Abstract
Background:
Surgery remains the mainstay of therapy for pancreatic head (PH) and periampullary carcinoma (PC) and provides the only chance of cure. Improvements of surgical technique, increased surgical experience and advances in anesthesia, intensive care and parenteral nutrition have substantially decreased surgical complications and increased survival. We evaluate the effects of reconstruction type, complications and pathological factors on survival and quality of life.
Materials and Methods:
This is a prospective study to evaluate the impact of various reconstruction methods of the pancreatic remnant after pancreaticoduodenectomy and the pathological characteristics of PC patients over 3.5 years. Patient characteristics and descriptive analysis in the three variable methods either with or without stent were compared with Chi-square test. Multivariate analysis was performed with the logistic regression analysis test and multinomial logistic regression analysis test. Survival rate was analyzed by use Kaplan-Meier test.
Results:
Forty-one consecutive patients with PC were enrolled. There were 23 men (56.1%) and 18 women (43.9%), with a median age of 56 years (16 to 70 years). There were 24 cases of PH cancer, eight cases of PC, four cases of distal CBD cancer and five cases of duodenal carcinoma. Nine patients underwent duct-to-mucosa pancreatico jejunostomy (PJ), 17 patients underwent telescoping pancreatico jejunostomy (PJ) and 15 patients pancreaticogastrostomy (PG). The pancreatic duct was stented in 30 patients while in 11 patients, the duct was not stented. The PJ duct-to-mucosa caused significantly less leakage, but longer operative and reconstructive times. Telescoping PJ was associated with the shortest hospital stay. There were 5 postoperative mortalities, while postoperative morbidities included pancreatic fistula-6 patients, delayed gastric emptying in-11, GI fistula-3, wound infection-12, burst abdomen-6 and pulmonary infection-2. Factors that predisposed to development of pancreatic leakage included male gender, preoperative albumin < 30g/dl, pre-operative hemoglobin < 10g/dl and non PJ-duct to mucosa type of reconstruction. The ampullary cancers presented at an earlier stage and had a better prognosis than pancreatic cancer and cholangiocarcinoma. Early stage (I and II), negative surgical margin, well and moderate differentiation and absence of lymph node involvement significantly predicted for longer survival.
Conclusions:
PJ duct-to-mucosa anastomosis was safe, caused least pancreatic leakage and least blood loss compared with the other methods of reconstruction and was associated with early return back to home and prolonged disease free and overall survival.
Keywords: Complication, mortality and survival, pancreaticojujenostomy duct to mucosa, periampullary cancer, postoperative pancreatic fistula, reconstruction
Introduction
Periampullary adenocarcinoma (PC) including ampulla, distal bile duct and second part of the duodenum is an important health problem in many industrialized nations.[1] Pancreatic adenocarcinoma is the fourth most common cause of cancer related death in the United States, with a yearly incidence of 11.7 deaths per 100,000 people. Despite advances in the diagnosis and treatment of pancreatic adenocarcinoma, the five-year overall survival rate remains a dismal 5%, in part because the majority of patients present with advanced disease that precludes curative therapy. The overall risk of developing pancreatic cancer rises after age 50 years, and the majority of patients are between 60 and 80 years old.[2] In Japan, a dramatic increase has been observed during the last decade and the lowest incidence worldwide is seen in India and the middle East.[3] Pancreaticoduodenectomy (PD) is the only potentially curative treatment for PC and surgical resection remains the only modality to offer the possibility of long term survival.[4] Survival is more favorable in patients with ampullary carcinoma whereas in patients with pancreatic carcinoma, surgery was considered as a waste of resources by some authors,[5] as the five year survival was 3.5%.[6] Subsequently, with improvement in the post-resection survival of patients with PC and use of PD for treatment of benign disease like chronic pancreatitis, focus shifted towards preventing the long-term sequelae of reconstruction. Currently, various reconstructive techniques are available, as suggested by surgeons from all over the world.[7]
In Egypt, patients usually present at a late stage, with the majority presenting with malignant obstructive jaundice, which is different from what is seen in other countries. We analyzed the effects of reconstructive methods of the pancreatic remnant after PD on survival of Egyptian patients.
Materials and Methods
This is a comparative prospective study of reconstruction methods after PD, conducted in the South Egypt Cancer Institute (SECI), Assiut University between November 2008 and July 2012.
Inclusion criteria
Patients aged less than 75 years with a clinical diagnosis of pancreatic head (PH) and PC, with adequate hepatic, renal and bone marrow function were included.
Exclusion criteria
Patients who were over 75 years, had received prior chemotherapy or radiotherapy, had cardiac disease or any medical condition likely to be life threatening within three months were excluded. After enrolment, at the time of exploration, patients were excluded if the tumor was advanced and unresectable, if there was evidence of metastatic disease or cirrhosis.
Reconstruction techniques
All anastomoses were done by hand sewn technique. Reconstructions involved: Reconstruction of the pancreatic remnant by three methods including pancreaticogastrostomy (PG), pancreaticojejunostomy (PJ) duct to mucosa and PJ dunking. All intra and postoperative complications in the three groups (PBD and no-PBD) were recorded. The pancreatic anastomosis was end to side or end to end PJ or PG. Details of reconstruction techniques are available in supplementary materials (3MB, pdf) online.
Reconstructive methods following pancreaticoduodenectomy
Evaluation
Patients were evaluated and compared according to the following: Length of time of operation, blood loss during surgery, feasibility of reconstruction techniques, post-procedure complications such as pancreaticoenteric anastomotic or PG anastomosis leak, hepaticojejunostomy leak, infectious complications, like wound infection and intra-abdominal abscess, and any other major complication.
Follow up
After surgery, the patients were followed up to detect complications, local recurrence, distant metastasis and survival rate. Ultrasound and non-enhanced CT scans were obtained at three and six months after and contrast radiography was obtained as needed to evaluate the anastomotic stenosis or early obstruction.
Statistical methods
Continuous variables were summarized by means. Categorical data were condensed by absolute and relative values. Cross tabulations were created to compare frequency distributions between groups. Multivariate analysis of variables was performed with logistic regression analysis test and multinomial logistic regression analysis test. t-test was used to assess whether the associations displayed in the cross tabulations were statistically significant. Disease free survival curves were estimated according to the Kaplan-Meier method. The global significance level for all statistical test procedures conducted was chosen as = 5%. All statistical analyses were conducted in an explorative manner. Thus, with consideration of the explorative character of the analysis, P values of P = 0.05 were interpreted as statistically significant test results. SPSS version 16 software (SPSS, Chicago, Illinois, USA) was used to collect data and perform logistic regression analysis. Mendeley desktop 1.6 version (©2008-2012 Mendeley Ltd) free software was used for references management.
Results
Patients’ characteristics are summarized in Table 1 [Figure 1].
Table 1.
Patient demographic characteristic
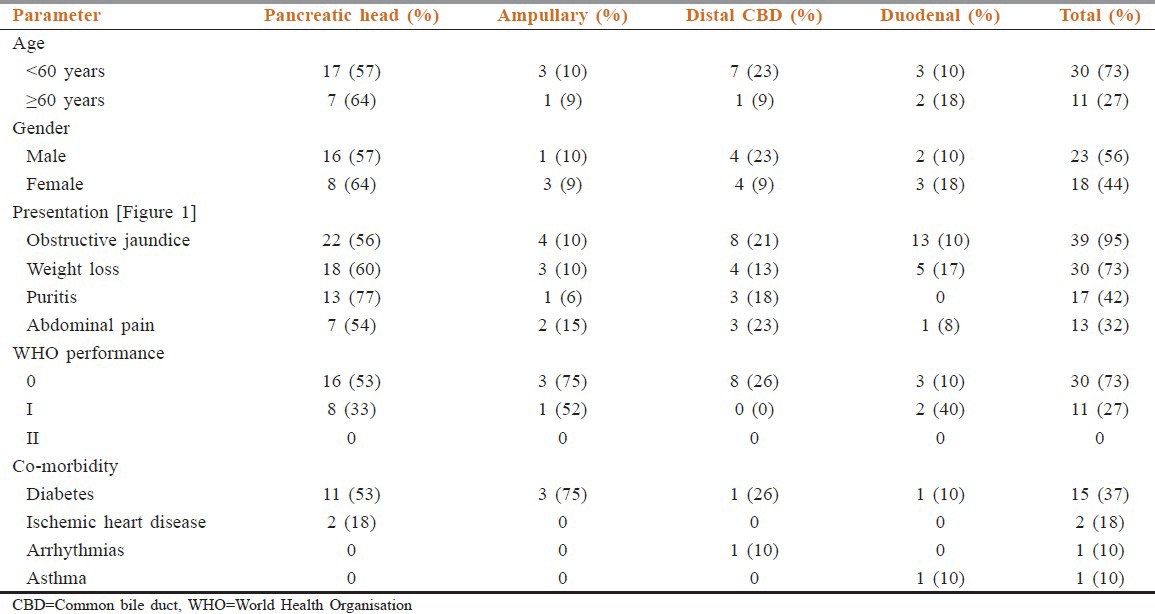
Figure 1.
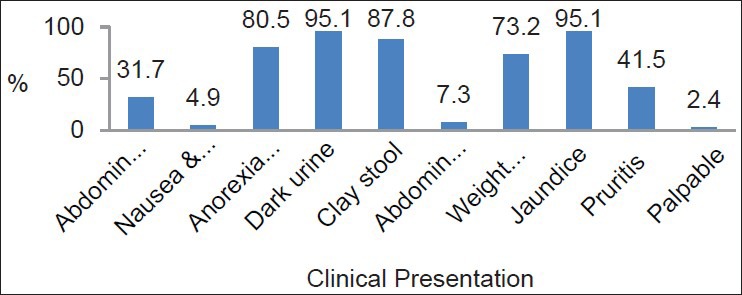
Clinical presentation of the studied sample
Preoperative abdominal ultrasonography showed PH mass in 16 patients (67%), and distended gallbladder in 35 patients (35%), while thin-cuts abdominal CT showed pancreatic head lesions in 21 cases (88%), one of which was intraductal papillary mucinous tumors. Vascular invasion was detected in two cases. Endoscopic retrograde cholangiopancreatography (ECRP) was attempted in 25 patients, biliary drainage and stenting was performed in 18 operable patients.
Reconstruction of the pancreatic remnant was of three types; PG in 15 cases (36.6%), telescoping PJ in 17 cases (41.5%) and PJ duct to mucosa in nine cases (22.0%) [Table 2].
Table 2.
Pancreatic reconstruction methods determinants*
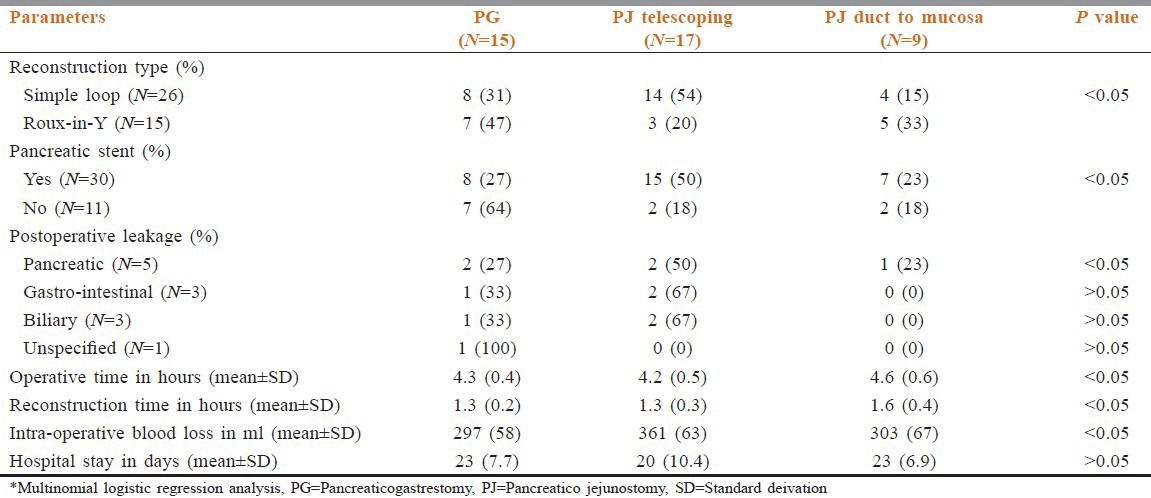
The PJ duct-to-mucosa caused significantly less leakage than the other methods of reconstruction. Duct-to-mucosa PJ was associated with longer operative and reconstructive times than other two methods while telescoping PJ required less operative time but longer reconstructive time than the PG. Telescoping of pancreas into the jejunum with stenting was safe, required a short operative time, and was the least difficult technique. The length of hospital stay was less in telescoping PJ than the other two types. The mean (±SD) length of hospital stay was PJ duct to mucosa 23 ± 6.9 days, PG 23 ± 7, and telescoping PJ 20 ± 10 [Table 2].
There were five post-operative mortalities (12.5%), and one patient died five months after surgery due to liver cirrhosis [Table 3]. The first postoperative mortality was on day 9 due to high grade pancreatic fistula (type-C) and biliary fistula after PG reconstruction and end to end hepaticojejunostomy, following redo PG and hepaticojejunostomy on day 7. The second patient died on the 14th post-operative day due to trickling of the gastric and bile juice from transhepatic biliary stent causing peritonitis. The third patient died on day 18 due to high grade pancreatic fistula (type-B) and gastrointestinal fistula after invagination PJ. The fourth patient developed gastrointestinal fistula on day 10, was explored on day 14 and had redo of gastrointestinal anastomosis, however died on day 22. The fifth patient died on day 35 due to intracranial hemorrhage.
Table 3.
Post-operative morbidity, mortality and follow up
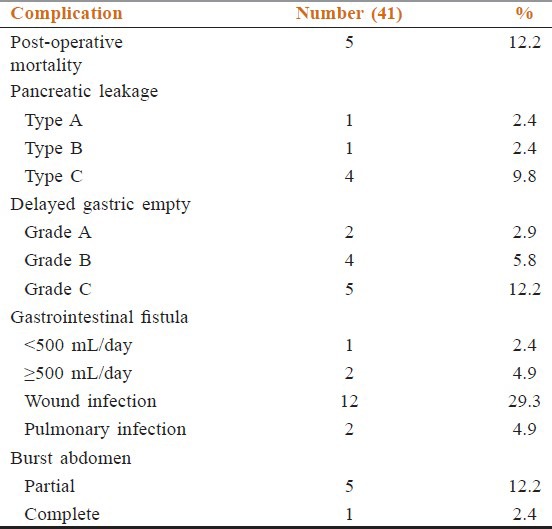
Post-operative morbidity in this study [Table 3] included pancreatic fistula in six cases (14.6%) as type-A one case (2.4%), type-B one case (2.4%) and type-C four cases (9.8%). The type-A pancreatic fistula was managed conservatively, however the type-B and type C pancreatic fistula required surgical exploration and redo. Delayed gastric emptying occurred in 11 cases (20.9%): Grade-A two cases (2.9%), grade-B four cases (5.8%), grade-C five cases (12.2%). Gastrointestinal fistula was noted in three cases (7.3%): one case (2.4%) low output fistula that closed with conservative therapy and two cases (4.9%) high output fistula that required surgical exploration and redo. One case of high output fistula was associated with type-C pancreatic fistula. Wound infection occurred in 12 patients (29.3%), all of whom required surgical debridement and daily dressing. Six patients (14.6%) developed burst abdomen: five (12.2%) partial, which were closed by secondary suturing after resolution of wound infection and the start of granulation tissue, and one complete (2.4%), which was closed by tension suturing. Pulmonary complications in the form of chest infection occurred in two patients (4.9%), which responded to antibiotics.
Regarding disease control, one patient had recurrence of tumor two months after surgical excision at the site of operation. One patient was suspected to have liver metastasis on abdominal ultrasound at the two year follow-up, however multislice-CT abdomen revealed that it was a simple liver cyst.
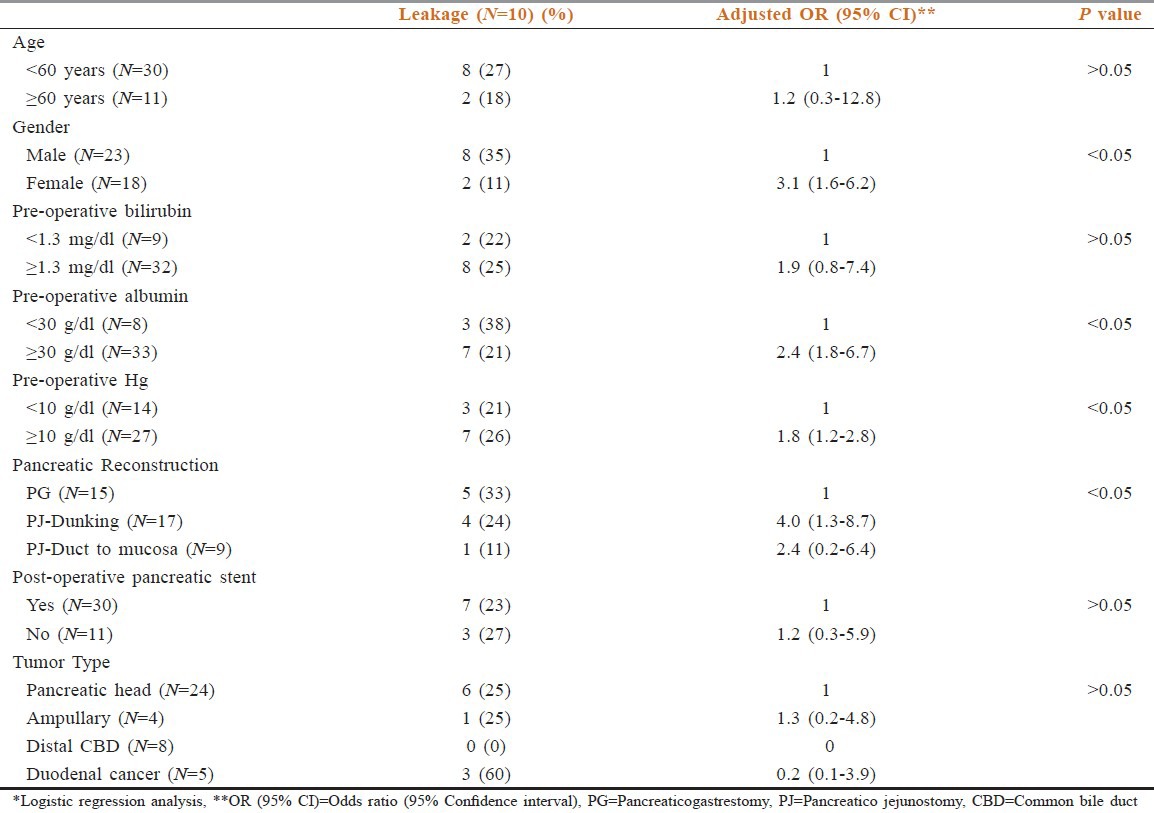
Regarding the impact of histological diagnosis and clinical factors on the development of pancreatic fistula, male gender, preoperative albumin <30 g/dl, pre-operative hemoglobin <10 g/dl and non PJ-duct to mucosa type of reconstruction appeared to predispose to an increased chance of pancreatic leakage. Age <60 yrs, preoperative direct bilirubin >1.3 mg/dl and non-stenting of the pancreatic duct intraoperatively caused a numerical but not statistically significant increase in pancreatic leakage [Table 4].
Table 4.
Fistula based on histological diagnosis and clinical factor*
In terms of histopathology, tumors of the head of the pancreas constituted 24 cases (58.5%), of which 23 were adenocarcinoma, and one patient had solid cystic papillary neoplasm. The remaining 17 cases were adenocarcinoma arising in distal bile duct-four (9.8%), ampulla-eight (19.5%) and duodenum-5 (12.2%). Regarding staging for pancreatic head, ampullary, distal CBD and duodenal cancer, the most common presentations were locally advanced stage II 20 cases (83%), early stage I five cases (63%), local stage II four cases (100%) and local stage II five cases (80%) respectively. Pancreatic head tumors presented commonly as large size T2 14 cases (58%), T3 nine cases (38%) and lymph node positive in 11 cases (58%) while ampullary cancer presented commonly as small size tumor T1 in two cases (25%), T2 in six cases (75%) and lymph node metastasis in two cases (25%). The P < 0.005 was statically significant in all above tumor pathological characteristics and staging.
Regarding the mortality rate in relation to the type of periampullary cancer [Figure 2], pancreatic head cancer had high mortality rate at 9.67%, duodenal cancer had mortality rate of 2.44% while the ampullary and distal common bile duct cancer had no mortality rate due to early clinical presentation and early pathological stage at presentation. Ampullary cancer had the highest median survival, while pancreatic head cancer had the lowest median survival rate [Figure 3]. Early stage (I and II), negative surgical margin, well and moderate differentiation and absence of lymph node involvement significantly predicted for longer survival [Table 5, Figures 4 and 5].
Figure 2.
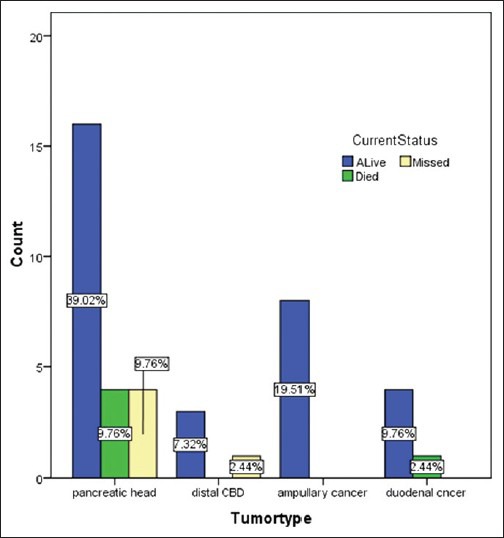
Mortality in relation to tumor type
Figure 3.
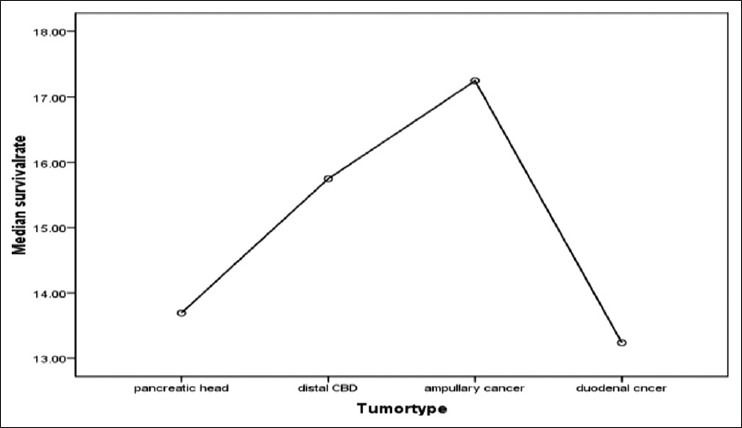
Median survival of different tumor types
Table 5.
Effect of tumor stage, lymph node status and surgical margin on survival*
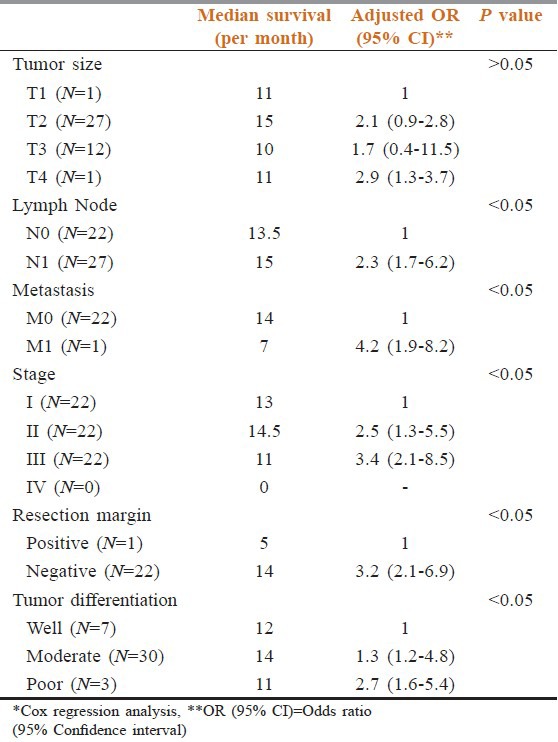
Figure 4.
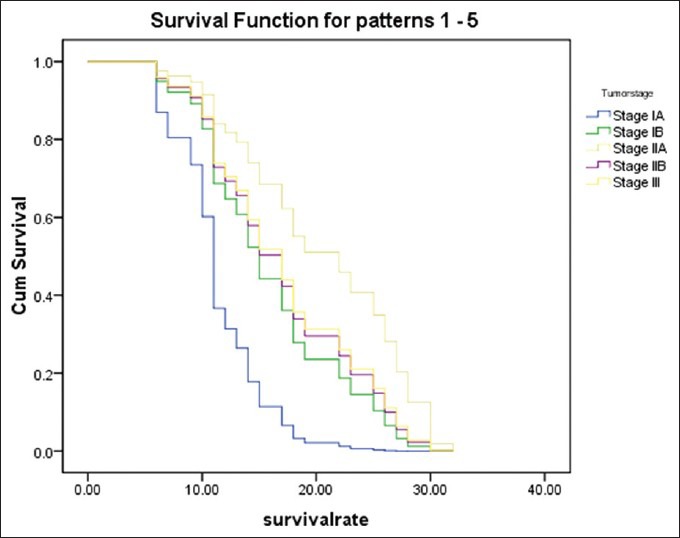
Survival rate of each tumor stage
Figure 5.
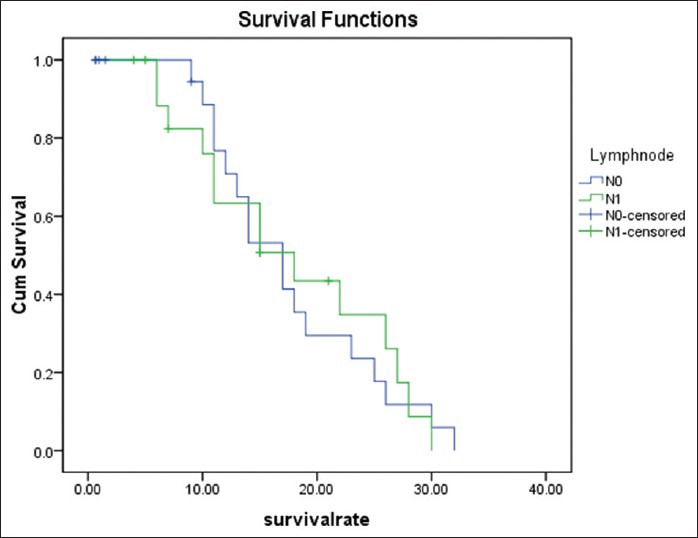
Survival rate in relation to lymph node metastasis
Discussion
Early disease and curative-intent surgery are the best predictors of outcome in periampullary cancer.[8] Although incidence is roughly equal for the sexes, African Americans seem to have a higher incidence of pancreatic cancer than white Americans.[9] The age and sex distribution in our study matched that described by Jamieson et al.[10]
There are two widely used methods to accomplish an end-to-side PJ after pancreaticoduodenectomy: Invagination PJ (or dunking the pancreatic remnant into the jejunum) or duct-to-mucosa PJ. Continuous duct-to-mucosa anastomosis is described as being safer with a significantly lower leakage rate of 1.6 to 2%.[11,12,13,14,15,16] Matsumoto et al.,[17] in a retrospective study of 100 patients, showed a pancreatic fistula rate of 4.2% after duct-to-mucosa anastomosis versus 26.4% after invagination anastomosis. Conversely, Marcus et al.,[18] reported that duct-to-mucosa anastomosis had lower pancreatic fistula rate in low-risk patients with dilated pancreatic duct or firm fibrotic pancreas, compared to the end-to-end anastomosis. Hosotani et al.[19] and Poon et al.[20] also concluded that a duct-to-mucosa anastomosis is safer. However, three RCTs,[21,22,23] showed similar pancreatic fistula rates between the PG and PJ anastomosis, and a recent meta-analysis concluded[24] that the two techniques of anastomosis were not different in terms of pancreatic fistula rate and overall morbidity rate. Nevertheless, the incidence of postoperative morbidity has remained unacceptably high. The lack of a uniform technique of performing PG anastomosis reproduces the same debate with PJ anastomosis (dunking vs. duct-to-mucosa). In a more recent trial,[25] the overall rate of pancreatic fistula was 17.8%, and the invagination method significantly decreased the rate of pancreatic fistula versus the duct-to-mucosa anastomosis (12% vs. 24%; P = 0.04).
The results of our study matched those of the above studies and confirmed that PJ-duct to mucosa is safer. The advantages of duct-to mucosa PJ over PG and invagination PJ are: (1) Simple, when MPD was dilated; (2) easy, no need of isolation of long segment pancreatic stump; (3) less pancreatic leakage; (4) less blood loss; (5) less biliary leakage and gastrointestinal leakage; (6) less hospital stay. Disadvantage was longer operative and reconstructive time.
Postoperative complication rate and pancreatic fistula development following pancreatogastroanastomosis and pancreatojejunoanastomosis has been reported to be similar.[26,27] The development of pancreatic fistula depends on the type of pancreatic anastomosis, the type of pancreatic remnant, the pancreatic duct size. Fuks and colleagues[28] reported a slightly higher rate of grade C anastomotic failures (4.8% in a series of 680 patients) using a variety of anastomotic techniques and a mortality rate of 40% associated with grade C anastomotic failures.[28] Veillette and associates[29] also recently reported a pancreatic anastomotic failure rate-related mortality rate of 9.3% after pancreaticoduodenectomy. Other authors have reported clinically significant (grade B or C) pancreatic fistula rates ranging from 11% to 15%.[30,31,32]
Delayed gastric emptying (DGE) is one of the most common complications after pancreatic head resection and contributes substantially to overall morbidity and to the impairment of preoperative quality of life.[33] DGE occurred in 36 (13.8%) of 260 patients. It should be noted that the criteria employed in the ISGPS definition are more restrictive than those used in earlier definitions, with the result that the proportion of DGE patients tends to be substantially higher than those described in earlier reports.[34] According to the current literature, only two studies have sought to evaluate the feasibility of the ISGPS classification of DGE.[35,36] The first of these reported an incidence of 42% (standard operative manoeuvres included PPPD and subtotal stomach preserving PD),[36] whereas the latter described an incidence of 33% after PPPD or classical Whipple PD.[35] Akizuki et al.,[36] showed no differences in postoperative hospital stay among DGE grades, whereas Park et al., showed a significantly prolonged hospital stay in DGE grade C patients. In another recent paper by Nikfarjam et al.,[37] the presence of postoperative morbidity was not associated with an increase in DGE.
In our study, the post-operative mortality was 12.5%. Pancreatic head cancer had the highest mortality rate at 9.6%, while ampullary cancer and distal CBD cancer had no mortality, due to early presentation. Survival was high in duct to mucosa PJ because of lowest leakage rate, less complications and short hospital stay. The mortality, morbidity and length of hospital stay in our study were high as compared to what has been previously reported in other studies. This could be due to the smaller number of cases in our study, improper selection of cases, malnutrition, late clinical presentation and hand sewing of the resection and anastomosis which were done by different teams. The relatively longer hospital stay in our study may be attributed to the low education level and low socioeconomic status of our patients.
Several authors have reported that the presence of postoperative complications can affect survival after pancreatic cancer resection.[38,39] In patients with complications, the median survival time was 12 months as compared to 18 months in patients without complications. We also found that postoperative complications adversely affect survival. The final pathologic diagnosis of the surgical specimens was adenocarcinoma in over 80.3% of patients. Both pancreatic duct adenocarcinoma and ampullary adenocarcinoma accounted for 34.4%, distal bile duct 5.7%, and duodenal adenocarcinoma 4.0%. The mean survival after PD in patients with pancreatic cancer was 22.6 months while the mean survival for ampullary adenocarcinoma was 31.4 months.[40] In our study as well, patients with ampullary cancer had the highest survival rate, while pancreatic head cancer had the lowest median survival rate. Other factors that significantly affected survival included stage (early stage i.e., I, II had median survival of 13,14.5 months, compared to stage III and IV at 11 months), status of the surgical margin (negative surgical margin had a median survival of 15 months as compared to a positive surgical margin), tumor differentiation (well and moderately differentiated tumors had high survival at 12,14 months compared to poor differentiated tumors at 11 months), no evidence of distant metastasis (median survival of 14 months compared to positive distant metastasis tumors, in which median survival was 7 months), and presence of lymph node metastasis (lower median survival). Although larger tumor size appeared to decrease survival, this result did not reach statistical significance in our study.
In our present study, 97.6% patients had adenocarcinoma, which included pancreatic head adenocarcinoma in 58.5% while ampullary, distal bile duct and duodenal carcinoma accounted for 19.5, 9.8, and 12.2% respectively. This was similar to what has been reported in a large retrospective study by Max Schmidt et al.[41] Van Roest et al. analyzed data from 121 patients who underwent resection for periampullary cancer and reported the overall three and five year survival rates of patients stratified according to the site of origin of the tumor were 26.1 and 17.9% (pancreatic head), 43.6 and 35.2% (ampulla of Vater), 73.7 and 53.6% (distal bile duct), and 71.1 and 44.4% in duodenal carcinoma, respectively. The corresponding median survival times were 15.0 months (pancreatic head), 31.9 months (ampulla of Vater), 102.0 months (distal bile duct), and 44.4 months (duodenum).[42] Pancreatic malignancies overall are associated with poor long-term prognosis. Five-year survival rates following pancreatic resection for pancreatic adenocarcinoma remain low (<20%), even in large volume institutions. This cohort had a median follow-up of nearly five years and an actuarial survival of 27%, which is comparable with the recently published MD Anderson series.[43] The five year survival for patients who underwent pancreaticoduodenectomy for periampullary malignancies other than pancreatic adenocarcinoma was 61%. This result is also in line with previously published results for periampullary carcinomas, including a recently published series from Birmingham which reported an actuarial five year survival of 60% for ampullary carcinoma following resection.[44] An involved pancreatic resection margin is associated with poor patient outcome.[45] A recent study reported a margin of ≥1.5 mm to be associated with better outcome.[46] If this is borne out by other investigators, a re-evaluation of the R0 margin will be required. The five year survival rate was highest at 23% for carcinoma of the ampulla (32.7%) and lowest for pancreatic head at 5.5%, which was a significant difference.[47] Cameron et al. reported a 19% actuarial five year survival in 89 patients undergoing PD. Various prognostic factors have been described including size > 2.5 cm, +ve nodes, vessel invasion,[48,49,50] high grade tumors,[49] positive margins,[50,51] perineural and duodenal invasion.[52]
Winter JM et al. demonstrated that pancreatic resection can be performed safely with significantly improved postoperative outcomes over time.[53] Intermediate survival (one-year survival after resection) improved over time from 58% in the first part of the study to 68% in the 2000s. Although some of the improvement could be explained by decreased perioperative mortality, additional factors likely played a role as well.[54] However, in the multivariate analysis, which included pathologic features such as histological grade and lymph node status, the decade of resection was not an important predictor of survival. Furthermore, after stratification by lymph node status, no difference was observed in long term survival between the three decades. Notably, the percentage of cancers that were poorly differentiated remained relatively constant, as did the median number of positive lymph nodes in the specimens with regional lymph node metastases. The authors opined that the observed trend toward more frequent metastases to regional lymph nodes likely resulted from improved examination techniques by experienced specialized pancreatic pathologists.[54]
Conclusion
The PJ duct-t-mucosa anastomosis is safe, causes least pancreatic leakage and least blood loss compared with the other methods of reconstruction and is associated with early return back to home and prolonged disease free and overall survival, however PG is associated with more functional deterioration. The ampullary cancers present at an earlier stage and, thus, have better prognosis than pancreatic cancer and cholangiocarcinoma. However, when controlled for stage, tumor type is not predictive of overall survival. Based upon our results, surgical resection for ampullary cancers mandates margin-negative resection, whereas anticipation of microscopically positive margins should not preclude resection in pancreatic and distal bile duct cancers.[SUPPORTING:1]
See online for supplementary materials
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer Statistics. Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Bose D, Katz MHG, Fleming JB. Pancreatic Adenocarcinoma. In: Feig BW, Ching CD, editors. The MD Anderson Surgical Oncology Hand book. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2012. pp. 472–90. [Google Scholar]
- 3.Yeo JL. Tumors of the gall bladder and bile ducts. In: Zinner MJ, Seymour I, Ellis SH, editors. Mingots Abdominal operations. 10th ed. New York: A Simon and Schuster Company; 1997. pp. 1835–54. [Google Scholar]
- 4.Yeo CJ, Sottn TA. Periampullary adenocarcenoma analysis of 5 year survivors. Ann Surg. 1998;227:821–31. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudjonsson B. Carcinoma of the pancreas: Critical analysis of costs, results of resections and the need standardized reporting. J Am Coll Surg. 1995;8:483–503. [PubMed] [Google Scholar]
- 6.Gudjonsson B. Cancer of the pancreas: 50 years of surgery. Cancer. 1987;60:2284–303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Peng S, Mou Y, Cai XPC. Binding pancreaticojejunostomy is a new technique to minimize leakage. Am J Surg. 2002;1833:283–5. doi: 10.1016/s0002-9610(02)00792-4. [DOI] [PubMed] [Google Scholar]
- 8.Shrikhande SV, Barreto SG. Surgery for Pancreatic Carcinoma: State of the Art. Indian J Surg. 2012;74:79–86. doi: 10.1007/s12262-011-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iii AB. NIH Public Access Pancreatic Adenocarcinoma; 2011. p. 89. [Google Scholar]
- 10.Jamieson NB, Denley SM, Logue J, MacKenzie DJ, Foulis AK, Dickson EJ, et al. A Prospective Comparison of the prognostic value of tumor-and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2011;18:2318–28. doi: 10.1245/s10434-011-1560-3. [DOI] [PubMed] [Google Scholar]
- 11.Fragulidis GP, Arkadopoulos N, Vassiliou I, Marinis A, Thedosopoulos T, Safyla V, et al. Pancreatic leakage after pancreaticoduodenectomy. Pancreas. 2009;38:e177–82. doi: 10.1097/MPA.0b013e3181b57705. [DOI] [PubMed] [Google Scholar]
- 12.Lee SE, Yang SH, Jang JY. Pancreatic fistula after pancreaticoduodenectomy: A comparison between the two pancreaticojejunostomy methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer: Interrupted vs. continuous stitches. World J Gastroenterol. 2007;13:5351–6. doi: 10.3748/wjg.v13.i40.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strasberg SM, Drebin JA, Mokadam NA, Green DW, Jones KL, Ehlers JP, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: Effect on anastomotic failure in the Whipple procedure. J Am Coll Surg. 2002;194:746–58. doi: 10.1016/s1072-7515(02)01202-4. [DOI] [PubMed] [Google Scholar]
- 14.Z’graggen K, Uhl W, Friess H, Büchler MW. How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg. 2002;9:733–7. doi: 10.1007/s005340200101. [DOI] [PubMed] [Google Scholar]
- 15.Peng SY, Wang JW, Lau WY, Cai XJ, Mou YP, Liu YB, et al. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy. A prospective randomized trial. Ann Surg. 2007;245:692–8. doi: 10.1097/01.sla.0000255588.50964.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Onishi S, Hanazaki K. Risk factors, predictors and prevention of pancreatic fistula formation after pancreatoduodenectomy. J Hepatobiliary Pancreat Surg. 2007;6:557–63. doi: 10.1007/s00534-007-1242-5. [DOI] [PubMed] [Google Scholar]
- 17.Peng SY, Mou YP, Liu YB, Su Y, Peng CH, Cai XJ, et al. Binding pancreaticojejunostomy: 150 consecutive cases without leakage. J Gastrointest Surg. 2003;7:898–900. doi: 10.1007/s11605-003-0036-6. [DOI] [PubMed] [Google Scholar]
- 18.Poon RT, Fan ST, Loch M, Ng KK, Yuen WK, Yeung C, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: A prospective randomized trial. Ann Surg. 2007;3:425–33. doi: 10.1097/SLA.0b013e3181492c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosotani R, Doi R, Imamura M. Duct-to-mucosa pancreaticojejunostomy reduces the risk of pancreatic leakage after pancreatoduodenectomy. World J Surg. 2002;26:99–104. doi: 10.1007/s00268-001-0188-z. [DOI] [PubMed] [Google Scholar]
- 20.Poon RT, Lo SH, Fong D, Fan ST, Wong J. Prevention of pancreatic anastomotic leakage after pancreaticoduodenectomy. Am J Surg. 2002;183:42–52. doi: 10.1016/s0002-9610(01)00829-7. [DOI] [PubMed] [Google Scholar]
- 21.Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, et al. A prospective randomized trial of pancreatogastrostomy or pancreatojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–8. doi: 10.1097/00000658-199510000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: Results of a comparative study. Ann Surg. 2005;242:767–71. doi: 10.1097/01.sla.0000189124.47589.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffas JP, Suc B, Msika S, Fourtanier G, Muscari F, Hay JM, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreaticoduodenectomy. Am J Surg. 2005;189:720–9. doi: 10.1016/j.amjsurg.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Wente N, Shrikhande SV, Müller MW, Diener MK, Seiler CM, Friess H, et al. Pancreaticojejunostomy versus pancreaticogastrostomy: Systematic review and meta analysis. Am J Surg. 2007;193:171–83. doi: 10.1016/j.amjsurg.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? Randomized, prospective, dual-institution trial. J Am Coll Surg. 2009;5208:738–47. doi: 10.1016/j.jamcollsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–92. doi: 10.1097/00000658-199510000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassi C, Falconi M, Molinari G, Salvia R, Butturini G, Sartori N, et al. Reconstruction by pancreatojejunostomy versus pancreatogastrostomy following pancreatectomy. Ann Surg. 2005;242:767–71. doi: 10.1097/01.sla.0000189124.47589.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuks D, Piessen G, Huet E, Tavernier M, Zerbib P, Michot F, et al. Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: Incidence, prognosis, and risk factors. Am J Surg. 2009;197:702–9. doi: 10.1016/j.amjsurg.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Veillette G, Dominguez I, Ferrone C, Thayer SP, McGrath D, Warshaw AL, et al. Implications and management of pancreatic fistulas following pancreaticoduodenectomy: The Massachusetts general hospital experience. Arch Surg. 2008;143:476–81. doi: 10.1001/archsurg.143.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: A report from the pancreatic anastomotic leak study Group. J Gastrointest Surg. 2007;11:1451–8. doi: 10.1007/s11605-007-0270-4. discussion 1459. [DOI] [PubMed] [Google Scholar]
- 31.Liang TB, Bai XL, Zheng SS. Pancreatic fistula after pancreaticoduodenectomy: Diagnosed according to International Study Group Pancreatic Fistula (ISGPF) definition. Pancreatology. 2007;7:325–31. doi: 10.1159/000105498. [DOI] [PubMed] [Google Scholar]
- 32.Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM., Jr Clinical and economic validation of the International StudyGroup of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443–51. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA. Fast-track recovery programme after pancreaticoduodenectomy reduced delayed gastric emptying. Br J Surg. 2008;95:1387–93. doi: 10.1002/bjs.6324. [DOI] [PubMed] [Google Scholar]
- 34.Malleo G, Crippa S, Butturini G, Salvia R, Partelli S, Rossini R, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: Validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. [Last accessed on 2012 Aug 8];HPB (Oxford) 2010 12:610–8. doi: 10.1111/j.1477-2574.2010.00203.x. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2999788andtool=pmcentrezandrendertype=abstract . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JS, Hwang HK, Kim JK, Cho SI, Yoon DS, Lee WJ, et al. Clinical validation and risk factors for delayed gastric emptying based on the International Study Group of Pancreatic Surgery (ISGPS) Classification. Surgery. 2009;146:882–7. doi: 10.1016/j.surg.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Akizuki E, Kimura Y, Nobuoka T, Imamura M, Nagayama M, Sonoda T, et al. Reconsideration of postoperative oral intake tolerance after pancreaticoduodenectomy: Prospective consecutive analysis of delayed gastric emptying according to the ISGPS definition and the amount of dietary intake. Ann Surg. 2009;249:986–94. doi: 10.1097/SLA.0b013e3181a63c4c. [DOI] [PubMed] [Google Scholar]
- 37.Nikfarjam M, Kimchi ET, Gusani NJ, Shah SM, Sehmbey M, Shereef S, et al. A reduction in delayed gastric emptying by classic pancreaticoduodenectomy with an antecolic gastrojejunal anastomosis and aretrogastric omental patch. J Gastrointest Surg. 2009;13:1674–82. doi: 10.1007/s11605-009-0944-1. [DOI] [PubMed] [Google Scholar]
- 38.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary ad- encarcinoma, part 2. Randomized control trial evaluating survival, mortality and morbid. Ann Surg. 2002;3:355–68. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kat’uchová J, Bober J, oňak J. Postoperative complications and survival rates for pancreatic cancer patients. [Last accessed on 2012 Jul 23];Wiener klinische Wochenschrift. 2011 123:94–9. doi: 10.1007/s00508-010-1513-z. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21253778 . [DOI] [PubMed] [Google Scholar]
- 40.Chan C, Franssen B, Rubio A, Uscanga L. Pancreaticoduodenectomy in a Latin American country: The transition to a high-volume center. [Last accessed on 2012 Aug 4];J Gastrointest Surg. 2008 12:527–33. doi: 10.1007/s11605-007-0274-0. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17763915 . [DOI] [PubMed] [Google Scholar]
- 41.Lockhart AC, Rothenberg ML, Berlin JD. Treatment for Pancreatic Cancer: Current Therapy and Continued Progress. Gastroenterol. 2005;128:1642–54. doi: 10.1053/j.gastro.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 42.van Roest MH, Gouw AS, Peeters PM, Porte RJ, Slooff MJ, Fidler V, et al. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: Perineural growth more important prognostic factor than tumor localization. [Last accessed on 2012 Aug 5];Ann Surg. 2008 248:97–103. doi: 10.1097/SLA.0b013e31817b6609. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18580212 . [DOI] [PubMed] [Google Scholar]
- 43.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Longterm survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Roest MH, Gouw AS, Peeters PM, Porte RJ, Slooff MJ, Fidler V, et al. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: Perineural growth more important prognostic factor than tumor localization. Ann Surg. 2008;248:97–103. doi: 10.1097/SLA.0b013e31817b6609. [DOI] [PubMed] [Google Scholar]
- 45.Bilimoria KY, Talamonti MS, Sener SF, Bilimoria MM, Stewart AK, Winchester DP, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg. 2008;207:510–9. doi: 10.1016/j.jamcollsurg.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 46.Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855–62. doi: 10.1200/JCO.2008.20.5104. [DOI] [PubMed] [Google Scholar]
- 47.Shyr YM, Su CH, Wu CW, Lui WY. Reappraisal of surgical risk and prognoses of periampullary lesions after PD. Zhonghua YI Xue Za Zhi (Taipei) 2001;64:84–94. [PubMed] [Google Scholar]
- 48.Sohn TA, Yeo CJ, Cameron JL, Geschwind JF, Mitchell SE, Venbrux AC, et al. Role of interventional radiologists in managing patients and complications. J Gastrointest Surg. 2003;7:290–19. doi: 10.1016/s1091-255x(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 49.Geer RJ, Brennan MF. Resection of pancreatic adenocarcinoma: Prognostic indicators for survival. Am J Surg. 1993;165:68–73. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 50.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. PD for cancer of the head of the pancreas 201 patients. Ann Surg. 1995;221:721–33. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willett CG, Lewandrowski K, Warshaw AL, Efird J, Compton CC. Resection margins in carcinoma of the head of the pancreas: Implications for radiation therapy. Ann Surg. 1993;217:144–8. doi: 10.1097/00000658-199302000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas: Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Winter JM, Brennan MF, Tang LH, D’Angelica MI, Dematteo RP, Fong Y, et al. Survival after resection of pancreatic adenocarcinoma: Results from a single institution over three decades. [Last accessed on 2012 Aug 10];Ann Surg Oncol. 2012 19:169–75. doi: 10.1245/s10434-011-1900-3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21761104 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reconstructive methods following pancreaticoduodenectomy


