Abstract
Human sulfatase 2 (SULF2) functions as an oncoprotein in hepatocellular carcinoma (HCC) development by promoting tumor growth and metastasis via enhancement of fibroblast growth factor-2/extracellular signal-regulated kinase and WNT/ β-catenin signaling. Recent results implicate that SULF2 activates the transforming growth factor beta (TGFB) and Hedgehog/GLI1 pathways in HCC. OKN-007 is a novel phenyl–sulfonyl compound that inhibits the enzymatic activity of SULF2. To investigate the antitumor effect of OKN-007 in HCC, we treated Huh7 cells, which express high levels of SULF2, with OKN-007 and found that it significantly promoted tumor cell apoptosis and inhibited cell proliferation, viability, and migration. To understand the action of OKN-007 on SULF2, we used Huh7 cells which normally express SULF2 and Hep3B cells that do not normally express SULF2. Utilizing Huh7 cells transfected with short hairpin RNA targeting SULF2 and transfection of Hep3B cells with a SULF2 plasmid to enhance SULF2 expression, we showed that the antitumor activity of OKN-007 was more pronounced in cells expressing SULF2. Furthermore, in vivo experiments verified that OKN-007 repressed tumor growth significantly. These results identify SULF2 as an important target of the antitumor effect of OKN-007. To determine the molecular mechanism of the antitumor effect of OKN-007, both TGFB1/SMAD and Hedgehog/GLI1 signaling pathway activity were measured by Western blot and SMAD- or GLI-reporter luciferase assays. We found that both signaling pathways were inhibited by OKN-007. Together, these results show that OKN-007 can suppress TGFB1/SMAD and Hedgehog/GLI1 signaling via its inhibition of SULF2 enzymatic activity. We conclude that OKN-007 or more potent derivatives may be promising agents for the treatment of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common malignant liver tumor and the third most frequent cause of death from cancer (Parkin et al., 2005; El-Serag and Rudolph, 2007). Only 10–20% of HCCs are diagnosed at an early stage; consequently, most patients are not candidates for curative therapy, such as liver resection or liver transplantation. Locoregional therapy using radiofrequency ablation or chemoembolization is palliative and results in only transient benefit (Sandhu et al., 2008; Faivre et al., 2011). Moreover, current systemic chemotherapy for HCC patients is of limited effectiveness (Roxburgh and Evans, 2008; Rahbari et al., 2011). There is, therefore, an urgent need for new and more effective targeted agents against HCC.
The human sulfatase 2 (SULF2) gene at 20q13 encodes an extracellular enzyme which catalyzes the removal of 6-O-sulfate groups from the heparan sulfate (HS) disaccharide units of heparan sulfate proteoglycans (HSPGs) (Morimoto-Tomita et al., 2002; Lai et al., 2008). HSPGs are present on the cell surface and in the extracellular matrix and act either as sequestration sites for growth factor and cytokine ligands or as coreceptors in multiple cell signaling pathways (Pye et al., 1998, 2000). Desulfation of HSPGs by SULF2 is presumed to decrease the affinity of HSPGs for a variety of signaling ligands, releasing them from sequestration sites and making them available for signaling (Uchimura et al., 2006; Rosen and Lemjabbar-Alaoui, 2010; Bret et al., 2011). In our previous study, SULF2 was found to promote proliferation and migration of HCC cells in vitro and to be correlated with larger tumor volume and poor survival in HCC xenografts in nude mice (Lai et al., 2010a). SULF2 is overexpressed in the majority of human HCCs and is associated with more rapid recurrence and a worse prognosis (Lai et al., 2010a). We have shown that FGF-mediated extracellular signal-regulated kinase (ERK) signaling and WNT/β-catenin signaling are enhanced by SULF2 (Lai et al., 2008, 2010a,b). We also recently found that both transforming growth factor beta (TGFB)1/SMAD2 signaling and Hedgehog/GLI1 signaling are enhanced by SULF2 during liver carcinogenesis and liver regeneration (unpublished data). SULF2 has also been shown to be upregulated in breast cancer and to have oncogenic effects in lung and pancreas cancer (Morimoto-Tomita et al., 2005; Nawroth et al., 2007; Lemjabbar-Alaoui et al., 2010). Given the oncogenic effects of SULF2 in cancer, it has been proposed as a target for cancer therapy (Lemjabbar-Alaoui et al., 2010; Rosen and Lemjabbar-Alaoui, 2010; Schelwies et al., 2010).
2,4-Disulfophenyl-N-tert-butylnitrone (designated OKN-007; formerly NXY-059) is a disulfonyl nitrone which has undergone extensive human safety studies in trials exploring its efficacy as a treatment for acute ischemic stroke and shown to be a safe compound (Shuaib et al., 2007; Diener et al., 2008; Cucchiara et al., 2009; Amaro and Chamorro, 2011). In earlier studies, phenyl-tert-butylnitrone, from which OKN-007 was derived, was shown to inhibit development of preneoplastic lesions in a rat model of liver carcinogenesis and also showed antineoplastic effects in an in vivo rat glioma model (Garteiser et al., 2010; He et al., 2011). Recently, OKN-007 has been shown to inhibit the enzymatic activity of SULF2 in both kidney and breast tumor cells (unpublished data). On the basis of its inhibitory activity against SULF2, we proposed the hypothesis that OKN-007 has an antitumor action against HCC.
In our studies to date, SULF2 has been shown to have an oncogenic effect in HCC via regulation of fibroblast growth factor-2 (FGF2)/ERK, WNT/ β-catenin, TGFB1/SMAD2 signaling, and Hedgehog (Hh) /GLI1 signaling (Lai et al., 2008, 2010a,b; Yang et al., 2011). We therefore tested the hypothesis that OKN-007 synchronously suppresses the activation of TGFB1/SMAD2 signaling and Hedgehog/GLI1 signaling via inhibition of SULF2, and consequently inhibits the progression of HCC. To test this hypothesis, we addressed the following questions: (1) What is the effect of OKN-007 on apoptosis, proliferation, viability, and migration of HCC cells? (2) Does the expression of SULF2 in HCC cells render them more susceptible to the antitumor effect of OKN-007? (3) Is the antitumor effect of OKN-007 mediated through inactivation of TGFB1/SMAD2 signaling and Hedgehog/GLI1 signaling in HCC cells?
MATERIALS AND METHODS
Chemicals and Antibodies
The OKN-007 compound (purity, >98.5%) was synthesized by Synterys (Union City, CA) and provided by the Oklahoma Medical Research Foundation. Recombinant human TGFB1 was obtained from R&D Systems (Minneapolis, MN). The BrdU ELISA kit was from Roche (Indianapolis, IN). The Caspase-Glo® 3/7 Assay kit and Apo-ONE® Homogeneous Caspase-3/7 Assay were from Promega (Madison, WI). Complete Mini Protease Inhibitor Mixture and 4,6-diamidino-2-phenylindole (DAPI), anti-β-actin primary antibody (A-5316), and anti-mouse secondary antibodies conjugated with HRP (A3673) were from Sigma Chemical (St. Louis, MO); anti-SMAD2 (#3103), and anti-GLI1 (#2534S) primary antibodies were from Cell Signaling (Beverly, MA); anti-rabbit secondary antibodies conjugated with HRP (ALI3403) were from Biosource (Carlsbad, CA), and the HyGLO HRP detection kit was from Denville (Metuchen, NJ). We generated a rabbit polyclonal antibody against SULF2 using a peptide from the SULF2 coding sequence (amino acids 421–444: HKRDNDKVDAQEENFLPKYQ RVKD, Genbank accession number NM_018837) (Lai et al., 2008). The pcDNA3.1 vector was obtained from Invitrogen (Carlsbad, CA) and the pSS-H1p vector was a kind gift from Dr. Daniel D. Billadeau (Mayo Clinic, Rochester, MN). The GLI-Luciferase reporter plasmid was kindly provided by Dr. Martin E. Fernandez-Zapico (Mayo Clinic, Rochester, MN). The SBE12-lux reporter was kindly provided by Dr. Edward B. Leof (Mayo Clinic, Rochester, MN).
HCC Cell Lines
The Hep3B and SNU387 HCC cell lines were obtained from the American Type Culture Collection (Manassas, VA). The Hep3B cell line was cultured in complete MEM medium with 10% fetal bovine serum (FBS). The SNU387 cell line was cultured in RPMI 1640 medium with 10% FBS. The Huh7 cell line was obtained from the Japan Health Science Research Resources Bank (HSRRB, Osaka, Japan) and was grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% FBS.
Establishment of SULF2 Stable Transfectant Clones
A recombinant plasmid expressing full-length SULF2 cDNA cloned into the pcDNA3.1 expression vector was used as a SULF2-expressing plasmid. Short hairpin RNAs (shRNA) targeting the SULF2 mRNA were cloned into the vector pSS-H1p. The sequences of shRNA targeting SULF2 mRNA include shRNA-a (AAGTACGTCCACAACCACA) and shRNA-b (AATGTGACTGTCACAAAAT). The vector pSS-H1p containing scrambled target sequence was used as controls. Hep3B cells were grown to 80–90% confluence and transfected with SULF2-expressing plasmid using FuGENE® 6 Transfection Reagent from Roche (Indianapolis, IN). Geneticin (G418) from Invitrogen (Carlsbad, CA) at a dose of 300 μg/mL was used to select stably SULF2-expressing clones. Similarly, Huh7 cells were transfected with both SULF2 shRNA-a and shRNA-b synchronously using FuGENE® 6. The stable transfectant clones were obtained after 2-week selection with 600 μg/mL G418.
Relative Quantitative Real-Time Reverse Transcription PCR
Total RNA was extracted from HCC cell lines using the RNeasy kit from Qiagen (Valencia, CA). cDNA synthesis was performed using the High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems (Carlsbad, CA) to transcribe 2 μg of total RNA. Real-time reverse transcription PCR (RT-PCR) analysis was performed with ABI TaqMan assays in an ABI 7300 system using the following profile: 95°C for 10 min followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. The following ABI TaqMan probes were used: 18s rRNA (Hs99999901_s1) and SULF2 (Hs 00378697_m1). Each measurement was performed six times.
Western Immunoblotting
Western immunoblotting was performed as described previously (Lai et al., 2010a). Thirty micrograms of protein was separated by denaturing gel electrophoresis. After transfer to PVDF membrane, blots were probed overnight with the following anti-human primary antibodies: anti-GLI1 antibody (1:1,000 dilution), anti-phospho-ERK (1:1,000 dilution), antitotal ERK (1:1,000 dilution), anti-phospho-SMAD2 (1:1,000 dilution), anti-SMAD2 (1:1,000 dilution), anti-SULF2 (1:1,000 dilution), and anti-β-actin antibody (1:1,000 dilution). Blots were then incubated with anti-rabbit or anti-mouse secondary antibodies conjugated with HRP (1:5,000 dilution), and signals were visualized using the HyGLO HRP detection kit from Denville (Metuchen, NJ). β-Actin was measured to control for equal loading.
Luciferase Reporter Assay
Huh7 cells plated in six-well plates at 80% confluence were transfected with the different reporter plasmids using FuGENE® 6 Transfection Reagent. Luciferase activity was measured using the Dual-Luciferase Reporter assay from Promega (Madison, WI) and normalized by protein quantification. Before the luciferase assay for TGFB1 signaling, Huh7 cells were incubated in DMEM with 1% FBS for 24 h and stimulated with 10 ng/mL of recombinant human TGFB1 for 72 h. Each data point represents an average of six independent transfections.
Cell Proliferation and Cell Viability Assays
HCC cells were seeded into 96-well plates at 3,000 cells per well for 24 h and then treated with different concentrations of OKN-007. After 5 days of OKN-007 treatment, the BrdU ELISA assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay were performed to assess cell proliferation and viability. All experiments were repeated at least six times.
Detection of Cell Apoptosis
Cell apoptosis was assessed by fluorescence microscopy to identify apoptotic nuclear changes (chromatin condensation and nuclear fragmentation) after staining cells with DAPI. HCC cells were plated in six-well plates at a density of 1.5 × 105 cells per well. After incubation at 37°C in 5% CO2 for 24 h, the media was changed. OKN-007 was then added at different concentrations, and the cells were incubated at 37°C in 5% CO2 for 24 h. Five micrograms of DAPI was added to each well, and the plates were incubated for 15 min at 37°C. The cells were then examined by fluorescence microscopy and apoptotic cells were counted. All experiments were repeated at least six times.
Caspase 3/7 Activity Assay
HCC cells were plated into black 96-well plates at a density of 5,000 cells per well and cultured for 24 h. OKN-007 was then added at different concentrations. After 48 h, the Caspase 3/7 activity assay was performed using the Apo-ONE® Homogeneous Caspase-3/7 Assay kit or the Caspase-Glo® 3/7 Assay kit. The results were obtained using a Fluoroskan or Luminoskan. All experiments were repeated independently at least six times.
Migration Assay
HCC cells were plated onto six-well plates and grown to confluence. Scratch wounds were made with a 1,000-μL pipette tip. The HCC cells were treated with different concentrations of OKN-007. At 0, 24, and 48 h after OKN-007 treatment, the wounds were photographed with a phase-contrast microscope. Cell migration was quantitated by measuring the width of the wounds. The experiments were repeated at least six times.
Statistical Analysis
All data represent at least three independent experiments and are expressed as means and standard errors of the mean. Differences between groups were compared using the Mann–Whitney U-test.
RESULTS
OKN-007 Induces Apoptosis and Inhibits Cell Proliferation, Viability, and Migration in Huh7 Cells Expressing High Levels of SULF2
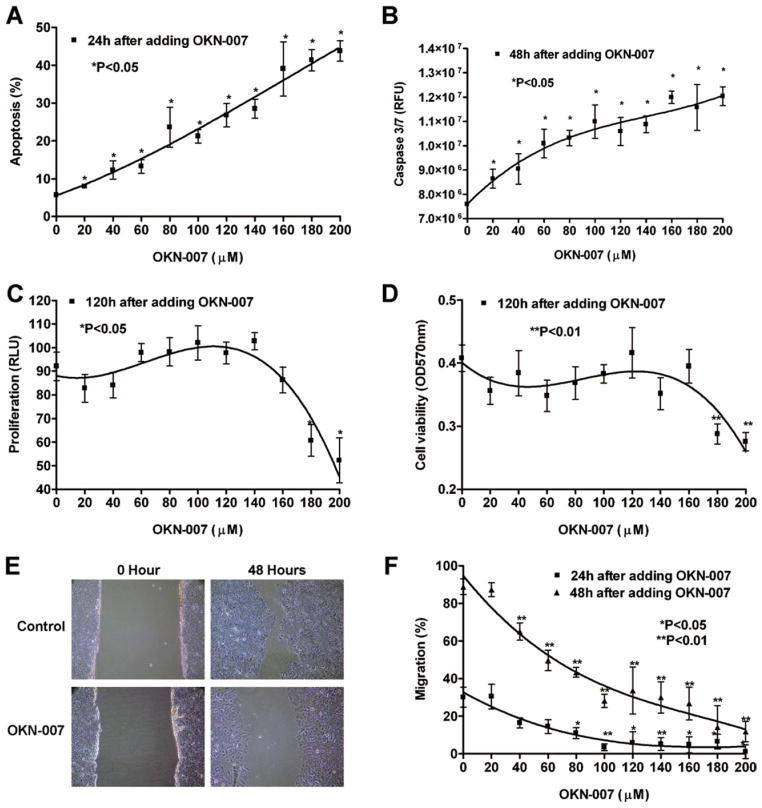
We have shown that Huh7 cells have a relative high level of SULF2 expression (Lai et al., 2010a). To investigate the antitumor effect of the SULF2 inhibitor OKN-007 in HCC, we treated Huh7 cells with increasing concentrations of OKN-007 (0–200 μM) for 24 h. Apoptosis was assessed by staining with DAPI followed by fluorescence microscopy. OKN-007 induced a dose-dependent increase in apoptosis of Huh7 cells (Fig. 1A; P < 0.05). Concurrent with OKN-007 induction of apoptosis, the activity of the proapoptotic caspases 3 and 7 also showed a dose-dependent increase over a 48-h period (Fig. 1B; P < 0.05). In similar experiments conducted over 5 days to assess the effect of OKN-007 on cell proliferation, BrdU incorporation was not affected until concentrations of 180 μM (P = 0.0084) and 200 μM (P = 0.008) were reached, indicating that proliferation of Huh7 cells is relatively resistant to suppression by OKN-007 (Fig. 1C). Similarly, Huh7 cell viability was relatively resistant to suppression by OKN-007, as measured by the MTT assay (Fig. 1D; P = 0.0021 for 180 μM; P = 0.0009 for 200 μM). In contrast, migration of Huh7 cells as assessed by a wound healing assay was more sensitive to treatment with OKN-007 (Figs. 1E and 1F).
Figure 1.
OKN-007 induces apoptosis and inhibits cell proliferation, viability, and migration in Huh7 cells, which express high levels of SULF2. (A) OKN-007 induced a dose-dependent increase in apoptosis of Huh7 cells as assessed by staining with DAPI followed by fluorescence microscopy. (B) The activity of the proapoptotic caspases 3 and 7 also showed a dose-dependent increase after OKN-007 treatment for 48 h. The Apo-ONE® Homogeneous Caspase-3/7 Assay kit was used and the unit of the results is RFU. (C) Cell proliferation as measured by BrdU incorporation was not affected by OKN-007 until concentrations of 180 and 200 μM were reached. (D) Huh7 cell viability was also relatively resistant to suppression by OKN-007, as measured by the MTT assay. (E) Images of wound-healing assays in Huh7 cells treated with control diluent or 200 μM OKN-007 at 0 and 48 h. (F) OKN-007 substantially decreased the rate of wound healing. OKN-007 treatment for both 24 and 48 h caused a significant dose-dependent decrease in migration of Huh7 cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Knockdown of SULF2 Suppressed the Antitumor Effect of OKN-007 in Huh7 Cells
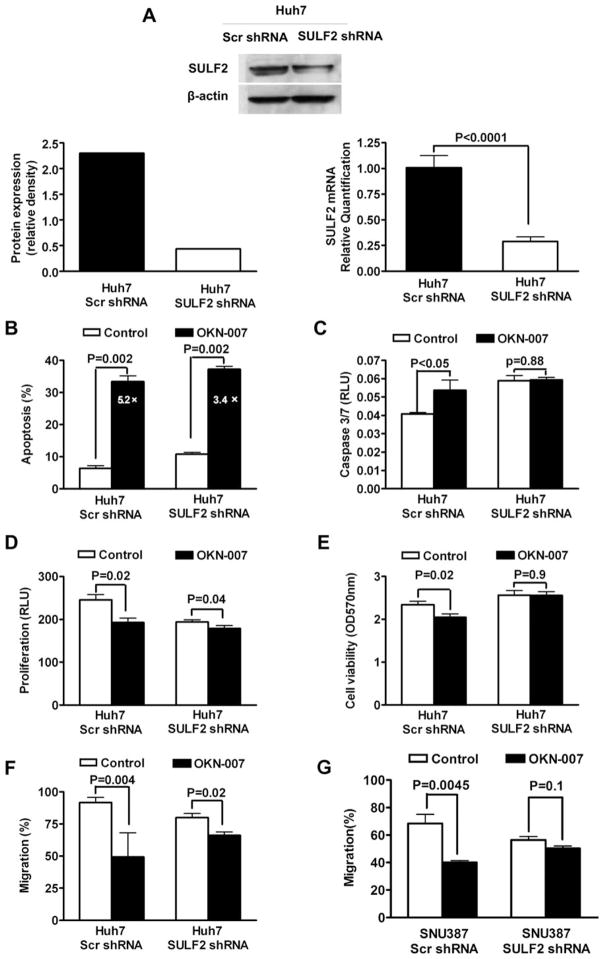
To determine whether the antitumor effect of OKN-007 on HCC occurs via inhibition of SULF2, we silenced the expression of SULF2 in Huh7 cells using plasmids expressing shRNAs targeting SULF2 mRNA (Huh7 SULF2 shRNA) and measured the antitumor effect of OKN-007. Measurement of SULF2 mRNA by quantitative RT-PCR (qRT-PCR) showed that SULF2 mRNA was reduced about 71% by stable transfection with SULF2 shRNAs (Fig. 2A; P < 0.0001). The immunoblotting results also confirmed that SULF2 protein expression of Huh7 SULF2 shRNA cells was substantially decreased by 77% compared with SULF2 protein expression in Huh7 cells stably transfected with scrambled shRNA (Huh7 Scr shRNA cells) (Fig. 2A).
Figure 2.
Knockdown of SULF2 suppresses the antitumor effect of OKN-007 in Huh7 cells. (A) Both SULF2 mRNA and protein were significantly downregulated by stable transfection with SULF2 shRNAs as assessed by qRT-PCR and caspase. (B) Knockdown of SULF2 resulted in an increased percent apoptosis in untreated Huh7 SULF2 shRNA cells and a lower fold increase in apoptosis after OKN-007 treatment (3.4-fold). In contrast, untreated control Huh7 Scr shRNA cells exhibited a lower percent apoptosis and were more sensitive to OKN-007-induced apoptosis, with a 5.2-fold increase in apoptosis after OKN-007 treatment. (C) Cells transfected with control scrambled shRNA exhibited a significant increase after treatment with OKN-007, whereas cells transfected with SULF2 shRNAs exhibited no significant difference in caspase 3/7 activity after OKN-007 treatment. The Caspase-Glo® 3/7 Assay kit was used and the unit of the results is RLU. (D) Knockdown of SULF2 decreased the basal rate of cell proliferation, and also substantially decreased the effect of OKN-007 on proliferation of Huh7 cells. (E) Basal cell viability of Huh7 cells was not significantly altered by transfection with shRNAs targeting SULF2. OKN-007 treatment of the SULF2 expressing control cells led to a significant decrease in cell viability, whereas the shRNA-transfected cells with suppressed SULF2 exhibited no change in cell viability on treatment with OKN-007. (F) OKN-007 treatment for 48 h substantially inhibited migration of control Huh7 Scr shRNA cells by 47%; in contrast, Huh7 SULF2 shRNA cells exhibited a much lower 17% inhibition of migration in response to OKN-007. (G) The inhibitory effect of OKN-007 on migration ability of SNU387 cells was decreased by silencing SULF2 expression with shRNAs targeting SULF2.
Measurement of OKN-007-induced apoptosis by DAPI staining and fluorescence microscopy showed that knockdown of SULF2 resulted in an increased percent apoptosis in untreated Huh7 SULF2 shRNA cells and a lower fold increase in apoptosis after OKN-007 treatment (3.4-fold). In contrast, untreated Huh7 Scr shRNA cells exhibited a lower percent apoptosis and were more sensitive to OKN-007-induced apoptosis, with a 5.2-fold increase in apoptosis after OKN-007 treatment (Fig. 2B). Similar results were obtained by the measurement of caspase 3/7 activity of Huh7 cells. Cells transfected with control-scrambled shRNA exhibited a significant increase (P < 0.05) after treatment with OKN-007, whereas cells transfected with SULF2 shRNAs exhibited no significant difference (P = 0.88) in caspase 3/7 activity after OKN-007 treatment (Fig. 2C).
Next, we assessed the effect of suppression of SULF2 expression on OKN-007-induced inhibition of cell proliferation. Knockdown of SULF2 decreased the basal rate of cell proliferation, and also substantially decreased the effect of OKN-007 on cell proliferation (Fig. 2D). A similar effect was shown on cell viability as measured by the MTT assay. Although basal cell viability of Huh7 cells transfected with control scrambled shRNA was not significantly altered by transfection with shRNAs targeting SULF2, OKN-007 treatment of the SULF2 expressing control cells led to a significant decrease in cell viability (P = 0.02), whereas the shRNA-transfected cells with suppressed SULF2 exhibited no change (P = 0.9) in cell viability on treatment with OKN-007 (Fig. 2E).
Finally, we assessed the effect of suppression of SULF2 expression on OKN-007-induced inhibition of HCC cell migration. OKN-007 treatment for 48 h substantially inhibited migration of control Huh7 Scr shRNA cells by 47% (P = 0.004), whereas in contrast Huh7 SULF2 shRNA cells exhibited a much lower 17% inhibition of migration (P = 0.02) in response to OKN-007 (Fig. 2F). Thus, the antimigratory effect of OKN-007 was much stronger in SULF2-expressing Huh7 Scr shRNA cells than in SULF2-suppressed Huh7 SULF2 shRNA cells. Similar results were found in SNU387 cells which express a relative high level of SULF2 (Lai et al., 2008). Knockdown of SULF2 markedly attenuated the inhibitory effect of OKN-007 on SNU387 cell migration (Fig. 2G; P < 0.0001).
Forced Expression of SULF2 in SULF2-Negative Hep3B Cells Sensitizes Hep3B Cells to OKN-007 Treatment
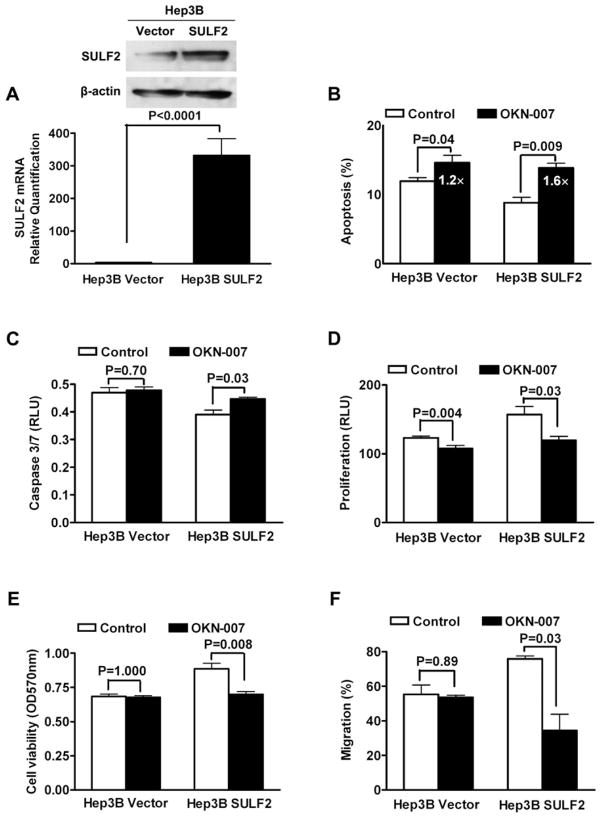
To investigate further whether SULF2 is the target of the antitumor effects of OKN-007 in HCC, we increased SULF2 expression in SULF2-negative Hep3B cells and assessed the antitumor effects of OKN-007. The SULF2 mRNA and protein levels of Hep3B cells were substantially increased by stable transfection with a SULF2-expressing plasmid (Hep3B SULF2 cells) compared with Hep3B cells transfected with empty plasmid vector (Hep3B Vector cells) (Fig. 3A; P < 0.0001).
Figure 3.
Forced expression of SULF2 in SULF2-negative Hep3B cells sensitizes Hep3B cells to OKN-007 treatment. (A) The mRNA and protein levels of SULF2 in Hep3B cells were significantly increased by transfection with a SULF2-expressing plasmid, as assessed by qRT-PCR and caspase. (B) Forced expression of SULF2 inhibited the basal level of apoptosis of Hep3B cells as assessed by DAPI staining and fluorescence microscopy. SULF2-transfected Hep3B SULF2 cells were substantially more sensitive to OKN-007-induced apoptosis, showing an almost 60% increase in apoptosis compared with the 22% induction of apoptosis in Hep3B Vector cells. (C) There was no substantial induction of caspase 3/7 activity by OKN-007 in Hep3B Vector cells, whereas Hep3B SULF2 cells exhibited a 15% increase in caspase 3/7 activity. The Caspase-Glo® 3/7 Assay kit was used and the unit of the results is RLU. (D) Forced expression of SULF2 promoted proliferation of Hep3B cells. Treatment with 200 μM OKN-007 for 5 days suppressed the proliferative effect of SULF2 in Hep3B cells. The effect of OKN-007 on proliferation of Hep3B SULF2 cells was substantially greater than the effect on SULF2-negative Hep3B Vector cells (reduction of 23% compared with reduction of 13%). (E) Assessment of cell viability using the MTT assay verified that overexpression of SULF2 increased the viability of Hep3B cells and that the effect of OKN-007 on viability of Hep3B cells was dependent on SULF2 expression. (F) Migration of Hep3B cells was significantly enhanced by overexpression of SULF2. Treatment with 200 μM OKN-007 had no significant effect on migration of Hep3B Vector cells, but substantially reduced the migration rate of Hep3B SULF2 cells by 55%.
Forced expression of SULF2 inhibited the basal level of apoptosis of Hep3B cells as assessed by DAPI staining and fluorescence microscopy (Fig. 3B), confirming the antiapoptotic effect of SULF2 in HCC. SULF2-transfected Hep3B SULF2 cells were substantially more sensitive to OKN-007-induced apoptosis, showing an almost 60% increase in apoptosis compared with the 22% induction of apoptosis in Hep3B Vector cells (Fig. 3B). A similar effect was observed with OKN-007 induction of proapoptotic caspase 3/7 activity; there was no substantial induction of caspase 3/7 activity by OKN-007 in Hep3B Vector cells (P = 0.7), whereas Hep3B SULF2 cells exhibited a 15% increase (P = 0.03) in caspase 3/7 activity (Fig. 3C).
Forced expression of SULF2 promoted proliferation of Hep3B cells, as assessed by BrdU incorporation. OKN-007 treatment (200 μM) for 5 days suppressed the proliferative effect of SULF2 in Hep3B cells. The effect of OKN-007 on Hep3B SULF2 cells was substantially greater than the effect on SULF2-negative Hep3B Vector cells (reduction of 23% compared to reduction of 13%) (Fig. 3D). There were similar effects of SULF2 and OKN-007 on cell viability as assessed by the MTT assay (Fig. 3E). Migration of Hep3B cells was significantly enhanced by over-expression of SULF2. OKN-007 (200 μM) had no significant effect on migration of Hep3B Vector cells (P = 0.89), but substantially reduced the migration rates of Hep3B SULF2 cells by about 55% (P = 0.03) (Fig. 3F).
The effects of OKN-007 on apoptosis, proliferation, cell viability, and migration are, therefore, all more pronounced in cells expressing SULF2, confirming that SULF2 is a major target for the effects of OKN-007.
OKN-007 Inactivates TGFB1/SMAD2 Signaling and Hedgehog/GLI1 Signaling in Huh7 Cells
To determine the specific molecular mechanisms of the antitumor effect of OKN-007 on HCC cells, we measured the effect of OKN-007 on the activity of two well-known oncogenic signaling pathways in HCC, including TGFB1/ SMAD2 signaling and Hedgehog/GLI1 signaling. First, luciferase expression was measured in SULF2-positive Huh7 cells transfected with luciferase reporter constructs responsive to activated TGFB1 pathway SMAD transcription factors or to the Hh pathway GLI transcription factors. OKN-007 treatment significantly suppressed SMAD-responsive luciferase activity (Fig. 4A; P = 0.03), as well as GLI-responsive luciferase activity (Fig. 4B; P = 0.03). Immunoblotting confirmed the concomitant suppression of the activated transcription factors pSMAD2 (Fig. 4A) and GLI1 (Fig. 4B). These data support the hypothesis that OKN-007 treatment suppresses the activation of signaling pathways and mediators that we have previously shown to be responsive to SULF2 expression, including TGFB1/SMAD2 and Hedgehog/GLI1.
Figure 4.
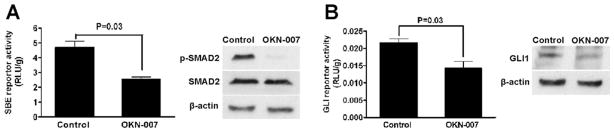
OKN-007 inhibits TGFB1/SMAD2 and Hedgehog/GLI1 signaling in Huh7 cells. (A) OKN-007 treatment was shown to repress the TGFB1/SMAD2 signaling pathway in Huh7 cells by both SBE reporter luciferase activity assay and immunoblotting for phospho-SMAD2. (B) OKN-007 treatment was shown to suppress the Hh/GLI1 signaling pathway in Huh7 cells by both a GLI reporter luciferase activity assay and immunoblotting for GLI1 protein.
OKN-007 Inhibits Tumorigenesis in Nude Mice in a SULF2-Dependent Manner
To verify the in vitro effect of OKN-007 in vivo, we inoculated 5 × 105 Huh7 SULF2 shRNA and Huh7 Scr shRNA cells subcutaneously into 22 nude mice each and observed the growth of the tumors over time. When the volume of xenografts reached 150–300 mm3, the nude mice were randomly assigned to two groups of 11 mice and treated respectively with 200 μM OKN-007 dissolved in phosphate-buffered saline (PBS) at a total dose of 50 mg/kg body weight in drinking water or the same volume of PBS for control. The mice were treated with PBS or OKN-007 in the drinking water daily for 1 week, every other day for 1 week, and every 3 days for two more weeks. Tumor size was measured with calipers every 3 days and the mice were sacrificed when the tumor volume reached 2,000 mm3. OKN-007 modestly repressed the growth of tumors with normal expression of SULF2 (Fig. 5A), but did not affect the growth of tumors derived from cells in which SULF2 expression was silenced (Fig. 5B). Further, knockdown of SULF2 rescued the growth inhibitory effect of OKN-007 (Fig. 5C). Analysis of tumor growth using a Kaplan–Meier curve showed that the median time to reach a tumor size of 2,000 mm3 was increased by 18 days in tumors derived from Huh7 Scr shRNA cells, indicating that SULF2 expression is required for the anti-HCC effect of OKN-007 in vivo.
Figure 5.
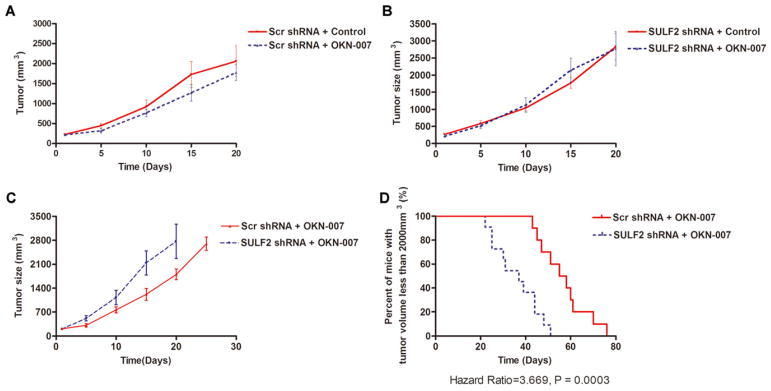
SULF2 enhances the efficacy of OKN-007 in growth inhibition of HCC xenografts. (A) OKN-007 represses tumor growth in mice bearing Huh7 xenografts (P = 0.0003). (B) In xenografts from Huh7 cells with suppressed expression of SULF2, there was no significant difference between tumor growth in the OKN-007 group versus the control group. (C) Knockdown of SULF2 blocked the growth inhibitory effect of OKN-007 in Huh7 xenografts. (D) Silencing SULF2 decreased the time to reach a tumor size of 2,000 mm3 (hazard ratio = 3.669; P = 0.0003), confirming that SULF2 is required for the antineoplastic function of OKN-007. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Multiple oncogenic signaling pathways have been implicated in the progression of HCC, a factor that potentially contributes to the failure of therapies that target either one pathway alone or a limited number of signaling pathways (Papoulas and Theocharis, 2009; Thomas, 2009; Whittaker et al., 2010). It is therefore critical to identify additional oncogenic targets for the treatment of HCC, particularly those targets that can regulate several oncogenic signaling pathways coordinately and/or synchronously. Such targets would have the potential for the creation of high-performance targeted agents for HCC therapy. Many studies have shown that significant amounts of the ligands for multiple signaling pathways can be sequestered by binding to HSPGs on the cell surface and in the extracellular matrix (Sanderson et al., 2005; Selleck, 2006; Ji et al., 2011). SULF2 is a novel member of a family of HS-degrading endosulfatases that can desulfate HS, releasing ligands bound to HSPGs and consequently modulating multiple signaling pathways (Rosen and Lemjabbar-Alaoui, 2010; Bret et al., 2011). We have previously shown, using gene expression microarray assays, that SULF2 expression is upregulated in the majority of human HCC tissues and human HCC cell lines and that HCC patients with increased SULF2 expression have higher recurrence rates and a worse prognosis after surgical resection. In in vitro and in vivo experiments, we have proven that SULF2 can promote HCC growth by enhancing FGF2 and WNT pathway signaling (Lai et al., 2008, 2010a). Examination of gene expression microarray data also identified a number of additional oncogenic signaling pathways which correlate with SULF2 expression (Yang et al., 2011). Moreover, data from our in vitro and in vivo experiments have also demonstrated that SULF2 can activate TGFB1/SMAD2 and Hedgehog/GLI1 signaling. Hence, we consider SULF2 as a potential target for HCC therapy that can simultaneously affect multiple signaling pathways.
OKN-007 is a disulfonyl derivative of the spin-trapping agent phenyl-tert-butylnitrone and its chemical structure has been reported by us (Floyd et al., 2008, 2011). OKN-007 has undergone extensive human safety testing and has been shown to be a safe compound, even at the relatively high micromolar concentrations (300 μM) at which it is active (Cheng et al., 2008). In the clinical trials of OKN-007 (NXY-059) for acute stroke (SAINT I and II), a serum concentration of 260 μM for OKN-007 was maintained, which was proven to be safe for patients (Shuaib et al., 2007; Diener et al., 2008; Cucchiara et al., 2009). We previously found that OKN-007 inhibits the enzymatic function of SULF2 in the HEK-293 human kidney cancer cell line and MCF-7 breast cancer cells. Additionally, in a glioma rat model imaged by magnetic resonance imaging, OKN-007 suppressed the growth of gliomas (Garteiser et al., 2010; He et al., 2011). OKN-007, or more potent derivatives currently under development, may therefore be a good candidate for targeted inhibition of SULF2 in cancer.
Given the evidence for antitumor activity and SULF2 inhibition of OKN-007, as well as our previous results on the oncogenic role of SULF2 in HCC, we hypothesized that inhibition of SULF2 would have an antitumor effect in HCC cells, particularly those expressing significant levels of SULF2. We therefore assessed the antitumor effect of OKN-007 in Huh7 cells, which express a high level of SULF2, and found that OKN-007 promoted apoptosis of Huh7 cells with a concomitant increase in caspase3/7 activity. OKN-007 also significantly inhibited cell proliferation, viability, and migration. All of the antitumor effects occurred in a dose-dependent manner, but the effects on apoptosis and migration occurred at lower doses than the effects on cell proliferation and cell viability. To investigate whether the anti-HCC effects of OKN-007 were directed specifically against SULF2, we assessed the antitumor effects of OKN-007 in Huh7 cells after silencing SULF2 expression. The results confirmed that suppression of SULF2 promoted cell apoptosis and suppressed proliferation, viability, and migration of Huh7 cells, and further, that cells with knockdown of SULF2 exhibited decreased sensitivity to the proapoptotic, antiproliferative, and antimigratory functions of OKN-007 in Huh7 cells. The effect of OKN-007 on migration of HCC cells expressing SULF2 was also confirmed using the SNU387 HCC cell line. On the other hand, forced expression of SULF2 in Hep3B cells with endogenous low expression of SULF2 confirmed the effects of SULF2 on the survival, growth, and migration of HCC cells. Further, Hep3B cells stably transfected with SULF2-expressing plasmid were more sensitive to OKN-007-induced facilitation of apoptosis and inhibition of cell proliferation, viability, and migration. Conversely, control Vector-transfected Hep3B cells were more resistant to the effects of OKN-007 on apoptosis, growth, and migration of tumor cells. These data strongly suggest that the effects of OKN-007 on the growth and migration of HCC cells are mediated, at least in part, through inhibition of SULF2.
To determine the specific molecular pathways inhibited by OKN-007, we assessed the effects of OKN-007 on the activation of two tumor-promoting signaling pathways known to be enhanced by SULF2, TGFB1/SMAD2, and Hedgehog/GLI1 signaling. OKN-007 synchronously inhibited both oncogenic signaling pathways in HCC cells.
Finally, to verify further the requirement for SULF2 in the anti-HCC function of OKN-007, we carried out an in vivo mouse xenograft experiment, examining the effect of OKN-007 on the growth of xenografts established from a control SULF2-expressing Huh7 cell line transfected with a Vector expressing a scrambled shRNA sequence compared with xenografts from Huh7 cells with stable knockdown of SULF2 expression. The results indicated that OKN-007 suppressed the growth of SULF2-expressing Huh7 HCC xenografts, but had no effect on xenografts with knockdown of SULF2. Thus, SULF2 expression is required for the effects of OKN-007 both in vitro and in vivo.
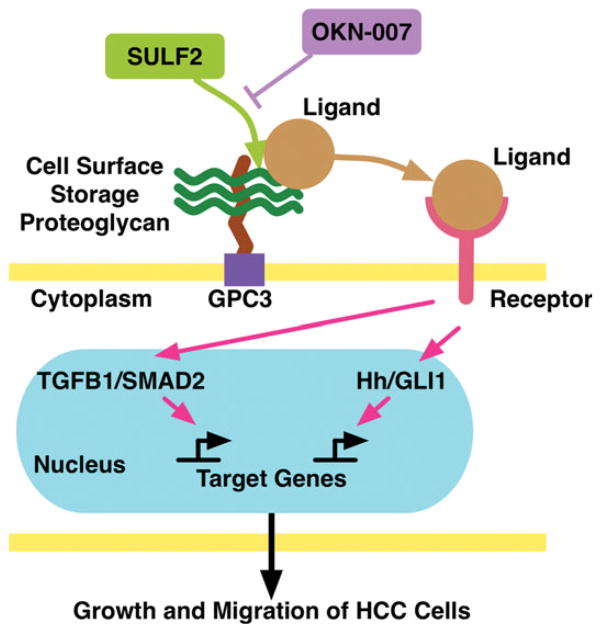
In conclusion, as shown in Figure 6, SULF2 is an oncogenic protein which promotes the progression of HCC by releasing ligands from HSPGs and regulating multiple cell signaling pathways. The SULF2 inhibitor, 2,4-disulfophenyl-N-tert-butylnitrone (OKN-007) induces apoptosis and inhibits growth and migration of HCC cells via inhibition of multiple oncogenic signaling pathways including TGFB1/SMAD2 and Hedgehog/ GLI1 signaling at concentrations which have been shown to be safe for health volunteers and patients with acute stroke. Given its antitumor activity and known safety in humans, OKN-007 may be a promising candidate for clinical evaluation as a targeted therapy for HCC.
Figure 6.
Working model for the effects of SULF2 and its inhibitor OKN-007 on HCC progression. Forced expression of SULF2 desulfates HS on HSPGs such as GPC3, which is highly expressed in HCC, releasing bound ligands. The ligands released by SULF2 are freed to bind with their cognate receptors and subsequently activate several signaling pathways, including TGFB1/SMAD2 signaling and Hh/GLI1 signaling, which promote the survival, growth, and migration of HCC cells. Inhibition of SULF2 by OKN-007 blocks the enzymatic function of SULF2 and prevents ligand release from HSPGs. Consequently, SULF2 inhibition can suppress the survival and oncogenic signaling pathways and inhibit the growth and migration of HCC cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Acknowledgments
Supported by: The National Institutes of Health to Lewis R. Roberts, Grant numbers: CA100882, CA128633, and CA165076; The Mayo Clinic Center for Cell Signaling in Gastroenterology, Grant number: P30DK084567; The Mayo Clinic Cancer Center, Grant number: CA15083; The Mayo Foundation.
The authors thank Dr. Daniel D. Billadeau for provision of the pSS-H1p vector, Dr. Chi-chung Hui for provision of the GLI-Luciferase reporter plasmid, Dr. Edward B. Leof for provision of the SBE12-lux reporter plasmid, and Tammy Szewczynski and Jennifer Rud for secretarial assistance. RAF is the Merrick Foundation Chair in Aging Research.
References
- Amaro S, Chamorro A. Translational stroke research of the combination of thrombolysis and antioxidant therapy. Stroke. 2011;42:1495–1499. doi: 10.1161/STROKEAHA.111.615039. [DOI] [PubMed] [Google Scholar]
- Bret C, Moreaux J, Schved JF, Hose D, Klein B. SULFs in human neoplasia: Implication as progression and prognosis factors. J Transl Med. 2011;9:72. doi: 10.1186/1479-5876-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YF, Jiang J, Hu P, Reinholdsson I, Guo W, Asenblad N, Nilsson D. Pharmacokinetics of 8-hour intravenous infusion of NXY-059: A phase I, randomized, double-blind (within dose panels), placebo-controlled study in healthy Chinese volunteers. Clin Ther. 2008;30:2342–2353. doi: 10.1016/j.clinthera.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Cucchiara B, Kasner SE, Tanne D, Levine SR, Demchuk A, Messe SR, Sansing L, Lees KR, Lyden P. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: Pooled analysis of placebo data from the Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke. 2009;40:3067–3072. doi: 10.1161/STROKEAHA.109.554386. [DOI] [PubMed] [Google Scholar]
- Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Shuaib A, Ashwood T, Wasiewski W, Alderfer V, Hardemark HG, Rodichok L. NXY-059 for the treatment of acute stroke: Pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Faivre S, Bouattour M, Raymond E. Novel molecular therapies in hepatocellular carcinoma. Liver Int. 2011;31:151–160. doi: 10.1111/j.1478-3231.2010.02395.x. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Kopke RD, Choi CH, Foster SB, Doblas S, Towner RA. Nitrones as therapeutics. Free Radic Biol Med. 2008;45:1361–1374. doi: 10.1016/j.freeradbiomed.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA, Chandru HK, He T, Towner R. Anti-cancer activity of nitrones and observations on mechanism of action. Anticancer Agents Med Chem. 2011;11:373–379. doi: 10.2174/187152011795677517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garteiser P, Doblas S, Watanabe Y, Saunders D, Hoyle J, Lerner M, He T, Floyd RA, Towner RA. Multiparametric assessment of the antiglioma properties of OKN007 by magnetic resonance imaging. J Magn Reson Imaging. 2010;31:796–806. doi: 10.1002/jmri.22106. [DOI] [PubMed] [Google Scholar]
- He T, Doblas S, Saunders D, Casteel R, Lerner M, Ritchey JW, Snider T, Floyd RA, Towner RA. Effects of PBN and OKN007 in rodent glioma models assessed by 1H MR spectroscopy. Free Radic Biol Med. 2011;51:490–502. doi: 10.1016/j.freeradbiomed.2011.04.037. [DOI] [PubMed] [Google Scholar]
- Ji W, Yang J, Wang D, Cao L, Tan W, Qian H, Sun B, Qian Q, Yin Z, Wu M, Su C. hSulf-1 gene exhibits anticancer efficacy through negatively regulating VEGFR-2 signaling in human cancers. PLoS One. 2011;6:e23274. doi: 10.1371/journal.pone.0023274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Sandhu DS, Yu C, Han T, Moser CD, Jackson KK, Guerrero RB, Aderca I, Isomoto H, Garrity-Park MM, Zou H, Shire AM, Nagorney DM, Sanderson SO, Adjei AA, Lee JS, Thorgeirsson SS, Roberts LR. Sulfatase 2 upregulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology. 2008;47:1211–1222. doi: 10.1002/hep.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Oseini AM, Moser CD, Yu C, Elsawa SF, Hu C, Nakamura I, Han T, Aderca I, Isomoto H, Garrity-Park MM, Shire AM, Li J, Sanderson SO, Adjei AA, Fernandez-Zapico ME, Roberts LR. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology. 2010a;52:1680–1689. doi: 10.1002/hep.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Sandhu DS, Yu C, Moser CD, Hu C, Shire AM, Aderca I, Murphy LM, Adjei AA, Sanderson S, Roberts LR. Sulfatase 2 protects hepatocellular carcinoma cells against apoptosis induced by the PI3K inhibitor LY294002 and ERK and JNK kinase inhibitors. Liver Int. 2010b;30:1522–1528. doi: 10.1111/j.1478-3231.2010.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemjabbar-Alaoui H, van Zante A, Singer MS, Xue Q, Wang YQ, Tsay D, He B, Jablons DM, Rosen SD. Sulf-2, a heparan sulfate endosulfatase, promotes human lung carcinogenesis. Oncogene. 2010;29:635–646. doi: 10.1038/onc.2009.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Uchimura K, Bistrup A, Lum DH, Egeblad M, Boudreau N, Werb Z, Rosen SD. Sulf-2, a proangiogenic heparan sulfate endosulfatase, is upregulated in breast cancer. Neoplasia. 2005;7:1001–1010. doi: 10.1593/neo.05496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth R, van Zante A, Cervantes S, McManus M, Hebrok M, Rosen SD. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS One. 2007;2:e392. doi: 10.1371/journal.pone.0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoulas M, Theocharis S. Primary liver tumors: Origin and target therapy. Expert Opin Ther Targets. 2009;13:957–965. doi: 10.1517/14728220903074588. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pye DA, Vives RR, Turnbull JE, Hyde P, Gallagher JT. Heparan sulfate oligosaccharides require 6-O-sulfation for promotion of basic fibroblast growth factor mitogenic activity. J Biol Chem. 1998;273:22936–22942. doi: 10.1074/jbc.273.36.22936. [DOI] [PubMed] [Google Scholar]
- Pye DA, Vives RR, Hyde P, Gallagher JT. Regulation of FGF-1 mitogenic activity by heparan sulfate oligosaccharides is dependent on specific structural features: Differential requirements for the modulation of FGF-1 and FGF-2. Glycobiology. 2000;10:1183–1192. doi: 10.1093/glycob/10.11.1183. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Mehrabi A, Mollberg NM, Muller SA, Koch M, Buchler MW, Weitz J. Hepatocellular carcinoma: Current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- Rosen SD, Lemjabbar-Alaoui H. Sulf-2: An extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets. 2010;14:935–949. doi: 10.1517/14728222.2010.504718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh P, Evans TR. Systemic therapy of hepatocellular carcinoma: Are we making progress? Adv Ther. 2008;25:1089–1104. doi: 10.1007/s12325-008-0113-z. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: Growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- Sandhu DS, Tharayil VS, Lai JP, Roberts LR. Treatment options for hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2008;2:81–92. doi: 10.1586/17474124.2.1.81. [DOI] [PubMed] [Google Scholar]
- Schelwies M, Brinson D, Otsuki S, Hong YH, Lotz MK, Wong CH, Hanson SR. Glucosamine-6-sulfamate analogues of heparan sulfate as inhibitors of endosulfatases. Chembiochem. 2010;11:2393–2397. doi: 10.1002/cbic.201000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleck SB. Signaling from across the way: Transactivation of VEGF receptors by HSPGs. Mol Cell. 2006;22:431–432. doi: 10.1016/j.molcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Thomas M. Molecular targeted therapy for hepatocellular carcinoma. J Gastroenterol. 2009;44:136–141. doi: 10.1007/s00535-008-2252-z. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Morimoto-Tomita M, Bistrup A, Li J, Lyon M, Gallagher J, Werb Z, Rosen SD. HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: Effects on VEGF, FGF-1, and SDF-1. Biomed Chromatogr Biochem. 2006;7:2. doi: 10.1186/1471-2091-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- Yang JD, Sun Z, Hu C, Lai J, Dove R, Nakamura I, Lee JS, Thorgeirsson SS, Kang KJ, Chu IS, Roberts LR. Sulfatase 1 and sulfatase 2 in hepatocellular carcinoma: Associated signaling pathways, tumor phenotypes, and survival. Genes Chromosomes Cancer. 2011;50:122–135. doi: 10.1002/gcc.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]