Abstract
The effective transport of photosensitizers (PS) across the membrane and the intracellular accumulation of PS are the most crucial elements in antimicrobial photodynamic therapy (aPDT). However, due to the morphological complexity of Gram-negative bacteria the penetration of PS is limited, especially hydrophobic PS. Electroporation (EP) could increase the effectiveness of aPDT, by promoting the formation of transient pores that enhance the permeability of the bacterial membrane to PS. In this study we evaluated the combination of aPDT mediated by the hydrophobic PS, hypericin and EP (aPDT/EP) against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli. These bacteria were exposed to light (590 nm) in the presence of hypericin (4µM), following electroporation. The results showed that aPDT/EP inactivated 3.67 logs more E. coli and 2.65 logs more S. aureus than aPDT alone. Based on these results we suggest that EP can potentiate the aPDT effect.
Keywords: antimicrobial photodynamic therapy, hypericin, electroporation, E. coli, S. aureus
1. Background
Antimicrobial photodynamic therapy (aPDT) is an emerging treatment for microbial infections, is a potential alternative to conventional antimicrobial agents [1]. aPDT can equally kill multi-drug resistant bacteria and no resistance mechanisms against it are yet known. aPDT is a multi-target process producing damage mainly to the cytoplasmic membrane leading to leakage of cellular contents and inactivation of membrane transport systems, and also to DNA [2] [3].
However, aPDT is limited against Gram-negative bacteria owing to the higher complexity of the cell wall that has a complex structure that consists of an inner cytoplasmic membrane and an outer membrane that are separated by the peptidoglycan-containing periplasm [4] forming an effective permeability barrier to many photosensitizers (PS) especially hydrophobic compounds [5]. Although cationic PS can effectively kill all classes of bacteria, it is still desirable to study methods to make non-cationic hydrophobic PS effective as well.
2. Aims
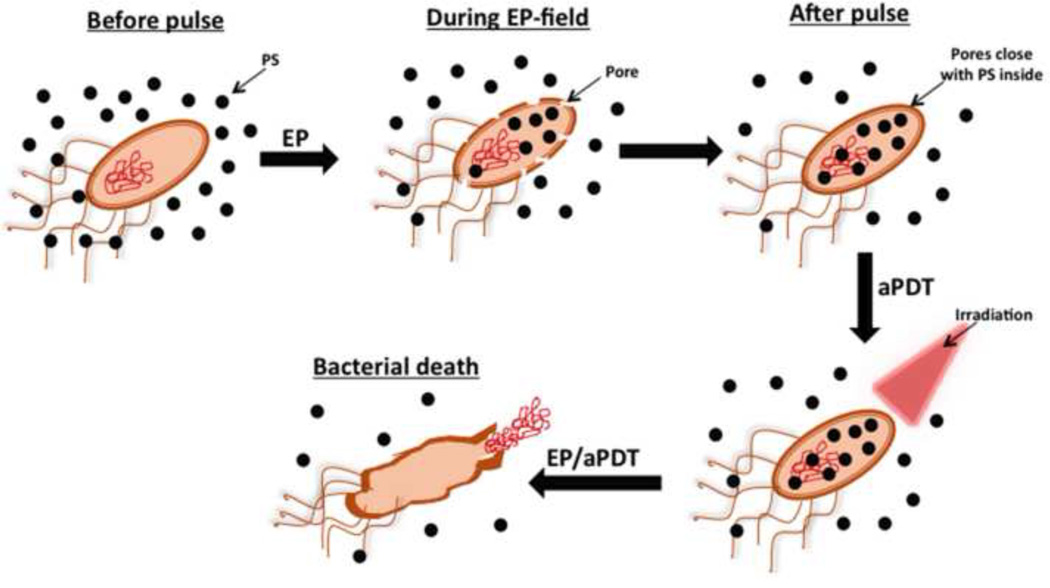
Electroporation (EP) [6] exposes the cell to short external electric pulses of high voltage, affecting the organization of cell wall and the plasma membrane forming transient permeable channels known as electropores that allow transport of various non-permeant molecules into the cell [7] (Fig. 1). An important advantage of EP is the enhanced selectivity of the treatment since the PS transportation rate is only elevated in the area of the electric field application [8, 9]. Several studies have shown that EP can potentiate the cytotoxicity of PDT, however those studies were applied against tumor cells [6–8, 10–13]. We here present the first study on electroporation combined with PDT applied to bacteria.
Figure 1. Electroporation in combination with aPDT.
The PS in solution is allowed to gain intracellular access to the bacteria after temporary electropores are created by the pulse. The pores close up without killing the bacteria but when red light is delivered the bacteria are killed by aPDT more than simply incubating the bacteria with PS alone.
3. Methods
2.1. Bacterial growth
Escherichia coli (ATCC #25922) and Staphylococcus aureus (ATCC #35556) were grown overnight in brain heart infusion medium (BHI) at 37°C and 100 rpm, then 1 mL of them was transferred into fresh BHI medium and left to grow aerobically at 37°C until the mid-logarithmic phase. The cells were harvested by centrifugation (10min, 3000rpm) and resuspended in sterile PBS at pH 7.0. The final bacterial concentration was 108 colony-forming units (CFU) mL−1.
2.2. Photosensitizer
Hypericin (Hy) was purchased from Sigma-Aldrich, (St Louis, MO). The stock solution of Hy (10 mM) was prepared in dimethylsulfoxide (DMSO) and stored at −20°C in the dark. All the experiments were performed by diluting the stock solution in PBS to 4µM (10µg/mL). The DMSO concentration did not have any anti-bacterial effect alone under these conditions.
2.3. Electroporation
Electroporation was performed by Easyject Optima– Equibio apparatus (St. Louis, MO) equipped with square-wave pulses, generating electrical pulses with the magnitude of 0–3000V, 10–600µs long, in the series of 1–99 pulses separated by the time interval of 100ms – 10s. The electroporation parameters were selected based on previously experiments (data not shown): 2 electric pulses at 1000 Vcm−1, 50µs long, 1-Hz frequency. We used cuvettes fitted with electrodes with 0.1 cm between embedded aluminum plates (catalog number P41050, Invitrogen, Grand Island, NY).
2.4. aPDT procedure
50 µL of bacterial suspensions with 1×108 cells and 50 µL of hypericin solution (4µM) were mixed and added into electroporation cuvette and subjected to the electroporation procedure. This suspension and a similar 100 µL of bacterial suspension with hypericin and no electroporation were incubated at 37°C in the dark for 10 minutes, and then irradiated with yellow light (590 nm) from a Lumacare LC122 lamp (fitted with a fiber optic filtered probe (570–630-nm) at different fluences (10–40 J/cm2) delivered at 100mW/cm2. Bacterial cells were serially 10-fold diluted with medium and the number of colonies counted after 24h incubation at 37°C. Control studies were performed (Table 1).
Table 1.
Parameters of the electroporation study
| Experiments | Electroporation (2 pulses / 1000 V cm−1) |
Light (590 nm) |
Hypericin (10 µg mL−1) |
|---|---|---|---|
| Control groups | X | ||
| X | |||
| X | X | ||
| 10 J cm−2 | |||
| 20 J cm−2 | |||
| 40 J cm−2 | |||
| aPDT groups | 10 J cm−2 | X | |
| 20 J cm−2 | X | ||
| 40 J cm−2 | X | ||
| EP/aPDT groups | X | 10 J cm−2 | X |
| X | 20 J cm−2 | X | |
| X | 40 J cm−2 | X | |
X: applied parameters
2.6. Statistics
Values are given as means and standard errors of four separate experiments. Differences between aPDT and EP/aPDT were tested for significance by one way ANOVA with Tukey post-hoc test. p-values of less than 0.05 were considered significant.
3. Results and discussion
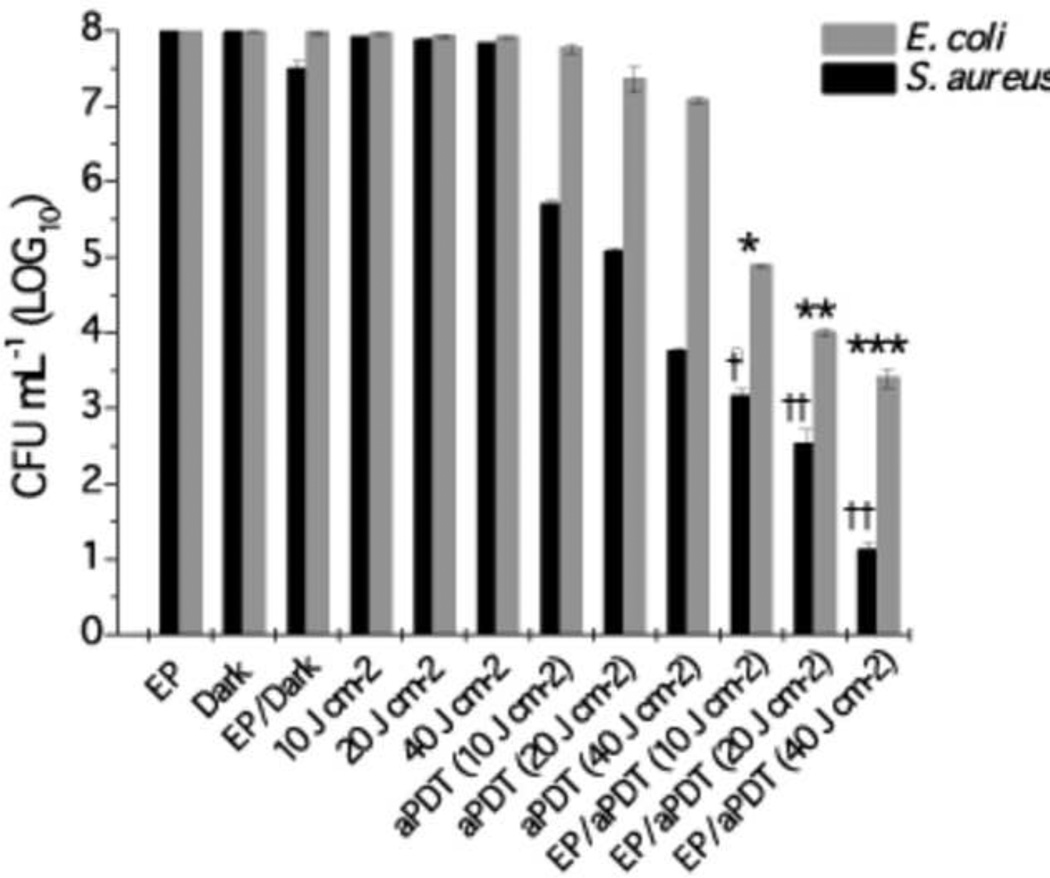
Electroporation parameters were selected based on the viability of the microorganisms after EP (data not shown), considering an excessively high electric field can cause irreversible damage to the plasma membrane of the cells. So, the selected field energy was high enough to enhance the hypericin penetration and below the level to needed to kill the bacterial cells (Figure 2).
Figure 2.
Synergism of electroporation and aPDT mediated by hypericin. Against S. aureus and E. coli. Values are means of four separate experiments and bars are standard deviations. †,*p < 0.05 versus aPDT alone at 10 J cm−2; ††, **p < 0.01 versus aPDT 20 J cm−2; †† p < 0.01 and ***p < 0.001 versus aPDT 40 J cm−2.
Hypericin is a plant derived phenanthroperylene quinone [14]. It possesses minimal dark toxicity, is not metabolized and is a photodynamically active molecule with red fluorescence [15]. The ability to generate singlet oxygen or reactive oxygen species upon irradiation also makes hypericin one of the most powerful natural PS [16, 17].
The control groups did not show significantly reduced bacterial viability (Fig 2), S. aureus (4.25 log reduction at 40J/cm2) was more susceptible to hypericin-PDT than E. coli (0.93 log reduction at 40J/cm2. A similar killing of these microorganisms was observed by Yow et al who evaluated the photodynamic antimicrobial effect of hypericin [16]. These results are consistent with the differences between Gram-positive bacteria with a relative porous layer of peptidoglycan that allows PS penetration and Gram-negative bacteria with a many-layered cell wall that acts as a permeability barrier [18]. Therefore EP was investigated to increase hypericin efficiency, especially against E. coli.
The combination of aPDT with EP potentiated the hypericin PDI effect on both bacteria more than aPDT alone (Fig 2). S. aureus showed significantly increased killing (2.5 log more than aPDT alone, p<0.05) at the lowest dose of 10J/cm2 and as the fluence increased the difference between aPDT alone and aPDT/EP became even more pronounced with 20J/cm2 giving 2.6 logs more killing (p<0.01) and 40J/cm2 also giving 2.6 logs more killing (p<0.01). For E. coli there was also a significantly increased killing of 2.4 log more than aPDT alone (p<0.05) at the lowest dose of 10J/cm2, and 20J/cm2 giving 3.3 logs more killing (p<0.01) and 40J/cm2 giving 3.5 logs more killing (p<0.001).
Importantly the relative effect of EP was greater in the case of the impermeable E. coli (at 40J/cm2 there was 3.5 logs more killing than PDT alone) than it was in the case of the permeable S. aureus (2.6 logs more killing than PDT alone).
Caveats to this study are that: (i) host cells should also be tested as well as bacteria to demonstrate selectivity for microbial cells; and (ii) hypericin is almost certainly not the best antimicrobial photosensitizer to combine with electroporation, and methylene blue should also be tested.
4. Conclusions
Electroporation increased the phototoxic effect of hypericin against E. coli and S. aureus because EP increased the extent to which the PS penetrated and accumulated into the bacterial cells. This suggests that EP could be applied to improve the delivery of PS, especially hydrophobic ones such as hypericin, into microorganisms resulting in an enhancement of aPDT.
Acknowledgments
Wanessa Melo expresses sincere thanks for the financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Research in the Hamblin laboratory was supported by US NIH grant R01AI050875.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang L, Dai T, Hamblin MR. Antimicrobial photodynamic inactivation and photodynamic therapy for infections. Methods Mol Biol. 2010;635:155–173. doi: 10.1007/978-1-60761-697-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavares A, et al. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar Drugs. 2010;8(1):91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schastak S, et al. Improved photoinactivation of gram-negative and gram-positive methicillin-resistant bacterial strains using a new near-infrared absorbing meso tetrahydroporphyrin: a comparative study with a chlorine e6 photosensitizer photolon. Methods Find Exp Clin Pharmacol. 2008;30(2):129–133. doi: 10.1358/mf.2008.30.2.1165448. [DOI] [PubMed] [Google Scholar]
- 5.Kharkwal GB, et al. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43(7):755–767. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labanauskiene J, et al. Enhancement of photodynamic tumor therapy effectiveness by electroporation in vitro. Medicina (Kaunas) 2009;45(5):372–377. [PubMed] [Google Scholar]
- 7.Labanauskiene J, Gehl J, Didziapetriene J. Evaluation of cytotoxic effect of photodynamic therapy in combination with electroporation in vitro. Bioelectrochemistry. 2007;70(1):78–82. doi: 10.1016/j.bioelechem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Kulbacka J, et al. The influence of electroporation on in vitro photodynamic therapy of human breast carcinoma cells. Folia Biol (Praha) 2011;57(3):112–118. [PubMed] [Google Scholar]
- 9.Kotulska M, Kulbacka J, Saczko J. Advances in photodynamic therapy assisted by electroporation. Curr Drug Metab. 2013;14(3):309–318. doi: 10.2174/1389200211314030006. [DOI] [PubMed] [Google Scholar]
- 10.Saczko J, et al. The effects of the electro-photodynamic in vitro treatment on human lung adenocarcinoma cells. Bioelectrochemistry. 2010;79(1):90–94. doi: 10.1016/j.bioelechem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Aihua Zhou ML, Baciu Cristina, Glück Brigitte, Berg Hermann. Membrane electroporation increases photodynamic effects. Journal of Electroanalytical Chemistry. 2000;486:220–224. [Google Scholar]
- 12.Kulbacka J, et al. Cellular stress induced by photodynamic reaction with CoTPPS and MnTMPyPCl in combination with electroporation in human colon adenocarcinoma cell lines (LoVo and LoVoDX) Cell Stress Chaperones. 2013 doi: 10.1007/s12192-013-0423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wezgowiec J, et al. Electric Field-Assisted Delivery of Photofrin to Human Breast Carcinoma Cells. J Membr Biol. 2013 doi: 10.1007/s00232-013-9533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk MWaH. Progress in the Chemistry of Second Generation Hypericin Based Photosensitizers. Current Organic Chemistry. 2011;15:3894–3907. [Google Scholar]
- 15.Kubin A, et al. Hypericin--the facts about a controversial agent. Curr Pharm Des. 2005;11(2):233–253. doi: 10.2174/1381612053382287. [DOI] [PubMed] [Google Scholar]
- 16.Yow CM, et al. Hypericin-mediated photodynamic antimicrobial effect on clinically isolated pathogens. Photochem Photobiol. 2012;88(3):626–632. doi: 10.1111/j.1751-1097.2012.01085.x. [DOI] [PubMed] [Google Scholar]
- 17.C Bernal JAOR, Guimarães APP, Ribeiro AO, de Oliveira KT, Imasato H, Perussi JR. Selective Photoinactivation of C. albicans and C. dubliniensis with Hypericin. LASER METHODS IN CHEMISTRY,BIOLOGY, AND MEDICINE. 2011;21(1):245–249. [Google Scholar]
- 18.Minnock A, et al. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44(3):522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]