Abstract
Plaque assays are a widely used method to quantify stocks of viruses. Although this method is well established for titrating viral stocks, it is time consuming and can take several days to complete. In this study, the creation and validation of a quantitative real-time PCR (qPCR) assay for enumerating virions of vaccinia virus is reported. PCR primers and a minor groove-binding probe were designed to hybridize to the DNA polymerase gene (E9L) from a number of different orthopoxviruses. The number of viral genomes determined using qPCR was approximately similar to results obtained using OD 260 measurements and a direct count of fluorescent virions by microscopy indicating that all three methods are comparable in their ability to quantify virions from a purified stock. In addition, this report describes methodologies to harvest and quantify, using the qPCR assay, three of the four types of vaccinia virions produced during morphogenesis: intracellular mature virions, cell-associated enveloped virions, and extracellular enveloped virions. Using these procedures a particle to plaque forming unit of 61:1, 14:1 and 6:1 was calculated for IMV, CEV and EEV respectively. These results show that qPCR can be used as a fast and accurate assay to quantify stocks of vaccinia virus over several orders of magnitude from both purified and unpurified stocks and should be applicable to other members of the orthopoxvirus genera.
Keywords: Vaccinia Virus, orthopoxvirus, qPCR, particle to PFU ratio
1. INTRODUCTION
Orthopoxviruses are large, dsDNA viruses that replicate entirely in the cytoplasm (Moss, 2007). Vaccinia virus (VV), the prototypical member of the poxvirus family, was used as a live vaccine during the eradication of variola virus, the causative agent of smallpox, and is still used to protect against its clandestine release (DesRoches, 2003). Although naturally occurring variola was eradicated, VV is still studied as a vaccine platform and more recently as a potential oncolytic virus for the treatment of various types of cancers (Breitbach et al., 2012; Kim and Gulley, 2012; Kirn and Thorne, 2009; Price et al., 2013). In addition, VV is widely used in laboratories to express proteins of interest in a eukaryotic background (Fuerst, Earl, and Moss, 1987; Mohamed and Niles, 2004) For these reasons work with VV has continued to flourish and therefore methods to rapidly and accurately titrate viral stocks are required.
VV has a complex morphogenesis producing two morphologically distinct, infectious forms; intracellular mature virus (IMV) and extracellular virus (EV) (Smith and Law, 2004). EV is derived from a subset of IMV that have received an extra envelope derived from the endomembrane system (Schmelz et al., 1994; Tooze et al., 1993). IMV represent the majority of progeny virions (~90 %) but are not released from the cell. Although EV represents the minority of EV produced, they are actively released from the cell and are responsible for cell-to-cell and systemic spread and of the infection (Appleyard, Hapel, and Boulter, 1971; Blasco and Moss, 1992; Boulter and Appleyard, 1973; Payne, 1980). EV can be further divided into two types, virions attached to the cell surface (CEV) and virions released from the cell surface (EEV). VV is now thought to transcribe over 200 ORFs during infection (Yang et al., 2010). Upwards of 100 of these ORFs are transcribed post genome replication when morphogenesis occurs. Mutagenic studies of genes involved in morphogenesis can have profound effects on the production of both IMV and EV and their infectivity. Therefore the accurate quantitation of the particle to plaque forming units of the various forms is required to fully understand the function of proteins involved in morphogenesis.
This report provides a protocol for the quantitation of VV from purified stocks and infected cell lysate using a TaqMan® qPCR assay. In addition, the results of quantifying VV by qPCR are compared with those obtained via plaque assay, OD260, and direct counting of fluorescent particles. Further, this manuscript shows that quantitation of the three forms of infectious vaccinia virus, EEV, CEV, and IMV, using qPCR, correlates with titration by plaque assay to a particle to PFU ratios of 6:1, 14:1, and 61:1, respectively.
2. MATERIALS AND METHODS
2.1. Cells, viruses and viral genomes
Monolayers of HeLa and BS-C-1 cells were maintained as described previously (Ward, 2005). The Western Reserve strain of VV (WR) was obtained from ATCC. The recombinant virus vA4-YFP has been described previously (Ward, 2005). Genome sequence data was downloaded from the Poxvirus Bioinformatics Resource Center at poxvirus.org. Plaque assays and virus purification were performed as described (Ward and Moss, 2001a). Genomic DNA from Modified Vaccinia Ankara (MVA), VV Lister (Elstree), VV IHD, VV Lederle-Chorioallantoic, VV New York City Board of Health (NYCBOH) (Wyeth, calf adapted), VV WR (tissue culture adapted), WR (mouse adapted), Cowpox Brighton Red, and Monkeypox Walter Reed Army Institute of Research (WRAIR) was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH.
2.2. qPCR
The sequence encoding the DNA polymerase from vaccinia virus strain WR (WR 065, Copenhagen designation E9L) was searched using Primer Express software (Applied Biosystems) for qPCR primer and probe sets. Suitable regions selected by the software were checked against an alignment of DNA polymerase genes from multiple orthopoxviruses and the region consisting of bp 2048–2118 from the VV WR genome was selected for a target sequence as it exhibits almost complete homology among the 14 orthopoxviruses aligned. The chosen target sequence yielded the following primer and probe sequences: E9L forward primer: CGGCTAAGAGTTGCACATCCA, E9L reverse primer: CTCTGCTCCATTTAGTACCGATTCT and a TaqMan® Minor Groove Binding (MGB) probe (E9L Probe) with the sequence AGGACGTAGAATGATCTTGTA (Applied Biosystems).
To quantify viral genomes, 1×105 HeLa cells were infected with an MOI of 5. Two hours post infection the inoculum was removed and 2ml of fresh media was added. The following day, infected cells were harvested by scraping and lysed by 3 cycles of snap freezing and thawing. Nucleic acids were extracted from a 100µ l aliquot of lysate using a Wizard® Minipreps DNA purification system according to the manufacturers instructions (Promega). After the final elution, DNA was concentrated by ethanol precipitation and resuspended in 50µ l of dH2O. For real-time qPCR assays, 5 µl of DNA extract was added to 25 µl 2× TaqMan Universal PCR Master Mix (Applied Biosystems) and 5 µl each of 50 µM E9L Forward Primer, 50 µM E9L Reverse Primer, and 2 µM E9L Probe. The volume was brought up to 50 µl with nuclease-free water (Invitrogen). All real-time qPCR assays were performed using an Applied Biosystems 7300 Real Time PCR System and the following protocol: initial denaturation step at 95° C for 10 min, followed by 40 cycles of denaturation for 15s at 95° C and annealing/extension for 1 min at 60° C.
2.3. qPCR standard/positive control
A plasmid containing the E9L open reading frame (ORF) was created for use as a positive control and to generate a standard curve for the qPCR assays. Primer pair AAGCTTATGGATGTTCGGTGC and GAGCTCTTATGCTTCGTAAAAT were used to amplify the E9L ORF. The PCR product was cloned into pCR2.1 using the TOPO TA cloning kit (Invitrogen) to produce pE9L. The integrity of the insert was confirmed by sequencing. Preparations of pE9L were digested with ScaI (New England Biolabs), and linearized plasmids separated by agarose gel electrophoresis. Following extraction from the gel and purification, the number of plasmid molecules was calculated based on an OD260 measurement.
2.4. Counting of Fluorescent Virions
Virions from cells infected with vA4-YFP were purified on CsCl gradients as described previously (Moss and Earl, 1998). Purified virions were sonicated on ice 4 times for 30 seconds using a cup sonicator to disassociate aggregated virions and diluted immediately 1:100 in 10 mM Tris pH 9.0. For visualization, diluted virus was further diluted 1:10 in ProLong Gold mounting media (Invitrogen) containing 5ug/ml Hoechst. One chamber of a C-Chip hemocytometer (InCyto) was filled with the diluted vA4-YFP and the ends sealed with nail polish. vA4-YFP virions were imaged and counted using a Leica DMIRB inverted fluorescent microscope with an attached cooled charge-coupled device (Cooke) that was controlled using Image-Pro Plus software (MeidaCybernetics).
2.5. Optical Density
The OD260 of the purified virions was measured using a Nano Drop 100 Spectrophotometer (Thermo Scientific). The number of virions was calculated using the conversion 1 OD at 260nm = 1.2 × 1010 virions (Joklik, 1962).
2.6. DNAse Treatment of Pure WR
10µ l of purified virions were treated with 1 unit of DNAse I (New England Biolabs) as per manufacturers instructions.
2.7. Quantifying EEV, CEV, and IMV
1 × 105 HeLa cells were infected with WR at a MOI of 5 for 2 hours. After which, the inoculum was removed and the cells were washed twice with media and incubated overnight. At 24h post infection, the media covering the infected monolayer was removed and saved (EEV). The cells were washed once with Dulbecco’s Modified Eagle medium (DMEM) and then overlaid with DMEM containing 0.001mg/ml trypsin (Worthington) and incubated for one hour to release CEV from the cell surface. The supernatant (CEV) was removed from the monolayer and fetal bovine serum was added to 5% to inactivate the trypsin. To harvest IMV, 1mL of 100mM Tris (pH 9.0) was added to the infected cell monolayer, and the cells were released by scraping and added to a microfuge tube. Tubes containing EEV and CEV were centrifuged at 18,000 × g for 1 min to pellet intact cells and cellular debris. Supernatants were transferred to fresh tubes and pellets were added to the tube containing the harvested cells. Cells were lysed by three rapid freeze/thaw cycles using a dry ice/ethanol bath and a 37° C water bath. The lysate was centrifuged at 18,000 × g for 1 min and the resulting pellet was ground in the tube with a micro pestle to ensure virion release. For trypsinization of IMV, an aliquot of 400µ L was removed and added to an equal volume of 0.25mg/ml trypsin and incubated at 37° C for 30 min after which the trypsin was inactivated with fetal bovine serum added to 5%. For DNAseI treatment, a 400µ l aliquot of the trypsinized IMV sample was removed and added to a tube containing 44µ l of 10× DNAse I Buffer and treated with 40 units of DNAse I.
3. RESULTS
3.1. Assay design and detection limits
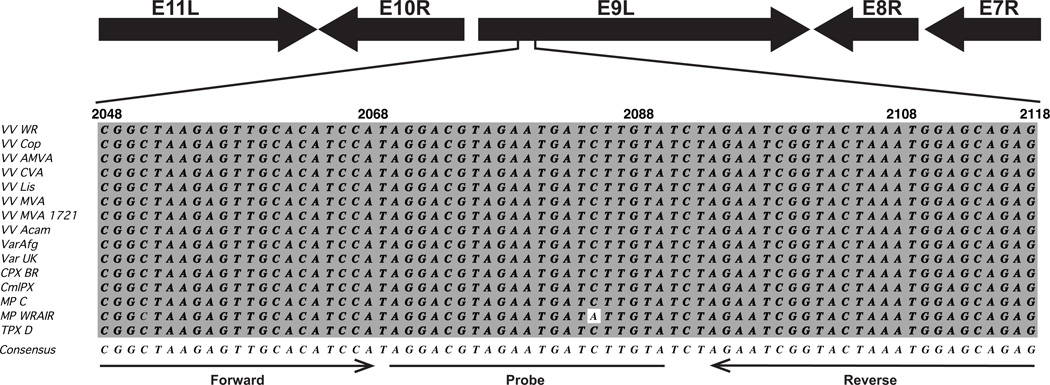
Design of a qPCR assay to quantify the number of VV genomes in a virus stock would provide a method that is faster and more versatile than traditional methods of virus quantitation. The number of VV genomes should be directly related to the number of viral particles because it is believed that there is only one copy of the genome in each particle (Condit, Moussatche, and Traktman, 2006). VV has a large dsDNA genome that is over 190 kb in length. The versatility of the assay could be increased if the same set of primers and probe could be used for multiple members of the Orthopoxvirus genus. Therefore the DNA polymerase gene 065 (Copenhagen designation E9L) of VV strain Western Reserve (WR) was chosen as the target for the qPCR assay because it is well conserved across several strains of VV and other members of the orthopoxviruses genus. Indeed, an alignment of WR 065 and its orthologs from 14 different orthopoxviruses revealed multiple regions of homology (not shown). Using Primer Express Software (Applied Biosystems), several potential target sequences in the WR 065 ORF were identified from which a qPCR primer and probe set could be constructed (not shown). After comparing the potential target sequences in WR 065 to the homology in the alignment of the DNA polymerase gene from 14 different orthopoxvirus, the region of bp 2048–2118 in WR 065 was selected as the target sequence. This region is well conserved across all of the compared strains and species. Indeed, looking at all of the aligned sequences, onlyMonkeypox WRAIR has a single nucleotide difference (Fig. 1). The following primer and probe set suggested by the software for the target sequence was used: forward primer: CGGCTAAGAGTTGCACATCCA, reverse primer: CTCTGCTCCATTTAGTACCGATTCT, TaqMan® Minor Groove Binding (MGB) probe with the sequence AGGACGTAGAATGATCTTGTA.
Figure 1.
Alignment of primers and probe sequences used in the qPCR assay. Top, diagram of the genomic region containing the E9L gene and showing the direction of the neighboring open reading frames. The relative position of the sequence used to design the qPCR assay is demarked. Bottom, alignment of bp 2068–2118 from WR within the 065 gene to several different species and strains of orthopoxviruses. Viruses listed are: VV strains; Western Reserve (VV WR), Copenhagen (VV Cop), Acambis 3000 Modified Virus Ankara (VV AMVA), Chorioallantois Virus Ankara (VV CVA), Lister (VV Lis), Modified Virus Ankara (VV MVA), Modified Virus Anakara-1721 (VV MVA 1721), Acambis 2000 (VV Acam); Variola virus strains; Afganistan 1970 (Var Afg), United Kingdom 1952 Butler (Var UK). Cowpox Virus Brighton Red (CPX BR), Camelpox M96 (CmlPX), Monkeypox Congo and Walter Reed Army Institute of Research (MPC and MP WRAIR respectively), and Taterapox virus Dahomey (TPX D). Bases identical to the consensus sequence (bottom) are shaded gray. The position and direction of primers and probe used in qPCR assays is indicated at the bottom.
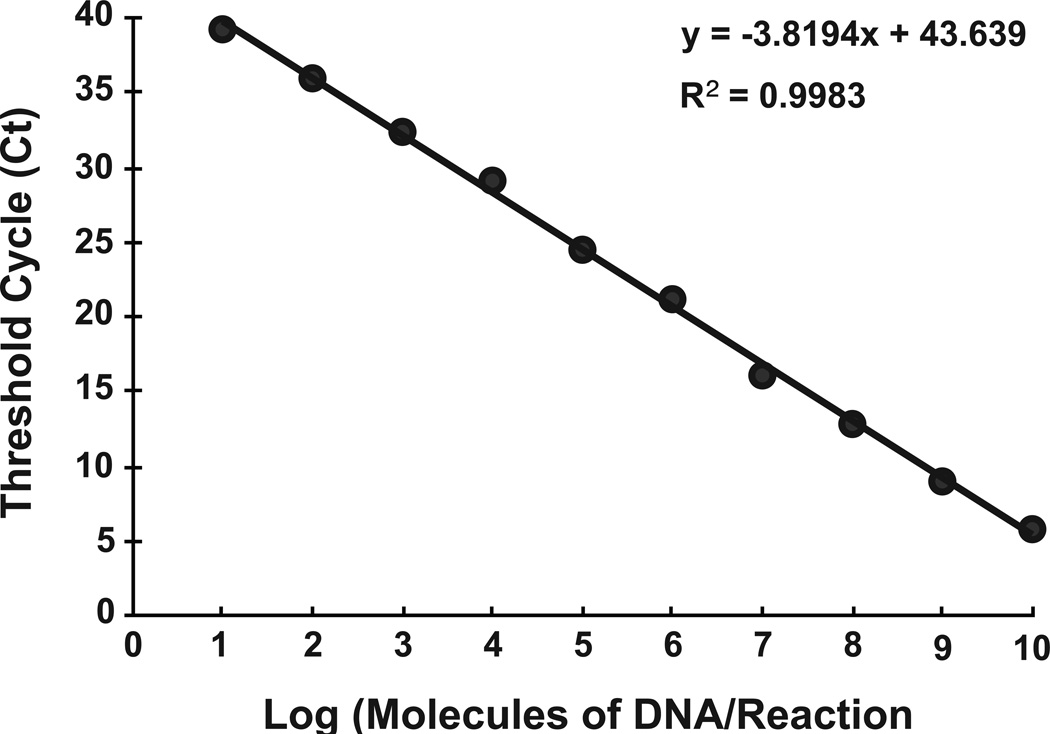
To validate the primer set, and to generate a standard curve, the entire coding sequence of WR 065 was cloned into a plasmid (pE9L). In an effort to emulate the viral genome, which is a linear dsDNA molecule, pE9L was linearized by restriction digest, which would remove supercoiling of the plasmid. The linearized plasmid was repurified, quantified, and 10 fold serial dilutions, between 10-1010 gene copies/reaction, were made and real-time qPCR assay was performed in triplicate for each dilution. By plotting the Ct values against the number of copies in each dilution, a linear regression line was drawn with an r2 value of 0.996 (Fig. 2) representing a strong linear relationship over 9 logs down to 10 copies/reaction.
Figure 2.
Standard curve for the developed qPCR assay. Ten-fold dilutions between 101 and 1010 molecules of pE9L were assayed using qPCR and the threshold number (Ct) calculated for each and graphed. The calculated Ct represents the average of 3 different assays. A best-fit line and the coefficient of determination (R2) were calculated.
3.2. Quantitation of VV genome from purified virus
The above result demonstrates that the novel qPCR assay is capable of detecting different concentrations of the plasmid template in a linear fashion between 10-1010 copies of the E9L gene. To determine if the assay was capable of quantifying the viral genome, DNA was extracted from VV that had been purified using a CsCl gradient. qPCR performed on the extracted DNA and a 10-fold dilution determined that there were 1.59 × 108 and 1.53 × 107 genome copies in each, respectively. These results verify that there is a linear correlation between the number of viral genomes and the Ct value obtained in the assay.
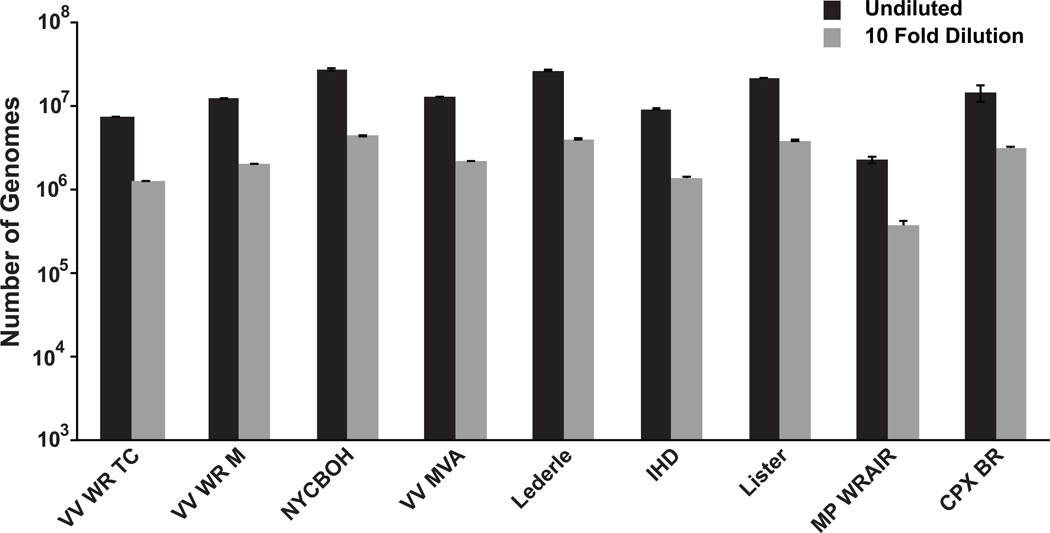
The primers for the assay were chosen based on their homology to the DNA polymerase gene from several different orthopoxviruses. Therefore the qPCR assay should be capable of quantifying the genomes from several different orthopoxviruses. To this end, purified genomic DNA from VV strains Lister, IHD, Lederle-Chorioallantoic, New York City Board of Health, and Modified Vaccinia Ankara was obtained to test in the qPCR assay. In addition two other orthopoxvirus genomes were tested, Cowpox Virus Brighton Red, and monkeypox virus Walter Reed Army Institute of Research 7–61. All of these showed an ~ 10 fold decrease in genome copy number by qPCR when diluted 10 fold (Fig. 3). Surprisingly, this included the genome from monkeypox, which is predicted to have a mismatched base with the probe sequence (Fig. 1).
Figure 3.
Quantitation of the genomes from 9 different orthopoxviruses. Purified genome from Vaccinia Virus strains; Western Reserve tissue culture adapted and muse adapted (VV WR TC, and VV WR M, respectively), New York City Board of Health (NYCBOH), modified virus Ankara (VV MVA), Lederle, International Health Department (IHD), Lister, in addition to cowpox strain Brighton Red (CPX BR), and monkeypox Walter Reed Army Institute of Research (MP WRAIR) were purchased from BEI resources. The preparations were used either undiluted or diluted 10 fold before being quantified using the qPCR assay.
3.3. Comparison of qPCR to three methods of virus titration
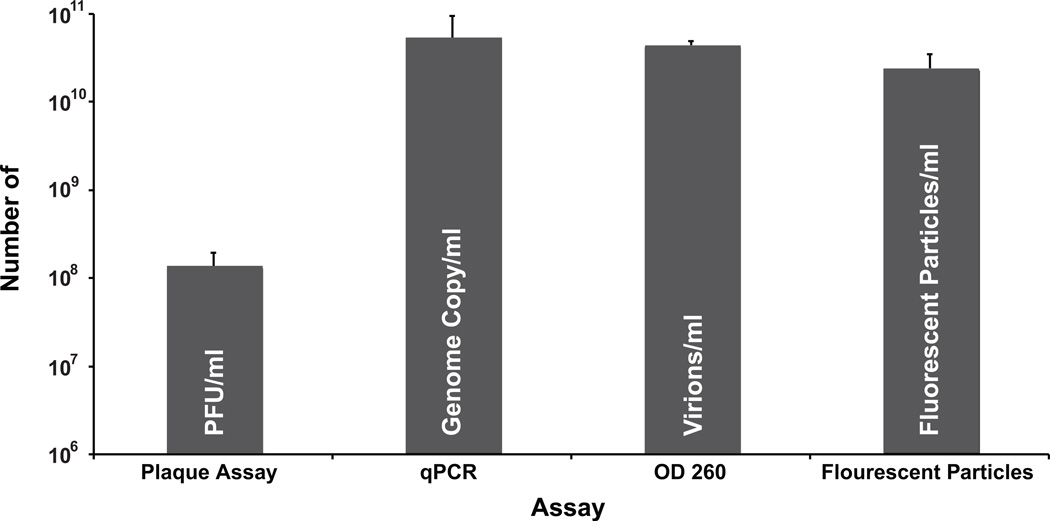
The results obtained with the qPCR assay were compared to those obtained through several other traditional methods of quantifying virus, namely plaque assay, OD 260 measurements, and direct particle count. VV was one of the first viruses accurately titrated by plaque assay (Moss, 2001). Indeed, VV, reproducibly produces plaques that are easily visualized by staining with crystal violet on monolayers of several different cell lines making it a widely used technique for determining viral titers. For comparison, gradient purified virus was titrated by either traditional plaque assay or by qPCR assay. The qPCR assay determined that there were 5.3 × 1010 genome copies/ml in the sample, while the plaque assay revealed that there was only 1.3 ×108 plaque forming units (PFU)/ml (Fig. 4) yielding a particle to PFU ratio of 408:1. This large discrepancy suggests that many of the particles in the purified virus preparation are either inactive or potentially aggregated together. Alternatively, there may be a large amount of unpackaged genome that co-purifies with the virions.
Figure 4.
Quantitation of purified VV vA4-YFP by four different methods. The same stock of purified vA4-YFP was quantified by plaque assay, qPCR assay, measuring OD260, and counting fluorescent particles using a hemocytometer as described in the materials and methods. All measurements are represented as /ml of the initial stock.
Optical density at 260nm, (OD260), is used for a variety of viruses to determine the amount of virions in a purified stock (Joklik, 1962; Mittereder, March, and Trapnell, 1996; Smith, Zweerink, and Joklik, 1969). For VV, it was determined empirically that there were 1.2 × 1010 “elementary bodies per ODU at 260 mµ” in a purified stock of virus (Joklik, 1962). This conversion was determined by directly counting the number of particles in a known volume using electron microscopy. By measuring the OD260 of the purified stock it was determined that it contained 4.4 × 1010 particles/ml (Fig. 4) resulting in a particle to PFU ratio of 338:1. A number that closely agrees with the results obtained using the qPCR assay.
VV produces brick shaped particles that are ~300 × 250 µm in size and are visible using light microscopy (Condit et al., 2006; Cudmore et al., 1995; Vanderplasschen and Smith, 1997). In addition, several recombinant VV have been made that express viral protein/fluorescent protein chimeras (Carter et al., 2003; Doceul et al., 2010; Hollinshead et al., 2001; Ward, 2005; Ward and Moss, 2001b). One such recombinant, vA4-YFP, has YFP attached to the C-terminus of the viral core protein A4 (Ward, 2005). This recombinant produces virions that fluoresce brightly making them readily visible by conventional fluorescent microscopy. One can directly count the number of virions in a purified virus prep of vA4-YFP using a hemocytometer and fluorescent microscopy. Using a sealed hemocytometer it was determined that there were 2.4 × 1010 particles/ml in the sample, a number that is in close agreement with both the qPCR and the OD260 measurement, but still 2 logs greater than that determined by plaque assay (Fig. 4).
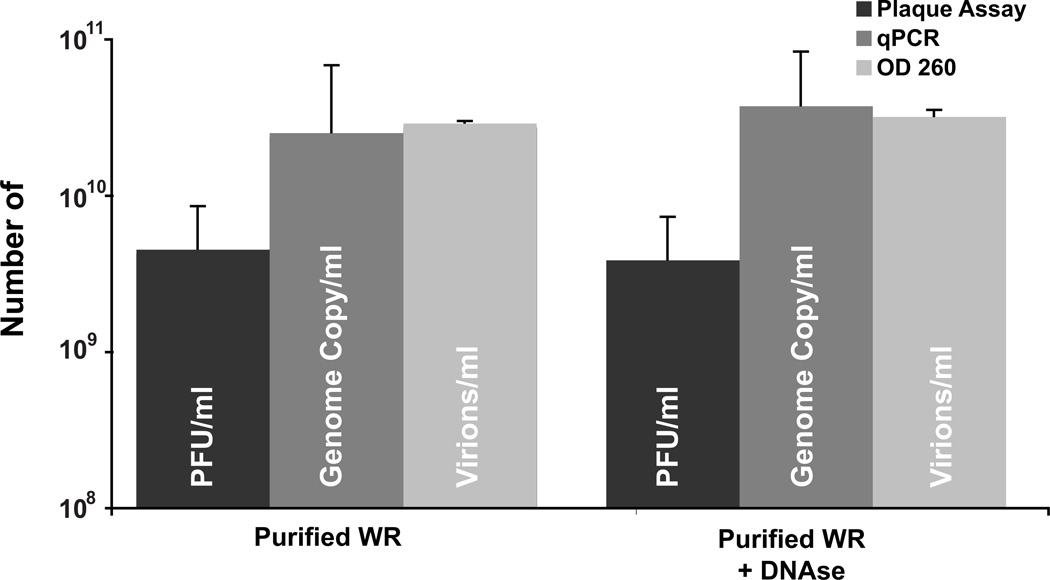
The authors can think of three reasons for the large discrepancy between the PFUs and the qPCR/OD260 measurements. It is possible that the vast majority of particles in the purified prep are either not infectious or aggregated together as this was an older preparation of purified virus. Alternatively, (but not exclusively) there could be a large amount of unpackaged viral DNA associated with the purified virions. To try and determine the extent unpackaged DNA contributed to genome copy in the qPCR assay, a fresh stock of purified virus was prepared and titered (Fig. 5). The fresh preparation resulted in a higher titer of 4.5 × 109 PFU/ml. Quantitation by either qPCR assay or OD260 yielded counts similar to the previous preparation of 2.5 × 1010 and 2.9 × 1010 respectively. The calculated particle to PFU ratio of 5:1 and 6:1 for qPCR and OD260 respectively, is similar to ones published previously for purified VV preparations (Joklik, 1962; Locker et al., 2000; Vanderplasschen and Smith, 1997). To determine the extent unpackaged DNA was quantified by the qPCR assay, an aliquot of the viral prep was treated with DNAse I, and quantitation was repeated using all three assays. The treatment had little to no affect on all three assays (Fig. 5) indicating that the additional DNA quantified by the qPCR and OD260 measurements is protected from DNAse I and likely packaged in virions that are not infectious.
Figure 5.
Quantitation of purified WR virions after DNAse treatment. The same stock of purified vA4-YFP was titered by plaque assay, qPCR and OD260, before and after a 30 min treatment with DNAse I. All measurements are represented as /ml of the initial stock.
3.4. Quantitation of VV from infected cell lysate
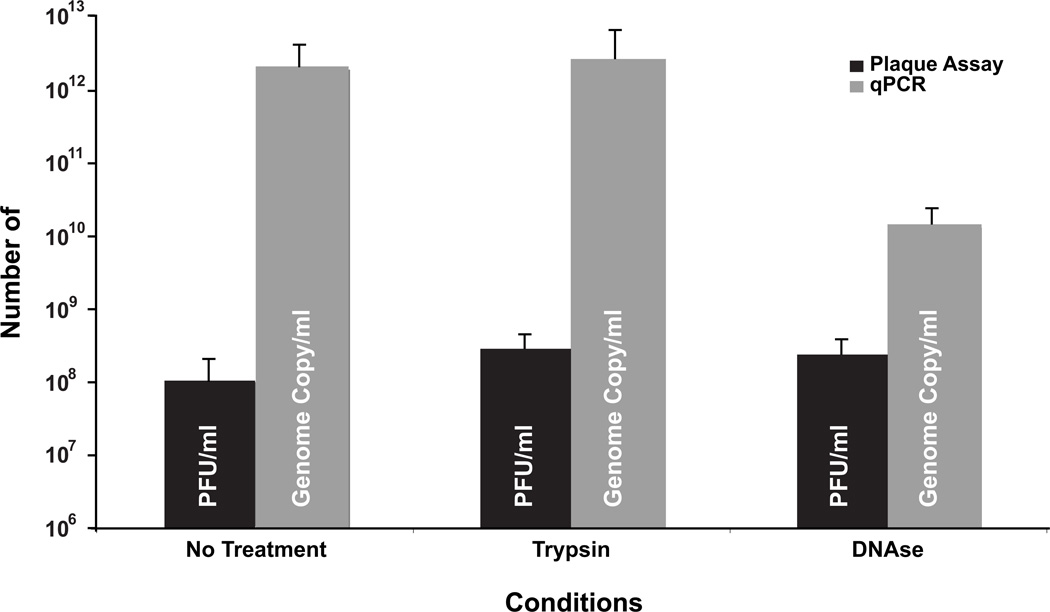
Whereas purified virus is used for some assays with vaccinia virus, stocks of virus are typically produced from a crude lysate of infected cells and consist mostly of IMV (Earl and Moss, 1991). These are not amenable to titration by measuring OD260 or direct counting of particles because the large amount of cell debris in the lysate would interfere with these assays. To determine if the qPCR assay was capable of quantifying genome copies from a crude lysate of infected cells, HeLa cells that had been infected with WR were lysed by snap freezing and thawing three times followed by grinding with micro pestle. Viral genomes were purified from an aliquot of the lysate and quantified by qPCR. In addition, the number of infectious virions in the lysate was determined by plaque assay. Using the qPCR assay, it was determined that there were 2.16 × 1012 copies of the genome per ml of lysate (Fig. 6). This represents a large difference (~4 logs) compared to 1 × 108 PFU per ml of lysate determined by plaque assay.
Figure 6.
Quantitation of intracellular mature virions (IMV) from cell lysate. IMV was harvested from HeLa cells infected with VV WR at an M.O.I. of 5 at 24 h.p.i. The lysate resulting from freeze/thaw lysis was quantified by both plaque assay and qPCR before and after treatment with trypsin and DNAse I. All measurements are represented as /ml of the initial lysate.
Moderate treatment of vaccinia virus lysates with trypsin can increase the number of PFU in the stock (Earl and Moss, 1991). To determine if trypsin would affect the qPCR assay, the lysate was treated with trypsin and the assays were repeated. As had been reported previously (Gifford and Klapper, 1969), treatment with trypsin increased the number of PFU in the stock by almost two-fold while having little effect on the number of genomes as determined by the qPCR assay (Fig. 6). Although the trypsin treatment gave a modest increase in PFUs, there was still a large difference between the number of genomes and the number of PFUs. To determine the extent unpackaged DNA contributed to the large number of genomes in the lysate, the trypsinized lysate was treated with DNAseI and the assays were repeated. DNAse treatment had little effect on the number of PFUs in the stock but reduced the number of genomes by more than 2 logs as determined by qPCR (Fig. 6) revealing that the vast majority of the viral DNA in the sample is not packaged.
3.5. Quantitation of EV
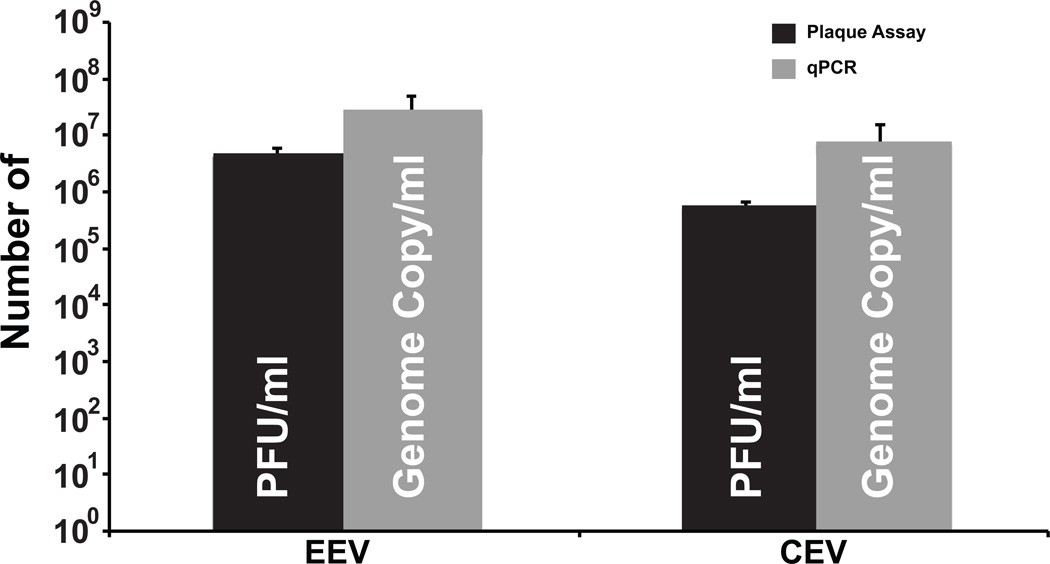
Orthopoxviruses have a complex morphogenesis and produce two morphologically distinct infectious forms, IMV and EV (Smith and Law, 2004). EV are derived from a subset of IMV that have received an additional envelope from post-Golgi compartments. EV are actively transported out of infected cells while IMV largely remain intracellular. EV can be further divided into two types, those found attached to the outer surface of infected cells (CEV) and those released from the cell surface (EEV). To look at the ratio of these two forms of virus and compare their PFU to genome copy, CEV and EEV were isolated from infected cells quantified by qPCR and plaque assay. Comparing the number of genomes determined by qPCR for EEV (Fig. 7) to the PFU resulted in a particle to PFU ratio of 6:1. This ratio is elevated for CEV (14:1) and likely reflects inactivation by the trypsin treatment used to release CEV from the cell surface. The number of EEV genomes is roughly 3 fold greater than CEV genomes suggesting that slightly more EV are released from the cell surface compared to those that remain attached.
Figure 7.
Quantitation of extracellular enveloped virions (EEV) and cell-associated virions (CEV). EEV and CEV from HeLa cells infected VV WR at an M.O.I. of 5 were harvested at 24 h.p.i. The amount of each was determined by plaque assay and qPCR.
4. DISCUSSION
Vaccinia virus is widely used in the laboratory as an expression vector for both molecular and immunological studies. Plaque assay has long been the preferred method of quantitation of VV, as well as many other viruses, because this method empirically determines the amount of infectious virus in a sample. In addition, the assay is simple, easily interpretable, and reliable. Despite this, the plaque assay does have several drawbacks. It is only applicable to viruses that can be grown in tissue culture adapted cells that form monolayers. The infection must damage the cells so that a visible plaque forms. Also, the assay can take several days to complete, is not amenable to automation, and only measures the amount of infectious virions produced. Therefore, virions that do not infect the assay cells remain unaccounted. Additional methods of quantitation for purified virions have been reported. These include measuring protein levels, optical density, and counting virion sized particles via electron microscopy. In this report, a real-time qPCR assay for quantifying the number of VV genomes is described. The number of genomes determined in the purified stock of VV was of the same magnitude as the number of particles in that sample as determined by either measuring the OD260 or directly counting the number of fluorescent particles (Fig. 4). This shows that qPCR can be used in place of these two methods. Whereas measuring OD260 or counting virions would be quicker than qPCR for determining the number of particles in a purified sample, these assays are inherently dependent on the purity of sample as impurities such as cellular debris and DNA would affect the readout. In contrast the qPCR assay is applicable to both pure and crude lysates of virus. Several PCR based assays have been developed for the identification of various orthopoxviruses but they are not specifically quantitative (Aitichou et al., 2008; Li et al., 2007; Saijo et al., 2008; Scaramozzino et al., 2007; Shchelkunov, Gavrilova, and Babkin, 2005). The qPCR assay described here shows linearity over 9 logs of genome copy (Fig. 2) and detected genomes from several different orthopoxviruses (Fig. 3) making it suitable for very sensitive quantitation of a broad range of these viruses.
Quantifying the number of genomes by qPCR from a purified virion preparation resulted in similar numbers of particles obtained by measuring OD260 and directly counting fluorescent virions. However, titration of the preparation by plaque assay yielded numbers of infectious virions that were much lower (Fig. 4). Considering that the DNAse treatment had little effect on the number of genome copies and the OD260 measurement in the purified virus preparation, it seems likely that these represent a large fraction of non-infectious virus. Surprisingly, there was also a very large difference between the number of genomes and PFUs from the cell lysate. This difference was drastically reduced when lysates were treated with DNAseI. Initially, 1 × 105 cells were infected with an MOI of 5 so that all of the cells should have been infected. Using the qPCR assay, it was determined that there was 2 × 1012 copies of the genome in the lysate and of these, 1.5 × 1010 copies were unaffected by DNAse treatment. This indicates that on average each cell produced 2 × 107 genomes, 1.5×105 of which were protected from the affects of DNAse. These results indicate that there is a large amount of viral genome that is made but not packaged. It is unclear why much of the genome is replicated if it is not going to be packaged. It is clear that for accurate results using qPCR, treatment of the cell lysate is required to remove unpackaged genomes.
When quantifying the amount of EV it was found that 17% of the EEV were infectious. In contrast, only 7% of the CEV where infectious. This could be due to the trypsin treatment used to release this form of EV from the cell surface. Regardless, it is surprising that by qPCR, ~20% of the total EV is stuck to the surface of the cell and is typically missed by only assaying the media from infected cells. Several mutants have been reported that affect EV production (Blasco and Moss, 1991; Engelstad and Smith, 1993; McIntosh and Smith, 1996; Parkinson and Smith, 1994; Roper and Moss, 1999; Roper et al., 1998; Sanderson, Hollinshead, and Smith, 2000; Wolffe, Isaacs, and Moss, 1993). As EV production is required for plaque formation, the qPCR assay should be useful for accurate quantitation of the numbers of virions produced by these mutations. In addition, some mutations affect not only the amount of EV produced but also their infectivity (McIntosh and Smith, 1996; Roper et al., 1998). The qPCR assay provides a quick and easy assay to determine the particle to PFU ratio to help understand the nature of these types of mutations.
In summary, the authors have developed a fast, broadly applicable, qPCR assay to quantitate the number of VV genomes in a sample. The assay is linear over 9 logs of genome copy and yields results similar to those obtained by OD260 and direct particle count.
Highlights.
-
! !
Quantitative real-time PCR assay for enumerating vaccinia virus particles
-
! !
Applicable to several different species of orthopoxviruses
-
! !
Yielded similar results to those obtained using OD260 and direct virion counting
-
! !
Protocol for isolating and enumerating the three forms of progeny orthopoxviruses, IMV, CEV, and EEV.
Acknowledgements
Parts of this work were funded by National Institute of Allergy and Infectious Diseases grant AI067391, AI081082 and contract AI50020.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitichou M, Saleh S, Kyusung P, Huggins J, O'Guinn M, Jahrling P, Ibrahim S. Dual-probe real-time PCR assay for detection of variola or other orthopoxviruses with dried reagents. Journal of virological methods. 2008;153:190–195. doi: 10.1016/j.jviromet.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard G, Hapel AJ, Boulter EA. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 1971;13:9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- Blasco R, Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 1991;65:5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco R, Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 1992;66:4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter EA, Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog. Med. Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- Breitbach CJ, Thorne SH, Bell JC, Kirn DH. Targeted and armed oncolytic poxviruses for cancer: the lead example of JX-594. Curr Pharm Biotechnol. 2012;13:1768–1772. doi: 10.2174/138920112800958922. [DOI] [PubMed] [Google Scholar]
- Carter GC, Rodger G, Murphy BJ, Law M, Krauss O, Hollinshead M, Smith GL. Vaccinia virus cores are transported on microtubules. J. Gen. Virol. 2003;84:2443–2458. doi: 10.1099/vir.0.19271-0. [DOI] [PubMed] [Google Scholar]
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- DesRoches P. Smallpox vaccination--implications for the occupational health professional. AAOHN journal : official journal of the American Association of Occupational Health Nurses. 2003;51:240–242. [PubMed] [Google Scholar]
- Doceul V, Hollinshead M, van der Linden L, Smith GL. Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science. 2010;327:873–876. doi: 10.1126/science.1183173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Moss B. Generation of recombinant vaccinia viruses. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Greene Publishing Associates & Wiley Interscience; 1991. pp. 16.17.1–16.17.16. [Google Scholar]
- Engelstad M, Smith GL. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology. 1993;194:627–637. doi: 10.1006/viro.1993.1302. [DOI] [PubMed] [Google Scholar]
- Fuerst TR, Earl PL, Moss B. Use of a hybrid vaccinia virus T7 RNA polymerase system for expression of target genes. Mol. Cell. Biol. 1987;7:2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford GE, Klapper DG. Effect of proteolytic enzymes on vaccinia virus replication. Arch Gesamte Virusforsch. 1969;26:321–333. doi: 10.1007/BF01250942. [DOI] [PubMed] [Google Scholar]
- Hollinshead M, Rodger G, Van Eijl H, Law M, Hollinshead R, Vaux DJ, Smith GL. Vaccinia virus utilizes microtubules for movement to the cell surface. J. Cell Biol. 2001;154:389–402. doi: 10.1083/jcb.200104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik WK. The purification of four strains of poxvirus. Virology. 1962;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Kim JW, Gulley JL. Poxviral vectors for cancer immunotherapy. Expert Opin Biol Ther. 2012;12:463–478. doi: 10.1517/14712598.2012.668516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Li Y, Ropp SL, Zhao H, Damon IK, Esposito JJ. Orthopoxvirus pan-genomic DNA assay. Journal of virological methods. 2007;141:154–165. doi: 10.1016/j.jviromet.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Locker JK, Kuehn A, Schleich S, Rutter G, Hohenberg H, Wepf R, Griffiths G. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Molecular biology of the cell. 2000;11:2497–2511. doi: 10.1091/mbc.11.7.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AA, Smith GL. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J. Virol. 1996;70:272–281. doi: 10.1128/jvi.70.1.272-281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. Journal of virology. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MR, Niles EG. Transient and inducible expression of vaccinia/T7 recombinant viruses. Methods Mol. Biol. 2004;269:41–50. doi: 10.1385/1-59259-789-0:041. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxviridae: The viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott-Raven Publishers; 2001. pp. 2849–2883. [Google Scholar]
- Moss B. Poxviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott, Williams & Wilkins; 2007. pp. 2905–2946. [Google Scholar]
- Moss B, Earl P. Expression of proteins in mammalian cells using vaccinia viral vectors. Overview of the vaccinia virus expression system. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology, Greene Publishing Associates. New York: Wiley Interscience; 1998. pp. 16.15.1–16.15.5. [Google Scholar]
- Parkinson JE, Smith GL. Vaccinia virus gene A36R encodes a M(r) 43–50 K protein on the surface of extracellular enveloped virus. Virology. 1994;204:376–390. doi: 10.1006/viro.1994.1542. [DOI] [PubMed] [Google Scholar]
- Payne LG. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Price PJ, Torres-Dominguez LE, Brandmuller C, Sutter G, Lehmann MH. Modified vaccinia virus Ankara: Innate immune activation and induction of cellular signalling. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Roper RL, Moss B. Envelope formation is blocked by mutation of a sequence related to the HKD phospholipid metabolism motif in the vaccinia virus F13L protein. J. Virol. 1999;73:1108–1117. doi: 10.1128/jvi.73.2.1108-1117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RL, Wolffe EJ, Weisberg A, Moss B. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J. Virol. 1998;72:4192–4204. doi: 10.1128/jvi.72.5.4192-4204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo M, Ami Y, Suzaki Y, Nagata N, Iwata N, Hasegawa H, Ogata M, Fukushi S, Mizutani T, Iizuka I, Sakai K, Sata T, Kurata T, Kurane I, Morikawa S. Diagnosis and assessment of monkeypox virus (MPXV) infection by quantitative PCR assay: differentiation of Congo Basin and West African MPXV strains. Japanese journal of infectious diseases. 2008;61:140–142. [PubMed] [Google Scholar]
- Sanderson CM, Hollinshead M, Smith GL. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particlesJGen. Virol. 2000;81:47–58. doi: 10.1099/0022-1317-81-1-47. [DOI] [PubMed] [Google Scholar]
- Scaramozzino N, Ferrier-Rembert A, Favier AL, Rothlisberger C, Richard S, Crance JM, Meyer H, Garin D. Real-time PCR to identify variola virus or other human pathogenic orthopox viruses. Clinical chemistry. 2007;53:606–613. doi: 10.1373/clinchem.2006.068635. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Sodeik B, Ericsson M, Wolffe EJ, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J. Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchelkunov SN, Gavrilova EV, Babkin IV. Multiplex PCR detection and species differentiation of orthopoxviruses pathogenic to humans. Molecular and cellular probes. 2005;19:1–8. doi: 10.1016/j.mcp.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Law M. The exit of vaccinia virus from infected cells. Virus. Res. 2004;106:189–197. doi: 10.1016/j.virusres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Smith RE, Zweerink HJ, Joklik WK. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969;39:791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. EurJCell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- Vanderplasschen A, Smith GL. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. Journal of virology. 1997;71:4032–4041. doi: 10.1128/jvi.71.5.4032-4041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BM. Visualization and characterization of the intracellular movement of vaccinia virus intracellular mature virions. J. Virol. 2005;79:4755–4763. doi: 10.1128/JVI.79.8.4755-4763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BM, Moss B. Vaccinia virus intracellular movement is associated with microtubules and independent of actin tails. J. Virol. 2001a;75:11651–11663. doi: 10.1128/JVI.75.23.11651-11663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BM, Moss B. Visualization of intracellular movement of vaccinia virus virions containing a green fluorescent protein-B5R membrane protein chimera. J. Virol. 2001b;75:4802–4813. doi: 10.1128/JVI.75.10.4802-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe EJ, Isaacs SN, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J. Virol. 1993;67:4732–4741. doi: 10.1128/jvi.67.8.4732-4741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Bruno DP, Martens CA, Porcella SF, Moss B. Simultaneous high-resolution analysis of vaccinia virus and host cell transcriptomes by deep RNA sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11513–11518. doi: 10.1073/pnas.1006594107. [DOI] [PMC free article] [PubMed] [Google Scholar]