Abstract
Background
Guidelines for referral of chronic aortic insufficiency (AI) patients for aortic valve replacement (AVR) suggest that surgery can be delayed until symptoms or reduction in left ventricular (LV) contractile function occur. The frequent occurrence of reduced LV contractile function after AVR for chronic AI suggests that new contractile metrics for surgical referral are needed.
Methods
In 16 chronic AI patients, cardiac MRI tagged images were analyzed before and 21.5 ± 13.8 months after AVR to calculate LV systolic strain. Average measurements of three strain parameters were obtained for each of 72 LV regions, normalized using a normal human strain database (n=63), and combined into a composite index (multi-parametric strain z score [MSZ]) representing standard deviation from the normal regional average.
Results
Preoperative global MSZ (72-region average) correlated with post-AVR global MSZ (R2 = .825, p < .001). Preoperative global MSZ also predicts improvement of impaired regions (N=271 regions from 14 AI patients, R2 = .392, p < .001). Preoperative MRI-based left ventricular ejection fraction (LVEF) is also predictive (r = .410, p < .001). Although global preoperative MSZ had a significantly higher correlation than preoperative LVEF with improvement of injured regions (p < .001), both measures convey the same phenomenon.
Conclusions
Global preoperative MRI-based multi-parametric strain predicts global strain postoperatively, as well as improvement of regions (n=72/LV) with impaired contractile function. Global contractile function is an important correlate with improvement in regionally impaired contractile function, perhaps reflecting total AI volume-overload burden (severity/duration of AI).
Introduction
ACC/AHA Clinical Guidelines1 for timing of referral of patients with chronic AI for AVR suggest surgery can be delayed until the occurrence of symptoms or reduction in LV contractile function. Despite reasonably aggressive thresholds for surgical referral that have led to seemingly early referral for AVR, we2 and others3–6 have documented that reduced LV contractile function following AVR for chronic AI is not uncommon. The frequent occurrence of reduced LV contractile function after AVR for chronic AI suggests that new contractile metrics to determine surgical referral may be needed.
There is substantial evidence that contractile injury in chronically dilated left ventricles occurs in a heterogeneous distribution,7, 8 suggesting that LV regions vary in their sensitivity to homogeneously directed global injury vectors. This heterogeneous distribution of contractile injury may contribute to delay in detection of irreversible LV injury since uninjured regions can compensate for “early-injury” regions thereby masking the detection of early onset irreversible regional injury by ejection fraction, which is the current clinical standard. Highly quantitative contractile indices that are sensitive to changes in regionally varying contractile function must be developed for clinical application in order to exploit and target these sentinel early-injury regions in timing surgical referral to prevent irreversible contractile injury.
The preoperative prediction of contractile recovery in individually impaired LV regions has always been important in patients with ischemic coronary artery disease due to the well-recognized regional variability of the influence of coronary atherosclerosis. With the recent demonstration of heterogeneity in LV contractile injury in two patient subpopulations whose injury vectors were believed to have globally uniform LV distributions7, 8, the examination of regional contractile indices has become a mandate in the clinical setting of any cardiac disease. The ability to accurately preoperatively quantify the expected regional contractile response to AVR may provide a means by which the timing of surgical intervention for chronic AI can be optimized. To this end, we utilized MRI-based multi-parametric strain analysis to assess LV regional recovery (72 regions/LV) after AVR by quantifying the differences between pre- and postoperative contractile function in preoperatively impaired regions.
Materials and Methods
Patient Characteristics
Eighteen patients with isolated grade 3+ to 4+ chronic AI and exhibiting no significant mitral regurgitation, aortic stenosis, or known coronary artery disease, contributed complete LV systolic strain information by cardiac tagged MRI both before and 21.5 ± 13.8 months after AVR. For comparison, a separate group of 63 healthy volunteers (29 male, 34 female; age 34 ± 11 years; no known cardiac disease) also contributed multi-parametric systolic strain data using the same method. Of the 18 AI patients, two were excluded because of poor image quality, leaving a total study group of 16 AI patients and 63 normal controls. Two AI patients were females and the remainder males; mean age before AVR was 45 ± 17 years. Seven patients were given St. Jude (St. Jude Medical, Inc.; St. Paul, MN) mechanical valves; five had Carpentier-Edwards (Edwards Lifesciences, Inc.; Irvine, CA) bovine pericardial valves; three had Ross pulmonary auto-graft procedures; and one had a homograft valve. Uniform myocardial protection was employed in all patients, including antegrade blood cardioplegia supplemented by intermittent retrograde blood cardioplegia infusions every 15–20 minutes.
The earliest any patient was scanned after surgery was 6 months; the furthest out was 46 months. Since the majority of ventricular recovery following AVR occurs within 2–3 months, this is not believed to have significantly affected the results. All patients were in normal sinus rhythm at the time of scan. Select clinical patient data are presented in Table 1. There is no significant difference between the studied patients’ paired pre- and postoperative systolic blood pressures. Although strain is inherently load dependent at the extremes of physiological blood pressures, the minor variation at the mid-range physiological pressures seen in this study is not likely to have affected the observed results. The Human Research Protection Office at Washington University (St. Louis, MO) approved this study beginning August, 1999, and renewed approval yearly to present. All subjects have given informed written consent. No sex-based or racial/ethnic-based exclusions were present during patient recruitment.
Table 1.
Chronic Aortic Insufficiency Patient Data
| Preoperative | Postoperative | |
|---|---|---|
| LV dimension (mm) | ||
| Systolic | 42 ± 10 | 31 ± 12* |
| Diastolic | 59 ± 9 | 44 ± 10* |
| LV volume (mL) | ||
| Systolic | 101 ± 58 | 61 ± 72* |
| Diastolic | 210 ± 77 | 123 ± 79* |
| LVEF (%) | 54 ± 11 | 56 ± 14 |
| NYHA (# patients)† | ||
| Class I | 7 | 8 |
| Class II | 7 | 5 |
| Class III | 2 | 0 |
| Blood pressure (mmHg) | ||
| Systolic | 133 ± 16 | 124 ± 14 |
| Diastolic | 68 ± 9 | 79 ± 9* |
| Heart Rate (bpm) | 67 ± 10 | 69 ± 8 |
Data expressed as mean ± standard deviation.
LV, left ventricular; LVEF, left ventricular ejection fraction.
p < .05 vs. preoperative.
Postoperative NYHA data unavailable for 3 patients
Cardiac Magnetic Resonance Imaging
All imaging studies were carried out using a 1.5T scanner (Siemens Medical Solutions USA, Malvern, PA). ECG-gated short-axis tagged MR images were acquired in multiple parallel planes starting at the level of the mitral valve and extending to the apex of the LV. Additionally, long-axis tagged images were acquired in four radially oriented planes separated by 45° and intersecting at the center of the LV. In each imaging plane, a spatial modulation of magnetization (SPAMM) radio-frequency tissue-tagging preparation9, 10 was applied, followed by a 2D balanced steady-state free precession cine image acquisition. Typical imaging parameters were: repetition time 32.4ms, echo time 1.52ms, field of view 350×350mm, flip angle 20°, tag spacing 8mm and slice thickness 8mm.
Strain Analysis
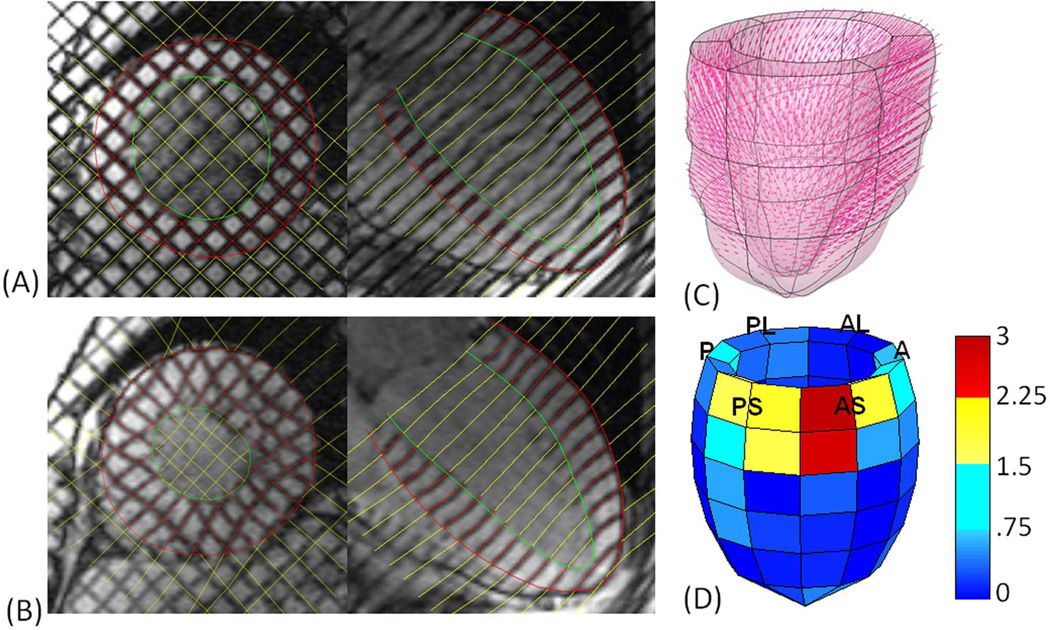
Strain is a measure of deformation that relates a deformed configuration to a reference configuration. A detailed account of our strain analysis has been previously reported11, 12, however a brief description is provided below. Short- and long-axis wall boundaries and tag lines were identified through one cardiac contraction, beginning at end-diastole (Figure 1A) and ending at end-systole (Figure 1B). The endocardial and epicardial walls were identified manually, and a semi-automated tag-finding algorithm based on pixel intensity was used to identify all tag lines at each time point in the short- and long-axis tagged MRI images (Figure 1A and 1B).
Figure 1. Image acquisition and post-processing steps.
Wall boundaries and tag lines were placed on each short- and long-axis image from end-diastole (A) to end-systole (B). Displacement vectors were calculated in three dimensions, relating tag line intersections from end-diastole to end-systole (C). A standard p-version finite element mesh was registered to the individual’s LV geometry. This analysis was used to fit the displacement components over the entire LV and calculate strain. Finally, normalized MSZs were calculated in each of 72 LV regions and plotted, as an LV from an example AI patient is shown (D). The LV labels refer to the 6 LV regions which each contain 12 smaller regions: P—posterior, PL—posterior lateral, AL—anterior lateral, A—anterior, AS—anterior septum and PS—posterior septum. Blue regions signify regions that have normal contractile function, while yellow and red regions signify increasing likelihood of contractile injury (red being worse than yellow).
For each tag line intersection within the myocardium, displacements were calculated from end-diastole to end-systole (Figure 1C). On the most basal short-axis image, the anterior and posterior intersection points of the right ventricular endocardium with the intraventricular septum were used as landmarks to create a standardized 18-region finite element model of the LV. A continuous representation of displacement components over the entire model was obtained from a least squares fitting of the measured displacement data utilizing the finite element software package StressCheck (ESRD Inc., St. Louis, MO). Using the results of this fitting, circumferential strain, longitudinal strain and the minimum principal strain angle were computed at each of 15,300 points for the entire LV. These point-wise values for each strain parameter were then averaged for each of 72 LV regions, which were created by subdividing each of the 18 regions into four regions based on the element coordinate values. Minimum principal strain angle is the angle between the plane formed by the circumferential and longitudinal axes and the axis of minimum principal strain13. In the LV, this angle directly reflects contractile function.
MRI-based Multi-parametric Strain Z score Analysis
Heterogeneous LV contractile injury is expected in patients with coronary artery disease, the distribution being dependent upon which coronary arteries have occlusive disease. Somewhat surprisingly, our lab and others have also shown a consistent pattern of heterogeneous contractile injury in patients with AI8 and with dilated cardiomyopathy7, 14, 15. Not only is normal human LV strain heterogeneous, but most LV strain injury also occurs in a heterogeneous distribution. These studies establish the critical necessity of utilizing normalized strain metrics (by way of comparison to a normal human strain database7, 16) in the analysis of regional myocardial contractile function.
Using the mean and standard deviation for each of the three strain parameters in each of 72 LV regions generated from the normal human strain database (n=63), the corresponding patient-specific regional strain value for each chronic AI patient was converted to a z score (standard deviation from the normal mean). The three mean systolic strain z scores from each region were then averaged into a single multi-parametric strain z score (MSZ), representing the contractile function of that region relative to normal. For instance, a region with a MSZ of 1.65 indicates the average multi-parametric systolic strain value in that region falls 1.65 standard deviations from the corresponding average of the normal human strain database (n=63). Regional MSZs (72 per LV) were plotted for each LV (Figure 1D).
A positive MSZ in a particular region indicates a systolic strain value that is less negative than the strain value of the corresponding region of the normal database, meaning myocardial contraction in that particular region is less than the normal average. Negative MSZs indicate the opposite. Thus, for positive MSZ values outside the normal range (approximately -1 to 1), greater MSZ values represent worse contractile function. In Figure 1D, blue regions signify regions that are not considered abnormal, while yellow and red regions signify contractile injury (red being worse than yellow).
MRI-based Ejection Fraction
Ejection fractions were obtained using the modified Simpson’s method to compute volumes from MRI long-axis images at both end-diastole and end-systole.
Statistical Analysis
To compare preoperative and postoperative MSZ, LVEF and volumes, paired-samples t tests were used. Pearson correlations coefficients were calculated to test associations between variables. Linear regression analysis (enter) was used to test the significance of the global average MSZ as a predictor for global and regional outcome. To compare the dependent correlations of regional improvement and preoperative global average MSZ with regional improvement and preoperative LVEF, the method proposed by Meng was used17. Continuous data are expressed as mean ± standard deviation, and all tests were two-tailed. A p-value of .05 or less was considered significant. All statistical computations were done using the statistical software package SPSS 19 (SPSS, Inc., Chicago, IL).
Results
Global Multi-parametric Strain
AVR was performed in a standard surgical referral group of 16 patients that did not necessarily have poor contractile function. Only four patients had preoperative MRI-based LVEFs below 50%. The LVEF increased in three of those patients by 7, 14, and 18 percent and decreased in the fourth by 16 percent. In the whole AI group for this particular study, neither MRI-based multi-parametric strain analysis nor MRI-based LVEF demonstrated significantly improved global contractile function after AVR (pre- vs. post-operative: MSZ: .46 ± .57 vs. .54 ± .82, p = .414; LVEF: 54 ± 11 vs. 56 ± 14, p = .358). No acute perioperative myocardial infarctions or low output syndrome was documented in this group of surgical patients.
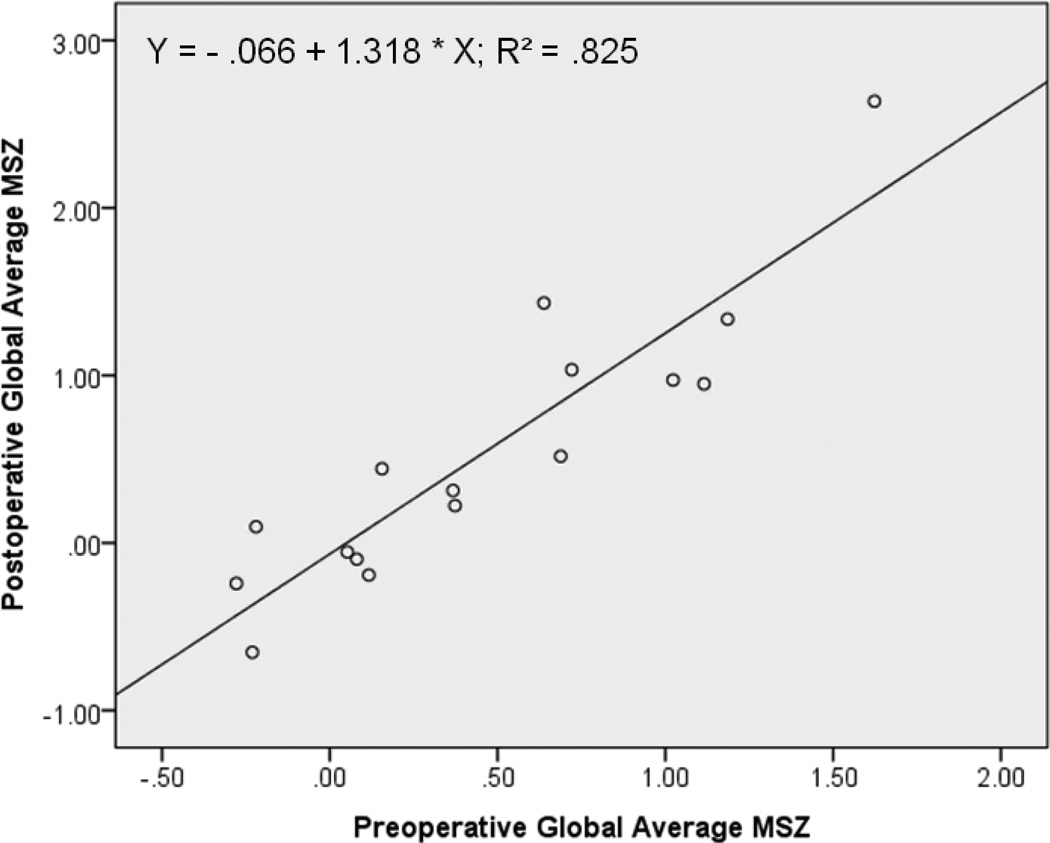
Preoperative global average (across the 72 LV regions) MSZ significantly correlated with the post-AVR value (r = .908, p < .001). Furthermore, preoperative global average MSZ predicts global average MSZ after AVR (R2 = .825, p < .001); as the preoperative global average MSZ increases by one unit (standard deviation), the post-AVR value is expected to increase by 1.318 units (Figure 2). In other words, the more the LV is injured before AVR, the more the LV is expected to be injured afterward. This finding is further supported by a similar correlation using LVEF measurements (pre- vs. post-operative LVEF; r = .632, p = .009).
Figure 2. Preoperative predicts postoperative global outcome.
For the AI patient study group (N=16), global average MSZs from before and after AVR are significantly correlated (r = .908, p < .001). For a preoperative global average MSZ = 1.25, the expected postoperative global average MSZ = − .066 + 1.318(1.25) = 1.58 > 1 → impaired LV. In other words, the greater the LV global injury is before AVR, the greater the LV injury is expected to be following surgery.
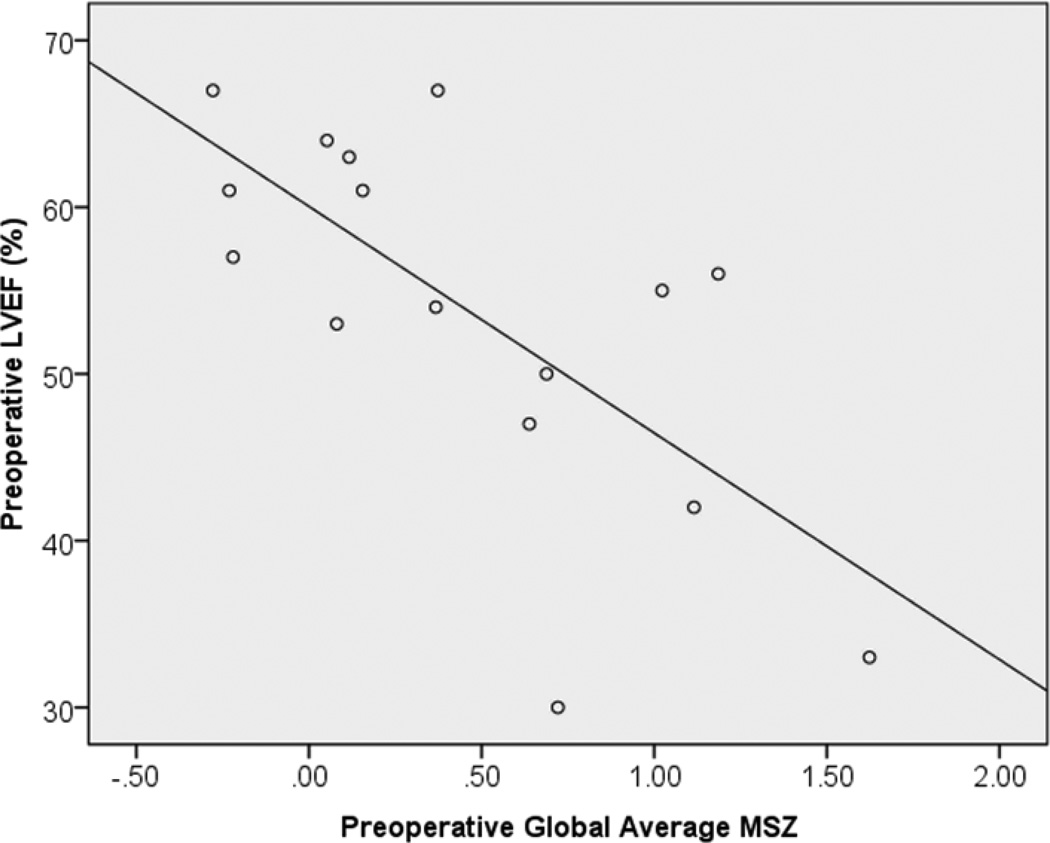
It is helpful to understand the relationship global MSZ has with the current clinical standard, LVEF. Figure 3 demonstrates the significant negative correlation between these two global contractility metrics (r = −.691, p = .003).
Figure 3. Preoperative global MSZ correlates with current clinical standard LVEF.
Higher global MSZs (indicating worse contractility) correlate with lower MRI-based LVEFs (r = −.691, p = .003).
Regional Multi-parametric Strain
Since LV regions with preoperatively normal contractility are not expected to improve following AVR, only regions that had a preoperative MSZ > 1 were included in the regional outcome analysis. Thus, 271 ‘preoperatively injured’ regions from 14 AI patients were tested for improvement.
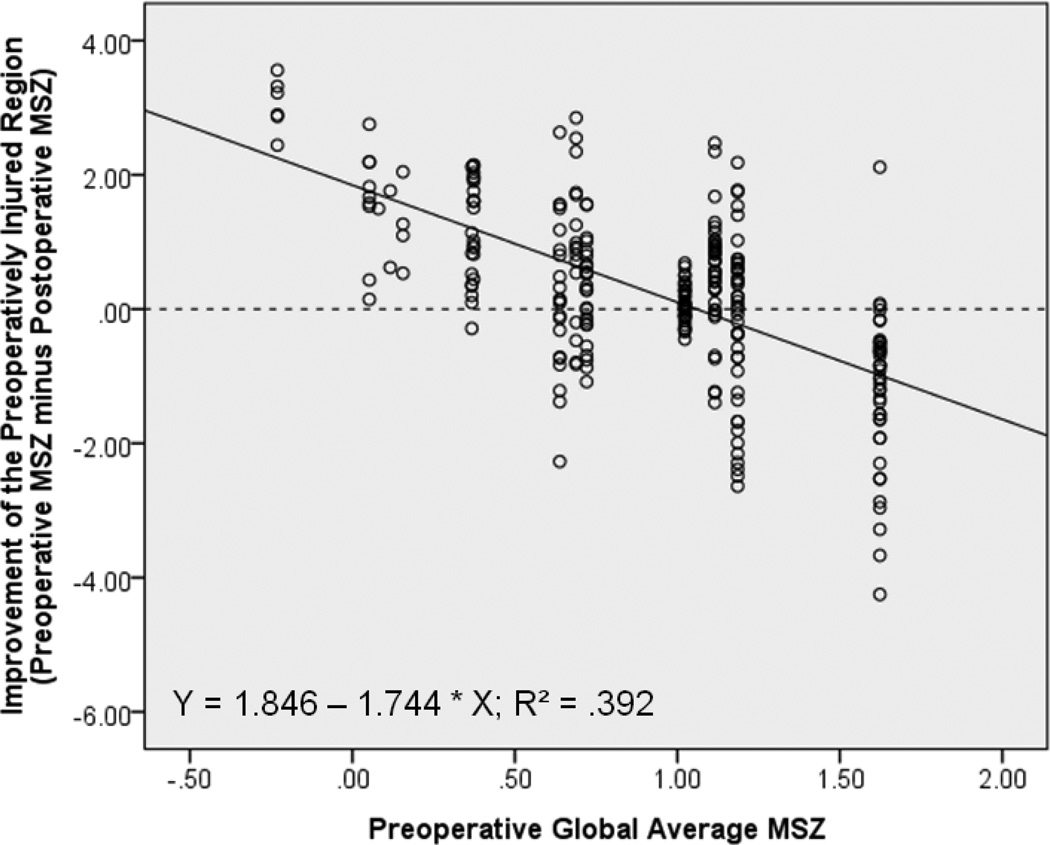
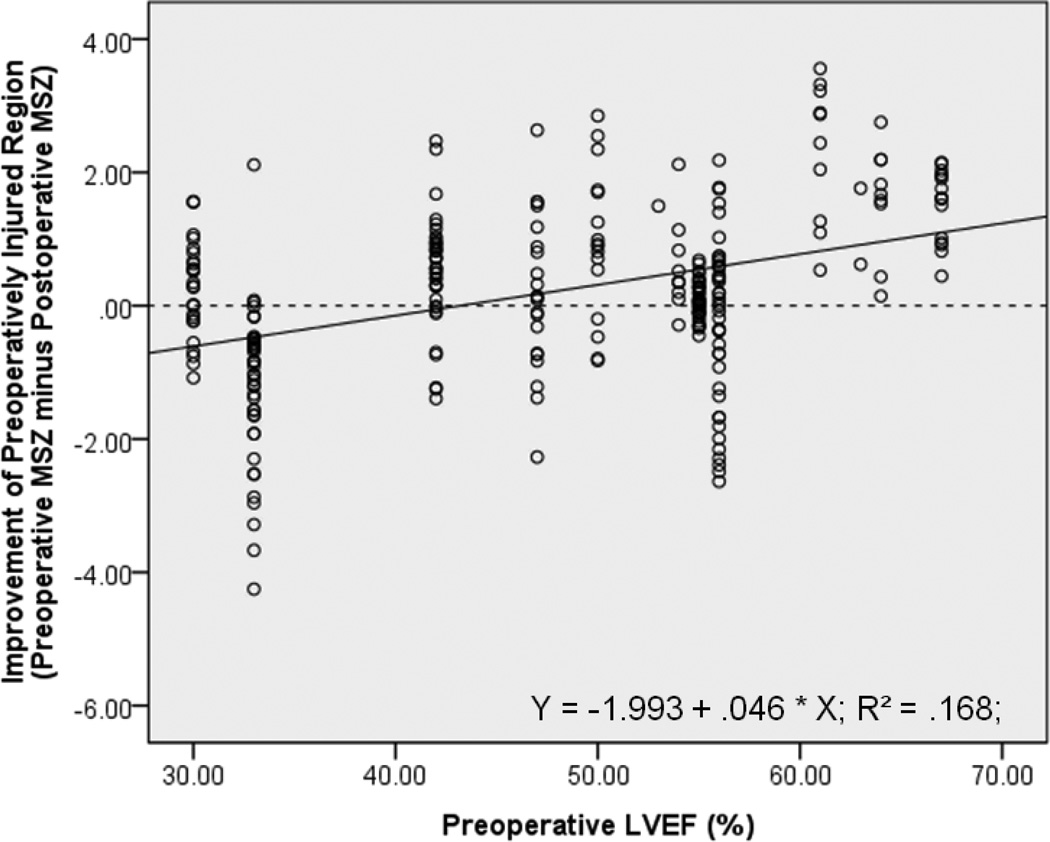
Figure 4 shows the significant correlation between regional improvement of preoperatively injured regions and the preoperative global average MSZ from the LV in which each region resides (r = −.626, p < .001). Preoperative global average MSZ in fact predicts regional improvement—as it increases by one unit, the expected improvement of any associated injured region is expected to decrease by 1.74 units. LVs that are more injured as a whole before AVR can expect to have less post-AVR improvement of impaired regions than LVs with more normal contractility (Figure 5).
Figure 4. Regional Improvement by MSZ.
For 271 preoperatively impaired regions (from 14 AI patients), the preoperative global average MSZ from the LV in which each region resides was significantly correlated with the improvement of that specific region following AVR. Y-Axis: ‘0’ = no improvement; positive values are improvement; negative values mean the region is faring worse after surgery.
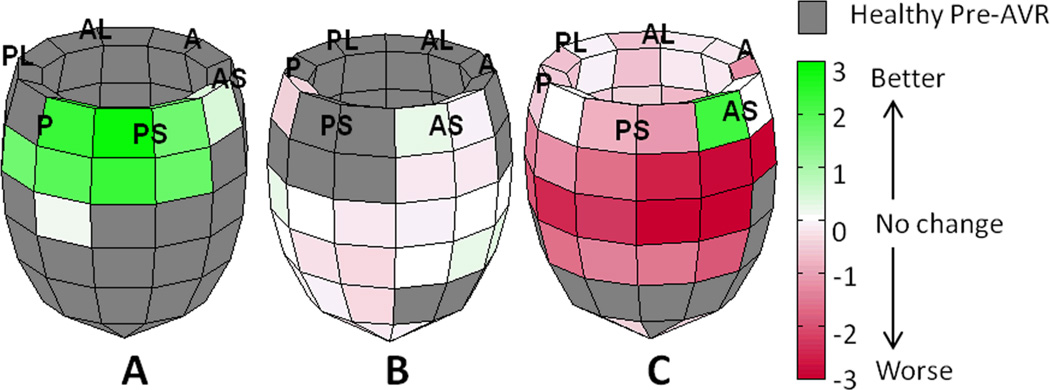
Figure 5. Post-AVR outcome models.
LV outcome models from three AI patients are shown with regional differences plotted as preoperative MSZ minus postoperative MSZ. The global average MSZ is a significant predictor of the amount of regional improvement after AVR (R2 = .392, p < .001). The preoperative global average MSZ from patients A, B, and C were .05, 1.02, and 1.62 respectively. The amount of regional improvement after AVR expected for any given region is as follows: Patient A, 1.846 - 1.744(.05) = 1.76 standard deviations (SD); Patient B, 1.846 - 1.744(1.02) = .07 SD; Patient C, 1.846 - 1.744(1.62) = −.98 SD. (P—posterior, PL—posterior lateral, AL—anterior lateral, A—anterior, AS—anterior septum and PS—posterior septum)
The significant correlation between improvement of preoperatively injured regions and corresponding preoperative global LV function, demonstrated using global MSZ as the contractile metric, is also supported when the metric is replaced with MRI-based LVEF (r = .410, p < .001, Figure 6). However, compared with preoperative LVEF, the preoperative global average MSZ correlates significantly better with the degree of regional improvement (p < .001)17.
Figure 6. Regional Improvement by LVEF.
For 271 preoperatively impaired regions (from 14 AI patients), the preoperative MRI-based LVEF from the ventricle in which each region resides is significantly correlated with the improvement of that specific region following AVR (r = .410, p < .001), though not as well the global average MSZ is (p < .001). Y-Axis: ‘0’ = no improvement; positive values are improvement; negative values mean the region is faring worse after surgery.
Conclusions
The combination of multiple, individually effective LV systolic strain metrics creates a single, more powerful16, 18, 19 composite index (MRI-based multi-parametric strain analysis). This is made possible by the “normalization” of individual strain parameters by a comparison to a normal human strain database with z score assignment. In the investigation of multiple patient subsets, including ischemic coronary artery disease16, AI2, 8, and dilated cardiomyopathy7, we utilized MRI-based multi-parametric strain analysis to populate a micro-regional LV multi-parametric strain grid. This clinically applicable approach quantifies “normalized” contractile function, especially applicable in patient subpopulations characterized by regionally varying myocardial contractile injury.
In the setting of chronic AI, our results suggest that LV regions with abnormal contractile function experience greater recovery if situated in an otherwise healthy, normally contracting LV as compared to being situated in an overall poorly contracting LV. Preserved global LV contractile function may simply be a marker of less overall exposure to the volume-overload physiology of AI. Total volume-overload burden is the product of the severity of the valvular insufficiency and the duration of the volume-overload exposure. The longer the exposure and the greater the valvular regurgitation, the greater the contractile injury that has been sustained by the exposed myocardium, and the more likely it is at least partially irreversible3, 4. Our search for surrogate metrics of total volume-overload burden is fueled by the unfortunate fact that total volume-overload burden is usually a clinically inestimable number since total time of exposure is almost always unknown and the degree of AI is dynamic and difficult to quantify. In any case, global LV contractile function may predict regional recovery simply because the presence of preserved global LV contractile function reflects short and/or less severe AI exposure.
On the other hand, globally reduced LV contractile function also clearly affects the mechanical milieu in which an injured LV region must attempt to recover following AVR. An LV that has sustained global contractile injury after prolonged severe volume-overload is uniformly dilated. Ventricular dilatation often persists to some degree following AVR for AI, despite the elimination of valvular insufficiency. This is especially true in left ventricles with poor contractile function3, 4. During early systole, the effect of an increase in LV dimension is an increase in systolic wall stress which therefore increases the afterload to LV ejection. Such a disadvantageous change in loading conditions cannot help but affect the degree of recovery of contractile function in preoperatively impaired LV regions.
Regardless of the mechanism involved, the ability of global LV contractile function to predict recovery of preoperatively impaired LV regions may have significant clinical implications. Many variables are factored into the risk/benefit ratio that determines whether surgical intervention is undertaken in chronic AI patients. When, for any number of reasons, the clinical assessment of risk is significantly elevated, the accurate weighing of true benefit is critical. This accurate determination of patient-specific benefit by the quantification of expected recovery of regional contractile function must become foundational in future clinical therapeutic algorithms, not only for chronic AI, but also for other clinical pathophysiological processes where residual LV contractile function determines symptoms and ultimately survival. Further investigation into the clinical application of quantitative methodologies to characterize and display (in a clinically meaningful fashion) regional contractile function is critical to the future application of high-risk surgical intervention. As the first highly quantitative clinically applicable methodology that is sensitive to patient-specific regionally varying differences in contractile function, MRI-based multi-parametric strain analysis is positioned to make significant contributions to future surgical therapy clinical algorithms.
This study is limited by the MRI data acquisition process and the complexity of the data analysis that have allowed only 16 AI patients (32 scans) to contribute to the outcome analysis (63 normal volunteers contributed to the normal human strain database). Continued efforts to fully automate the data analysis may extend this investigative tool to larger patient populations.
Further, we acknowledge that cardiac MRI has inherent limitations making it less than ideal in many clinical settings. When hospitalized patients are too critically ill to be moved to an MRI scanner, the portability of echocardiography offers clear clinical advantages. Also, the high volume of echocardiography performed at most institutions continues to give it a slight cost advantage. Even so, cardiac MRI remains the gold standard in regard to high-resolution fiducial accuracy in temporally describing dynamic cardiac geometry, which we believe outweighs the cost difference.
Our findings suggest the composite of “normalized” strain metrics embodied in MRI-based multi-parametric strain analysis may comprise a clinically applicable surrogate for the patient-specific total AI burden (severity times duration) sustained by patients who present with chronic AI. The fact that global MSZ correlates with post-AVR recovery of individually injured regions suggests that global MSZ may represent a metric by which patients can be positioned on the reversible/irreversible injury spectrum.
If this metric is a true surrogate of the patient’s proximity to irreversible LV injury, then the MSZ representing the threshold between reversible and irreversible injury need only be determined by the prospective application of this clinical metric in large AI patient study groups. Such a threshold would provide a contractile metric that, when combined with left ventricular dimensional measurements, may have the greatest potential to reduce post-AVR residual contractile dysfunction by appropriately timing surgical intervention.
Acknowledgments
Funding Sources: This work was supported in part by funding from the National Institutes of Health (HL064869 and HL069967) and by the American Association for Thoracic Surgery’s Summer Intern Scholarship.
Footnotes
Presentation: This work was presented at the American Heart Association, Cardiovascular Surgery Scientific Session November 12 – 16, 2011 in Orlando, Florida.
Conflict of Interest
Michael K. Pasque, MD, Brian P. Cupps, PhD, and Washington University may receive income based on a license of related technology by the University to CardioWise, LLC. CardioWise, LLC did not support this work.
References
- 1.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48(3):e1–e148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Pomerantz BJ, Wollmuth JR, Krock MD, et al. Myocardial systolic strain is decreased after aortic valve replacement in patients with aortic insufficiency. Ann Thorac Surg. 2005;80(6):2186–2192. doi: 10.1016/j.athoracsur.2005.05.095. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Picone AL, McIntosh CL, et al. Survival and functional results after valve replacement for aortic regurgitation from 1976 to 1983: impact of preoperative left ventricular function. Circulation. 1985;72(6):1244–1256. doi: 10.1161/01.cir.72.6.1244. [DOI] [PubMed] [Google Scholar]
- 4.Bonow RO, Rosing DR, Maron BJ, et al. Reversal of left ventricular dysfunction after aortic valve replacement for chronic aortic regurgitation: influence of duration of preoperative left ventricular dysfunction. Circulation. 1984;70(4):570–579. doi: 10.1161/01.cir.70.4.570. [DOI] [PubMed] [Google Scholar]
- 5.Chaliki HP, Mohty D, Avierinos JF, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation. 2002;106(21):2687–2693. doi: 10.1161/01.cir.0000038498.59829.38. [DOI] [PubMed] [Google Scholar]
- 6.Fioretti P, Roelandt J, Bos RJ, et al. Echocardiography in chronic aortic insufficiency. Is valve replacement too late when left ventricular end-systolic dimension reaches 55 mm? Circulation. 1983;67(1):216–221. doi: 10.1161/01.cir.67.1.216. [DOI] [PubMed] [Google Scholar]
- 7.Joseph S, Moazami N, Cupps BP, et al. Magnetic resonance imaging-based multiparametric systolic strain analysis and regional contractile heterogeneity in patients with dilated cardiomyopathy. J Heart Lung Transplant. 2009;28(4):388–394. doi: 10.1016/j.healun.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knutsen AK, Ma N, Taggar AK, et al. Heterogeneous distribution of left ventricular contractile injury in chronic aortic insufficiency. Ann Thorac Surg. 2012 doi: 10.1016/j.athoracsur.2011.12.067. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171(3):841–845. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 10.Axel L, Dougherty L. Heart wall motion: improved method of spatial modulation of magnetization for MR imaging. Radiology. 1989;172(2):349–350. doi: 10.1148/radiology.172.2.2748813. [DOI] [PubMed] [Google Scholar]
- 11.Moulton MJ, Creswell LL, Downing SW, et al. Spline surface interpolation for calculating 3-D ventricular strains from MRI tissue tagging. Am J Physiol. 1996;270(1 Pt 2):H281–H297. doi: 10.1152/ajpheart.1996.270.1.H281. [DOI] [PubMed] [Google Scholar]
- 12.Moustakidis P, Cupps BP, Pomerantz BJ, et al. Noninvasive, quantitative assessment of left ventricular function in ischemic cardiomyopathy. J Surg Res. 2004;116(2):187–196. doi: 10.1016/j.jss.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Cupps BP, Pomerantz BJ, Krock MD, et al. Principal strain orientation in the normal human left ventricle. Ann Thorac Surg. 2005;79(4):1338–1343. doi: 10.1016/j.athoracsur.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Potter DD, Araoz PA, Ng LL, et al. Cardiotropin-1 and myocardial strain change heterogeneously in cardiomyopathy. J Surg Res. 2007;141(2):277–283. doi: 10.1016/j.jss.2006.12.539. [DOI] [PubMed] [Google Scholar]
- 15.Young AA, Dokos S, Powell KA, et al. Regional heterogeneity of function in nonischemic dilated cardiomyopathy. Cardiovasc Res. 2001;49(2):308–318. doi: 10.1016/s0008-6363(00)00248-0. [DOI] [PubMed] [Google Scholar]
- 16.Cupps BP, Bree DR, Wollmuth JR, et al. Myocardial viability mapping by magnetic resonance-based multiparametric systolic strain analysis. Ann Thorac Surg. 2008;86(5):1546–1553. doi: 10.1016/j.athoracsur.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111:172–175. [Google Scholar]
- 18.Bree D, Wollmuth JR, Cupps BP, et al. Low-dose dobutamine tissue-tagged magnetic resonance imaging with 3-dimensional strain analysis allows assessment of myocardial viability in patients with ischemic cardiomyopathy. Circulation. 2006;114(1 Suppl):I33–I36. doi: 10.1161/CIRCULATIONAHA.105.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cupps BP, Taggar AK, Reynolds LM, et al. Regional myocardial contractile function: multiparametric strain mapping. Interact Cardiovasc Thorac Surg. 2010;10(6):953–957. doi: 10.1510/icvts.2009.220384. [DOI] [PMC free article] [PubMed] [Google Scholar]