Abstract
Peste des petits ruminants (PPR) is an acute, highly contagious, world organization for animal health (OIE) notifiable and economically important transboundary viral disease of sheep and goats associated with high morbidity and mortality and caused by PPR virus. PPR is considered as one of the main constraints in augmenting the productivity of small ruminants in developing countries and particularly severely affects poor farmer’s economy. The disease is clinically manifested by pyrexia, oculo-nasal discharges, necrotizing and erosive stomatitis, gastroenteritis, diarrhoea and bronchopneumonia. The disease can be diagnosed from its clinical signs, pathological lesions, and specific detection of virus antigen/antibodies/genome in the clinical samples by various serological tests and molecular assays. PPR is the one of the priority animal diseases whose control is considered important for poverty alleviation in enzootic countries. Availability of effective and safe live attenuated cell culture PPR vaccines and diagnostics have boosted the recently launched centrally sponsored control programme in India and also in other countries. This review article primarily focus on the current scenario of PPR diagnosis and its control programme with advancement of research areas that have taken place in the recent years with future perspectives.
Keywords: PPR, Symptoms, Diagnosis, Vaccines, Immunity, Control programme
Introduction
Peste des petits ruminants (PPR), is an acute, highly contagious, world organization for animal health (WOAH-OIE) notifiable and economically important transboundary viral disease of sheep and goats associated with high morbidity and mortality. Clinically, the disease resembles rinderpest (RP) in cattle and is characterized by high fever (pyrexia), conjunctivitis, oculo-nasal discharges, necrotizing and erosive stomatitis, diarrhea [151], and bronchopneumonia followed by either death of the animal or recovery from the disease [56]. The causative agent, PPR virus (PPRV) is an enveloped RNA virus belongs to the genus Morbillivirus of the family Paramyxoviridae (sub family Paramyxovirinae) under the order Mononegavirales [58] with other members of the genus, which include rinderpest virus (RPV), measles virus (MV), canine distemper virus (CDV), phocine distemper virus (PDV) and dolphin and porpoise morbillivirus (DMV) [20]. The virus is a pleomorphic particle with a lipoprotein membrane enveloping a ribo-nucleoprotein core, which contains RNA genome [62]. The genome is a negative sense single stranded-RNA, approximately 16 Kilo bases (kb) long with negative polarity [29]. The genes are arranged in the order of 3′ N–P/C/V–M–F–H–L 5′ [6, 46] and separated by inter-genic region [46] and the nucleotides follows the “rule-of-six” [19]. It is divided into six transcriptional units encoding two non-structural proteins (V, C) and six structural proteins: the surface glycoproteins which include fusion (F) and Haemagglutinin (H) proteins, the matrix protein (M), the nucleoprotein (N), and the phosphoprotein (P) which forms the polymerase complex in association with large (L) protein [36, 42, 61, 129]. The PPRV is genetically grouped into four lineages (I, II, III, and IV) based on the F and N gene sequences analyses [14, 39, 70, 128]. Lineages I–III circulate in Africa, while lineage IV is generally found in Asia [39, 128]. However, a recent appearance of lineage IV, which was associated with a large epizootic in Morocco, undermines the probable risk of introduction this lineage to Europe [77] and other parts of the world. PPR was first reported in the Ivory Coast, West Africa [56], and later from other parts of the world namely sub-Saharan Africa, the Middle East and Indian subcontinent [128, 130]. Spread of disease to a number of new countries in Africa and Asia with involvement of various lineage of PPRV is a cause of global concern especially recent introduction of Asian lineage in some African countries and presence of PPR in Europe through Western Turkey [1, 17, 77, 102]. This transboundary nature of the disease is one of the main constraints in augmenting the productivity of small ruminants in enzootic regions including the parts of Africa, the Middle East and the parts of Asia. Recent developments in the diagnosis of PPR, prospects for improved diagnosis and vaccine development for disease control have been reviewed earlier [17, 43, 45, 125]. However, the present review article comprehend the current scenario of PPR diagnosis and control with advancement of research areas that have taken place in the recent years particularly on diagnostic approaches and control measures with economic impact and future perspectives.
Geographical distribution
PPR, otherwise called as ‘Goat Plague’, is an acute, highly contagious and transboundary viral disease of sheep and goats and causing high morbidity and mortality with major constraints in the productivity of small ruminants in parts of world. The first authentic and scientific description of disease was reported during 1942. At that time Gargadennec and Lalanne [56], reported an epidemic disease in Ivory Coast (Cote d’Ivoire) of West Africa, which was clinically similar to rinderpest (RP) but was affecting only small ruminants, while in-contact cattle remained apparently healthy. The disease used to be called as ‘Kata’, ‘Psuedorinderpest’, ‘Pneumoenteritis complex’ and ‘Stomatitis-pneumoenteritis syndrome’ [26] in French speaking countries (Francophone) of West Africa. Based on outbreaks of disease in Senegal in 1871 and in French Guinea in 1927 {quoted by Curasson [37]}, it was believed that PPR might be much more historical than it has been thought of. The disease soon spread to neighbouring African countries like Nigeria, Senegal and Ghana. Till early 1980s, definite outbreaks of the disease were reported from different parts of West Africa [24, 63, 94, 109, 150]. Until 1984, PPR was regarded as a disease of West African countries, however, its presence in Sudan [50, 151] that year marked beginning of the spread of PPR. Since the later part of 1980s, the PPR further spread to countries of central and eastern Africa and parts of Asia. Evidence of PPR infection based on either serological tests and/or clinical investigations has been reported from several other countries [77].
Though there is no evidence of first appearance of PPR in India, its presence was first reported in 1987 [127]. The first confirmed outbreak of PPR in sheep with 25 % mortality was reported in Arasur village, Villipuram district of Tamil Nadu during 1987, where characteristic clinical signs pertaining to PPR were noticed [127]. Before 1987, rinderpest (RP) was believed to be the cause of infection in sheep and goats [96]. Whether, some of these infection were due to PPR is not clear. However, the emergence of PPR after the eradication of RP makes us speculate that some of the earlier outbreaks in sheep and goats could be due to PPR rather than RP. Unfortunately, there is not enough data to substantiate this speculation. Subsequent to this outbreak there were only two outbreak reports, from Andhra Pradesh during 1987 [146]. Nevertheless there is even possibility that in the absence of proper diagnostics, the disease could have been misdiagnosed in favour of other pneumonic diseases such as small ruminant rinderpest, pasteurellosis, contagious caprine pleuropneumonia, which precluded and delayed its recognition. An extensive clinical survey of PPRV infection could have been difficult up to this point as the importance of the disease was not known besides the non availability of diagnostic tests in different state diagnostic laboratories. Until severe epidemics swept through the rest of India in 1994 and onwards the disease was thought to be restricted in Southern India. Since then the disease became enzootic in many northern states of India [92, 95] causing huge economic losses to the small ruminant industry. In Maharashtra, the first PPR outbreak in goats was reported in 1994 [72]. Outbreaks in other species like buffaloes have also been reported in 1997 [60]. Now the disease is enzootic and outbreaks occurs throughout India almost all season of the year [8–10, 68, 73, 87, 95, 97, 134]. It is still not clear whether the apparent geographical spread of the disease in the last two decades is real or just a reflection of increased awareness, wider availability of diagnostic tools or even a change in the virulence of the virus. It seems most likely that combinations of these factors are responsible for the present knowledge of the disease distribution.
Diagnosis
PPR is tentatively diagnosed by clinical observations, characteristic symptoms, epidemiology, post-mortem lesions and laboratory confirmation by using various serological and molecular techniques. Serological tests such as agar gel immuno-diffusion test (AGID)/AGPT [101], counter-immuno-electrophoresis (CIE) [101] and indirect ELISA, although popular in earlier days for tentative diagnosis, do not differentiate PPR and RP infections [98, 104]. Advent of cell culture and molecular biological techniques has allowed development of specific, rapid and sensitive diagnostics. In general, virus isolation, AGID/AGPT [104], CIE [86], nucleic acid hybridization [41, 105], hemagglutination using piglet [160] or chicken [126] red blood cells, immuno histochemical detection [118], serum or virus neutralization test (VNT) and ELISA using PPRV-specific monoclonal antibodies (MAbs) are used for diagnosis of PPR.
Therefore, specific diagnosis of PPRV infection can be achieved by cDNA hybridization [41, 105, 127, 149], mobility of N protein [40, 149], neutralization test [28, 116, 149] and MAb-based ELISA [81, 82, 135, 136], RT-PCR [7, 53] and Real time RT-PCR [12, 13, 18]. A battery of serological tests and molecular assays are available to detect and identify PPRV antigen/nucleic acid and antibodies. Most of the conventional tests are time consuming, labor intensive, less sensitive and not rapid and therefore, not suitable for primary diagnosis but useful in secondary confirmatory testing and retrospective epidemiological studies. To overcome the drawbacks associated with these tests, the recent molecular biology tools and techniques like real time RT-PCR and loop mediated isothermal amplification (LAMP) assays [79] have been used for the rapid and sensitive detection of PPRV RNA from clinical samples.
Clinical signs and pathological lesions
PPR can be diagnosed tentatively based on clinical and pathological lesions. Clinical manifestation of PPR is more or less similar to RP except for the remarkable affinity of the PPRV to the lung tissues. Clinically, the disease is characterized by high fever (pyrexia), oculonasal discharges, necrotizing and erosive stomatitis, gastro enteritis, diarrhoea and bronchopneumonia, followed by either death of the animal or recovery from the disease [56]. The clinical features or signs are better described under experimental condition rather than the natural infection. Both in natural as well experimental PPR infection, the incubation period ranged from 2 to 6 days [74, 75, 95, 153]. In experimentally infected kids, the first clinical sign of pyrexia (40.6–44.2 °C) was observed on days 5, followed by diarrhea (between day 5 and 8) and death within 10 days [143]. Further, Kumar et al. [75] observed rise in the temperature at 3rd days post infection (dpi) (104.5 °F) to 10th dpi with general congestion, erosion and ulceration of oral mucosa in the challenged goats. The different stages of the disease are (i) incubation period, (ii) Prodromal phase (febrile), (iii) mucosal phase (ocular and nasal discharges, hyperaemia of conjunctiva and mucosa of anterior nares, and erosions on the tongue, palate, lips and other parts of the oral mucosa—Fig. 1), (iv) diarrhoeal stage and (v) in non-fatal cases, ‘recovery stage’ in which, sheep and goats that recover from PPR develop an active lifelong immunity. PPR may manifest in different forms like per acute, acute and a mild depending on the severity of the disease [103]. Further, the severity of disease depends on various factors namely the PPRV virulence/lineage [33], species, breed, immune status of animals, age of the animals, etc. [142]. PPR is frequently confused with other diseases that present fever and grossly similar clinical signs, especially when it is newly introduced. The disease with similar signs for differential diagnosis are Bluetongue (BT), Contagious ecthyma (Orf), Foot and mouth disease (FMD), Contagious caprine pleuropneumonia (CCPP), Pasteurellosis etc., Sometimes, mixed infection of PPR and goat pox or sheep pox or Orf or BT occurs [88, 93, 119]. Earlier, a mixed viral infection of PPR and adenovirus was also confirmed in goats in separate outbreaks in Nigeria [59]. Recently, Toplu et al. [152] reported dual infection of fetal and neonatal small ruminants with border disease virus (BDV) and PPRV.
Fig. 1.
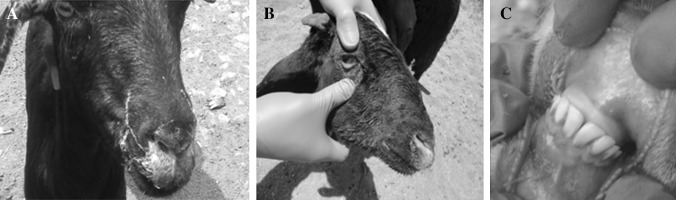
PPR virus infected animals showing nasal discharge (a), congestion of conjunctiva (b) and Bran-like deposits-discrete tiny necrotic ulceration or foci in the mucous membrane (c)
The pathology of PPR is characterized and dominated by retrogressive and necrotic changes in lymphoid tissues and epithelial cells of gastrointestinal and respiratory systems. Various reports have described gross pathological changes in naturally occurring PPR in goats and sheep [4, 74–76, 153, 154]. The prominent lesions in PPR infected animals include, consolidation, changes in colour of lungs and sometimes, frothy mucus is observed in cut pieces of lung on squeezing, antero-ventral areas of right lung are frequently involved; areas of lungs become dark red or purple, firm to touch mainly in the anterior and cardiac lobes [75]. Consolidation of lobes of lungs (Fig. 2a) and occlusion of airway caused by secondary bacterial pneumonia are common [76]. Congested alveolar border was found to be one of the most characteristic clinical and pathological changes of PPR in goats [74, 153, 154]. The involvement of respiratory system in PPR is remarkable and pneumonia is a predominant sign in PPR. Most reports found lung involvement in almost more than 90 % of cases dying during outbreak of PPR [4, 74, 100, 153, 154]. Bronchopneumonia is a constant lesion, with possibility of pleuritis and hydrothorax. Lymph nodes associated with lung (mediastinal) and intestine (mesenteric) are most commonly affected which are generally enlarged, oedematous and congested (Fig. 2b). Spleen was congested and engorged, and at times showed petechiae on the capsular surface. Severe congestion and necrotic lesions in gastro-intestinal tract are normal feature. Necrotic or haemorrhagic enteritis or congestion around the ileo-caecal valve, at the caeco-colic junction and in the rectum are seen usually. In the posterior part of colon and rectum, discontinuous streaks of congestion (“Zebra” stripes or “Zebra markings”) on the mucosal folds are observed, which are typical of PPR (Fig. 2c).
Fig. 2.
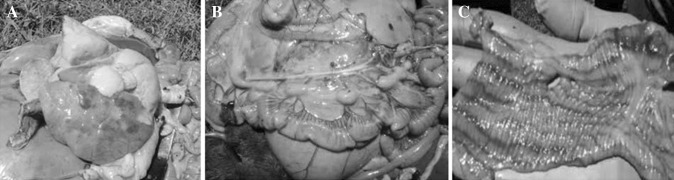
Post-mortem lesion showing congestion and consolidation of lobes of lung (a), enlarged oedematous and congested intestinal mesenteric lymph nodes (b) and colon showing discontinuous streaks of congestion and haemorrhages (Zebra markings) on the mucosal folds (c)
Conventional tests/assays
PPRV-specific antibodies have been used for the detection of virus antigen in tissue, swabs, conjunctival smears and formalin-fixed tissues by various researcher in different assays/tests [82, 99, 118, 147]. Earlier, AGID/AGPT was a frequently used method for the detection of RPV and PPRV antigens in the clinical and post-mortem samples [3]. CIE was comparatively more sensitive and rapid method than that of AGPT, but failed to differentiate between PPRV and RPV infection [48, 86, 98]. Indirect immunofluorescence test (IFAT) and Immunoperoxidase test (IPT) utilizing polyclonal and MAb provides rapid detection of virus antigen in situ in the infected cells or tissues samples [49, 86]. IPT could be a test of choice for localization of PPRV antigen in samples where antigen has not been reported previously.
Among all the methods, isolation of the virus remains the “gold standard” for diagnosis of PPR. The PPRV can be isolated and grown in vitro in primary bovine and sheep cells [49] as well as established cell lines such as Vero (African green monkey kidney) cells [78] and Marmoset B-lymphoblastoid-B95a cells [145]. The virus manifests specific cytopathic effect (CPE) after 3–5 days of infection, which include initial rounding of the infected cells in grape-bunch-like clusters, followed by vacuolation, granulation of the cell cytoplasm, fusion of the monolayer cells and formation of syncytia, which are characteristics of PPRV. Virus isolation cannot always be done as routine diagnostic assays because they are time-consuming and cumbersome and require cell culture facilities, and they are not as sensitive as RT-PCR [27]. VNT was performed for confirmation and differentiation of RP and PPR viruses [55, 78], either in tubes or in microtitre plates (micro-VNT) for the detection of PPRV antibodies in serum samples [116, 149]. Differential neutralization test is one of the important means to distinguish RP and PPR viruses [116, 149] and a micro-VNT has been employed to measure the infectivity titre of RPV and PPRV in calf kidney, sheep kidney and Vero cells [116]. Although PPRV clinically and antigenically related to RPV, PPRV is distinguishable serologically not only from RPV but also from MV and CDV [58]. Parallel titration of unknown sera for different antibody responses against PPR and RP viruses has been shown to detect specific etiology in earlier days [150].
Enzyme-linked immunosorbent assay (ELISA)
Researchers are working towards the development of molecular diagnostics for early and specific diagnosis of PPR. As a rapid, simple and sensitive assay, ELISA has been widely used in serological profiling of PPRV in mass screening of samples for sermonitoring/serosurveillance or clinical prevalence. Various workers have used MAb produced against PPRV and RPV for detection of antibodies and antigens in ELISA [2, 83, 117]. Saliki et al. [118] and Anderson and Mckay [2], used neutralizing MAbs against H protein of PPRV for specific detection of PPRV antibodies in competitive ELISA (c-ELISA) and blocking ELISA (B-ELISA) [83, 118, 131, 136] for antibody detection. Use of the virus neutralising MAb was more advantageous as it produced better correlation between VNT and ELISA [83, 117]. The sensitivity and specificity of B-ELISA was found to be 90.4 and 98.8 %, respectively when compared to VNT [117]. Anderson and McKay [2] used MAb-based c-ELISA for measurement of antibodies to PPR and RP viruses in sheep, goat and cattle in African countries. Libeau et al. [83] used anti ‘N’ MAb to PPRV in a c-ELISA, which was 94.5 % sensitive and 99.4 % specific in comparison to VNT. Singh et al. [136] developed a MAb-based c-ELISA for the detection of PPRV antibodies using a virus neutralising MAb directed against an epitope in ‘H’ protein specific MAb (4B11) to PPRV [131] and cell culture propagated vaccine virus antigen [144]. This c-ELISA had high diagnostic specificity (99.8 %) and sensitivity (90.5 %) for detection of PPRV antibody in convalescent sera, when compared with VNT and also the commercially available kit, where, it had high diagnostic sensitivity (92.2 %) and specificity (98.4 %) [133, 136]. This assay is currently being employed extensively throughout India for monitoring/serosurveillance of PPR. Further, a polyclonal antibody based indirect ELISA was also developed for detection of antibodies to PPRV in the serum samples of goats and sheep using Vero cell culture propagated purified PPRV antigen. This assay had a high degree of specificity and sensitivity (Dsp 95.09, Dsn 90.81 %) and (Dsp 100, Dsn 80 %), when compared with c-ELISA [136] and VNT, respectively. This may be a good alternative tool to c-ELISA for seroepidemiological surveys [15].
Further, MAb-based immunocapture ELISA [82] and sandwich ELISA (s-ELISA) [135] have been used extensively for detection of PPRV antigen in clinical specimens. The immunocapture ELISA was developed in the world reference laboratory (CIRAD–EMVT, France) and is an internationally accepted assay for PPRV antigen detection [82]. This assay uses a biotinylated anti-‘N’ MAb against a cross-reactive epitope of RP/PPRV to capture or detection of PPRV antigen in clinical samples. Similarly, the s-ELISA kit developed at Division of Virology, IVRI, Mukteswar, India uses a MAb (4G6) directed against an epitope of N protein of PPRV [133], which is the routinely being used for clinical prevalence or detection of PPRV antigen in clinical specimens in India [135]. This assay was efficacious, with diagnostic sensitivities (89 %) and specificities (93 %), comparable to the immunocapture-ELISA [83]. Further, assay using multiple antigenic peptides (MAPs), as isotope, for detection of PPRV antibodies in serum samples has also been developed for serosurveillance and seromonitoring. Three MAPs specific to PPRV polypeptides were used which reacted specifically to PPRV antibodies giving high ELISA titres were selected.
Molecular diagnostic techniques
PPR is a major problem in African and Asian continents and some of the researchers working towards molecular diagnostic approaches for early and specific diagnosis. Due to improved understanding of the viral genome and molecular biological techniques, nucleic acid-based specific and sensitive assays were also developed [41, 43, 105, 128].
Nucleic acid hybridization
RT-PCR, simple and aqueous phase ELISA (SNAP-ELISA) and nucleic acid hybridization have also been used for the detection and differentiation of PPRV [35, 43, 53, 54, 105, 127]. RT-PCR and cDNA hybridization techniques are the two sensitive means to diagnose the PPRV infection, but are time consuming and cumbersome for routine diagnosis with a large sample size. Nucleic acid hybridization is also suitable for providing diagnosis utilizing field materials, which are either collected from the putrefied or semi-putrefied carcasses or get putrefied during transit period [41, 105]. Diallo et al. [41]. developed 32P labelled cDNA probes derived from N gene of the RP and PPR viruses for differentiation of these viral infections without the need of virus isolation. cDNA probes targeting other genes such as M, F and P were non-specific [41]. Despite the high sensitivity, radiolabelled probes were not widely used because of short half life of 32P and the requirements of fresh specimens and isotopes handling facility. This led to the development of non-radioactive probes using biotinylated DNA or digoxigenin (DIG) labeled oligonucleotides [43, 105]. This assay was very specific and rapid but its sensitivity was not less than radioactive labeled probes [43].
Polymerase chain reaction
Further, the nucleic acid hybridization using radiolabeled or biotinylated cDNA probes, competitive and immunocapture ELISAs and differential immuno-histochemical staining using MAbs are less sensitive than PCR for the detection of viruses. Of late, RT-PCRs have been reported for detection and differential diagnosis of RP and PPR viruses in clinical specimens. Forsyth and Barrett [53] developed genus specific and universal morbillivirus primers to distinguish among known morbilliviruses. Various primers sets derived from ‘N’, ‘F’ and ‘P’ genes were described to detect and differentiate between RP and PPR by various workers [21, 35, 53]. The PCR techniques have been developed targeting F gene [53], N gene [35, 57], M gene [7, 57] and H gene [14, 69] and used for specific detection of PPRV from clinical samples. A PCR for PPRV/RPV using PPR virus-specific external protein F gene primer (F1 and F2) [53] has gained great importance for differential diagnosis and for epidemiological studies. However, this assay may not always be suitable for diagnosis of every virus strain, variant or isolate, as changes at the 3′ end of the primer binding sites, as a result of variation between strains in the immunogenic protein coding region, may yield a false-negative result. A two-step RT-PCR has been shown to useful for the rapid detection of virus specific RNA in the samples submitted for laboratory diagnosis [35, 57, 128]. Further, PCR strategies targeting M and N gene have been developed for detection and differentiation of PPRV in sheep and goats. In a similar direction, a RT-PCR assay based on the M and N gene has been developed [7, 57], which was employed successfully for the direct detection of PPRV in the clinical samples [7]. The one-step multiplex RT-PCR based on N and M genes for use in differential diagnosis of PPR from RP in a single reaction, which has the potential to replace the existing F gene based PCR for diagnosis of PPR was developed [7]. This was an improved assay over the existing assays for the simultaneous detection and differentiation of PPRV. A highly sensitive N-gene based RT-PCR-ELISA for the detection and differentiation of PPRV has also been developed using DIG-labelled RT-PCR product [122]. The assay can detect viral RNA in the infected tissue culture fluid with a titre as low as 0.01 TCID50/100 μl. PCR-ELISA is 10,000 times more sensitive than the detection of RT-PCR product for diagnosis of PPR in early as well as late phases of the disease and also used for the differential diagnosis of PPR [122].
As an improvement over conventional PCR approaches, the real-time PCR techniques targeting either N or M gene using TaqMan hydrolysis probe, SYBR Green with melting curve analysis have been in use for rapid, highly sensitive and specific detection and quantitation of PPRV. Earlier, N gene based one step TaqMan real-time RT-PCR has been developed for detection of PPRV nucleic acid in the clinical samples with high sensitivity [18]. Recently, M gene based two step TaqMan hydrolysis probe [12] and one step real-time RT-PCR based on SYBR Green chemistry [13] have been optimized for specific detection of PPRV in clinical samples. Some of earlier described RT-PCR assays are delineated in the OIEs Manual of Diagnostic Tests and Vaccines for Terrestrial Animals [103]. A duplex real-time qRT-PCR assay was also developed for a simple and rapid diagnosis of PPR [107]. In the recent past, as a field application diagnostic tool, a simple, rapid, specific and highly sensitive novel approach called as LAMP have also been developed targeting N gene [38, 79] and evaluated using field clinical samples. This assay appears to be potential as rapid and sensitive diagnostic tool for its application in less equipped rural diagnostics laboratory settings. These assays have the advantage of avoiding an extra step of agarose gel analysis.
Penside tests
A simple dot-ELISA has also been developed using either anti-M protein MAb [99] or anti-N protein MAb [130] for the detection of PPRV antigen in tissue homogenate/swab materials of sheep and goats origin. This test has been found to be useful for screening of large number of clinical samples and suitable for animal disease investigation laboratories in the field and could be used as a penside test for diagnosis of PPR. This assay had relative diagnostic sensitivity (82.5 %) and specificity (91 %) compared to s-ELISA for PPR diagnosis [130]. Lateral flow test for detection of PPRV antigen and antibody was also developed, but not up to the mark in regular usage. However, this test has the advantages of quick, easy to perform and does not involve technical skill or expertise and hence user friendly, useful as penside diagnostic test. Similarly, Dipsticks was developed for detection of PPRV in a test sample by using the unlabeled capture MAb directed to Matrix protein of PPRV is immobilized on the test zone of dipstick and the mobile detector reagent, which comprises combination of PPRV ‘H’ and ‘N’ MAbs, labeled with colloidal gold particles, is impregnated on the conjugate. Raj et al. [111] developed immunofiltration and antigen-competition ELISA methods for detection of PPRV antigen, which showed a sensitivity of 80 % and specificity of 100 %. These two tests can serve as a screening (immunofiltration) and confirmatory (antigen-competition ELISA) test, respectively, in the diagnosis of PPR in sheep or goats. Moreover, this test has the advantages of being quick, easy to perform and does not require technical skill or expertise and hence user friendly.
Recombinant antigen based Assays
Due to the advancement in rDNA technology, gene expression technology, production of recombinant viral proteins has become easier and more efficient. The ease of genetic modification, the yield of recombinant proteins and the maintenance of post-translational modifications often determine the choice of the host systems. Several attempts have been made since long to express various viral proteins in different expression systems viz., bacterial, mammalian, yeast and insect cells and to assess potential use of recombinant proteins in various diagnostic assays. In this direction, Ismail et al. [65] cloned and sequenced the cDNA of the N gene of the Nigeria 75/1 strain of PPRV. The recombinant baculovirus expressed N protein in insect cell system was used successfully as a coating antigen in ELISA for serodiagnosis of PPRV. Libeau et al. [83] developed a c-ELISA based on the reaction between a MAb and a recombinant N protein of the PPRV. They used recombinant N protein of Nigeria 75/1 strain of PPRV expressed in baculovirus as antigen and reported that the c-ELISA is as sensitive and specific as VNT, with the added advantage that it uses an antigen that is safe and can be produced in large quantities. Choi et al. [31] developed a rapid c-ELISA for diagnosis and surveillance of PPR. This assay detects PPRV antibodies in serum samples by quantifying the amount of MAb after 30 min of incubation of a serum-MAb conjugate mixture on plates coated with a PPRV recombinant N protein (rPPRV-N) expressed in baculovirus system. The expressed PPRV F protein in bacterial [51] and eukaryotic [80] system showed antigenicity and immunogenicity [159]. Further, the expressed truncated and full-length N protein of PPRV in E. coli showed reactivity in s-ELISA and tested as a coating antigen in c-ELISA for serological diagnosis of PPR infection [161]. Recently, Liu et al. [84] produced polyclonal antibodies against the recombinant truncated PPRV M protein expressed in E. coli and checked its specificity in western blot and immunofluorescence. These assays are safe and better alternatives to live PPRV antigen in ELISA for clinical or sero-surveillance of PPR in enzootic or non-enzootic countries.
Prevention and control
For the proper control of PPR, there is need of strong support of diagnostic methods and proper, timely vaccination of the susceptible population. Hence, the availability of attenuated cell culture vaccine and various diagnostic techniques/kits for the diagnostic of PPR favours strong recommendation put forward for the control program.
Prophylaxis
PPR is one of the priority animal diseases whose control is considered important for poverty alleviation in Africa and Southern Asia. Thus its control is a major goal for programmes aim at poverty alleviation. The only way to control PPR is by vaccination. For prevention of PPR, Gargannec and Lallane [56] tried formalized rinderpest spleen with inconclusive results. Mornet et al. [94] used lapinised RP vaccine (LRPV) for control of PPR in a few goats with some success, but found that LRPV did not prevent mortality in goats, however other causes of mortality were not ruled out in this study. Bourdin et al. [25] successfully employed tissue culture rinderpest virus (TCRPV) in protecting goats in Benin Republic and Senegal. Based on encouraging results for several years, OIE since 1972 recommended the use of TCRPV for PPR prophylaxis in west Africa, which was continued for long time. The vaccine was successfully used to control PPR in west African and other African countries. Considering the close antigenic relationship between RPV and PPRV, the live attenuated RP vaccine was tested in goats for vaccination against PPR and that provided a protection for a period of 1 year [151]. Therefore, earlier the disease was controlled in different parts of the world by using Plowright and Ferris [106] TCRP vaccine, which is a heterologous vaccine. This TCRP vaccine has earlier been used to protect against PPR but the use of TCRP vaccine to control PPR was later banned in all animal species world-wide so as to achieve the status of rinderpest-free country or zone following the OIE pathway [2], after the launch of rinderpest eradication programme, which stimulated the development of homologous PPR vaccine(s) by the world community. Hence the practice of heterologous PPR control was abolished in most countries.
The first homologous PPR vaccine was developed using live attenuated Nigerian strain PPRV Nig 75/1 after 63 passages in Vero cells produced a solid immunity for 3 years [45, 47]. During 1975, this virus was isolated from a dead PPRV infected goat in Nigeria [148]. Several vaccine trials had been conducted during 1989–1996 which demonstrated the efficacy of this vaccine in 98,000 sheep and goats in the field. The vaccine was safe under field conditions even for pregnant animals and induced immunity in 98 % of the vaccinated animals [47]. The vaccinated animals did not develop any disease following challenge with virulent PPRV strains and thereby this vaccine was used worldwide (Africa, Middle East and Sothern Asia) for effective control of PPR. In a cross protection study, PPR vaccine was found to protect cattle effectively against RP [34]. It has been demonstrated that freeze-drying of this vaccine in an excipient containing trehalose makes it very thermostable and it resists temperature up to 45 °C for a period of 14 days with minimal loss of potency [159]. The genetic diversity among the PPR viruses occurring in different geographic locations has already been established [39, 128]. Although the vaccine developed by Diallo et al. [47] may induce protection against different lineages of PPRVs, use of such a vaccine may introduce a new live virus of different genetic makeup which does not exist in Indian or Asian animal population. Similarly, three other homologous PPR vaccines using Indian isolates of PPRV(goat origin-Sungri-1996 and Coimbatore(CBE)-1997; sheep origin-Arasur-1987) have been developed and evaluated recently by Indian veterinary research institute (IVRI) and Tamil Nadu university of veterinary and animal science (TANUVAS), Chennai [121]. It is necessary that each animal to be vaccinated should receive a minimum recommended dose (OIE) i.e., 103TCID50. To this under field condition, maintenance of cold-chain is essential for these vaccines which requires highest recurrent cost. Therefore to determine the optimal conditions for storage and its use in the field level, information on the stability of the vaccine is of great importance. A homologous live attenuated PPR vaccine (Sungri/96 strain) developed at Division of Virology, IVRI, Mukteswar has been tested extensively in in-house as well as by field trials and has been found to be safe and potent in small ruminants [130, 132] and about 90 % of the animals have been found to have protective levels of antibodies under field conditions [144]. Using this vaccine, studies have been undertaken with respect to thermostability, pathogenicity and immunogenicity at various in vitro passages [112, 120, 123, 144]. The vaccine virus has been proven to be completely safe as it failed to revert back to virulence even after passage in natural hosts for five times [144] and was found to induce only a transient lymphopenia, which may not induce biologically significant immunosuppression [112]. Thus, this PPR vaccine (Sungri/96) is safe for mass vaccination campaign under field conditions and is presently used throughout India to vaccinate sheep and goats with great efficacy and has potentiality to use effectively against lineage IV virus circulating in Indian sub-continents and in other countries in Asia. The third and fourth live attenuated vaccines were PPRV Arasur/87 (sheep origin) and CBE/97 (goat origin), respectively, which were developed by TANUVAS, Chennai, India [104]. These vaccines are being used in southern states of India. The CPE pattern of this virus differs from PPR Sungri/96 [130]. However these vaccines provide satisfactory protection against virulent PPRV Izatnagar/94 strain [120]. These are also equally safe and protective as Sungri/96 in sheep and goats and suited for commercial vaccine production.
A major disadvantage when using classical live attenuated vaccine is that the antibody responses are indistinguishable from natural infection. This makes seroepidemiological surveillance of the disease impossible in enzootic areas where a vaccination program has been or is being implemented. A way to combine both the activities, viz., vaccination and serosurveillance, for the better management of the disease would be the use of DIVA vaccines, which enable differentiation between infected and vaccinated animals [155]. Faris et al. [52] conducted cross-sectional epidemiological study followed by vaccination with Nigeria 75/1 vaccine, produced at National Veterinary Institute (NVI) in Debre-Zeit, Ethiopia and postvaccinal serum antibody assessment against PPR in small ruminant population of Awash Fentale District, Afar, Ethiopia, during September 2006–June 2007. The postvaccination seroconversion rate in the population was found to be 61.13 %, indicating a relatively weak herd immunity. The main reason for the low sero-conversion was speculated to be the thermolabile nature of the vaccine and this signifies the need for thermostable vaccine that could potentially increase the herd immunity.
Another disadvantage of this vaccine is to maintain the cold chain as these vaccine strains are labile to high temperature. There are only few reports regarding intrinsic thermo stabilization of animal viral vaccines. The notable ones are against New castle disease and RP [113]. The earliest reports on the thermo-stability of the cell culture adapted RPV were of Plowright and Ferris [106] and Johnson [66]. In the former study, mutant clones of RPV were selected by growing the virus for six passages at 40 °C with an increment of temperature 0.5 °C/passage. It was seen that compared to the vaccine virus, thermo-resistant virus multiplied to satisfactory titers at higher temperatures (40 °C). On evaluation of both viruses, it was seen that immunogenic protein profiles are similar, indicating that there was no change with regard to these characteristics but there were minor changes observed in the amino acid sequences on the basis of nucleotide sequence analysis [113]. A lyophilized thermo-stable Vero cell-adapted RP vaccine, stabilized with lactalbumin hydrolysate (LAH) and sucrose, was tested for safety, serological response and suitability for use with an abbreviated cold-chain under field conditions in Nigeria. The un-refrigerated vaccine retained a titer of 3.69 log10 TCID50/dose through day 34 [89, 90]. Sarkar et al. [123] have also reported the thermo-tolerance of an indigenous live attenuated PPR vaccine (Sungri/96) in India.
Based on this approach, the IVRI, Mukteswar, India have been successful in developing intrinsic live attenuated Thermo-adapted (Ta) PPR vaccines (goat and sheep isolates) employing thermo-adapted Vero cell line, which is adapted to grow at 40 °C [14, 114]. The vaccines have undergone successful in-house trials in both the sheep and goats either as single [125, Balamurugan et al., unpublished data], or in combination with sheeppox or goatpox as bivalent or Orf as trivalent vaccines (unpublished data). Further, Riyesh et al. [114] evaluated the efficacy of different stabilizers on the thermostability of this Ta PPR vaccines. These Ta vaccines can be used as alternatives to existing vaccines in tropical countries for the control of the disease as they are considerably more stable at ambient temperatures [114]. Similarly, another promising research at IVRI, Mukteswar with regard to the PPR vaccine (PPRV-Sungri/96) is the production of thermo-stable vaccine stabilized with appropriate stabilizers or heavy water (D2O) [124]. In this study, application of deuterium for enhancing the thermostability of PPR vaccine has been evaluated using heavy water as reconstituting diluent. Further, the ability of D2O–MgCl2 and conventional saline diluents were tested for increasing the thermostability of live attenuated PPR vaccine [124].
Economical and simpler process of production of thermo-stable live attenuated RP and PPR vaccine has been reported using trehalose dihydrate as stabilizer [159]. It has been found that PPRV requires higher levels of trehalose than that of RP virus to attain same degree of thermo-tolerance. The new methods for freeze drying of conventional vaccines for RP and PPR have added new dimension in increasing thermostability of these vaccines [90, 159]. Mariner et al. [89] developed a method for freeze drying wherein the moisture content of vaccine was greatly reduced thereby increasing thermostability of TCRP vaccine. Similarly, Worral et al. [159] reported an ultra rapid method (Xerovac) for the dehydration and preservation of live attenuated RP and PPR vaccines.
To protect goats from PPR and Goat Pox (GP), a combined live attenuated PPR and Capripox virus vaccine of sheep origin had been used in Cameroon [91]. Further, considering the similar geographic distribution of both PPR and goat or sheep pox virus infection as well as occurrence of mixed infections due to these viruses, use of a combined vaccine for control of these infections particularly in the endemic areas will be a good option. This approach would assist to a great extent in the mass immunization programs. Vero cell-attenuated homologous PPR and GP vaccines using Indian isolates [64]. The efficacy of the combined vaccine (PPR and GP) in goats showed that bivalent vaccine induced protective immune response against homologous challenge in goats. Thus, they concluded that both PPR and GP vaccine viruses are compatible with each other for making a live bivalent vaccine that could control PPRV and GPV infections in same geographic region [64]. Similarly, the combined sheep pox and PPR vaccine was prepared in lyophilized form containing recommended doses of both vaccine viruses [Romanian Fanar (RF) strain and PPRV-Sungri/96 strain] and Safety and immunogenicity of this combined vaccine was evaluated in sheep [30].
Another issue in PPR vaccination is the time of vaccination of young animals. There are few reports on duration of persistence of maternal antibodies in lambs/kids born to vaccinated dams. Maternal antibodies in young animals were detectable up to 6 months of age but fell below the protection threshold level at 3.5 and 4.5 months in lambs and kids, respectively [5, 23]. Similarly, the neutralizing maternal antibodies were detectable up to 4 months compared to 3 month with competitive ELISA [5, 81]. These findings lead to suggestion that lambs and kids from immunized or exposed dams should be vaccinated at 4 and 5 months of age, respectively [5]. Recently, Balamurugan et al. [11]., showed maternal antibodies in kids were detectable up to 6 months with a declining trend from the third month onwards and receded below the protective level by the fourth month in goats vaccinated with lineage IV vaccine.
Recombinant vaccines
Two external glycoproteins, the F and H proteins of the viruses are responsible for inducing protection against the disease in animals. The H and F protein genes of several morbilliviruses have been expressed in various vector systems and they can be used as effective sub-unit vaccines. Following this approach, the F protein of PPRV was inserted into the genome of an attenuated capripox vaccine virus candidate [22]. The resulting recombinant virus expressed the PPRV F protein on the surface of infected cells, which was recognized by an anti-F MAb. The recombinant virus was then tested in goats and shown to be effective in protecting inoculated animals against PPR at a dose as low as 0.1 plaque forming units (pfu). A similar vaccine which expressed the PPRV H protein was also produced and it is effective at a minimal dose of 10 pfu [44]. The duration of immunity provided by these two vaccines and also the effect of capripox pre- immunity over the vaccination have not yet been determined. Prasad et al. [108] developed an edible vaccine using HN gene of PPRV using binary vector pBI121 mobilized into Agrobacterium tumefaciens strain GV3 101 and subsequently expressed in pigeonpea. The goat immunized with baculovirus expressed recombinant HN glycoprotein of PPRV, produced immune response against PPRV and antibodies generated in immunized animals could neutralize both PPRV and RPV in vitro [140]. Recombinant Bombyx mori nucleopolyhedroviruses (BmNPV) expressed the immunodominant ectodomains of F glycoprotein of PPRV and the H protein of RPV, on the budded virions as well as the surface of the infected host cells and the recombinant virus particles induced immune response in mice against PPRV or RPV [110]. The transiently expressed PPRV HN protein in mammalian CV-1 cells was found to be biologically active in possessing hemadsorption and neuraminidase activities while RPV H protein exhibited neuraminidase activity but was deficient in hemadsorption activity [126]. Furthermore it was found that the transiently expressed PPRV F protein could bring about both fusion and hemifusion whereas the RPV F protein could only bring about hemifusion and fusion required the presence of an attachment protein-HN [126]. The successful generation of transgenic peanut plants and immune responses to HN protein of PPRV in sheep has also been reported [71].
Further, to facilitate the serosurveillance a marker vaccine and a companion test that can detect infection in vaccinated animals (DIVA) are the key. Therefore, though current live attenuated vaccine is efficacious against the clinical disease, to facilitate the serosurveillance and seromonitoring, a recombinant marker vaccine (either positive or negative marker) approach may be beneficial [125]. The development of reverse genetics technology for negative strand RNA viruses has given us another means of producing marker vaccines to combat viral disease such as PPR. One of these recombinant vaccines has the RP vaccine virus genome as the backbone into which the matrix (M), the F and H protein genes of RPV were replaced by those of PPRV [85]. The resulting chimeric virus proved safe and effective vaccine which could protect goats against virulent challenge with PPRV. The widespread use of such vaccines, along with the diagnostic tests to identify their serological signature, would greatly improve the surveillance capabilities for disease preparedness and emergency prevention procedures. The chimeric vaccine can be used in any enzootic country without compromising the global RP sero-surveillance effort since all antibody tests are based either on N or H virus proteins which are quite distinguishable serologically between RP and PPR viruses. The widespread use of such vaccines, along with the diagnostic tests to identify their serological signature, would greatly improve the surveillance capabilities for disease preparedness and emergency prevention procedures.
Although, goats vaccinated with a recombinant vaccinia virus (VACV) expressing the H and F genes of RPV developed neutralizing antibodies to RPV, and not to PPRV, they were completely protected against challenge with virulent PPRV [115, 162]. Goats were also protected against a lethal challenge of PPRV following vaccination with a recombinant capripoxvirus containing either the F or H gene of RPV [115]. Similarly recombinant VACV and capripox virus expressing the H and F genes of RPV were reported to protect goats against PPRV challenge [67, 115]. Recombinant adenovirus expressing F and H fusion proteins of PPRV induces both humoral and cell-mediated immune responses in goats [157]. Further, Wang et al. [158], constructed a suicidal DNA vaccine based on the Semliki forest virus (SFV) replicon and tested for its ability to induce immunogenicity in a mouse model. They injected pSCA1-H plasmid intramuscularly into BALB/c mice thrice at 2-week intervals and evaluated the immunogenicity of pSCA1-H, by ELISA, microneutralization and lymphocyte proliferation assays. This suicidal DNA vaccine could be a promising new approach for vaccine development against PPR.
Immunity
In morbilliviruses, the surface glycoproteins, H and F are highly immunogenic and confer protective immunity. PPRV is antigenically closely related to RPV and antibodies against PPRV are both cross-neutralising and cross-protective [150]. The VACV and capripox expressing H and F glycoproteins PPRV has been shown to protect goats against PPR [67, 115]. A VACV double recombinant expressing H and F glycoproteins of RPV has been shown to protect goats against PPR [67] though the animals developed virus-neutralising antibodies only against RPV and not against PPRV. Capripox recombinants expressing the H or F protein of RPV or the F protein of PPRV conferred protection against PPR in goats, but without production of PPRV-neutralizing antibodies [115] or PPRV antibodies detectable by ELISA [22]. Results of these studies suggested that cell-mediated immune responses could play a crucial role in the protection. Further, goats immunized with a recombinant baculovirus expressing H protein generated both humoral and cell-mediated immune responses [138, 141] and were also RPV cross reactive suggesting that the H protein presented by the baculovirus recombinant ‘resembles’ the native protein present on PPRV [140]. Further, lymphoproliferaltive responses were demonstrated against PPRV-H and RPV-H antigens [138–141] and mapping of N-terminal T cell determinant and a C-terminal domain harboring potential T cell determinant(s) was carried out [139, 140]. Though the sub-set of T cells (CD4+ and CD8+ T cells) in PBMC that responded to the recombinant protein fragments and the synthetic peptide could not be determined, this could potentially be a CD4+ helper T cell epitope, which has been shown to harbor an immunodominant H restricted epitope in mice [140]. Identification of B- and T cell epitopes on the protective antigens of PPRV would open up avenues to design novel epitope based vaccines against PPR. In a study involving antigenic and immunogenic properties of B cell epitopes in N protein of PPRV, 4 domains (A-I, A-II, C–I and C-II) were identified by using MAbs. Blocking and competitive ELISA revealed that epitopes on domain A-II and C-II were immunodominant. The A-II domain was shown to cross react with RPV antibodies [32].
Treatment
Since PPR is a viral disease, there is no specific treatment for this disease. Post-exposure therapeutic approaches for PPR infections are not mentioned much in the literature. However, treatment of affected animals by administration of antibiotics (long acting oxytetracycline, chlortetracycline) to prevent secondary bacterial infections and anti-diarrhoeal medicines has been practiced with supportive therapy (B-complex and Dextrose saline) for 5–7 days, which may be useful to reduce the severity of the disease. Treatment and management of clinical cases of PPR or in the event of outbreaks in sheep and goats is necessary in order to minimize the economic losses to farmers.
Control measures
The control of PPR can be ensured only through the implementation of effective prophylactic measures. All the sheep and goats of the affected flock should be under quarantine for at least 1 month after the last clinical case. Animal movements have to be strictly controlled in the area of the infection. Unfortunately, such sanitary and control measures are difficult to maintain in a vast country with difficult terrain where PPR is endemic like in India. Therefore, the only effective way to control PPR is by mass vaccination of the animals and quarantine measures and use of an effective vaccine against PPR is the only solution to control disease effectively. Some of the other control measures (sanitary prophylaxis) are strict quarantine and control of animal movements, quarantine of newly purchased or newly arriving goats/sheep for at least 2–3 weeks and know the health status and the source of any new animal(s) brought into the flock, migratory flocks are threat to local sheep and goat therefore contact may be avoided, effective cleaning and disinfection of contaminated areas of all premises with lipid solvent solutions of high or low pH and disinfectants including physical perimeters, equipment and clothing, dead animal/carcases should be burnt/buried deeply, monitor animals closely and frequently for any developing illness or signs of disease, isolate any sick animals from the flock and contact the Veterinarian immediately to examine sick animals in the herd/flock, use separate facilities and staff to handle isolated animals, educate and train the employees about PPR and the signs of illness and monitoring of wild and captive animals, especially in contact with sheep and goats.
Economic impact
Presence of disease can limit trade and export, import of new breeds and development of intensive livestock production, which in turn diminish the consumption of animal protein in human. The turnover rate of small ruminant populations is much faster than that of larger livestock, so vaccination has to be used more intensively and more frequently. But, if this can be achieved within a programme of progressive control, losses could be minimized and certain areas should be able to be freed from PPR. Cost benefit analysis is the most important criteria for launching any disease control programme. Epidemics of PPR have enormous consequences in terms of the dramatic effects this disease can bring about on livestock productivity and the high costs of control or eradication. Epidemics affect not only individual farmers but also the agricultural industry and as a consequence, the national economy. PPR is present in countries, which are either developing or under-developed thereby adding to the economic woes. An estimate in the absence of authentic data on the losses in India due to PPR is an arbitory.
Small ruminants such as sheep and goats are numerically and economically important livestock species in developing countries like India. India is a vast country with a population of 140.5 million goats and 71.6 million sheep (2007 18th Livestock census, DADF, GOI, www.dahd.nic.in) as against approximately 2.1 billions world population of sheep and goats, are contributing much to small, marginal and landless rural farmers to sustain their livelihood. Economic loss due to PPR is not quantified comprehensively so far either at the national or regional/state levels. As per rough estimate, the disease cause annual loss to the tune of 1, 800 million INR (39 million US$) [16, 156] or 4,000 million INR (DADF, Government of India, GOI, www.dahd.nic.in), or value of 4610.3 million (3,323.2 millions USD for goats and for sheep 1,287.1 million USD) with 28.39 % losses due to PPR in small ruminants [130] in India. Due to the occurrence of other diseases, the economic impacts of PPR are probably under estimated, but it is believed that PPR is one of the major constraints of small ruminant productivity. The quantification of economic losses due to any disease in animals is very important since it helps in prioritizing the research on animal health issues; and designing appropriate control programme for PPR.
Control strategies
For the proper control of PPR, there is need of strong support of diagnostic methods and proper, timely vaccination of the susceptible population upon understanding the epidemiology of the disease are imperative. Hence, the availability of attenuated cell culture vaccines [144] and various diagnostic techniques/kits [135, 136] for the diagnostic of PPR, public and regulatory concern along with control measure and strategies favours for a strong recommendation of National control programme (NCP) for PPR in India in order to alleviate the poverty in the country initially and continent later [130]. Control strategies may vary from country to country as per the prevalence of disease but in developing or under-developed countries the choices are limited. In India too, stamping out by slaughter is not feasible, both for economic and sentimental reasons. Vaccination has become a recommended tool to support control and eradication efforts and limit the economic losses due to PPR [130, 137]. This way, at least the immediate loss could be prevented and the small and marginal farmers rearing sheep and goats will be benefited. Therefore, PPR control and eradication depends mainly on rapid and accurate diagnosis or surveillance/monitoring and implementation of prompt vaccination programme. Recent success with the rinderpest eradication programme (NPRE) in the country has provided the confidence that is required to launch a similar programme with PPR too. All the elements (potent vaccine, kits for disease diagnosis and serosurveillance and the tested infrastructure, etc.) required for a control programme are available [130, 137], which have further been recommended for a collaborative nation-wide program implemented by state Animal Husbandry Departments under the direction of the Department of Animal Husbandry, Dairying and Fisheries (DADF, GOI) and the co-operation of the local public under the guidance from the policy maker at the centre.
Vaccination against PPR has been practiced in some states of India since 2002 to control the disease [130]. The DADF, Government of India had launched a NCP for PPR that would be run in three phases during India’s 11th (2007–2012) and 12th (2012–2017) 5-year plan periods with an aim to control and eradicate this disease from India. Accordingly, this proposed programme has been initiated during the year 2010–2011 with a sum of Rs. 43.25 crore. The situation in India is improving as a result of progressive mass vaccination. The disease incidence has been in decline over the past 5 years as per analysis of outbreak data available in National Animal Disease Referral Expert System (NADRES), PD_ADMAS (unpublished data). In India, decreased numbers of outbreaks as well as changes in the severity of disease patterns recently observed might be due to the effectiveness of live attenuated vaccines, timely vaccination of sheep and goats, and circulation of a single Asian lineage IV PPRV, since the disease was first reported in India [14]. Currently vaccination programs are being implemented in some states of India which will alter PPR epidemiology, particularly distribution of the disease and pattern of disease [8, 134].
Currently three live attenuated PPR vaccines (Sungri/96, Arasur/87 and CBE/97 stains) are available, of which, Sungri/96, developed by IVRI, Mukteswar has undergone extensive field trial [130, 132, 137]. These vaccines may be sufficient to protect against the circulating field isolates/strains of PPRV in India and provide long-term (more than 6 years) protective immunity [120]. These vaccines can be used for the control and eradication of the disease not only from India but also from other Asian and some African countries following the example of the global RP eradication programme (GREP), as now Lineage IV virus expand its geographical locations.
Vaccination strategies for the control of PPR would be slightly different from vaccination programmes for RP. A mass vaccination campaign to cover 80 % herd or flock immunity would be needed to account for the population dynamics of sheep and goats, disparities in sheep and goats husbandry practices and the agro-climatic conditions affecting the pattern of disease [137]. The slaughtering of male goats at an early age combined with the high fecundity of the caprine species results in replacement of population (~30–40 % naïve population appears) every year. Initially, in order to reduce economic losses due to PPRV, intensive vaccination of the entire population within a specified area would need to be undertaken. Subsequent vaccinations would then be performed on younger animals at approximately 6 months of age [137]. The maternal antibodies in kids were detectable up to 6 months with a declining trend from the third month onwards and receded below the protective level by the fourth month in vaccinated and infected goats and PPR vaccination is recommended in kids, aged 4 months and born to immunized or exposed goats to avoid window of susceptibility in kids to PPRV and the effort to eliminate PPR infection from susceptible populations [11]. The 4–6 months old young ones in and around the vaccinated flocks will be few not even >30 % of the population at that point of time. The vaccinated flocks available will be 70–80 % and provides the herd or flock immunity. Vaccinated, infected and recovered animals are protected from re-infection for the remainder of their lives. In other way, vaccinations should be focused initially on high-risk group animals, for example young animals (6 months–1 year), goat population than sheep and migratory flocks [137] in suitable period (preferably during lean periods). Alternatively, intensive vaccinations can be carried out based on populations to make disease free areas (zone) by identifying the areas of infection and implementing vaccinations followed by screening, testing and over all revaccination if required in those areas as reported earlier [137]. It is hoped that PPR in the direction of rinderpest will be eradicated in India within a decade or few more years.
Future perspectives
PPR is an important animal viral disease of sheep and goats, which now threatens the billion-strong small ruminant population in Africa, the Middle and Near East, South-West and Central Asia. PPR is the one of the priority animal diseases whose control is considered important for poverty alleviation. But still PPR is a poorly recognized disease, particularly with regard to epidemiological features such as transmission dynamics under different production systems that is important to support control policy decisions. A great deal of more research into this aspect of the disease is need of an hour. The fact that PPRV can infect cattle, buffaloes and camels gives PPR an even higher priority, particularly in the current situation where RP has been eradicated. Though, natural transmission of the virus occurs in other species of animals like cattle, buffaloes and camels, generally not affected with clinical form of disease but seroconvert. The availability of an effective marker vaccine along with its companion serological tests will greatly assist in designing effective control programmes in future. Now the disease has been brought under control in goats and sheep by available effective and safe live attenuated cell culture PPR vaccines. The vaccines are effective in the face of natural outbreaks or experimental challenge and significantly reduce the mortality. The present scenario of PPR in India warrants the studies to be undertaken with the objective to know the effect of agro climatic changes on the occurrence of PPR in small ruminants in different agro-climatic zones and to analyze the relationship of disease occurrence and risk factors to formulate modules for forecasting and forewarning. The epidemiology of PPR is likely to change due to vaccination as the disease occurs more severely in the naive population only. More research is needed on the host-virus interaction through cellular receptor, immunological events including protective mechanisms, development of marker vaccine to differentiate between virulent and vaccine virus antibodies and also on development of thermostable vaccine. Analytical study about incidence of disease would be extremely useful and elicit widespread interest by providing sufficient additional information, especially in the epidemiology of the disease, which is important to support control policy decisions.
Acknowledgments
The assistance rendered by Dr. R. Apsana, Ph.D, Scholar, Department of Microbiology, Veterinary College (KVAFSU), Bangalore, India is gratefully acknowledged. All the authors who contributed to the understanding of the PPRV pathobiology especially in diagnosis and prophylaxis of PPR irrespective of their citation being figured or not in this review are acknowledged. Further, authors apologize for noninclusion of some of the references, if any, which is unintentional.
References
- 1.Albina E, Kwiatek O, Minet C, Lancelot R, de Servan Almeida R, Libeau G. Peste des petits ruminants, the next eradicated animal disease? Vet Microbiol. 2013;165(1–2):38–44. doi: 10.1016/j.vetmic.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J, Mckay JA. The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implications to rinderpest control programmes. Epidemiol Infect. 1994;112(7):225–231. doi: 10.1017/s0950268800057599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appel MJG, Gibbs EPJ, Martin SJV, Meulen T, Rima BK, Stephensen, Taylor WP. Morbillivirus diseases of animals and man. In: Kurstak E, Kurstak C, editors. Comparative diagnosis of viral diseases. IV. New York: Academic Press; 1981. pp. 235–297. [Google Scholar]
- 4.Aruni AW, Latha PS, Mohan AC, Chitravelu P, Ambumani SP. Histopathological study of a natural outbreak of peste des petits ruminants in goats in Tamil Nadu. Small Rumin Res. 1998;28:233–240. [Google Scholar]
- 5.Ata FA, AI-Sumry HS, King GJ, Ismaili SI, Ata AA. Duration of maternal immunity to peste des petits ruminants. Vet Rec. 1989;124(32):590–591. doi: 10.1136/vr.124.22.590. [DOI] [PubMed] [Google Scholar]
- 6.Bailey D, Banyard AC, Dash P, Ozkul A, Barrett T. Full genome sequence of peste des petits ruminants virus, a member of the Morbillivirus genus. Virus Res. 2005;110:119–124. doi: 10.1016/j.virusres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Balamurugan V, Sen A, Saravanan P, Singh RP, Singh RK, Rasool TJ, Bandyopadhyay SK. One-step multiplex RT-PCR assay for the detection of Peste-des-petits-ruminants virus in clinical samples. Vet Res Commun. 2006;30:655–666. doi: 10.1007/s11259-006-3331-3. [DOI] [PubMed] [Google Scholar]
- 8.Balamurugan V, Krishnamoorthy P, Veeregowda BM, Sen A, Rajak KK, Bhanuprakash V, Gajendragad MR, Prabhudas K. Seroprevalence of Peste des petits ruminants in cattle and buffaloes from Southern Peninsular India. Trop Anim Health Prod. 2012;44:301–306. doi: 10.1007/s11250-011-0020-1. [DOI] [PubMed] [Google Scholar]
- 9.Balamurugan V, Saravanan P, Sen A, Rajak KK, Bhanuprakash V, Krishnamoorthy P, Singh RK. Sero-epidemiological study of peste des petits ruminants in sheep and goats in India between 2003 and 2009. Rev Sci Technol. 2011;30(3):889–896. doi: 10.20506/rst.30.3.2087. [DOI] [PubMed] [Google Scholar]
- 10.Balamurugan V, Saravanan P, Sen A, Rajak KK, Venkatesan G, Krishnamoorthy P, Bhanuprakash V, Singh RK. Prevalence of peste des petits ruminants among sheep and goats in India. J Vet Sci. 2012;13(3):279–285. doi: 10.4142/jvs.2012.13.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balamurugan V, Sen A, Venkatesan G, Rajak KK, Bhanuprakash V, Singh RK. Study on passive immunity: time of vaccination in kids born to goats vaccinated against peste des petits ruminants. Virol Sin. 2012;27:228–233. doi: 10.1007/s12250-012-3249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balamurugan V, Sen A, Venkatesan G, Yadav V, Bhanot V, Bhanuprakash V, Singh RK. Application of semi-quantitative M gene-based hydrolysis probe (TaqMan) real-time RT-PCR assay for the detection of peste des petits ruminants virus in the clinical samples for investigation into clinical prevalence of disease. Transbound Emerg Dis. 2010;57:383–395. doi: 10.1111/j.1865-1682.2010.01160.x. [DOI] [PubMed] [Google Scholar]
- 13.Balamurugan V, Sen A, Venkatesan G, Yadav V, Bhanot V, Bhanuprakash V, Singh RK. A rapid and sensitive one step-SYBR green based semi quantitative real time RT-PCR for the detection of peste des petits ruminants virus in the clinical samples. Virol Sin. 2012;27(1):1–9. doi: 10.1007/s12250-012-3219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balamurugan V, Sen A, Venkatesan G, Yadav V, Bhanot V, Riyesh T, Bhanuprakash V, Singh RK. Sequence and phylogenetic analyses of the structural genes of virulent isolates and vaccine strains of peste des petites ruminants virus from India. Transbound Emerg Dis. 2010;57:352–364. doi: 10.1111/j.1865-1682.2010.01156.x. [DOI] [PubMed] [Google Scholar]
- 15.Balamurugan V, Singh RP, Saravanan P, Sen A, Sarkar J, Sahay B, Rasool TJ, Singh RK. Development of an Indirect ELISA for the detection of antibodies against peste des petits ruminants virus in small ruminants. Vet Res Commun. 2007;31:355–364. doi: 10.1007/s11259-006-3442-x. [DOI] [PubMed] [Google Scholar]
- 16.Bandyopadhyay SK. 14th Annualconference and national seminar on management of viral diseases with emphasis on global trade and WTO regime, 18–20th January. Bangalore, India: Indian Virological Society; 2002. The economic appraisal of PPR control in India. [Google Scholar]
- 17.Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol. 2010;91:2885–2897. doi: 10.1099/vir.0.025841-0. [DOI] [PubMed] [Google Scholar]
- 18.Bao J, Li L, Wang Z, Barrett T, Suo L, Zhao W, Liu Y, Liu C, Li J. Development of one-step real-time RT-PCR assay for detection and quantitation of peste des petits ruminants virus. J Virol Methods. 2008;148(1–2):232–236. doi: 10.1016/j.jviromet.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Baron MD, Barrett T. Sequencing and analysis of the nucleocapsid (N) and polymerase (L) genes and the extragenic domains of the vaccine strain of rinderpest virus. J Gen Virol. 1995;76:593–603. doi: 10.1099/0022-1317-76-3-593. [DOI] [PubMed] [Google Scholar]
- 20.Barrett T, Amarel-Doel C, Kitching RP, Gusev A. Use of the polymerase chain reaction in differentiating rinderpest field virus and vaccine virus in the same animals. Rev Sci Tech. 1993;12(3):865–872. doi: 10.20506/rst.12.3.734. [DOI] [PubMed] [Google Scholar]
- 21.Barrett T, Amarel-Doel C, Kitching RP, Gusev A. Use of the polymerase chain reaction in differentiating rinderpest field virus and vaccine virus in the same animals. Rev Sci Tech. 1993;12:865–872. doi: 10.20506/rst.12.3.734. [DOI] [PubMed] [Google Scholar]
- 22.Berhe G, Minet C, Le Goff C, Barrett T, Ngangnou A, Grillet C, Libeau G, Fleming M, Black DN, Diallo A. Development of a dual recombinant vaccine to protect small ruminants against peste-des-petits-ruminants virus and capripoxvirus infections. J Virol. 2003;77(2):1571–1577. doi: 10.1128/JVI.77.2.1571-1577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bidjeh KC, Diguimbaya P, Hendrikx V, Debet D, Tchari, Naissinger S. Maternal immunity in young goats or sheep whose dams were vaccinates with anti-peste des petits ruminants. Cahiers Agric. 1999;3:219–222. [Google Scholar]
- 24.Bonniwell MA. The use of tissue culture rinderpest vaccine (TCRV) to protect sheep and goats against “peste des petits ruminants” in the Ashanti region of Ghana. Rev Sci Technol. 1980;92:1233–1238. [Google Scholar]
- 25.Bourdin P, Rioche M, Laurent A. Emploi d’un vaccin antibovipestique produit sur culture cellaire dans la prophylaxie de la peste des petits ruminants an Dahomey—note préliminaire Revue d’Elevage et de Medecine Vétérénary des Pays Tropicaux. 1970;23:295–300. [PubMed]
- 26.Braide VB. Peste des petits ruminants. World Anim Rev. 1981;39:25–28. [Google Scholar]
- 27.Brindha K, Raj GD, Ganesan PI, Thiagarajan V, Nainar AM, Nachimuthu K. Comparison of virus isolation and polymerase chain reaction for diagnosis of peste des petits ruminants. Acta Virol. 2001;45(3):169–172. [PubMed] [Google Scholar]
- 28.Chandran NDJ, Kumanan K, Venkateswan RA. Differentiation of peste des petits ruminants and rinderpest viruses by neutralization indices using hyperimmune rinderpest antiserum. Trop Anim Health Prod. 1995;27:89–92. doi: 10.1007/BF02236317. [DOI] [PubMed] [Google Scholar]
- 29.Chard LS, Bailey DS, Dash P, Banyard AC, Barrett T. Full genome sequences of two virulent strains of peste-des-petits ruminants virus, the Coted’Ivoire 1989 and Nigeria 1976 strains. Virus Res. 2008;136:192–197. doi: 10.1016/j.virusres.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary SS, Pandey KD, Singh RP, Verma PC, Gupta PK. A vero cell derived combined vaccine against sheep pox and peste des petits ruminants for sheep. Vaccine. 2009;27(19):2548–2553. doi: 10.1016/j.vaccine.2009.01.104. [DOI] [PubMed] [Google Scholar]
- 31.Choi KS, Nah JJ, Ko YJ, Kang SY, Jo NI. Rapid competitive enzyme-linked immunosorbent assay for detection of antibodies to peste des petits ruminants virus. Clin Diagn Lab Immunol. 2005;12(4):542–547. doi: 10.1128/CDLI.12.4.542-547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi KS, Nah JJ, Ko YJ, Kang SY, Yoon KJ, Jo NI. Antigenic and immunogenic investigation of B-cell epitopes in the nucleocapsid protein of peste des petits ruminants virus. Clin Diagn Lab Immunol. 2005;12(1):114–121. doi: 10.1128/CDLI.12.1.114-121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couacy-Hymann E, Bodjo C, Danho T, Libeau G, Diallo A. Evaluation of the virulence of some strains of peste-des-petits-ruminants virus (PPRV) in experimentally infected West African dwarf goats. Vet J. 2007;173:178–183. doi: 10.1016/j.tvjl.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Couacy-Hymann E, Bidjeh K, Angba A, Domenech J, Diallo A. Protection of goats against rinderpest by vaccination with attenuated peste des petits ruminants virus. Res Vet Sci. 1995;59(2):106–109. doi: 10.1016/0034-5288(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 35.Couacy-Hymann E, Roger F, Hurard C, Guillou JP, Libeau G, Diallo A Rapid and sensitive detection of peste des petits ruminants virus by a polymerase chain reaction assay. J Virol Methods. 2002;100:17–25. doi: 10.1016/s0166-0934(01)00386-x. [DOI] [PubMed] [Google Scholar]
- 36.Crowley JC, Dowling PC, Menonna J, Silverman JI, Schuback D, Cook SD, Blumberg BM. Sequence variability and function of measles 3′ and 5′ ends and intercistronic regions. Virology. 1988;164:498–506. doi: 10.1016/0042-6822(88)90564-8. [DOI] [PubMed] [Google Scholar]
- 37.Curasson G. Traite de Pathologie Exotique Veterinaire et Comparee/G. Curasson. Vigot Freres Paris. 1936;1:28–302. [Google Scholar]
- 38.Dadas RC, Muthuchelvan D, Pandey AB, Rajak KK, Sudhakar SB, Shivchandra SB, Venkatesan G. Development of loop-mediated isothermal amplification (LAMP) assay for rapid detection of peste des petits ruminants virus (PPRV) genome from clinical samples. Indian J Comp Microbiol Immunol Infect Dis. 2012;33(1&2):7–13. [Google Scholar]
- 39.Dhar P, Sreenivasa BP, Barrett T, Corteyn M, Singh RP, Bandyopadhyay SK. Recent epidemiology of peste des petits ruminants virus (PPRV) Vet Microbiol. 2002;88:153–159. doi: 10.1016/s0378-1135(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 40.Diallo A, Barret T, Lefevre PC, Taylor WP. Comparison of proteins induced in cells infected with RP and PPR viruses. J GenVirol. 1987;68:2033–2038. doi: 10.1099/0022-1317-68-7-2033. [DOI] [PubMed] [Google Scholar]
- 41.Diallo A, Barrett T, Barbras M, Shaila MS, Taylor WP. Differentiation of rinderpest and peste des petits ruminants viruses using specific cDNA clones. J Virol Methods. 1989;23:127–136. doi: 10.1016/0166-0934(89)90126-2. [DOI] [PubMed] [Google Scholar]
- 42.Diallo A, Barrett T, Barbron M, Meyer G, Lefevre PC. Cloning of nucleocapsid protein gene of peste des petits ruminants virus: relationship to other morbilliviruses. J Gen Virol. 1994;75:233–237. doi: 10.1099/0022-1317-75-1-233. [DOI] [PubMed] [Google Scholar]
- 43.Diallo A, Libeau G, Couacy-Hymann E, Barbron M. Recent developments in the diagnosis of rinderpest and peste des petits ruminants. Review. Vet Microbiol. 1995;44(2–4):307–317. doi: 10.1016/0378-1135(95)00025-6. [DOI] [PubMed] [Google Scholar]
- 44.Diallo A, Minet C, Berhe G, Le Goff C, Black DN, Fleming M, Barrett T, Grillet C, Libeau G. Goat immune response to capripox vaccine expressing the hemagglutinin protein of peste des petits ruminants. Ann NY Acad Sci. 2002;969:88–91. doi: 10.1111/j.1749-6632.2002.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 45.Diallo A, Minet C, Le Goff C, Berhe G, Albina E, Libeau G, Barrett T. The threat of peste des petits ruminants: progress in vaccine development for disease control. Vaccine. 2007;25(30):5591–5597. doi: 10.1016/j.vaccine.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Diallo A. Morbillivirus group: genome organization and proteins. Vet Microbiol. 1990;23:55–163. doi: 10.1016/0378-1135(90)90145-l. [DOI] [PubMed] [Google Scholar]
- 47.Diallo A, Taylor WP, Lefevre PC, Provost A. Attenuation d’une souche de la peste des petits ruminants candidat pour un vaccine homologue vivant. Rev Elev Med Vet Pays Trop. 1989;42(3):311–319. [PubMed] [Google Scholar]
- 48.Durojaiye OA, Taylor WP. Application of counterimmunoelectrophoresis to the serology of Peste des petits ruminants. Rev Elev Med Pays Trop. 1984;37(3):272–276. [PubMed] [Google Scholar]
- 49.Durojaiye OA. Application of a precipitinogen inhibition test in the detection of antibody to peste des petits ruminants virus. Rev Elev Med Vet Pays Trop. 1987;40(1):17–20. [PubMed] [Google Scholar]
- 50.El Hag B, Taylor TW. Isolation of PPR virus from Sudan. Res Vet Sci. 1984;36:1–4. [PubMed] [Google Scholar]
- 51.Fan Y, Dou YX, Liang ZX, Luo XN, Cheng Y, Cai XP. Cloning of F gene of peste des petits ruminants virus Nigeria75/1 strain and its expression in Escherichia coli. Chinese Vet Sci. 2009;39(6):503–509. [Google Scholar]
- 52.Faris D, Yilkal A, Berhec G, Kelay B. Seroprevalence and sero-conversion after vaccination against peste des petits ruminants in sheep and goats from Awash Fentale District, Afar, Ethiopia. Prev Vet Med. 2012;103:157–162. doi: 10.1016/j.prevetmed.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Forsyth MA, Barrett T. Detection and differentiation of rinderpest and peste des petits ruminants viruses in diagnostic and experimental samples by polymerase chain reaction using P and F gene-specific primers. Virus Res. 1995;39:151–163. doi: 10.1016/0168-1702(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 54.Forsyth MA, Parida S, Alexandersen S, Belsham GJ, Barrett T. Rinderpest virus lineage differentiation using RT-PCR and SNAP-ELISA. J Virol Methods. 2003;107(1):29–36. doi: 10.1016/s0166-0934(02)00186-6. [DOI] [PubMed] [Google Scholar]
- 55.Furley CW, Taylor WP, Obi TU. An outbreak of peste des petits ruminants in a zoological collection. Vet Rec. 1987;121:443–447. doi: 10.1136/vr.121.19.443. [DOI] [PubMed] [Google Scholar]
- 56.Gargadennec L, Lalanne A. La peste des petits ruminants. Bull Serve Zootech Epizoot Afr Occid Fr. 1942;5:16–21. [Google Scholar]
- 57.George A, Dhar P, Sreenivasa BP, Singh RP, Bandyopadhyay SK. The M and N genes-based simplex and multiplex PCRs are better than the F or H gene-based simplex PCR for peste-des-petits-ruminants virus. Acta Virol. 2006;50(4):217–222. [PubMed] [Google Scholar]
- 58.Gibbs EP, Taylor WP, Lawman MJ, Bryant J. Classification of peste des petits ruminants virus as the fourth member of the genus Morbillivirus. Intervirology. 1979;11(5):268–274. doi: 10.1159/000149044. [DOI] [PubMed] [Google Scholar]
- 59.Gibbs EPJ, Taylor WP, Lawman MJP. The isolation of adenovirus from goats affected with peste des petits ruminants in Nigeria. Res Vet Sci. 1977;23:331–335. [PubMed] [Google Scholar]
- 60.Govindarajan R, Koteeswaran A, Venugopalan AT, Shyam G, Shaouna S, Shaila MS, Ramachandran S. Isolation ofpestes des petits ruminants virus from an outbreak in Indian buffalo (Buhalus huhalis) Vet Rec. 1997;141(22):573–574. doi: 10.1136/vr.141.22.573. [DOI] [PubMed] [Google Scholar]
- 61.Haas L, Baron MD, Liess B, Barrett T. Editing of morbillivirus P gene transcripts in infected animals. Vet Microbiol. 1995;44:299–306. doi: 10.1016/0378-1135(95)00024-5. [DOI] [PubMed] [Google Scholar]
- 62.Haffar A, Libeau G, Moussa A, Cècile M, Diallo A. The matrix protein gene sequence analysis reveals close relationship between PPR virus and dolphin morbillivirus. Virus Res. 1999;64:69–75. doi: 10.1016/s0168-1702(99)00080-5. [DOI] [PubMed] [Google Scholar]
- 63.Hamdy FM, Dardiri AH, Nduaka O, Breese SS, Ihemelandu EC. Etiology of the stomatitis pneumoenteritis complex in Nigerian dwarf goats. Can J Comp Med. 1976;40:276–284. [PMC free article] [PubMed] [Google Scholar]
- 64.Hosamani M, Singh SK, Mondal B, Sen A, Bhanuprakash V, Bandyopadhyay SK, Yadav MP, Singh RK. A bivalent vaccine against goat pox and peste des petits ruminants induces protective immune response in goats. Vaccine. 2006;24(35–36):6058–6064. doi: 10.1016/j.vaccine.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 65.Ismail TM, Yamanaka MK, Saliki JT, el-Kholy A, Mebus C, Yilma T. Cloning and expression of the nucleoprotein of peste des petits ruminants virus in baculovirus for use in serological diagnosis. Virology. 1995;208(2):776–778. doi: 10.1006/viro.1995.1210. [DOI] [PubMed] [Google Scholar]
- 66.Johnson RH. Rinderpest in tissue culture. III. Use of the attenuated strain as a vaccine for cattle. Br Vet J. 1962;118:141–150. [Google Scholar]
- 67.Jones L, Giavedoni L, Saliki JT, Brown C, Mebus C, Yilma T. Protection of goats against peste des petits ruminants with a vaccinia virus double recombinant expressing the F and H genes of rinderpest virus. Vaccine. 1993;11:961–964. doi: 10.1016/0264-410x(93)90386-c. [DOI] [PubMed] [Google Scholar]
- 68.Joshi VB, Nagal KB, Sharma M, Katoch RC, Batta MK, Sharma AK. Peste des petits ruminants (PPR) among Gaddi sheep and goats in Himachal Pradesh. Indian J Anim. Sci. 1996;66:1126–1127. [Google Scholar]
- 69.Kaul R. Hemagglutinin gene based molecular epidemiology of PPR virus. Dissertation, Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh, India; 2004.
- 70.Kerur N, Jhala MK, Joshi CG. Genetic characterizationof Indian peste des petits ruminants virus (PPRV) by sequencing and phylogenetic analysis of fusion protein and nucleoprotein gene segments. Res Vet Sci. 2008;85:176–183. doi: 10.1016/j.rvsc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Khandelwal A, Renukaradhya GJ, Rajasekhar M, Sita GL, Shaila MS. Immune responses to hemagglutinin-neuraminidase protein of peste des petits ruminants virus expressed in transgenic peanut plants in sheep. Vet Immunol Immunopathol. 2011;140(3–4):291–296. doi: 10.1016/j.vetimm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 72.Kulkarni DD, Bhikane AU, Shaila MS, Veralakshi P, Apte MP, Narladkar W. Peste des petits ruminants in goats. Vet Rec. 1996;136:87. doi: 10.1136/vr.138.8.187. [DOI] [PubMed] [Google Scholar]
- 73.Kumar N, Chaubey KK, Chaudhary K, Singh SV, Sharma DK, Gupta VK, Mishra AK, Sharma S. Isolation, identification and characterization of a peste des petits ruminants virus from an outbreak in Nanakpur, India. J Virol Methods. 2013;189(2):388–392. doi: 10.1016/j.jviromet.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Kumar P, Kumar R, Sharma AK, Tripathi BN. Pathology of peste des petits ruminants (PPR) in goats and sheep: spontaneous study. Indian J Vet Pathol. 2002;26(1&2):15–18. [Google Scholar]
- 75.Kumar P, Tripathi BN, Sharma AK, Kumar R, Sreenivasa BP, Singh RP, Dhar P, Bandyopadhyay SK. Pathological and immunohistochemical study of experimental peste des petits ruminants virus infection in goats. J Vet Med B. 2004;51(4):153–159. doi: 10.1111/j.1439-0450.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- 76.Kumar P. Pathological studies on Peste des petits ruminants (PPR) in goats and sheep. Dissertation, Indian Veterinary Rsearch institute, Izatnagar. 2001. p. 63.
- 77.Kwiatek O, Ali YH, Saeed IK, Khalafalla AI, Mohamed OI, Obeida AA, Rahman MBA, Osman HM, Taha KM, Abbas Z, Harrak ME, Lhor Y, Diallo A, Lancelot R, Albina E, Libeau G. Asian lineage of peste des petits ruminants virus, Africa. Emerg Infect Dis. 2011;17:1223–1231. doi: 10.3201/eid1707.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lefevre PC, Diallo A. Peste des petits ruminants. Rev Sci Technol. 1990;9:951–965. doi: 10.20506/rst.9.4.532. [DOI] [PubMed] [Google Scholar]
- 79.Li L, Bao J, Wu X, Wang Z, Wang J, Gong M, Liu C, Li J. Rapid detection of peste des petits ruminants virus by a reverse transcription loop-mediated isothermal amplification assay. J Virol Methods. 2010;170(1–2):37–41. doi: 10.1016/j.jviromet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 80.Liang ZX, Qiang Z, Fan Y, Dou YX, Luo XN, Cheng Y, Cai XP. Construction and expression of the eukaryotic expression vector with F gene of peste des petits ruminants virus. Chinese Vet Sci. 2009;39(5):405–409. [Google Scholar]
- 81.Libeau G, Diallo A, Calvez D, Lefevre PC. A competitive ELISA using anti-N monoclonal antibodies for specific detection of RP antibodies in cattle and small ruminants. Vet Microbiol. 1992;31:147–160. doi: 10.1016/0378-1135(92)90073-3. [DOI] [PubMed] [Google Scholar]
- 82.Libeau G, Diallo A, Colas F, Guerre L. Rapid differential diagnosis of RP and PPR using an immunocapture ELISA. Vet Rec. 1994;134:300–304. doi: 10.1136/vr.134.12.300. [DOI] [PubMed] [Google Scholar]
- 83.Libeau G, Prehaud C, Lancelot R, CoLas F, Guerre L, Bishop DH, Diallo A. Development of a competitive ELISA for detecting antibodies to the PPR virus using a recombinant nucleoprotein. Res Vet Sci. 1995;58:50–55. doi: 10.1016/0034-5288(95)90088-8. [DOI] [PubMed] [Google Scholar]
- 84.Liu F, Wu X, Li L, Liu Z, Wang Z. Expression, purification and characterization of two truncated peste des petits ruminants virus matrix proteins in Escherichia coli, and production of polyclonal antibodies against this protein. Protein Expr Purif. 2013;91(1):1–9. doi: 10.1016/j.pep.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 85.Mahapatra M, Parida S, Baron MD, Barrett T. Matrix protein and glycoproteins F and H of peste-des-petits-ruminants virus function better as a homologous complex. J Gen Virol. 2006;87(7):2021–2029. doi: 10.1099/vir.0.81721-0. [DOI] [PubMed] [Google Scholar]
- 86.Majiyagbe KA, Nawathe DR, Abegunde A. Rapid diagnosis of PPR infection, application of immuno-etectro-osmophoresis (IEOP) technique. Rev Elev Med Vet Pays Trop. 1984;37:11–15. [PubMed] [Google Scholar]
- 87.Majumder P. Studies on an incidence of peste-des-petits ruminants in the regional goat breeding farm at Debipur, Tripura. Indian J Anim Health. 1997;36(2):169–171. [Google Scholar]
- 88.Malik YS, Singh D, Chandrashekar KM, Shukla S, Sharma K, Vaid N, Chakravarti S. Occurrence of dual infection of peste-des-petits-ruminants and goatpox in indigenous goats of central India. Transbound Emerg Dis. 2011;58(3):268–273. doi: 10.1111/j.1865-1682.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- 89.Mariner JC, House JA, Sollod AE, Stem E, Van den Ende M, Mebus CA. Comparison of the effect of various chemical stabilizers and lyophilization cycles on the thermostability of a vero cell-adapted rinderpest vaccine. J Vet Microbiol. 1990;21:195–209. doi: 10.1016/0378-1135(90)90032-q. [DOI] [PubMed] [Google Scholar]
- 90.Mariner JC, van den Ende M, House JA, Mebus CA. The serological response to a thermostable vero cell-adapted rinderpest vaccine under field conditions in Niger. J Vet Microbiol. 1990;22:119–127. doi: 10.1016/0378-1135(90)90099-h. [DOI] [PubMed] [Google Scholar]
- 91.Matrencher A, Zoyem N, Diallo A. Experimental study of a mixed vaccine against peste des petits ruminants and capripox infection in goats in northern Cameroon. Small Rumin Res. 1997;26:39–44. [Google Scholar]
- 92.Mondal AK, Chottopadhyay AP, Sarkar S, Saha GR, Bhowmik MK. Report on epizootiological and clinicopathological observations on peste des petits ruminants (PPR) of goats in West Bengal. Indian J Anim Health. 1995;34:145–148. [Google Scholar]
- 93.Mondal B, Sen A, Chand K, Biswas SK, De A, Rajak KK, Chakravarti S. Evidence of mixed infection of peste des petits ruminants virus and bluetongue virus in a flock of goats as confirmed by detection of antigen, antibody and nucleic acid of both the viruses. Trop Anim Health Prod. 2009;41(8):1661–1667. doi: 10.1007/s11250-009-9362-3. [DOI] [PubMed] [Google Scholar]
- 94.Mornet P, Orue 1, Gilbert Y, Thiery G, Sow Mamadou. La Peste des Petits ruminants en Mrique occidentale francaise : ses rapports avec la Peste bovine. Rev Elev Med Vet Pays Trop.1956; 9: 313-42.
- 95.Nanda YP, Chatterjee A, Purohit AK, Diallo A, Innui K, Sharma RN, Libeau G, Thevasagayam JA, Bruning A, Kiching RP, Anderson J, Barrett T, Taylor WP. The isolation of peste des petits ruminants virus from Northern India. Vet Microbiol. 1996;51:207–216. doi: 10.1016/0378-1135(96)00025-9. [DOI] [PubMed] [Google Scholar]
- 96.Narayanaswamy M, Ramani K. Preliminary studies on rinderpest virus isolated from outbreaks in sheep in Mysore State. Indian Vet J. 1973;50:829–832. [Google Scholar]
- 97.Nayak BC, Patro DN, Tripathy SB, Pradhan RK, Mohapatra PK, Moharana HK, Mohanty DN. Incidence of peste des petits ruminants (PPR) in goats in Orissa. Indian Vet J. 1997;74:346–348. [Google Scholar]
- 98.Obi TU, McCullough KC, Taylor WP. The production of peste des petits ruminants hyperimmune sera in rabbits and their application in virus diagnosis. J Vet Med. 1990;37:345–352. doi: 10.1111/j.1439-0450.1990.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 99.Obi TU, Ojeh CK. Dot enzyme immuno assay for visual detection of Peste des Petits ruminants virus antigen from infected caprine tissues. J Clin Microbiol. 1989;27:2096–2099. doi: 10.1128/jcm.27.9.2096-2099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Obi TU, Ojo MO, Durojaiye OA, Kasali OB, Akpavie S, Opasina DB. Peste des petits ruminants (PPR) in goats in Nigeria: clinical, microbiological and pathological features. Zentralbl Veterinarmed B. 1983;30:751–761. doi: 10.1111/j.1439-0450.1983.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 101.Obi TU, Pattrick D. The detection of peste des petits ruminants (PPR) virus antigen by agar gel precipitation test and counter immunoelectrophoresis. J Hyg(Lond). 1984;93:579–586. doi: 10.1017/s0022172400065165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.OIE-Peste des petits ruminants (PPR) in Morocco, OIE Alert Message. 2008. MAR 23-07-08.
- 103.OIE . World organisation for animal health-manual of diagnostic tests and vaccines for terrestrial animals. Paris: OIE; 2013. Chapter 2.7.11; pp. 1–14. [Google Scholar]
- 104.Palaniswami KS, Thangavelu A, Velmurugan R. Development of thermostable peste des petits ruminants (PPR) virus vaccine and assessment of molecular changes in the F gene. In: Makkar HPS, Villjoen GJ, editors. Applications of gene-based technologies for improving animal production and health in developing countries. Netherlands: IAEA, Springer; 2005. pp. 673–678. [Google Scholar]
- 105.Pandey KD, Baron MD, Barrett T. Differential diagnosis of rinderpest and PPR using biotinylated cDNA probes. Vet Rec. 1992;131:199–200. doi: 10.1136/vr.131.9.199. [DOI] [PubMed] [Google Scholar]
- 106.Plowright W, Ferris RD. Studies with rinderpest virus in tissue culture. III. The stability of cultured virus and its use in virus neutralization tests. Arch Gesamte Virusforsch. 1962;11:516–533. doi: 10.1007/BF01241304. [DOI] [PubMed] [Google Scholar]
- 107.Polci A, Cosseddu GM, Ancora M, Pinoni C, ElHarrak M, Sebhatu TT, Ghebremeskel E, Sghaier S, Lelli R, Monaco F. Development and preliminary evaluation of a new real-time RT-PCR assay For detection of peste des petits ruminants virus genome. Transbound Emerg Dis. 2013 doi: 10.1111/tbed.12117. [DOI] [PubMed] [Google Scholar]
- 108.Prasad V, Satyavathi VV, Sanjaya KM, Valli, Abha K, Shaila MS, Lakshmi Sita G. Expression of biologically active Haemagglutonin-neuraminidase protein of peste des petits ruminants virus in transgenic pigeon pea (Cajanus cajan (L) Millsp) Plant Sci. 2004;166:199–205. [Google Scholar]
- 109.Provost A, Maurice YB. Protection antipestic conférée aux bovins parle virus de la rougéole II. Application aux veaux nés de mères elles-mêmes vaccines avec la souche MB 113Y. Rev Elev Méd Vét Pays Trop. 1971;24:167–172. [PubMed] [Google Scholar]
- 110.Rahman MM, Shaila MS, Gopinathan KP. Baculovirus display of fusion protein of peste des petits ruminants virus and hemagglutination protein of rinderpest virus and immunogenicity of the displayed proteins in mouse model. Virology. 2003;317(1):36–49. doi: 10.1016/j.virol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 111.Raj GD, Rajanathan TM, Kumar CS, Ramathilagam G, Hiremath G, Shaila MS. Detection of peste des petits ruminants virus antigen using immunofiltration and antigen-competition ELISA methods. Vet Microbiol. 2008;129(3–4):246–251. doi: 10.1016/j.vetmic.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 112.Rajak KK, Sreenivasa BP, Hosamani M, Singh RP, Singh SK, Singh RK, Bandyopadhyay SK. Experimental studies on immunosuppressive effectsof peste des petits ruminants (PPR) virus in goats. Comp Immunol Microbiol Infect Dis. 2005;28:287–296. doi: 10.1016/j.cimid.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 113.Raut A, Singh RK, Malik M, Joseph MC, Bakshi CS, Suryanarayana VV, Butchaiah G. Development of a thermoresistant tissue culture rinderpest vaccine virus. Acta Virol. 2001;45(4):235–241. [PubMed] [Google Scholar]
- 114.Riyesh T, Balamurugan V, Sen A, Bhanuprakash V, Venkatesan G, Yadav V, Singh RK. Evaluation of efficacy of stabilizers on the thermostability of live attenuated thermo-adapted Peste des petits ruminants vaccines. Virol Sin. 2011;26(5):324–337. doi: 10.1007/s12250-011-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Romero CH, Barrett T, Kitching RP, Bostock C, Black DN. Protection of goats against Peste des Petits ruminants with recombinant capripoxvirus expressing the fusion and haemagglutonin protein genes of rinderpest virus. Vaccine. 1995;44:151–163. doi: 10.1016/0264-410x(95)80008-2. [DOI] [PubMed] [Google Scholar]
- 116.Rossiter PB, Jessett DM, Taylor WP. Microneutralization systems for use with different strains of peste des petits ruminants virus and rinderpest virus. Trop Anim Health Prod. 1985;17(2):75–81. doi: 10.1007/BF02360775. [DOI] [PubMed] [Google Scholar]
- 117.Saliki J, Libeau G, House JA, Mebus A, Dubov EJ. Monoclonal antibody based blocking ELISA for specific detection and titration of PPR virus antibody in caprine and ovine sera. J Clin Microbiol. 1993;31:1075–1082. doi: 10.1128/jcm.31.5.1075-1082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saliki JT, House JA, Mebus CA, Dubovi EJ. Comparison of monoclonal antibodies-based sandwich ELISA and virus isolation for detection of PPR virus in goats tissue and secretion. J Clin Microbiol. 1994;32:1349–1353. doi: 10.1128/jcm.32.5.1349-1353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saravanan P, Balamurugan V, Sen A, Sarkar J, Sahay B, Razak KK, Hosamani M, Rasool TJ, Yadav MP, Singh RK. A mixed infection of peste des petits ruminants and Orf in a goat farm, Shahjhahanpur, India. Vet Rec. 2007;160(12):410–412. doi: 10.1136/vr.160.12.410. [DOI] [PubMed] [Google Scholar]
- 120.Saravanan P, Balamurugan V, Sen A, Sreenivasa BP, Singh RP, Bandyopadhyay SK, Singh RK. Long term immune response of goats to a vero cell adapted live attenuated homologous PPR vaccine. Indian Vet J. 2010;87:1–3. [Google Scholar]
- 121.Saravanan P, Sen A, Balamurugan V, Rajak KK, Bhanuprakash V, Palaniswami KS, Nachimuthu K, Thangavelu A, Dhinakarraj G, Hegde R, Singh RK. Comparative efficacy of peste des petits ruminants (PPR) vaccines. Biologicals. 2010;38(4):479–485. doi: 10.1016/j.biologicals.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 122.Saravanan P, Singh RP, Balamurugan V, Dhar P, Sreenivasa BP, Muthuchelvan D, Sen A, Aleyas AG, Singh RK, Bandyopadhyay SK. Development of a N gene-based PCR-ELISA for detection of Peste-des-petits-ruminants virus in clinical samples. Acta Virol. 2004;48(4):249–255. [PubMed] [Google Scholar]
- 123.Sarkar J, Sreenivasa BP, Singh RP, Dhar P, Bandyopadhyay SK. Comparative efficacy of various chemical stabilizers on the thermostability of a live-attenuated peste des petits ruminants (PPR) vaccine. Vaccine. 2003;21:4728–4735. doi: 10.1016/s0264-410x(03)00512-7. [DOI] [PubMed] [Google Scholar]
- 124.Sen A, Balamurugan V, Rajak KK, Chakravarti S, Bhanuprakash V, Singh RK. Role of heavy water in biological sciences with an emphasis on thermostabilization of vaccines. Expert Rev Vaccines. 2009;8(11):1587–1602. doi: 10.1586/erv.09.105. [DOI] [PubMed] [Google Scholar]
- 125.Sen A, Saravanan P, Balamurugan V, Rajak KK, Sudhakar SB, Bhanuprakash V, Parida S, Singh RK. Vaccines against peste des petits ruminants virus. Expert Rev Vaccines. 2010;7:785–796. doi: 10.1586/erv.10.74. [DOI] [PubMed] [Google Scholar]
- 126.Seth S, Shaila MS. The hemagglutinin-neuraminidase protein of peste des petitsruminants virus is biologically active when transiently expressed in mammalian cells. Virus Res. 2001;75(2):169–177. doi: 10.1016/s0168-1702(01)00238-6. [DOI] [PubMed] [Google Scholar]
- 127.Shaila MS, Purushothaman V, Bhavasar D, Venugopal K, Venkatesan RA. Peste des petits ruminants in India. Vet Rec. 1989;125:602. [PubMed] [Google Scholar]
- 128.Shaila MS, Shamaki D, Forsyth MA, Drallo A, Groatley L, Kitching RP, Barrett T. Geographic distribution and epidemiology of peste des petits ruminants viruses. Virus Res. 1996;43:149–153. doi: 10.1016/0168-1702(96)01312-3. [DOI] [PubMed] [Google Scholar]
- 129.Sidhu MS, Husar W, Cook SD, Dowling PC, Udem SA. Canine distemper terminal and intergenic non-protein coding neucleotide sequences: completion of the entire CDV genome sequence. Virology. 1993;193:66–72. doi: 10.1006/viro.1993.1103. [DOI] [PubMed] [Google Scholar]
- 130.Singh RK, Balamurugan V, Bhanuprakash V, Sen A, Saravanan P, Yadav MP. Control and Eradication of peste des petits ruminants in sheep and goats in India: possibility. Vet Ital. 2009;45:449–462. [PubMed] [Google Scholar]
- 131.Singh RP, Bandyopadhyay SK, Sreenivasa BP, Dhar P. Production and characterization of monoclonal antibodies to peste des petits ruminants (PPR) virus. Vet Res Commun. 2004;28(7):623–639. doi: 10.1023/b:verc.0000042875.30624.67. [DOI] [PubMed] [Google Scholar]
- 132.Singh RP, De UK, Pandey KD. Virological and antigenic characterization of two peste des petits ruminants (PPR) vaccine viruses of Indian origin. Comp Immunol Microbiol Infect Dis. 2010;33(4):343–353. doi: 10.1016/j.cimid.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 133.Singh RP, Saravanan P, Sreenivasa BP, Shah LC, Singh RK, Bandyopadhyay SK. Comparison of diagnostic efficacy of a monoclonal antibody based competitive-ELISA test with similar commercial test for the detection of antibodies to peste des petits ruminants (PPR) virus. Vet Res Commun. 2006;30:325–330. doi: 10.1007/s11259-006-3192-9. [DOI] [PubMed] [Google Scholar]
- 134.Singh RP, Saravanan P, Sreenivasa BP, Singh RK, Bandyopadhyay SK. Prevalence and distribution of peste des petits ruminants (PPR) virus infection in small ruminants in India. Rev Sci Technol. 2004;23:807–819. doi: 10.20506/rst.23.3.1522. [DOI] [PubMed] [Google Scholar]
- 135.Singh RP, Sreenivasa BP, Dhar P, Bandyopadhyay SK. Development A sandwich-ELISA for the diagnosis of Peste des petits ruminants (PPR) infection in small ruminants using anti-nucleocapsid protein monoclonal antibody. Arch Virol. 2004;149:2155–2170. doi: 10.1007/s00705-004-0366-z. [DOI] [PubMed] [Google Scholar]
- 136.Singh RP, Sreenivasa BP, Dhar P, Shah LC, Bandyopadhyay SK. Development of a monoclonal antibody based competitive-ELISA for detection and titration of antibodies to peste des petits ruminants (PPR) virus. Vet Microbiol. 2004;98:3–15. doi: 10.1016/j.vetmic.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 137.Singh RP. Control strategies for peste des petits ruminants in small ruminants of India. Rev Sci Technol. 2011;30(3):879–887. doi: 10.20506/rst.30.3.2079. [DOI] [PubMed] [Google Scholar]
- 138.Sinnathamby G, Naik S, Renukaradhya GJ, Rajasekhar M, Nayak R, Shaila MS. Recombinant hemagglutinin protein of rinderpest virus expressed in insect cells induces humoral and cell mediated immune responses in cattle. Vaccine. 2001;19(28–29):3870–3876. doi: 10.1016/s0264-410x(01)00127-x. [DOI] [PubMed] [Google Scholar]
- 139.Sinnathamby G, Nayak R, Shaila MS. Mapping of T-helper epitopes of Rinderpest virus hemagglutinin protein. Viral Immunol. 2001;14(1):83–92. doi: 10.1089/08828240151061446. [DOI] [PubMed] [Google Scholar]
- 140.Sinnathamby G, Renukaradhya GJ, Rajasekhar M, Nayak R, Shaila MS. Immune responses in goats to recombinant hemagglutinin-neuraminidase glycoprotein of peste des petits ruminants virus: identification of a T cell determinant. Vaccine. 2001;19:4816–4823. doi: 10.1016/s0264-410x(01)00210-9. [DOI] [PubMed] [Google Scholar]
- 141.Sinnathamby G, Renukaradhya GJ, Rajasekhar M, Nayak R, Shaila MS. Recombinant hemagglutinin protein of rinderpest virus expressed in insect cells induces cytotoxic T-cell responses in cattle. Viral Immunol. 2001;14(4):349–358. doi: 10.1089/08828240152716592. [DOI] [PubMed] [Google Scholar]
- 142.Soundararajan C, Sivakumar T, Ramesh S, Muthukrishnan S, Palanidorai R. Peste des petits ruminants among sheep and goats in an organized farm in Tamil Nadu. Indian Vet J. 2006;83(10):1045–1047. [Google Scholar]
- 143.Sreenivasa BP, Dhar P, Singh RP, Bandyopadhyay SK. Development of peste des petits ruminants (PPR) challenge virus from a field isolate. Presented at XIV annual conference and national seminar on management of viral diseases with emphasis on global trade and WTO regime of Indian Virological Society. January 18–20, 2002, IVRI, Hebbal, Bangalore, India.
- 144.Sreenivasa BP, Dhar P, Singh RP, Bandyopadhyay SK. Evaluation of an indigenously developed homologous live-attenuated cell culture vaccine against peste des petits ruminants of small ruminants. In: Proceedings of XX annual conference of Indian Association of Veterinary microbiologists, Immunologists and specialists in infectious diseases and National symposium on trends in vaccinology for animal diseases. October 14–16, 2000, GBPUA&T, Pantnagar, India.
- 145.Sreenivasa BP, Singh RP, Mondal B, Dhar P, Bandyopadhyay SK. Marmoset B95a cells: a sensitive system for cultivation of peste des petits ruminants (PPR) virus. Vet Res Commun. 2006;30(1):103–108. doi: 10.1007/s11259-005-3200-5. [DOI] [PubMed] [Google Scholar]
- 146.Sudharshana KJ, Rajasekhar M, Upadhye AS. Prevalence of peste des petits ruminants and rinderpest antibodies in small ruminants. Indian Vet J. 1995;72(12):1246–1250. [Google Scholar]
- 147.Sumption KJ, Aradom G, Libeau G, Wilsmore AJ. Detection of peste des petits ruminants virus antigen in conjunctival smears of goats by indirect immunofluorescence. Vet Rec. 1998;142(16):421–424. doi: 10.1136/vr.142.16.421. [DOI] [PubMed] [Google Scholar]
- 148.Taylor WP, Abegunde A. The isolation of peste des petits ruminants virus from Nigerian sheep and goats. Res Vet Sci. 1979;26(1):94–96. [PubMed] [Google Scholar]
- 149.Taylor WP, Abusaidy S, Barret T. The epidemiology of PPR in the sultanate of Oman. Vet Microbiol. 1990;22:341–352. doi: 10.1016/0378-1135(90)90021-m. [DOI] [PubMed] [Google Scholar]
- 150.Taylor WP. Serological studies with the virus of peste des petits ruminants in Nigeria. Res Vet Sci. 1979;26:236–242. [PubMed] [Google Scholar]
- 151.Taylor WP. The distribution and epidemiology of peste des petits ruminants in the sultanate of oman. Vet Microbiol. 1984;22:341–352. doi: 10.1016/0378-1135(90)90021-m. [DOI] [PubMed] [Google Scholar]
- 152.Toplu N, Oguzoglu TC, Albayrak H. Dual infection of fetal and neonatal small ruminants with border disease virus and peste des petits RUMINANTS virus (PPRV): neuronal tropism of PPRV as a novel finding. J Comp Pathol. 2012;146:289–297. doi: 10.1016/j.jcpa.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 153.Tripathi BN, Chattopadhyay SK, Parihar NS. Clinical and pathological studies on peste des petits ruminants-an emerging disease in Indian goats. In: Proceeding of International Conference on Goats, Beijing, China. 1996; 742–44.
- 154.Tripathi BN, Chhattopadhyay SK, Parihar NS. Clinicopathological studies on an outbreak of peste des petits ruminants in goats and sheep. Indian J Vet Pathol. 1996;20:99. [Google Scholar]
- 155.Van Oirschot JT. DIVA vaccines that reduce virus transmission. J Biotechnol. 1999;73:195–205. doi: 10.1016/s0168-1656(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 156.Venkataramanan R, Bandyopadhyay SK, Oberoi MS. Present status and strategies for the control of transboundary and other economically important animal diseases in India: a Review. Indian J Ani Sci. 2005;75(4):456–464. [Google Scholar]
- 157.Wang Y, Liu G, Chen Z, Li C, Shi L, Li W, Huang H, Tao C, Cheng C, Xu B, Li G. Recombinant adenovirus expressing F and H fusion proteins of peste des petits ruminants virus induces both humoral and cell-mediated immune responses in goats. Vet Immunol Immunopathol. 2013;154(1–2):1–7. doi: 10.1016/j.vetimm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 158.Wang Y, Liu G, Shi L, Li W, Li C, Chen Z, Jin H, Xu B, Li G. Immune responses in mice vaccinated with a suicidal DNA vaccine expressing the hemagglutinin glycoprotein from the peste des petits ruminants virus. J Virol Methods. 2013;193(2):525–530. doi: 10.1016/j.jviromet.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 159.Worrall EE, Litamoi JK, Seck BM, Ayele TG. Xerovac: an ultra rapid method for the dehydration and preservation of live attenuated rinderpest and peste des petits ruminants vaccines. Vaccine. 2000;19(7–8):834–839. doi: 10.1016/s0264-410x(00)00229-2. [DOI] [PubMed] [Google Scholar]
- 160.Wosu LO. Agglutination of red blood cells by peste des petits ruminants (PPR) virus Niger. Vet J. 1985;14:54–58. [Google Scholar]
- 161.Yadav V, Balamurugan V, Bhanuprakash V, Sen A, Bhanot V, Venkatesan G, Riyesh T, Singh RK. Expression of Peste des petits ruminants virus nucleocapsid protein in prokaryotic system and its potential use as a diagnostic antigen or immunogen. J Virol Methods. 2009;162(1–2):56–63. doi: 10.1016/j.jviromet.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 162.Yamanouchi K, Barrett T. Progress in the development of a heat-stable recombinant rinderpest vaccine using an attenuated vaccinia virus vector, review. Rev Sci Technol. 1994;13(3):721–735. doi: 10.20506/rst.13.3.790. [DOI] [PubMed] [Google Scholar]


