Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by a prominent loss of nigrostriatal dopamine (DA) neurons with an accompanying neuroinflammation. The peptide angiotensin II (AngII) plays a role in oxidative-stress induced disorders and is thought to mediate its detrimental actions via activation of AngII AT1 receptors. The brain renin-angiotensin system is implicated in neurodegenerative disorders including PD. Blockade of the angiotensin converting enzyme or AT1 receptors provides protection in acute animal models of parkinsonism. We demonstrate here that treatment of mice with the angiotensin converting enzyme inhibitor captopril protects the striatum from acutely administered 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrine (MPTP), and that chronic captopril protects the nigral DA cell bodies from degeneration in a progressive rat model of parkinsonism created by the chronic intracerebral infusion of 1-methyl-4-phenylpyridinium (MPP+). The accompanying activation of microglia in the substantia nigra of MPP+-treated rats was reduced by the chronic captopril treatment. These findings indicate that captopril is neuroprotective for nigrostriatal DA neurons in both acute and chronic rodent PD models. Targeting the brain AngII pathway may be a feasible approach to slowing neurodegeneration in PD.
Keywords: Parkinson’s disease, captopril, angiotensin converting enzyme, dopamine neurodegeneration, mice, rats, MPTP, MPP+, microglia, osmotic minipump
INTRODUCTION
Parkinson’s disease (PD) is a devastating progressive neurodegenerative disorder characterized by severe loss in motor function due to the extensive degeneration of the nigrostriatal dopamine (DA) neurons. The exact cause(s) of neurodegeneration remain to be determined but defective mitochondria, oxidative stress and inflammatory responses likely play prominent roles (Appel, et al., 2009, German, et al., 2012, Hirsch and Hunot, 2009).
Oxidative stress and inflammation are major contributors to hypertension and cardiovascular disease. The peptide angiotensin II (AngII) plays a prominent role in cardiovascular disease and other oxidative stress-related disorders. AngII, via actions on the AngII AT1 receptors, is a potent inducer of reactive oxygen species (ROS), oxidative stress and inflammation in many cells including immune cells and neurons (Bernstein, et al., 2013, Coleman, et al., 2013, Labandeira-Garcia, et al., 2012). This occurs predominantly by way of AT1 receptor activation of the NADPH-oxidase (Nox) system with formation of superoxide and subsequently other oxidant molecules (Dikalov, 2011).
The brain possesses a complete renin-angiotensin system (RAS), reviewed in (Labandeira-Garcia, et al., 2012, Saavedra, 2012). Most of the actions of AngII in the brain are mediated by AngII AT1 receptors (AT1R) and AT2 receptors (AT2R) which reside on neurons and non-neuronal cells. Much of what is known about the brain RAS has evolved from research on its role in cardiovascular regulation by the brainstem nuclei. Considerably less is known about its function in other brain regions. However, RAS components are found throughout the brain, particularly in the basal ganglia (McKinley, et al., 2003). AngII is formed by the sequential activity of renin and the angiotensin-converting enzyme (ACE) on the precursor angiotensinogen (McKinley, et al., 2003). ACE activity is considerably higher in the substantia nigra (SN) and striatum as compared with other brain regions and AngII receptors co-localize with nigral DA neurons (Arregui and Barer, 1980, Joglar, et al., 2009, Rodriguez-Perez, et al., 2010). AngII alters DA function by modifying striatal DA synthesis, storage and release (Dwoskin, et al., 1992, Jenkins, et al., 1996, Mertens, et al., 2009, Rodriguez-Perez, et al., 2012). Thus, AngII actions impact DA function.
The brain RAS is implicated in neurodegenerative disorders including PD, stroke, and Alzheimer’s disease (AD) (Saavedra, 2012). In humans, ACE activity is increased in the cerebrospinal fluid of PD and AD patients which is thought to reflect a response to increased brain inflammation (Konings, et al., 1994). A genetic polymorphism in the ACE gene is associated with increased risk of PD (Lin, et al., 2002, Lin, et al., 2007). In animal models, pharmacological studies document a role for AngII in mediating inflammation and damage through the AT1 receptor (Benicky, et al., 2009, Marchesi, et al., 2008, Saavedra, et al., 2006, Sanchez-Lemus, et al., 2009, Schulz and Heusch, 2006). Blockade of ACE, using captopril or perindopril has been shown to exert neuroprotective effects in the striatum and the SN of mice treated acutely with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Jenkins, et al., 1999, Munoz, et al., 2006) or in rats receiving an acute intracerebral (icv) infusion of 6-hydroxydopamine (Kurosaki, et al., 2004, Lopez-Real, et al., 2005).
The purpose of the present study was to examine the neuroprotective effects of the ACE inhibitor captopril in an acute as well as a progressive rodent model of PD. While there is evidence that acute treatments with ACE inhibitors or AngII AT1R antagonists are neuroprotective in acute treatment paradigms, it is not known if these compounds can protect in a chronic progressive PD model. A progressive PD model was created by chronic intracerebral ventricular (icv) infusion of 1-methyl-4-phenylpyridinium (MPP+) in the rat. In this model, there is a progressive loss of striatal DA and tyrosine hydroxylase (TH) and of nigral DA neurons (Sonsalla, et al., 2012, Yazdani, et al., 2006). We also extended our studies to examine ACE activity, antioxidant pathways and microglia response in these PD models. Our findings demonstrate that captopril provides neuroprotection in both animal models, and suggest that reducing the microglial response may, in part, underlie its neuroprotective properties.
METHODS
Animals
Male Swiss-Webster mice (Taconic Farms, Germantown, NY) weighing 30-35 g were used for the MPTP studies. Male Sprague–Dawley rats (weighing ~300 g at the beginning of the study) (Taconic Farms, Germantown, NY) were used for the MPP+ infusion studies. All animals were maintained on a 12-h light–dark cycle with food and water available ad libitum. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the animal care committee of Rutgers-RWJMS. The number of mice used ranged from 4-8 mice/group and the number of rats for the neurochemical studies ranged from 8-10 rats/group. The actual number/group is indicated in the figure legends.
MPTP treatment in mice
Mice were treated with (1) vehicle/vehicle (saline); (2) captopril/vehicle (single s.c. injection of captopril at 20 mg/kg 30 min before vehicle); (3) vehicle/MPTP (single acute s.c. injection of 30 mg/kg MPTP as free base) and (4) captopril/MPTP (single s.c. injection of captopril 20 mg/kg 30 min before MPTP). Mice were euthanized 15 h, 3 days or 7 days after treatment. We and others have shown that the mouse MPTP model produces extensive loss of striatal nerve terminals and of nigral DA neurons (Albers, et al., 1996, German, et al., 1996, Giovanni, et al., 1994, Liang, et al., 1996). The dose of captopril was selected based on previous studies (Munoz, et al., 2006). MPTP-HCl and captopril were from Sigma-Aldrich.
Surgeries and MPP+ infusions in rats
Vehicle (sodium-iodide) or MPP+-iodide (Sigma-Aldrich) dissolved in sterile saline was infused into the left cerebral ventricle through a cannula which was connected by tubing to an Alzet osmotic minipump (model 2ML4) implanted subcutaneously as previously described (Sonsalla, et al., 2012, Yazdani, et al., 2006, Zeevalk, et al., 2007). Stereotaxic cannula placement was at coordinates relative to bregma: anterior -0.5 mm, lateral left +1.4 mm, depth −3.9 mm (Paxinos and Watson, 1986). Stereotaxic surgery and minipump placement were performed under ketamine/metetomidine anesthesia (100 mg/kg and 0.6 mg/kg, respectively). Following the surgical procedure, the rats were treated with atipamezole (1 mg/kg s.c.) to reverse anesthesia. Rats also received rimadyl (5 mg/kg s.c., before surgery and 24 h later), and ampicillin administered under anesthesia (100 mg/kg i.m.) and again 24 h later (50 mg/kg, s.c.). MPP+-iodide was infused at a dose of 75 μg/day for 28 days with a drug delivery rate of 2.5 μl/h. Animals were killed 27-28 days after surgery. Captopril was administered twice daily (7.5 mg/kg/injection s.c.) starting on the day of MPP+ infusion.
Neurochemistry
In rats used for neurochemistry and midbrain DA cell counts, brains were rapidly removed and sectioned at mid-hypothalamus. Left and right striata were dissected from the forebrain, weighed and frozen at −80°C until analyzed. The hindbrain containing the SN was immersion fixed in paraformaldehyde for immunohistochemistry. In mice, the striata were dissected from the brain and frozen until analyzed. Monoamines and metabolites were measured using HPLC/EC and TH protein was measured with an ELISA technique as is routinely performed in our laboratory (Sonsalla, et al., 2012). ACE activity was measured in tissue homogenates as described previously (Schwager, et al., 2006); glutathione peroxidase (GPx) activity was measured by the Sigma assay kit (product number CGP-1); superoxide dismutase (SOD) was measured by the Sigma assay kit (product number 19160) and proteosomal activity was measured by the proteosome 20S assay (product number BML-AK740-0001; Enzo Life Sciences).
Brain MPP+ Measurements
Mice received vehicle or captopril (20mg/kg s.c.) 30 min before MPTP and were euthanized 1.5 h after MPTP administration. Striata and cortex were dissected out and frozen until assayed. MPP+ concentrations were measured using procedures established in our laboratory (Giovanni, et al., 1991).
Immunohistochemistry for nigral DA cells and activated microglia
The hindbrain in rats was used for performing SN TH+ cell counts using immunohistochemical methods as described previously (Sonsalla, et al., 2012, Yazdani, et al., 2006). Briefly, 30-μm-thick coronal sections were cut through the entire rostral-caudal extent of the SN. Every fourth section through the SN was stained with an antibody against TH (1:4000; Protos Biotech Corp.) to identify the DA neurons. Unbiased stereology techniques were used to count TH+ cells in the SN as previously described (Sonsalla, et al., 2012).
Some rats were transcardially perfused for evaluating microglial response and TH staining in both the striatum and the SN as previously described (Sonsalla, et al., 2012). Cryostat sections from the striatum and the SN were immunostained with an antibody against ED1 (1:100; AbD Serotec) to identify activated microglia. Some sections were double labeled by immunofluorescence for TH and ED1. Digital photographs were taken using a Zeiss Axioplan microscope (Carl Zeiss, Inc.) equipped with an epi-fluorescence illuminator and Axiovision software.
Stereology
StereoInvestigator software (version 9.0. MicroBrightfield Inc., Williston, VT) was used to count TH-immunoreactive (TH-IR) SN cells. Cells were counted with a 40× objective using a Leica DMRE microscope. The cell counting frame was 50 × 50 × 5 μm with a 2 μm upper and lower guard zone. A cell was defined as TH-IR somata with a clearly visible unstained nucleus. The TH cell counts were taken from 6 sections, spaced 4 apart (120 μm) and 200–250 cells were counted in the SN on the left side of each brain ipsilateral to the lateral ventricular MPP+ infusion. The SN region was defined according to previous anatomical demarcation in the rat (German and Manaye, 1993).
Statistical analysis
Differences among the means in the MPTP-treated mice were analyzed using one-way analysis of variance (ANOVA). In rats with a unilateral lesion, two-way ANOVA was initially used to evaluate the data. Two-way ANOVA revealed significant differences between treatment groups and sides (left lesioned and right non-lesioned sides). One-way ANOVA on data from the right unlesioned sides of the rats revealed no significant differences across treatment groups allowing use of one-way ANOVA for comparisons in the left lesioned side across treatment groups. When ANOVA showed significant differences, comparisons between means were tested by the Tukey–Kramer or Bonferroni multiple comparisons post hoc test. In all analyses, the null hypothesis was rejected at the 0.05 level. All values are expressed as the mean ± SEM.
RESULTS
Captopril attenuates MPTP-induced loss of striatal DA, DOPAC and TH in the acute parkinsonian mouse model
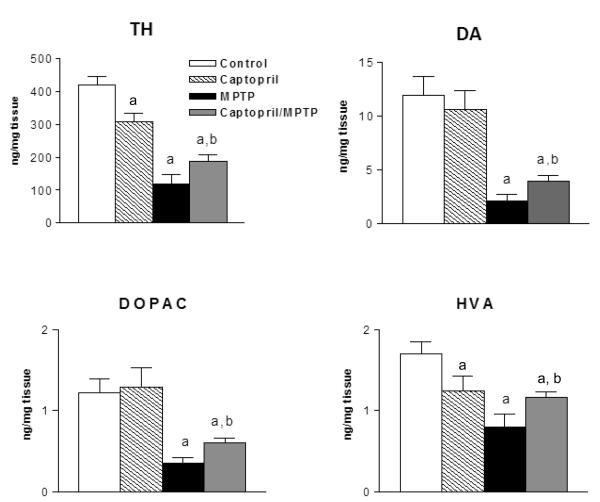
To determine a neuroprotective effect of captopril, mice were treated acutely with captopril and MPTP. Seven days after the acute treatment paradigm, there were significant reductions in striatal DA (−90 %), DOPAC (−71%) and TH (−82 %) in MPTP-treated mice as compared to control values (Fig.1). A single injection of captopril significantly attenuated the MPTP-induced reduction of DA (-52 %), DOPAC (−51%) and TH (−56 %) levels. These findings show a neuroprotective effect of captopril in the mouse striatum. Mice treated with captopril alone had small but significant reductions in striatal TH protein and HVA content.
Figure 1. Captopril treatment attenuates the decreases in striatal DA neurochemistry produced by MPTP treatment in mice.
Mice were treated with vehicle or captopril (20 mg/kg s.c.) 30 min before MPTP (single 30 mg/kg s.c. injection) and euthanized 7 days later. Results are the mean ± SEM of n=4 for controls and captopril alone, and n=8 for MPTP and MPTP plus captopril. ap<0.05 from controls; bp<0.05 from MPTP group.
In mice treated acutely with MPTP, captopril does not modify MPP+ concentrations in the brain, inhibit MAO-B activity in brain homogenates or prevent uptake of 3H-DA into striatal synaptosomes
Because MPTP requires conversion to the actual neurotoxicant MPP+ by monoamine oxidase B (MAO-B), we needed to determine if captopril had any effect on MPP+ formation in the mouse brain, on MAO-B activity, or on the DA transporter. As shown in Table 1, in MPTP-treated mice that had been pre-treated with vehicle or captopril, striatal MPP+ levels were similar. Additionally, MPP+ levels in cortex were similar in both groups as well although they were approximately one-third of the amount measured in the striatum. As might be expected, MAO-B activity in brain homogenates was not altered by concentrations up to 1 mM captopril (data not shown). Likewise, captopril, at concentrations up to 1 mM, did not impair the uptake of 3H-DA into striatal synaptosomes (data not shown). These findings indicate that captopril does not interfere with the formation of MPP+ in the brain or of its accumulation within DA neurons.
Table 1.
Captopril does not alter MPP+ formation from MPTP in mouse brain. Mice were treated with captopril (20 mg/kg s.c.) 30 min before MPTP (30 mg/kg s.c.) and were euthanized 1.5 h after MPTP administration. Results are the mean MPP+ concentration ± SEM from 4 mice/group.
| Treatment | MPP+ (μg/g tissue) | |
|---|---|---|
| Striatum | Cortex | |
| Vehicle + MPTP | 7.9 ± 0.7 | 2.4 ± 0.4 |
| Captopril + MPTP | 7.4 ± 0.9 | 2.3 ± 0.5 |
In the progressive rat model produced by chronic MPP+ infusion, captopril attenuates the loss of nigral TH+ cells
We used a rat model in which MPP+ is chronically infused into the left cerebral ventricle. Rats were euthanized at 27-28 days after pump implantation. At this time point, TH+ cells in the ipsilateral SN of rats infused with MPP+ were reduced by 69% (Table 2). Chronic treatment of the MPP+-treated rats with captopril (7.5 mg/kg, twice daily) significantly attenuated the TH+ cell loss to only 24%, indicating that captopril provides neuroprotection to DA cell bodies in the chronic progressive MPP+ model. Counting of the Nissl-stained SN neurons in these animals has confirmed that this is loss of neurons and not just a loss of TH staining in the SN of the MPP+-treated rats (Yazdani, et al., 2006).
Table 2.
Captopril reduces MPP+-induced loss of TH+ neurons in substantia nigra (SN) of rats. Rats received continuous icv infusion of MPP+ (75 μg/day, left cerebral ventricle) for 27−28 days and were killed at the end of the infusion period. Some rats were injected with captopril (twice daily, 7.5 mg/kg/injection s.c.) for the duration of treatment. Results are the mean number of TH+ neurons in the left SN ± SEM. N/group is in parentheses.
p<.001 vs. naives;
p<.01 vs. MPP+ group.
Captopril does not affect weight gain in rats treated chronically with MPP+
Shown in Figure 2 are the weight gains in rats treated with vehicle, MPP+ and captopril/MPP+. Captopril did not significantly affect weight gain in the rats. While there was a slight loss of weight in all three groups during the first few days, this is a result of the surgical procedure. It can be seen that the slope of the lines for all 3 groups are similar, indicating that the rate of weight gain in the rats was similar across the groups. These data indicate that neither MPP+ nor captopril had adverse effects on weight gain.
Figure 2. Captopril does not alter weight gain in rats receiving icv MPP+.
Results are the average weight of rats treated with vehicle, MPP+ or captopril/MPP+ (8-10 rats/group). No significant differences in weights were observed between the groups at any of the time points. Moreover, the slopes of the lines are similar for all three groups.
In the chronic progressive parkinsonian model, captopril does not attenuate MPP+− induced loss of striatal TH and DA in rats
In contrast to the protection seen in the SN, captopril treatment did not significantly modify the MPP+-induced neurochemical changes in the striatum. The chronic icv MPP+ infusion produced significant unilateral reductions in the contents of TH (−34%), DA (−54%), DOPAC (−76%), and HVA (−44%) in the left striatum (ipsilateral to icv infusion) of the rats (Fig.3). Chronic captopril treatment did not significantly modify the MPP+-induced reductions. Moreover, serotonin was not reduced by MPP+ treatment, demonstrating the selectivity of this dose of MPP+ toward DA neurons as we have previously reported (Sonsalla, et al., 2012, Yazdani, et al., 2006). Striatal TH and DA are not reduced in sham-treated rats, indicating that cannula placement into the ventricle does not damage DA nerve terminals (data not shown; see also Yazdani et al., 2006). Taken together, these data indicate that captopril protection is exerted at the level of the cell bodies in the SN.
Figure 3. Captopril treatment does not modify MPP+ - induced changes in striatal DA neurochemistry.
Data are the mean ± SEM presented as percent of control (n = 5 naives), MPP+ (n=10), and captopril/MPP+ (n=10) rats. ap<0.05 from controls.
In the chronic progressive parkinsonian model, captopril attenuates microglia response in the SN of MPP+-treated rats
To evaluate the microglial response, brain sections containing the SN or striatum were immunostained with an antibody to ED1, which detects activated microglia, and counterstained with a TH antibody to detect DA neurons. In these experiments, we examined immunofluorescence in two rats treated with MPP+ and two rats treated with captopril/MPP+. Immunohistochemical staining revealed robust ED1 immunostaining in the lesioned striatum of the MPP+-treated rats especially near the ventricle (Fig. 4A). Very few ED1 immunostained cells are seen in the right striatum, consistent with the lack of damage in the non-lesioned striatum. Also, in the right unlesioned striatum, there is intense TH immunostaining whereas in the left lesioned striatum, TH immunostaining is markedly reduced. In rats treated with MPP+ and captopril, the ED1 immunostaining in cells in the left striatum was similar to that seen in rats treated with only MPP+ (data not shown). In the SN, ED1 immunostained cells were prevalent in the lesioned side of MPP+-treated rats (Fig. 4B). In rats treated with MPP+ and captopril, there were markedly fewer ED-1 immunostained cells. We also examined the striatum for expression of the microglia marker Mac-1 and the astrocytic marker GFAP. MPP+ treatment produced a significant increase in GFAP and Mac-1 expression (~3-fold), which was not modified by captopril treatment (data not shown). While further studies are needed to explore the microglia response, the activation of microglia in the SN of the captopril/MPP+ -treated rats appears much less than that in the MPP+-treated rats.
Figure 4. Captopril reduces the microglia response in the SN ipsilateral to the i.c.v. MPP+ infusion.
Photomicrographs are from one of two rats evaluated in each group. ED-1 staining for macrophages/microglia is in red. TH+ staining for DA neurons and fibers is in green. Panel 4A. Note intense ED-1 staining in the left lesioned striatum of the MPP+-treated rat but absence of staining in the right striatum. Also note reduced TH+ immunostaining in lesioned side. Panel 4B. Representative photomicrographs of the lesioned SN from one of two rats treated with MPP+ and from one of two rats treated with captopril and MPP+. Bottom photomicrographs are at a higher magnification. Note the large number of ED-1 stained microglia in the lesioned SN and the fewer ED-1 stained cells in the lesioned SN of the rat treated with MPP+ and captopril.
Acute treatment of mice with MPTP produces transient elevation in striatal angiotensin converting enzyme (ACE) activity
To determine if striatal damage modifies the activity of ACE or of antioxidant enzymes, we assayed the activities of ACE, glutathione peroxidase (GPx), superoxide dismutase (SOD) and proteosome 20S in the striatum at early time points after MPTP administration. As shown in Fig. 5, ACE activity was elevated by 43% at 15 hours but had returned to control values by 3 days after MPTP treatment. This treatment paradigm had little effect on GPx, SOD or proteosomal function (data not shown) although DA was significantly reduced at 15 hr (50%) and 3 days (74%) after MPTP administration, indicating that the MPTP treatment produced a substantial DA lesion.
Figure 5. ACE activity in mouse striatum is significantly increased after MPTP treatment.
Mice were treated with a single 30 mg/kg s.c. injection of MPTP and were killed 15 h, 30 h or 3 days later. Results are the mean ± SEM of 6-7 mice/group. ACE activity was increased at 15 hr after MPTP treatment, ap<0.05 from controls.
DISCUSSION
Here we demonstrate that acute captopril treatment attenuates the reduction of striatal DA measures produced by acute MPTP treatment in the mouse. We also show that the chronic treatment of rats with captopril attenuates the loss of nigral DA cell bodies in the progressive MPP+ rat model of parkinsonism. The reduced loss of TH+ neurons in the captopril/MPP+ treated rats was accompanied by a reduced microglia response in the SN in these rats. These data indicate that captopril is protective for DA neurons in an acute model as well as in a chronic progressive mode of parkinsonism. Furthermore, ACE activity is transiently increased in mice treated acutely with MPTP although no discernible changes in the antioxidant enzymes (GPx, SOD) or proteosomal activity were seen at these early time points.
Our data demonstrate that blocking ACE activity with captopril provides good protection in the striatum and SN against the neurotoxic effects of MPTP/MPP+. The extent of striatal protection by captopril in the mice treated acutely with MPTP in our study is similar to that observed by Munoz and colleagues (2006) who used the same doses of captopril and MPTP per day but extended their treatment paradigm to 5 days. In their experiments nigral DA cell loss was reduced from ~50% in MPTP-treated mice to only ~24% in mice treated with captopril and MPTP (Munoz, et al., 2006). In our progressive rat MPP+ model, captopril treatment produced protection in the SN that was similar to that seen in the mouse MPTP studies (e.g., from a 70% reduction in TH+ cells in rats treated with only MPP+ to a 24% loss in rats treated with captopril and MPP+; see Table 2). However, in the chronic progressive MPP+ rat model, captopril did not attenuate striatal reductions in TH, DA or its metabolites as it did in the MPTP treated mouse (Figs. 1,3). A similar finding was observed in our recent work showing neuroprotection with caffeine (Sonsalla et al., 2012). Chronic caffeine treatment in the drinking water provided protection to nigral cell bodies but not striatal DA terminals in the progressive MPP+ model. Reasons for this disparity in the striatum of the acute MPTP mouse model and the progressive MPP+ model may reflect the acute vs chronic administration paradigms for the neurotoxicant and captopril and/or the mode of administration of the neurotoxicant (systemic s.c. MPTP vs icv MPP+). It may also be possible that with the icv route of MPP+ administration, the striatum is exposed to much higher MPP+ concentrations than the SN, concentrations that overwhelm all antioxidant pathways. Additionally, the striatal DA terminals may be more vulnerable than the nigral DA cell bodies to toxic insult. The latter possibility is supported by several examples of pharmacological agents that provide better protection in the SN than the striatum from systemically administered MPTP (Carta, et al., 2009, Dehmer, et al., 2000, Nomura, et al., 2011, Pierri, et al., 2005, Yu, et al., 2008). Moreover, in mice with targeted mitochondrial damage to DA neurons, loss of striatal DA markers precede and are generally more severe than loss of nigral DA cell bodies (Pickrell, 2011). Impairment of mitochondrial function by MPP+ is a principal mechanism by which it causes neurodegeneration (Vyas, et al., 1986). MPP+ also impairs mitochondrial transport in DA axons, providing another mechanism for greater damage in striatal DA axons than in cell bodies (Kim-Han, et al., 2011). Other possibilities include differences in number, rate and duration of glial cell activation in the two brain regions or in brain region differences in production of pro- and anti-inflammatory cytokines or other pro- and anti-oxidant molecules. In a study with rosiglitazone (an agonist at peroxisome proliferators-activated receptor), there was complete protection of nigral DA neurons with minimal protection of striatal DA terminals in the MPTP/probenecid model (Schintu, et al., 2009). This protection was attributed to the marked attenuation of the SN microglia response. Our findings would also suggest an attenuation of the microglia response in MPP+-treated rats receiving captopril. The differential effect of captopril in the striata of the acute MPTP model and the progressive chronic MPP+ model needs further study. .
Captopril protection is not due to altered MPTP pharmacokinetics or impairment of the uptake of MPP+ into dopamine neurons. In the acute MPTP mouse model, striatal MPP+ concentrations were not reduced by captopril treatment indicating its administration to mice did not interfere with the pharmacokinetics of MPTP or of its conversion by MAO-B, findings that are consistent with a previous report (Munoz, et al., 2006). Captopril at concentrations of up to 1 mM did not significantly alter MAO-B activity in brain homogenates or prevent 3H-DA accumulation in striatal synaptosomes (unpublished data). Moreover, the lack of striatal protection by captopril in the chronic MPP+ rat model also indicates that captopril or a metabolite did not interfere with MPP+ uptake by striatal DA nerve terminals.
The mechanism(s) by which ACE inhibition provides neuroprotection likely involve the reduction of AngII actions on the AT1 receptors. In parkinsonian models, neuronal loss and neuroinflammation are reduced by pharmacological inhibition or genetic deletion of the AT1 receptor (Garrido-Gil, et al., 2012, Grammatopoulos, et al., 2007, Joglar, et al., 2009). Excessive AT1R stimulation is associated with enhanced inflammation in both the periphery and the CNS where it can cause neuronal damage (Saavedra, 2012).
Considerable evidence suggests that reducing AngII actions on AT1 receptors reduces oxidative stress primarily by impairing Nox activity and the production of superoxide and other pro-oxidant molecules (Hernandes and Britto, 2012). AngII activates Nox in neurons by a mechanism that involves the translocation of the p47phox subunit from the cytosol to the plasma membrane where it associates with the membrane components of Nox (p22phox and gp91phox) forming the active enzyme (Coleman, et al., 2013, Garrido and Griendling, 2009).
Reducing AngII effects within the basal ganglia could provide protection via one or more mechanisms of action and via different cell types. Nigral DA neurons express various subunits of Nox and nearly all DA neurons express AT1 receptors thus allowing for actions of AngII directly on DA neurons (Garrido, et al., 2013, Grammatopoulos, et al., 2007, Rodriguez-Perez, et al., 2010). In a DA cell line (N27 cells), which expresses Nox, AT1 receptor antagonism reduces MPP+-induced ROS in a manner similar to that seen with pharmacological inhibition or siRNA silencing of Nox providing a functional link between AT1 receptors, Nox activity and DA function (Garrido-Gil, et al., 2012, Rodriguez-Perez, et al., 2012). Considerable cross-talk exists between mitochondria and Nox with the ROS generated by either source having the capability to activate ROS production by the other pathway (Dikalov, 2011). This process becomes particularly detrimental to DA neurons in which mitochondrial dysfunction occurs, as by MPP+ in animal PD models or in PD patients presenting with dysfunctional mitochondria (Meredith, et al., 2008, Schapira, 2012). One can envision a cycle of events by which mitochondrial dysfunction leads to increased Nox activity and elevation of ROS within the DA neurons.
Reducing AngII actions in microglial cells can reduce their activation state and the production of ROS. Microglial cells are activated by insults to the brain including injury and neurodegenerative disorders (German, et al., 2012). In this reactive state, they function to clear debris by phagocytic engulfment, clear extracellular glutamate to prevent excitotoxicity, and generate pro-oxidant molecules. However, reactive microglia can also actively destroy neurons by phagocytosis and the production of pro-inflammatory molecules. AT1 receptors are highly expressed on activated microglia and AngII via AT1 receptor activation is a powerful inducer of Nox activity in microglia (Block, et al., 2006). Activation of AT1 receptors on microglia enhances DA cell death in mesencephalic cultures (Rodriguez-Pallares, et al., 2008). Thus, a reduction in AngII actions on microglia cells could reduce their activation state, reduce the production of superoxide and other pro-inflammatory molecules and lessen damage to DA neurons. The reduced number of activated microglia in our progressive MPP+ model suggests that captopril may have exerted at least some of its beneficial actions via effects on microglia.
Astrocyte function can also be modified by captopril treatment. Astrocytes are the main source of angiotensinogen, the precursor of AngII (Milsted, et al., 1990). Furthermore, astrocytes maintain extracellular glutamate levels via regulation of the astrocyte glutamate transporter. In primary neuron-astrocyte cultures exposed to oxygen-glucose deprivation, blockade of AT1 receptors or gene knockdown improves neuronal viability and reduces the elevation in extracellular glutamate levels by actions which cause an up-regulation of the glutamate transporter in the astrocytes (Wu, et al., 2010). Extracellular nigral glutamate levels are elevated in animal PD models (Ochi, et al., 2004) and excessive stimulation of the nigral glutamatergic N-methyl-D-aspartate (NMDA) receptors located on DA neurons can be excitotoxic, especially to metabolically compromised DA neurons as in the MPP+-treated rats. Selective blockade of nigral NMDA receptors protects DA neurons from metabolic stress, indicating the importance of nigral glutamate and excitotoxicity to DA neurons (Zeevalk, et al., 2000). Further research is needed to determine if reducing AngII improves astrocytic uptake of glutamate and reduces excitotoxic damage to nigral DA neurons.
We found striatal ACE activity is increased at 15 hr but not at 3 days after MPTP. This observation indicates a short-term elevation and return to normal within hours after MPTP treatment. Because microglia activation occurs over several days, it appears that microglia may not be the source of this increase in ACE activity. Further research is needed to determine the cell type and the mechanisms for this up-regulation of ACE activity.
Captopril, other ACE inhibitors, and AT1R antagonists are used in the treatment of hypertension. A reduction in blood pressure is oftentimes seen in PD patients. Thus, it remains to be determined if reducing AngII actions is a reasonable approach for protection of DA neurons in PD. The doses of captopril used in our studies are about 7.5 fold higher than the doses used by humans for the control of blood pressure. In humans, the daily dose of captopril ranges from 20-150 mg (Hilal-Dandan, 2011). A 150 mg dose given to a 70 kg human would equate to 2 mg/kg/day. In our rats, the daily dose was 15 mg/kg/day, which is 7.5-fold higher than the recommended highest dose administered to humans. The ACE inhibitor perindopril has been tested in PD patients and has been shown to improve motor function at doses that have minimal effects on blood pressure; however, long term studies were not evaluated for preservation of DA neurons (Reardon, et al., 2000). Understanding the mechanism(s) of action of this class of drugs clearly warrants further investigation.
In summary, captopril treatment provided protection in the acute MPTP mouse model and the progressive MPP+ rat model. Further research is needed to identify not only the mechanism(s) of neuroprotection but also the cell types that are involved.
Highlights.
Chronic icv MPP+ infusion produces loss of nigrostriatal dopamine neurons
Captopril protects dopamine neurons in the mouse MPTP model and the progressive rat MPP+ model
Angiotensin converting enzyme activity is transiently elevated in mice treated with MPTP
Acknowledgments
Grant Support: Research was funded by an NIH/NINDS grant award (R21NS058329), NIEHS Center Grant (P30ES005022), a New Jersey Health Foundation Award, and the James Webb Fund of the Dallas Foundation.
Footnotes
Present address: Weill Cornell Medical College, 407 East 61st Street, New York, NY 10065
References
- 1.Albers DS, Zeevalk GD, Sonsalla PK. Damage to dopaminergic nerve terminals in mice by combined treatment of intrastriatal malonate with systemic methamphetamine or MPTP. Brain Research. 1996;718:217–220. doi: 10.1016/0006-8993(96)00135-7. [DOI] [PubMed] [Google Scholar]
- 2.Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson's disease and amyotrophic lateral sclerosis: are we listening? Trends in Immunology. 2009;31:7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arregui A, Barer GR. Chronic hypoxia in rats: alterations of striato-nigral angiotensin converting enzyme, GABA, and glutamic acid decarboxylase. J Neurochem. 1980;34:740–743. doi: 10.1111/j.1471-4159.1980.tb11206.x. [DOI] [PubMed] [Google Scholar]
- 4.Benicky J, Sanchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cellular & Molecular Neurobiology. 2009;29:781–792. doi: 10.1007/s10571-009-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein KE, Ong FS, Blackwell W-LB, Shah KH, Giani JF, Gonzalez-Villalobos RA, Shen XZ, Fuchs S. A Modern Understanding of the Traditional and Nontraditional Biological Functions of Angiotensin-Converting Enzyme. Pharmacological Reviews. 2013;65:1–46. doi: 10.1124/pr.112.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, Wilson B, Yang J, Hong JS. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. Faseb J. 2006;20:251–258. doi: 10.1096/fj.05-4553com. [DOI] [PubMed] [Google Scholar]
- 7.Carta AR, Kachroo A, Schintu N, Xu K, Schwarzschild MA, Wardas J, Morelli M. Inactivation of neuronal forebrain A receptors protects dopaminergic neurons in a mouse model of Parkinson's disease. J Neurochem. 2009;111:1478–1489. doi: 10.1111/j.1471-4159.2009.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman CG, Wang G, Faraco G, Marques Lopes J, Waters EM, Milner TA, Iadecola C, Pickel VM. Membrane trafficking of NADPH oxidase p47(phox) in paraventricular hypothalamic neurons parallels local free radical production in angiotensin II slow-pressor hypertension. Journal of Neuroscience. 2013;33:4308–4316. doi: 10.1523/JNEUROSCI.3061-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- 10.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radical Biology and Medicine. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwoskin LP, Jewell AL, Cassis LA. DuP 753, a nonpeptide angiotensin II-1 receptor antagonist, alters dopaminergic function in rat striatum. Naunyn-Schmiedebergs Archives of Pharmacology. 1992;345:153–159. doi: 10.1007/BF00165730. [DOI] [PubMed] [Google Scholar]
- 12.Garrido-Gil P, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Involvement of PPAR- in the neuroprotective and anti-inflammatory effects of angiotensin type 1 receptor inhibition: effects of the receptor antagonist telmisartan and receptor deletion in a mouse MPTP model of Parkinson's disease. Journal of Neuroinflammation. 2012;9:38. doi: 10.1186/1742-2094-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Molecular and Cellular Endocrinology. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrido P, Velenauela R, Villar-Cheda B, Lanciego JL, Labandeira-Garcia JL. Expression of angiotensinogen and receptors for angiotensin and prorenin in the monkey and human susbstantia nigra: an intracellular renin-angiotensin system in the nigra. Brain Structure and Function. 2013;218:373–388. doi: 10.1007/s00429-012-0402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.German DC, Eagar T, Sonsalla P. Parkinson's disease: A role for the immune system. Current Molecular Pharmacology. 2012;5:340–349. [PubMed] [Google Scholar]
- 16.German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. Journal of Comparative Neurology. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- 17.German DC, Nelson EL, Liang CL, Speciale SG, Sinton CM, Sonsalla PK. The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration. 1996;5:299–312. doi: 10.1006/neur.1996.0041. [DOI] [PubMed] [Google Scholar]
- 18.Giovanni A, Sieber BA, Heikkila RE, Sonsalla PK. Correlation between the neostriatal content of the 1-methyl-4-phenylpyridinium species and dopaminergic neurotoxicity following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to several strains of mice. J Pharmacol Exp Ther. 1991;257:691–697. [PubMed] [Google Scholar]
- 19.Giovanni A, Sieber BA, Heikkila RE, Sonsalla PK. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine>. Part 1: Systemic administration. Journal of Pharmacology & Experimental Therapeutics. 1994;270:1000–1007. [PubMed] [Google Scholar]
- 20.Grammatopoulos TN, Jones SM, Ahmadi FA, Hoover BR, Snell LD, Skoch J, Jhaveri VV, Poczobutt AM, Weyhenmeyer JA, Zawada WM. Angiotensin type I receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Molecular Neurodegeneration. 2007:2. doi: 10.1186/1750-1326-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandes MS, Britto LRG. NADPH Oxidase and Neurodegeneration. Current Neuropharmacology. 2012;10:321–327. doi: 10.2174/157015912804143540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilal-Dandan R. Renin and Angiotensin1. In: Brunton LL, Knollmann BC, editors. Goodman & Gillman's The Pharmacological Basis of Therapeutics. 12e. McGraw-Hill; New York: 2011. B. A. C. [Google Scholar]
- 23.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurology. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins TA, Allen AM, Chai SY, MacGregor DP, Paxinos G, Mendelsohn FA. Interactions of angiotensin II with central dopamine. Advances in Experimental Medicine & Biology. 1996;396:93–103. doi: 10.1007/978-1-4899-1376-0_10. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins TA, Wong JY, Howells DW, Mendelsohn FA, Chai SY. Effect of chronic angiotensin-converting enzyme inhibition on striatal dopamine content in the MPTP-treated mouse. J Neurochem. 1999;73:214–219. doi: 10.1046/j.1471-4159.1999.0730214.x. [DOI] [PubMed] [Google Scholar]
- 26.Joglar B, Rodriguez-Pallares1 J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson's disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem. 2009;109:656–669. doi: 10.1111/j.1471-4159.2009.05999.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim-Han JS, Antenor-Dorsey JA, O'Malley KL. The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. Journal of Neuroscience. 2011;31:7212–7221. doi: 10.1523/JNEUROSCI.0711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konings CH, Kuiper MA, Bergmans PL, Grijpma AM, van Kamp GJ, Wolters EC. Increased angiotensin-converting enzyme activity in cerebrospinal fluid of treated patients with Parkinson's disease. Clinica Chimica Acta. 1994;231:101–106. doi: 10.1016/0009-8981(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 29.Kurosaki R, Muramatsu Y, Imai Y, Kato H, Araki T. Neuroprotective effect of the angiotensin-converting enzyme inhibitor perindopril in MPTP-treated mice. Neurological Research. 2004;26:644–657. doi: 10.1179/016164104225015949. [DOI] [PubMed] [Google Scholar]
- 30.Labandeira-Garcia JL, Rodriguez-Pallares J, Rodriguez-Perez AI, Garrido-Gil P, Villar-Cheda B, Valenzuela R, Guerra MJ. Brain angiotensin and dopaminergic degeneration: relevance to Parkinson's disease. Am J Neurodegener Dis. 2012;1:226–244. [PMC free article] [PubMed] [Google Scholar]
- 31.Liang CL, Sinton CM, Sonsalla PK, German DC. Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration. 1996;5:313–318. doi: 10.1006/neur.1996.0042. [DOI] [PubMed] [Google Scholar]
- 32.Lin JJ, Yueh KC, Chang DC, Lin SZ. Association between genetic polymorphism of angiotensin-converting enzyme gene and Parkinson's disease. J Neurol Sci. 2002;199:25–29. doi: 10.1016/s0022-510x(02)00081-3. [DOI] [PubMed] [Google Scholar]
- 33.Lin JJ, Yueh KC, Lin SZ, Harn HJ, Liu JT. Genetic polymorphism of the angiotensin converting enzyme and L-dopa-induced adverse effects in Parkinson's disease. Journal of the Neurological Sciences. 2007;252:130–134. doi: 10.1016/j.jns.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Real A, Rey P, Soto-Otero R, Mendez-Alvarez E, Labandeira-Garcia JL. Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism. Journal of Neuroscience Research. 2005;81:865–873. doi: 10.1002/jnr.20598. [DOI] [PubMed] [Google Scholar]
- 35.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends in Pharmacological Sciences. 2008;29:367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 36.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY, Mendelsohn FAO. The brain renin-angiotensin system: location and physiological roles. International Journal of Biochemistry & Cell Biology. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 37.Meredith GE, Sonsalla PK, Chesselet M-F. Animal models of Parkinson's disease progression. Acta Neuropathologica. 2008;115:385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mertens B, Massie A, Michotte Y, Sarre S. Effect of nigrostriatal damage induced by 6-hydroxydopamine on the expression of glial cell line-derived neurotrophic factor in the striatum of the rat. Neuroscience. 2009;162:148–154. doi: 10.1016/j.neuroscience.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 39.Milsted A, Barna BP, Ransohoff RM, Brosnihan KB, Ferrario CM. Astrocyte cultures derived from human brain tissue express angiotensinogen mRNA. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5720–5723. doi: 10.1073/pnas.87.15.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz A, Rey P, Guerra MJ, Mendez-Alvarez E, Soto-Otero R, Labandeira-Garcia JL. Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology. 2006;51:112–120. doi: 10.1016/j.neuropharm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MCG, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid Hydrolysis Generates Brain Prostaglandins That Promote Neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochi M, Shiozaki S, Kase H. Adenosine A(2A) receptor-mediated modulation of GABA and glutamate release in the output regions of the basal ganglia in a rodent model of Parkinson's disease. Neuroscience. 2004;127:223–231. doi: 10.1016/j.neuroscience.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in steriotaxic coordinates. Academic Press; Orlando, FL: 1986. [Google Scholar]
- 44.Pickrell AM, Pinto M, Hida A, Moraes CT. Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson's disease. Journal of Neuroscience. 2011;31:17649–17658. doi: 10.1523/JNEUROSCI.4871-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierri M, Vaudano E, Sager T, Englund U. KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacology. 2005;48:517–524. doi: 10.1016/j.neuropharm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Reardon KA, Mendelsohn FA, Chai SY, Horne MK. The angiotensin converting enzyme (ACE) inhibitor, perindopril, modifies the clinical features of Parkinson's disease. Australian & New Zealand Journal of Medicine. 2000;30:48–53. doi: 10.1111/j.1445-5994.2000.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Pallares J, Rey P, Parga JA, Munoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiology of Disease. 2008;31:58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Perez AI, Valenzuela R, Villar-Cheda B, Guerra MJ, Labandeira-Garcia JL. Dopaminergic neuroprotection of hormonal replacement therapy in young and aged menopausal rats: role of the brain angiotensin system. Brain. 2012;135:124–138. doi: 10.1093/brain/awr320. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Perez AI, Valenzuela R, Villar-Cheda B, Guerra MJ, Lanciego JL, Labandeira-Garcia JL. Estrogen and angiotensin interaction in the substantia nigra. Relevance to postmenopausal Parkinson's disease. Experimental Neurology. 2010;224:517–526. doi: 10.1016/j.expneurol.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Saavedra JM. Angiotensin II AT(1) receptor blockers ameliorate inflammatory stress: a beneficial effect for the treatment of brain disorders. Cellular and Molecular Neurobiology. 2012;32:667–681. doi: 10.1007/s10571-011-9754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saavedra JM, Benicky J, Zhou J. Mechanisms of the Anti-Ischemic Effect of Angiotensin II AT( 1 ) Receptor Antagonists in the Brain. Cellular & Molecular Neurobiology. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Lemus E, Benicky J, Pavel J, Saavedra JM. In vivo Angiotensin II AT1 receptor blockade selectively inhibits LPS-induced innate immune response and ACTH release in rat pituitary gland. Brain, Behavior, & Immunity. 2009;23:945–957. doi: 10.1016/j.bbi.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schapira AHV. Mitochondrial diseases. Lancet. 2012;379:1825–1834. doi: 10.1016/S0140-6736(11)61305-6. [DOI] [PubMed] [Google Scholar]
- 54.Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. European Journal of Neuroscience. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- 55.Schulz R, Heusch G. Angiotensin II type 1 receptors in cerebral ischaemia-reperfusion: initiation of inflammation. Journal of Hypertension - Supplement. 2006;24:S123–129. doi: 10.1097/01.hjh.0000220417.01397.6a. [DOI] [PubMed] [Google Scholar]
- 56.Schwager SL, Carmona AK, Sturrock ED. A high-throughput fluorimetric assay for angiotensin I-converting enzyme. Nature Protocols. 2006;1:1961–1964. doi: 10.1038/nprot.2006.305. [DOI] [PubMed] [Google Scholar]
- 57.Sonsalla PK, Wong L-Y, Harris SL, Richardson JR, Khobahy I, Li W, Shrikanth B, German DG. Caffeine treatment prevents nigral dopamine neuron loss in a progressive rat model of Parkinson's disease. Experimental Neurology. 2012;234:482–487. doi: 10.1016/j.expneurol.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vyas I, Heikkila RE, Nicklas WJ. Studies on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: inhibition of NAD-linked substrate oxidation by its metabolite, 1-methyl-4-phenylpyridinium. J Neurochem. 1986;46:1501–1507. doi: 10.1111/j.1471-4159.1986.tb01768.x. [DOI] [PubMed] [Google Scholar]
- 59.Wu X, Kihara T, Hongo H, Akaike A, Niidome T, Sugimoto H. Angiotensin receptor type 1 antagonists protect against neuronal injury induced by oxygen-glucose depletion. British Journal of Pharmacology. 2010;161:33–50. doi: 10.1111/j.1476-5381.2010.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yazdani U, German DC, Liang CL, Manzino L, Sonsalla PK, Zeevalk GD. Rat model of Parkinson's disease: chronic central delivery of 1-methyl-4-phenylpyridinium (MPP+) Experimental Neurology. 2006;200:172–183. doi: 10.1016/j.expneurol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Yu L, Shen H-Y, Coelho JE, Araujo IM, Huang Q-Y, Day Y-J, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Muller CE, Linden J, Cunha RA, Chen J-F. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Annals of Neurology. 2008;63:338–346. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]
- 62.Zeevalk GD, Manzino L, Sonsalla PK. NMDA receptors modulate dopamine loss due to energy impairment in the substantia nigra but not striatum. Experimental Neurology. 2000;161:638–646. doi: 10.1006/exnr.1999.7283. [DOI] [PubMed] [Google Scholar]
- 63.Zeevalk GD, Manzino L, Sonsalla PK, Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson's disease. Experimental Neurology. 2007;203:512–520. doi: 10.1016/j.expneurol.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]