charge leading to rapid clearance from the bloodstream as well as distinct liver pathology37 as seen in the current study by strong liver transduction, increased liver transaminase levels, as well as pathologic liver histology. In contrast, the electrostatically coated adenovirus complex is no longer negatively charged as described by Vetter et al.2 and the avoidance of liver toxicity observed in our study may be explained by preventing activation of Kupffer cells and the induction of proinflammatory processes.
One approach to improve viral oncolytic therapy is its combination with standard anticancer therapies such as radiotherapy38 as we and others have shown before.1,29,39 Thus, in the next step we addressed the question whether the advantage in tumoral transduction efficiency is able to also improve therapeutic radionuclide application in liver cancer xenografts, after it had previously been demonstrated after local administration of a NIS-expressing adenovirus that the outcome of combined radiovirotherapy is highly dependent upon the viral dose that is delivered to the tumor40 possibly based on increased tumoral transduction subsequently leading to decelerated radioiodine efflux. In the current study, NIS-mediated radiotherapy using a replication-deficient dendrimer-coated adenovirus showed a significant delay in tumor growth that was associated with markedly improved survival as compared with control mice treated with saline only. A comparable delay in tumor growth has been reached by injection of the oncolytic replication-selective dc300/GE11Ad5-E1/AFP-E3/NIS. Furthermore, the effects of oncolysis and radiation therapy have been used synergistically and tumor-specific oncolysis was shown to be further enhanced by combination with NIS-mediated radiotherapy. Potent and selective systemic anti-tumoral efficacy was demonstrated. Radiovirotherapy using PAMAM-G2-PEG-GE11 instead of PAMAM-G51 for coating of our replication-selective Ad5-E1/AFP-E3/NIS demonstrated further deceleration of tumor growth as well as improved survival of mice. The synergistic therapeutic effectiveness of the combination therapy may also allow a reduction of the doses usually applied in individual single therapies and thereby reduce potential toxic side effects as it was shown before.41 Moreover, Trujillo et al.42 recently showed after intratumoral application of a replication-selective adenovirus carrying the NIS gene that a minimal applied dose of 37 MBq 131I is required for radiovirotherapy in a murine xenograft model in order to improve efficacy of oncolytic virotherapy alone and that the doses needed to achieve reduced tumor growth and extended survival in mice are scaled well within doses currently clinically used for the treatment of thyroid cancer patients. With regards to potential clinical application of adenovirus-based NIS gene therapy it is noticeable that at the Mayo Clinic (Rochester, MN, USA) the first NIS-expressing adenovirus is currently Food and Drug Administration approved for a human clinical trial in patients with locally recurrent prostate cancer.43
In conclusion we developed a new adenovirus-based vector by EGFR-targeted dendrimeric surface modification that retained the superior characteristics of dendrimer coating described in our former study1 and additionally improved its biodistribution and selective transduction efficiency of peripheral tumor tissues upon systemic vector administration by EGFR-specific targeting. The ability of the coated vector to improve NIS gene delivery to EGFR-expressing tumor cells, combined with its reduced hepatic tropism and toxicity profile, which warrants further investigation in more advanced tumor models, highlights its potential as a prototype virus for future clinical investigation.
Materials and methods
Cell culture. The human hepatocellular cell line HuH7 (JCRB 0403), the human ovarian carcinoma cell line SKOV-3 (ATCC, HTB-77), and the human hepatocellular cell line HepG2 (ATCC, HB-8065) were cultured as described previously.2 Flow cytometry analysis of EGFR levels was carried out as described previously.2
Recombinant adenovirus production and coating with EGFR-specific dendrimer. The replication-deficient adenovirus Ad5-CMV/NIS carrying the hNIS gene under the control of the unspecific cytomegalovirus promoter44 and the replication-selective adenovirus Ad5-E1/AFP-E3/NIS were generated as described previously. In Ad5-E1/AFP-E3/NIS replication is controlled by cloning the E1A region under control of the liver cancer-specific mouse AFP promoter and the hNIS gene is inserted in the E3 region under control of the replication-dependent E3 promoter.29 As a control, a replication-deficient adenovirus carrying the hNIS gene under the control of the AFP promoter Ad5-AFP/NIS was used as described previously.45 The replication-selective human recombinant type 5 adenovirus Ad5-E1/AFP-RSV/NIS (1.1 × 1012 particles = 1.0 × 1010 plaque forming units) replicating under control of the mouse AFP promoter and expressing the human NIS gene under control of the unspecific RSV promoter was also developed by ViraQuest (North Liberty, IA, USA).
Synthesis of dendrimers PAMAM-G2-PEG-GE11, PAMAM-G2-PEG-Cys,2 and adenoviral surface modification1 were carried out as described previously. Dendrimer coating of the virus with 10 ng PAMAM-G2-PEG-GE11 is indicated in writing by the prefix dc10/GE11, with 300 ng PAMAM-G2-PEG-GE11 by the prefix dc300/GE11, and with 300 ng PAMAM-G2-PEG-Cys by the prefix dc300/Cys.
Adenovirus-mediated NIS gene delivery in vitro. In vitro infection, iodide uptake experiments, and measurement of cell viability were carried out as described previously.1
In vivo NIS gene transfer and radioiodine biodistribution studies. Establishment of HuH7 xenografts29 and in vivo NIS gene transfer1 were carried out as described previously. The experimental protocol was approved by the regional governmental commission for animals (Regierung von Oberbayern, Munich, Germany).
Four days after systemic adenovirus injection mice received 18.5 MBq 123I intraperitoneally (i.p.) and radioiodine biodistribution was monitored by serial gamma camera imaging as described previously.46 A subset of control mice has additionally been pretreated with the competitive NIS-inhibitor perchlorate (NaClO4). Quantification of regions of interest and dosimetric calculations were carried out as described previously.1
Three days after systemic injection of Ad5-E1/AFP-RSV/NIS or dc300/GE11Ad5-E1/AFP-RSV/NIS mice received 10 MBq 124I i.p. and radioiodine biodistribution was monitored by a 15-minute-static acquisition, 3 hours postinjection using a micro PET system (Inveon, SIEMENS Preclinical Solutions, Erlangen, Germany). A subset of mice was pretreated i.p. with 0.25 mg of the EGFR-specific antibody cetuximab (Erbitux; Merck, Darmstadt, Germany) 24 hours before adenovirus administration. Mean tumoral radioiodine uptake was calculated in megabequerel per milliliter (MBq/ml) by manually placing 3D regions of interest in the tumor.
Ex vivo analysis. NIS mRNA expression levels of livers, tumors, and nontarget organs (kidney, spleen, lungs) were analyzed ex vivo via quantitative real-time PCR as described previously.14 For analysis of hepatotoxicity, uncoated Ad5-E1/AFP-RSV/NIS or dendrimer-coated dc300/GE11Ad5-E1/AFP-RSV/NIS were injected i.v. and 3 days thereafter, mice were killed and blood serum samples were collected to assess alanine aminotransferase and aspartate aminotransferase levels (measured at the Department of Clinical Biochemistry and Pathobiochemistry, Klinikum rechts der Isar, Munich, Germany). Subsequently, liver tissues were harvested and embedded in paraffin for hematoxylin and eosin (H/E) staining.
Radiovirotherapy study in vivo. HuH7 xenografts were established in four groups of mice. The first group was used as control and injected i.v. with saline only (NaCl-control, n = 8). A second group received a single i.v. injection of 1 × 109 plaque forming units of the dendrimer-coated replication-selective dc300/GE11Ad5-E1/AFP-E3/NIS (dc300/GE11Ad5-E1/AFP-E3/NIS, virotherapy, n = 8). The third group received a single i.v. injection of 1 × 109 plaque forming units of the dendrimer-coated replication-deficient dc300/GE11Ad5-AFP/NIS and 3 days later a single i.p. dose of 55.5 MBq 131I (dc300/GE11Ad5-AFP/NIS + 131I, radiotherapy, n = 7). The fourth group received a single i.v. injection of 1 × 109 plaque forming units of the dendrimer-coated replication-selective dc300/GE11Ad5-E1/AFP-E3/NIS and 3 days later a single i.p. dose of 55.5 MBq 131I (dc300/GE11Ad5-E1/AFP-E3/NIS + 131I, radiovirotherapy, n = 10). Tumor measurements were performed twice weekly thereafter. Tumor volume was estimated using the equation: tumor volume = length × width × height × 0.52. Mice were followed for a total of 100 days or until tumor burden was such that animals had to be killed (≥1500 mm3).
Statistical methods. All in vitro experiments were carried out in triplicates. Results are represented as means ± SD of triplicates. Statistical significance of in vitro and in vivo experiments was tested using Student's t-test (two-tailed; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
SUPPLEMENTARY MATERIAL Figure S1. Radioiodine biodistribution in untreated control mice. Figure S2. NIS mRNA expression in non-target organs.
Acknowledgments
The authors are grateful to Sissy M. Jhiang (Ohio State University, Columbus, OH, USA) for supplying the full-length human NIS cDNA and to Richard D. Anderson (Viraquest, North Liberty, IA, USA) for the synthesis of the adenovirus vectors. We thank Florian Kreppel (University of Ulm, Ulm, Germany) for helpful discussions and providing the SKOV-3 cells; as well as Peter B. Luppa (TU Munich, Munich, Germany) for blood serum analysis. We also thank Sybille Reder (TU Munich, Munich, Germany) for assistance with PET-imaging studies, Wolfgang Rödl (Ludwig-Maximilians University, Munich, Germany) for conjugate synthesis as well as Doris Mayr for liver histology analysis. This study was supported by a grant from the Deutsche Forschungsgemeinschaft SFB 824 to C.S. and M.O., a grant from the Wilhelm-Sander-Stiftung (2008.037.1) and a grant from the Deutsche Forschungsgemeinschaft within the SPP 1629 (SP 581/6-1) to C.S., a grant from the Center for Nanoscience (CeNS) to M.O. as well as the excellence cluster Nanosystems Initiative Munich to E.W. The authors declare no conflict of interest.
Supplementary Material
Radioiodine biodistribution in untreated control mice.
NIS mRNA expression in non-target organs.
References
- Grünwald GK, Vetter A, Klutz K, Willhauck MJ, Schwenk N, Senekowitsch-Schmidtke R, et al. Systemic image-guided liver cancer radiovirotherapy using dendrimer-coated adenovirus encoding the sodium iodide symporter as theranostic gene. J Nucl Med. 2013;54:1450–1457. doi: 10.2967/jnumed.112.115493. [DOI] [PubMed] [Google Scholar]
- Vetter A, Virdi KS, Espenlaub S, Rödl W, Wagner E, Holm PS, et al. Adenoviral vectors coated with PAMAM dendrimer conjugates allow CAR independent virus uptake and targeting to the EGF receptor. Mol Pharm. 2013;10:606–618. doi: 10.1021/mp300366f. [DOI] [PubMed] [Google Scholar]
- Choi JW, Lee JS, Kim SW, Yun CO. Evolution of oncolytic adenovirus for cancer treatment. Adv Drug Deliv Rev. 2012;64:720–729. doi: 10.1016/j.addr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Kim PH, Sohn JH, Choi JW, Jung Y, Kim SW, Haam S, et al. Active targeting and safety profile of PEG-modified adenovirus conjugated with herceptin. Biomaterials. 2011;32:2314–2326. doi: 10.1016/j.biomaterials.2010.10.031. [DOI] [PubMed] [Google Scholar]
- Campos SK, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Curr Gene Ther. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MR, Parker AL, Bradshaw AC, Baker AH. Manipulation of adenovirus interactions with host factors for gene therapy applications. Nanomedicine (Lond) 2012;7:271–288. doi: 10.2217/nnm.11.186. [DOI] [PubMed] [Google Scholar]
- Rojas JJ, Gimenez-Alejandre M, Gil-Hoyos R, Cascallo M, Alemany R. Improved systemic antitumor therapy with oncolytic adenoviruses by replacing the fiber shaft HSG-binding domain with RGD. Gene Ther. 2012;19:453–457. doi: 10.1038/gt.2011.106. [DOI] [PubMed] [Google Scholar]
- Yao X, Yoshioka Y, Morishige T, Eto Y, Narimatsu S, Kawai Y, et al. Tumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapy. Mol Ther. 2011;19:1619–1625. doi: 10.1038/mt.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao XL, Nakagawa S, Gao JQ. Current targeting strategies for adenovirus vectors in cancer gene therapy. Curr Cancer Drug Targets. 2011;11:810–825. doi: 10.2174/156800911796798896. [DOI] [PubMed] [Google Scholar]
- Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11:689–708. doi: 10.1677/erc.1.00600. [DOI] [PubMed] [Google Scholar]
- de Bruin K, Ruthardt N, von Gersdorff K, Bausinger R, Wagner E, Ogris M, et al. Cellular dynamics of EGF receptor-targeted synthetic viruses. Mol Ther. 2007;15:1297–1305. doi: 10.1038/sj.mt.6300176. [DOI] [PubMed] [Google Scholar]
- Harvey TJ, Burdon D, Steele L, Ingram N, Hall GD, Selby PJ, et al. Retargeted adenoviral cancer gene therapy for tumour cells overexpressing epidermal growth factor receptor or urokinase-type plasminogen activator receptor. Gene Ther. 2010;17:1000–1010. doi: 10.1038/gt.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Abei M, Fukuda K, Nakamura K, Murata T, Wakayama M, et al. EpCAM- and EGFR-targeted selective gene therapy for biliary cancers using Z33-fiber-modified adenovirus. Int J Cancer. 2011;129:1244–1253. doi: 10.1002/ijc.25758. [DOI] [PubMed] [Google Scholar]
- Klutz K, Schaffert D, Willhauck MJ, Grünwald GK, Haase R, Wunderlich N, et al. Epidermal growth factor receptor-targeted (131)I-therapy of liver cancer following systemic delivery of the sodium iodide symporter gene. Mol Ther. 2011;19:676–685. doi: 10.1038/mt.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhao R, Wu X, Sun Y, Yao M, Li J, et al. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J. 2005;19:1978–1985. doi: 10.1096/fj.05-4058com. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Morris JC. The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol (Oxf) 2002;57:559–574. doi: 10.1046/j.1365-2265.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- Hingorani M, Spitzweg C, Vassaux G, Newbold K, Melcher A, Pandha H, et al. The biology of the sodium iodide symporter and its potential for targeted gene delivery. Curr Cancer Drug Targets. 2010;10:242–267. doi: 10.2174/156800910791054194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzweg C, Morris JC. Gene therapy for thyroid cancer: current status and future prospects. Thyroid. 2004;14:424–434. doi: 10.1089/105072504323150732. [DOI] [PubMed] [Google Scholar]
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigant F, Descamps D, Jullienne B, Esselin S, Connault E, Opolon P, et al. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol Ther. 2008;16:1474–1480. doi: 10.1038/mt.2008.132. [DOI] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Huard J, Lochmüller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- Green NK, Morrison J, Hale S, Briggs SS, Stevenson M, Subr V, et al. Retargeting polymer-coated adenovirus to the FGF receptor allows productive infection and mediates efficacy in a peritoneal model of human ovarian cancer. J Gene Med. 2008;10:280–289. doi: 10.1002/jgm.1121. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Hemminki A. Adenoviruses for treatment of cancer. Ann Med. 2005;37:33–43. doi: 10.1080/07853890410018934. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim PH, Kim SW, Yun CO. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials. 2012;33:1838–1850. doi: 10.1016/j.biomaterials.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15:1034–1044. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- Grünwald GK, Klutz K, Willhauck MJ, Schwenk N, Senekowitsch-Schmidtke R, Schwaiger M, et al. Sodium iodide symporter (NIS)-mediated radiovirotherapy of hepatocellular cancer using a conditionally replicating adenovirus. Gene Ther. 2013;20:625–633. doi: 10.1038/gt.2012.79. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, et al. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- Tallone T, Malin S, Samuelsson A, Wilbertz J, Miyahara M, Okamoto K, et al. A mouse model for adenovirus gene delivery. Proc Natl Acad Sci USA. 2001;98:7910–7915. doi: 10.1073/pnas.141223398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair GE, Dixon SC, Griffiths SA, Zajdel ME. Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res. 1989;14:339–346. doi: 10.1016/0168-1702(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Jauregui-Osoro M, Sunassee K, Weeks AJ, Berry DJ, Paul RL, Cleij M, et al. Synthesis and biological evaluation of [(18)F]tetrafluoroborate: a PET imaging agent for thyroid disease and reporter gene imaging of the sodium/iodide symporter. Eur J Nucl Med Mol Imaging. 2010;37:2108–2116. doi: 10.1007/s00259-010-1523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AJ, Jauregui-Osoro M, Cleij M, Blower JE, Ballinger JR, Blower PJ. Evaluation of [18F]-tetrafluoroborate as a potential PET imaging agent for the human sodium/iodide symporter in a new colon carcinoma cell line, HCT116, expressing hNIS. Nucl Med Commun. 2011;32:98–105. doi: 10.1097/MNM.0b013e3283419540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani SJ, Mezhir JJ, Roizman B, Weichselbaum RR. ReVOLT: radiation-enhanced viral oncolytic therapy. Int J Radiat Oncol Biol Phys. 2006;66:637–646. doi: 10.1016/j.ijrobp.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- Trujillo MA, Oneal MJ, McDonough SJ, Morris JC. Viral dose, radioiodide uptake, and delayed efflux in adenovirus-mediated NIS radiovirotherapy correlates with treatment efficacy. Gene Ther. 2013;20:567–574. doi: 10.1038/gt.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–5742. [PubMed] [Google Scholar]
- Trujillo MA, Oneal MJ, McDonough S, Qin R, Morris JC. A steep radioiodine dose response scalable to humans in sodium-iodide symporter (NIS)-mediated radiovirotherapy for prostate cancer. Cancer Gene Ther. 2012;19:839–844. doi: 10.1038/cgt.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Institute of Health 2012Gene Therapy and Radioactive Iodine in Treating Patients With Locally Recurrent Prostate Cancer That Did Not Respond to External-Beam Radiation Therapyhttp://clinicaltrials.gov/ct/show/NCT00788307.
- Spitzweg C, Dietz AB, O'Connor MK, Bergert ER, Tindall DJ, Young CY, et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther. 2001;8:1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- Klutz K, Willhauck MJ, Wunderlich N, Zach C, Anton M, Senekowitsch-Schmidtke R, et al. Sodium iodide symporter (NIS)-mediated radionuclide ((131)I, (188)Re) therapy of liver cancer after transcriptionally targeted intratumoral in vivo NIS gene delivery. Hum Gene Ther. 2011;22:1403–1412. doi: 10.1089/hum.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willhauck MJ, Sharif Samani BR, Gildehaus FJ, Wolf I, Senekowitsch-Schmidtke R, Stark HJ, et al. Application of 188rhenium as an alternative radionuclide for treatment of prostate cancer after tumor-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab. 2007;92:4451–4458. doi: 10.1210/jc.2007-0402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Radioiodine biodistribution in untreated control mice.
NIS mRNA expression in non-target organs.