Abstract
CD4+CD25+FoxP3+ regulatory T cells (Tregs) are critical for immune homeostasis and tolerance. However, because of their capacity to suppress antigen presenting cells (APC), T and B cells, Tregs could also inhibit protective immune responses to viruses and vaccines. Several viruses have been shown to exploit Tregs to evade immune response. By modulating APC and in particular by weakening the functions of dendritic cells such as their ability to secrete polarizing cytokines and expression of co-stimulatory molecules, viruses could support differentiation and expansion of Tregs. Of note, as a proof of concept, depletion of Tregs significantly enhanced the protective immune response to viruses and vaccines suggesting that Tregs are viable targets to enhance immunogenicity of vaccines. As Treg depletion or inhibition of their functions could lead to deleterious autoimmune and inflammatory disorders, any Treg-based approach for vaccination should not aim at depletion of Tregs and inhibition of their functions should be transient. Recent studies have targeted the interaction between CCR4 expressed on Tregs and its ligands CCL22 and CCL17 to inhibit transiently the recruitment of Tregs at the site of immunization. Importantly, use of CCR4 antagonists as ‘molecular adjuvants’ in vivo in experimental models, amplified cellular and humoral immune responses when injected in combination with various vaccine antigens. The significant adjuvant activity observed in diverse models without noticeable side effects provided strong evidence that CCR4 is a sustainable target for rational adjuvant design.
Keywords: Vaccine, Adjuvant, CCR4, Regulatory T cells, Tregs, CCL22, CCL17
Introduction
Vaccines play an indispensible role in the protection against various infectious diseases (virus, bacteria or protozoa) by eliciting antigen-specific T cell and humoral (antibody) responses. Vaccination programs helped to eradicate smallpox and rinderpest diseases and poliomyelitis is in the verge of eradication. Currently, inactivated vaccines (e.g. rabies, influenza, foot and mouth disease), live attenuated vaccines (e.g. poliomyelitis, measles, mumps, rubella, rota, influenza; and infectious bursal disease virus, Newcastle disease virus and infectious bronchitis virus vaccines of birds); and recombinant protein vaccines (e.g. hepatitis B) are licensed for use in humans and animals. In addition, genetically modified vaccines, virus-like particles and DNA vaccines are in various stages of pre-clinical and clinical trials [41].
Adjuvants play an important role to enhance the magnitude and duration of immunity to vaccines. Live vaccines do not require adjuvants as self-replicating pathogens provide required signals for the activation of immune system. However, inactivated vaccines and recombinant protein vaccines require adjuvants to boost the immunogenicity of vaccine antigens. Currently, irrespective of vaccine candidate, aluminum adjuvants are the only adjuvant licensed for human use worldwide. Other adjuvants such as oil-in-water emulsions, liposomes, toll-like receptor 4 (TLR4) agonists are licensed only for particular vaccine combinations [1, 41]. However, aluminum adjuvants induce only humoral but not cellular immune responses. Also, effective and protective vaccines are not yet available for many of the emerging and re-emerging viral diseases. Therefore, there is an obvious need for the identification of novel molecular adjuvants that induce both cellular and humoral immune responses. This review presents an overview of role of regulatory T cells (Tregs), the immunosuppressors, in the immune response to viruses, and identification and validation of CCR4 as one of the Treg-based adjuvant targets to boost protective immunity to viral diseases and vaccines.
Immune response to vaccines
The ability of a vaccine to confer protection is evaluated on the basis of specificity of immune response, the magnitude and duration of immunity it elicits. In general, the steps and process of mounting immune response to vaccines follow the same pattern as that of infection with pathogens. Thus, cooperation of the members of innate immune compartment such as antigen presenting cells (APC) and NK cells, and adaptive immune compartment (T and B cells) is critical for eliciting protective immune response to vaccines. Cellular immunity plays an important role in the clearance of pathogen-infected cells while antibodies clear cell-free pathogens.
Upon immunization, vaccine antigens are recognized by professional APC such as dendritic cells (DC), macrophages and monocytes. Vaccine antigens express various pathogen-associated molecular patterns and are recognized by diverse pattern-recognition receptors (PRRs) of APC. These PRRs include TLRs, C-type lectin receptors (CLRs such as Dectin-1, Dectin-2, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin or DC-SIGN and mannose receptors), NOD-like receptors (NLRs) and retinoid acid-inducible gene-I (RIG-I)-like receptors (RLRs). While TLRs and CLRs are present both on the surface and endosomes of the APC, NLRs and RLRs are intra-cellular in their location. Signalling by these PRRs activate canonical and non-canonical NF-κB and inflammasome activation resulting in phagocytosis of antigens, secretion of immunomodulatory and inflammatory cytokines, chemokines and production of pathogen-killing molecules. Thus, diversity of PRRs and their location ensure optimal activation of innate immune cells [20, 42].
The activation and differentiation of antigen-specific T cells require four different signals. The signal ‘zero’ represents the recognition of pathogens by APC via PRRs that leads to phagocytosis/endocytosis of antigens and activation of APC. The endocytosed external antigens are cleaved into smaller peptides by proteolytic enzymes such as cathepsins. These antigenic peptides are then loaded onto MHC class II molecules and transported to surface of APC for the presentation to CD4+ T cells. The antigenic peptides are recognized by T cell receptor (TCR)-CD3 complex (‘signal 1’). The antigens that are produced endogenously because of replication of pathogens within APC, are routed to proteasomes for cleavage and presentation via MHC class I to CD8+ T cells (or cytotoxic T lymphocytes, CTLs). In addition, by a process called ‘cross-presentation’, DC could also present external antigens in the context of MHC class I molecules to CD8+ T cells.
The activation of APC by pathogens also leads to enhanced surface expression of various co-stimulatory molecules such as CD80, CD86, CD40 and adhesion molecules CD54 and CD58 that provide co-stimulatory signals (‘signal 2’) to T cells for their activation. In addition, the ‘signal 3’ in the form of immunomodulatory or polarizing cytokines secreted by APC determines the polarization of CD4+ T cells into distinct subtypes such as Th1, Th2, Th17 or induced Tregs [63, 68]. CD4+ T cells provide help for CD8+ T cells and activated CTLs clear infected cells via cytotoxic programs. CD4+ T cell help is also crucial for B cell activation and antibody production [1].
Th1 cells express transcription factors T-bet and signal transducer and activator of transcription 4 (STAT4) and produce primarily interferon-γ (IFN-γ) and interleukin-2 (IL-2). IL-12 produced by APC plays an important role in the differentiation of Th1 cells. Th1 immune responses are primarily cell-mediated and IFN-γ secreted by Th1 cells activates macrophages to clear intra-cellular pathogens. Th2 cells express GATA-binding protein 3 and STAT6 and produce IL-4, IL-5 and IL-13. They are important for B cell differentiation and clearance of extra-cellular pathogens and parasites. Th17 cells express retinoic acid-related orphan receptor C (RORC) and STAT3 and produce IL-17A/F, IL-22, IL-21, granulocyte–macrophage colony-stimulating factor and chemokine CCL20. IL-21 and transforming growth factor-β (TGF-β) are key cytokines that mediate differentiation of human Th17 cells. IL-6 and IL-1β expand Th17 cells and IL-23 stabilizes the differentiated Th17 population. Th17 cells are important for protection against several extra-cellular pathogens including Klebsiella and Candida. Tregs are immunosuppressors and are positive for transcription factor forkhead box P3 (FoxP3). They play a regulatory role to check the activation of immune cells and to prevent inflammation-associated tissue damage.
Regulatory T cells
Tregs are critical for immune homeostasis and immune tolerance. Deficiency of Tregs or defects in their functions is associated with autoimmune diseases and inflammation. In human, deficiency of Tregs due to FoxP3 mutation results in immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, a severe autoimmune inflammatory disorder.
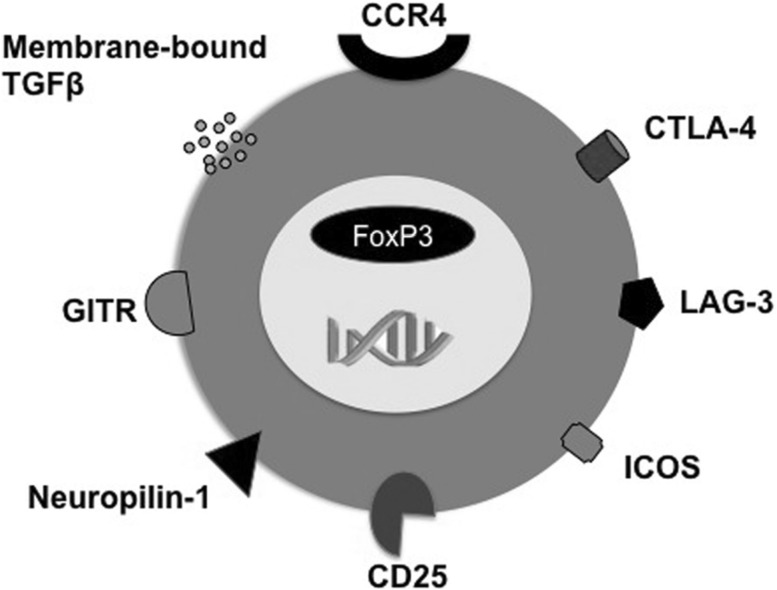
Tregs represent minor population within CD4+ T cells and are <1–2 % of CD4+ T cells. Phenotypically, Tregs are CD4+CD25high FoxP3+ and constitutively express cytotoxic T-lymphocyte antigen-4 (CTLA-4) and inducible T-cell costimulator [43, 44] (Fig. 1). FoxP3 governs the functions of Tregs and inflammatory cytokines could render Tregs dysfunctional [6]. Tregs could be ‘natural’ that are derived from the thymus, or ‘induced’ or ‘adaptive’ that are differentiated in the periphery from naïve CD4+ T cells under the influence of APC and TGF-β. As TGF-β is critical cytokine for the expression of both FoxP3 and RORC, the inflammatory cytokines in the microenvironment determine the stability of induced Tregs. Induced Tregs present phenotype similar to that of natural Tregs. However, natural Tregs are neuropilin-1+ and are functionally stable [66]. In addition, FoxP3 gene is widely demethylated in natural Tregs. Natural Tregs are mostly specific for self-antigens while induced Tregs are specific for foreign antigens including pathogens.
Fig. 1.
Some of the surface features of regulatory T cells
Although FoxP3 is a bona fide marker of Tregs in mice, in human it is also expressed transiently in recently activated CD4+ T cells. Thus, based on the expression pattern of FoxP3, three subpopulations of CD4+ T cells have been identified in the peripheral human blood [33]. They are potently suppressive CD45RA−FoxP3hiCD4+ activated and CD45RA+FoxP3loCD4+ resting Tregs, and cytokine-secreting non-suppressive CD45RA−FoxP3loCD4+ non-Tregs.
Functions of regulatory T cells
Several mutually nonexclusive cellular and molecular mechanisms have been proposed for Tregs. The immunosuppressive functions Tregs are mediated by both soluble factors and cell-associated molecules. CTLA-4, lymphocyte-activation gene-3, neuropilin-1 are major cell-associated molecules of Tregs while TGF-β, IL-10, perforins and granzymes are soluble factors implicated in Treg functions [3, 26, 43–45, 54]. Tregs could directly inhibit the activation and proliferation of effector T cells or indirectly by suppressing APC functions. Thus, Tregs suppress the maturation of DC, expression of MHC and co-stimulatory molecules; and inflammatory cytokines and chemokines [4, 5, 10, 18, 29, 32, 35–37, 46, 51, 53, 60, 65]. Thus, by suppressing the APC functions, Tregs inhibit the ability of APC to polarize effector T cells. Tregs could induce cytotoxic programs and cause apoptosis of target cells via granzymes and perforins and CD18-CD54 mediated adhesion molecule-mediated interaction [16]. Tregs induce catabolism of tryptophan by inducing indoleamine 2,3-dioxygenase in DC via CTLA-4-CD80/CD86 interaction. Tryptophan catabolism leads to the release of kynurenines that are potent immune suppressors [13]. In addition, Tregs also suppress the activation of macrophages, monocytes and B cells [27, 55, 67].
Regulatory T cell suppress protective immune responses to viruses and vaccines
Tregs are present both in secondary lymphoid tissues and in peripheral tissues such as skin. Tregs are important to prevent tissue damage associated with excessive inflammation following viral infection as indicated by the studies in dengue virus-infected patients and in several experimental models of virus infections including respiratory syncytial virus (RSV) and herpes simplex virus-1 (HSV-1) [14, 24, 28, 61].
However, by their ability to suppress APC, T and B cells, Tregs could also inhibit protective immune responses to viral infections. Several viruses such as retroviruses, herpes, influenza, RSV, rotavirus, dengue virus, hepatitis B virus and coronavirus have been shown to exploit Tregs to evade immune responses (Table 1). By modulating the APC and in particular by weakening the functions of DC such as their ability to secrete polarizing cytokines and expression of co-stimulatory molecules, viruses could support expansion of natural Tregs or promote differentiation of induced Tregs from naïve T cells at the expense of differentiation towards effector T cells. Alternatively, viruses could directly target Tregs via TLR-stimulation or by direct infection as in the case of feline immunodeficiency virus (FIV). By this pathway, viruses enhance the proliferation of Tregs and their suppressive functions by augmenting the expression of molecules that are implicated in immunosuppressive functions such as membrane-bound TGF-β. Tregs have been shown to modulate the virus-specific primary and memory CD4+ and CD8+ T cell responses, their proliferation, and effector cell number. Thus Tregs delay the clearance of viruses and enhance their persistence [2, 9, 14, 21–23, 28, 30, 31, 50, 57, 58].
Table 1.
Viral diseases in which role of Tregs were studied
On the contrary, depletion of Tregs by using anti-CD25 antibody (PC61) before infection with HSV-1, enhanced the HSV-1-specific CTL responses [50]. Also, depletion of memory Tregs or effector Tregs in mice before secondary infection with influenza led to increased magnitude of antigen-specific memory CD8+ T cell responses and airway cytokine and chemokine expression [9]. Similarly, in a neonatal murine model of rotavirus infection, depletion of natural Tregs before virus infection led to augmented CD4+ and CD8+ T cell responses to virus, such as proliferation and IFN-γ secretion. Interestingly, in the same model, proliferation of CD19+ B cells was also increased [22].
Tregs also diminish immune response to vaccines and in particular by hampering the development of effective immune responses following immunization with poorly immunogenic vaccines. The majority of work on role of Tregs in vaccination was done in diverse tumor models [40]. Immune response to HSV-1 DNA and peptide vaccines and inactivated Japanese encephalitis virus vaccine in mice were compromised by the presence of Tregs [25, 56], and depletion of Tregs enhanced the CTL response to vaccines and conferred greater protection upon viral challenge [56]. Enhanced immunogenicity and CD4+ T cell responses upon Treg depletion was also observed in the case of hepatitis B virus recombinant subunit protein vaccine and flu-vaccines (either UV-inactivated influenza PR8/A/34 virus vaccine or specific oligosaccharides scGOS/lcFOS/pAOS of influenza virus) [34, 48, 59]. Analysis of cellular and cytokine profiles from influenza-vaccinated individuals also suggested that Tregs and TGF-β contribute to inhibition of anti-influenza antibody responses following vaccination [64]. Also, tick-borne encephalitis or hepatitis B vaccine nonresponder individuals had increased IL-10-producing FoxP3+ Tregs upon vaccination [15].
Targeting Tregs in vaccination: small molecule CCR4 antagonists as molecular adjuvants
Since activation state of DC at the time of encounter with vaccine antigens determines the outcome of the immune responses, limiting the influence of Tregs on DC at this juncture could lead to an enhanced immune response to poor immunogenic vaccine candidates [8]. Although depletion of Tregs by targeting CD25 or the molecules implicated in Treg functions (such as CTLA-4) or inhibition of FoxP3 by using a peptide inhibitor P60, provide proof of principle for targeting Tregs in vaccination, such approaches might not be useful for human. The major reason is that depletion of Tregs or blocking their functions could break immune tolerance and cause autoimmune diseases and inflammatory disorders. The appearance of localized autoimmune disease has been reported in mice as a consequence of Treg depletion [49, 52]. Therefore, any approach that targets Tregs for vaccination purposes should not deplete Tregs and inhibition of their functions should be of transient in nature.
Small molecule antagonist-based approach has been explored to target the interaction between chemokines and their receptors to inhibit transiently the recruitment of Tregs at the site of immunization [7, 12, 38]. Up to 90 % of human Tregs and 15–20 % murine Tregs express CCR4, a chemokine receptor absent on naïve T cells [19]. CCR4 is a receptor for CCL22 and CCL17, the chemotactic agents for Tregs both in vitro and in vivo. Both the chemokines are produced at huge amounts (in nanogram quantities) by DC upon activation and promote the contact between DC and CCR4+ T cells. Monoclonal antibody to CCL22 significantly blocked the migration of Tregs [11], thus providing evidence that Tregs could be targeted via chemokine system. Small molecular weight antagonists to CCR4 (Fig. 2) identified by in silico approach blocked the migration of CCR4+ Tregs in vitro and in vivo and enhanced DC-mediated human CD4+ T cell proliferation in vitro [7, 62] (Fig. 3). Importantly, use of CCR4 antagonists as ‘molecular adjuvants’ in vivo in experimental models, amplified cellular and humoral immune responses when injected in combination with mycobacterium, hepatitis B virus or Plasmodium yoelii vaccine antigens [7, 12]. In addition, when combined with anti-tumor vaccines, CCR4 antagonists induced antigen-specific CD8+ T cells and tumor immunity against self-antigens [39] but did not prevent division of Tregs [47]. The CCR4 antagonists did not alter the absolute number of Tregs and compared to longer half-life (about 2–3 weeks) of monoclonal antibodies that were used to deplete Tregs or to inhibit their functions, the half-life of CCR4 antagonists is <24 h. Also, in mice injected with CCR4 antagonists, biological markers of autoimmune disease were not noticed [39]. The significant adjuvant activity observed in diverse models without noticeable side effects provided strong evidence that CCR4 is a sustainable adjuvant target for prophylaxis and therapeutic vaccines. These data also provided a pointer that structural biology, in silico technology and immunology tools could be combined to identify potent molecular adjuvants that might have a wide range utility for vaccines.
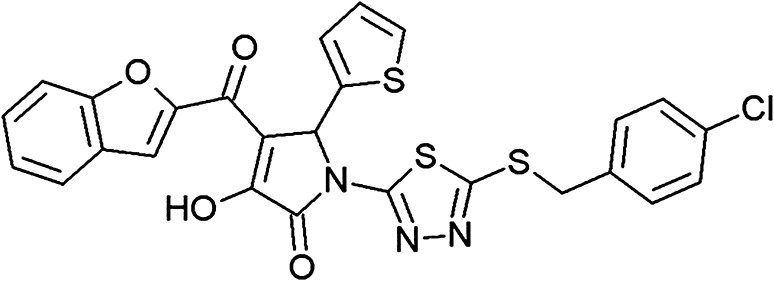
Fig. 2.
The chemical structure of AF-399/42018025 (C26H16CIN3O4S3), one of the CCR4 antagonists that demonstrated adjuvant features. The chemical name of the molecule is 4-(1-benzofuran-2-ylcarbonyl)-1-{5-[4-chlorobenzyl)sulfanyl]-1,3,4-thiadiazol-2-yl}-3-hydroxy-5-(2-thienyl)-1,5- dihydro-2H-pyrrol-2-one
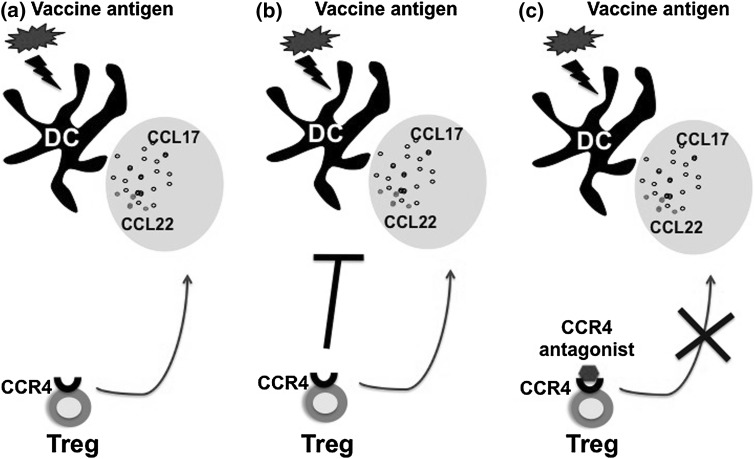
Fig. 3.
A model describing the CCR4 antagonist-based approach to target Treg-DC interaction in vaccination. a DC undergo maturation and activation upon receiving the signals from vaccine antigens. These DC secrete large amounts of chemokines CCL17 and CCL22 that attract CCR4-positive Tregs to the proximity of DC. b The migrated Tregs inhibit the activation and functions of DC and hence dampen the immune response to vaccines. c The small molecule antagonists to CCR4 block the interaction of CCR4 with CCL17 and CCL22 thus prevent the inhibitory action of Treg on DC. As a consequence, DC undergo complete activation process and induce maximum immune response to vaccines
Perspective
Although experimental models provided evidence that targeting Tregs would enhance the magnitude and duration of protective immunity to vaccines and pathogens, the translation of these results to clinics is hindered due to the fact that tilting the balance of Tregs would be deleterious. Also, Tregs share most of the surface markers that are common with effector T cells and hence many of the strategies that target Tregs lack specificity. The CCR4 is also expressed by Th2 cells and by a small subset of Th17 cells. Thus, CCR4 antagonists might inhibit antibody responses if used in the context of booster immunization as they might prevent interaction of DC with memory Th2 cells. As naïve T cells and Th1 subsets lack CCR4, the small molecule antagonists to CCR4 could be used to enhance cellular immune responses, which are crucial in viral diseases and in cancers. As DC are highly concentrated at epidermis and dermis, CCR4 antagonists could be used with vaccines for microneedle-based immunization [17]. Further work on pharmacokinetics of CCR4 antagonists, optimum dose-finding study and experiments in non-human primates are necessary before carrying CCR4 antagonists to the clinics and their use as vaccine adjuvant.
Acknowledgments
Thanks to all my colleagues and collaborators who contributed to evaluation of CCR4 antagonists in vaccination. Supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Université Pierre et Marie Curie, Université Paris Descartes and grant from the Indo-French Center for Promotion of Advanced Research (CEFIPRA, Reference No: 4803-1).
References
- 1.Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 2009;30:287–295. doi: 10.1016/j.tips.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Amoah S, Holbrook BC, Yammani RD, Alexander-Miller MA. High viral burden restricts short-lived effector cell number at late times postinfection through increased natural regulatory T cell expansion. J Immunol. 2013;190:5020–5029. doi: 10.4049/jimmunol.1200971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre S, Tough DF, Lacroix-Desmazes S, Kaveri SV, Bayry J. Surveillance of antigen-presenting cells by CD4 + CD25 + regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications. Am J Pathol. 2009;174:1575–1587. doi: 10.2353/ajpath.2009.080987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayry J, Triebel F, Kaveri SV, Tough DF. Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4 + CD25 + regulatory T cells. J Immunol. 2007;178:4184–4193. doi: 10.4049/jimmunol.178.7.4184. [DOI] [PubMed] [Google Scholar]
- 5.Bayry J, Kaveri SV, Tough DF. Do activated human dendritic cells diminish the suppressive functions of CD4 + , CD25 + regulatory T cells? Arthritis Rheum. 2007;56:3874–3876. doi: 10.1002/art.23028. [DOI] [PubMed] [Google Scholar]
- 6.Bayry J, Siberil S, Triebel F, Tough DF, Kaveri SV. Rescuing CD4 + CD25 + regulatory T-cell functions in rheumatoid arthritis by cytokine-targeted monoclonal antibody therapy. Drug Discov Today. 2007;12:548–552. doi: 10.1016/j.drudis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Bayry J, Tchilian EZ, Davies MN, Forbes EK, Draper SJ, Kaveri SV, Hill AV, Kazatchkine MD, Beverley PC, Flower DR, Tough DF. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc Natl Acad Sci USA. 2008;105:10221–10226. doi: 10.1073/pnas.0803453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayry J, Flower DR, Tough DF, Kaveri SV. From ‘perfect mix’ to ‘potion magique’-regulatory T cells and anti-inflammatory cytokines as adjuvant targets. Nat Rev Microbiol. 2008;6:C1. doi: 10.1038/nrmicro1681-c1. [DOI] [PubMed] [Google Scholar]
- 9.Brincks EL, Roberts AD, Cookenham T, Sell S, Kohlmeier JE, Blackman MA, Woodland DL. Antigen-specific memory regulatory CD4 + Foxp3 + T cells control memory responses to influenza virus infection. J Immunol. 2013;190:3438–3446. doi: 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cederbom L, Hall H, Ivars F. CD4 + CD25 + regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 12.Davies MN, Bayry J, Tchilian EZ, Vani J, Shaila MS, Forbes EK, Draper SJ, Beverley PC, Tough DF, Flower DR. Toward the discovery of vaccine adjuvants: coupling in silico screening and in vitro analysis of antagonist binding to human and mouse CCR4 receptors. PLoS One. 2009;4:e8084. doi: 10.1371/journal.pone.0008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 14.Fulton RB, Meyerholz DK, Varga SM. Foxp3 + CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J Immunol. 2010;185:2382–2392. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garner-Spitzer E, Wagner A, Paulke-Korinek M, Kollaritsch H, Heinz FX, Redlberger-Fritz M, Stiasny K, Fischer GF, Kundi M, Wiedermann U. Tick-borne encephalitis (TBE) and hepatitis B nonresponders feature different immunologic mechanisms in response to TBE and influenza vaccination with involvement of regulatory T and B cells and IL-10. J Immunol. 2013;191:2426–2436. doi: 10.4049/jimmunol.1300293. [DOI] [PubMed] [Google Scholar]
- 16.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Hegde NR, Kaveri SV, Bayry J. Recent advances in the administration of vaccines for infectious diseases: microneedles as painless delivery devices for mass vaccination. Drug Discov Today. 2011;16:1061–1068. doi: 10.1016/j.drudis.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4 + CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–5298. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- 19.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kastenmuller W, Gasteiger G, Subramanian N, Sparwasser T, Busch DH, Belkaid Y, Drexler I, Germain RN. Regulatory T cells selectively control CD8 + T cell effector pool size via IL-2 restriction. J Immunol. 2011;187:3186–3197. doi: 10.4049/jimmunol.1101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim B, Feng N, Narváez CF, He XS, Eo SK, Lim CW, Greenberg HB. The influence of CD4 + CD25 + Foxp3 + regulatory T cells on the immune response to rotavirus infection. Vaccine. 2008;26:5601–5611. doi: 10.1016/j.vaccine.2008.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo Y, Ueno Y, Kobayashi K, Kakazu E, Shiina M, Inoue J, Tamai K, Wakui Y, Tanaka Y, Ninomiya M, Obara N, Fukushima K, Ishii M, Kobayashi T, Niitsuma H, Kon S, Shimosegawa T. Hepatitis B virus replication could enhance regulatory T cell activity by producing soluble heat shock protein 60 from hepatocytes. J Infect Dis. 2010;202:202–213. doi: 10.1086/653496. [DOI] [PubMed] [Google Scholar]
- 24.Lee DC, Harker JA, Tregoning JS, Atabani SF, Johansson C, Schwarze J, Openshaw PJ. CD4 + CD25 + natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J Virol. 2010;84:8790–8798. doi: 10.1128/JVI.00796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Chen H, Wu N, Fan D, Liang G, Gao N, An J. Characterization of immune responses induced by inactivated, live attenuated and DNA vaccines against Japanese encephalitis virus in mice. Vaccine. 2013;31:4136–4142. doi: 10.1016/j.vaccine.2013.06.099. [DOI] [PubMed] [Google Scholar]
- 26.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, Tybulewicz V, Vignali D, Clynes R. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig-Portugall I, Hamilton-Williams EE, Gottschalk C, Kurts C. Cutting Edge: CD25 + regulatory T cells prevent expansion and induce apoptosis of B cells specific for tissue autoantigens. J Immunol. 2008;181:4447–4451. doi: 10.4049/jimmunol.181.7.4447. [DOI] [PubMed] [Google Scholar]
- 28.Lühn K, Simmons CP, Moran E, Dung NT, Chau TN, Quyen NT, le Thao TT, Van Ngoc T, Dung NM, Wills B, Farrar J, McMichael AJ, Dong T, Rowland-Jones S. Increased frequencies of CD4 + CD25(high) regulatory T cells in acute dengue infection. J Exp Med. 2007;204:979–985. doi: 10.1084/jem.20061381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahnke K, Ring S, Johnson TS, Schallenberg S, Schönfeld K, Storn V, Bedke T, Enk AH. Induction of immunosuppressive functions of dendritic cells in vivo by CD4 + CD25 + regulatory T cells: role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37:2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 30.Maizels RM, Smith KA. Regulatory T cells in infection. Adv Immunol. 2011;112:73–136. doi: 10.1016/B978-0-12-387827-4.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller MM, Fogle JE, Tompkins MB. Infection with feline immunodeficiency virus directly activates CD4 + CD25 + T regulatory cells. J Virol. 2013;87:9373–9378. doi: 10.1128/JVI.00996-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4 + CD25 + T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 33.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4 + T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AV. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J Immunol. 2005;175:7264–7273. doi: 10.4049/jimmunol.175.11.7264. [DOI] [PubMed] [Google Scholar]
- 35.Navarrete AM, Meslier Y, Teyssandier M, André S, Delignat S, Triebel F, Kaveri SV, Lacroix-Desmazes S, Bayry J. CD4 + CD25 + regulatory T cells modulate human dendritic cell chemokines via multiple mechanisms. Arthritis Rheum. 2009;60:2848–2849. doi: 10.1002/art.24784. [DOI] [PubMed] [Google Scholar]
- 36.Navarrete AM, Delignat S, Teillaud JL, Kaveri SV, Lacroix-Desmazes S, Bayry J. CD4 + CD25 + regulatory T cell-mediated changes in the expression of endocytic receptors and endocytosis process of human dendritic cells. Vaccine. 2011;29:2649–2652. doi: 10.1016/j.vaccine.2011.01.095. [DOI] [PubMed] [Google Scholar]
- 37.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3 + natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–8. [DOI] [PMC free article] [PubMed]
- 38.Othy S, Topcu S, Kaveri SV, Bayry J. Effect of CC chemokine receptor 4 antagonism on the evolution of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2012;109:E2412–E2413. doi: 10.1073/pnas.1209124109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E, Batteux F, Sandoval F, Adotevi O, Chiu C, Garcia S, Tanchot C, Lone YC, Ferreira LC, Nelson BH, Hanahan D, Fridman WH, Johannes L, Tartour E. A CCR4 antagonist combined with vaccines induces antigen-specific CD8 + T cells and tumor immunity against self antigens. Blood. 2011;118:4853–4862. doi: 10.1182/blood-2011-01-329656. [DOI] [PubMed] [Google Scholar]
- 40.Pere H, Tanchot C, Bayry J, Terme M, Taieb J, Badoual C, Adotevi O, Merillon N, Marcheteau E, Quillien VR, Banissi C, Carpentier A, Sandoval F, Nizard M, Quintin-Colonna F, Kroemer G, Fridman WH, Zitvogel L, Oudard SP, Tartour E. Comprehensive analysis of current approaches to inhibit regulatory T cells in cancer. Oncoimmunology. 2012;1:326–333. doi: 10.4161/onci.18852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3 + regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 45.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, Verdaguer J, Santamaria P. CD40 ligation releases immature dendritic cells from the control of regulatory CD4 + CD25 + T cells. Immunity. 2003;19:877–889. doi: 10.1016/S1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 47.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, Umansky V, Bazhin AV. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133:98–107. doi: 10.1002/ijc.27990. [DOI] [PubMed] [Google Scholar]
- 48.Surls J, Nazarov-Stoica C, Kehl M, Casares S, Brumeanu TD. Differential effect of CD4 + Foxp3 + T-regulatory cells on the B and T helper cell responses to influenza virus vaccination. Vaccine. 2010;28:7319–7330. doi: 10.1016/j.vaccine.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 49.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4 + CD25 + T cells regulate virus-specific primary and memory CD8 + T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4 + T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taguchi O, Takahashi T. Administration of anti-interleukin-2 receptor alpha antibody in vivo induces localized autoimmune disease. Eur J Immunol. 1996;26:1608–1612. doi: 10.1002/eji.1830260730. [DOI] [PubMed] [Google Scholar]
- 53.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Q, Bluestone JA. The Foxp3 + regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4 + CD25 + Foxp3 + regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toka FN, Suvas S, Rouse BT. CD4 + CD25 + T cells regulate vaccine-generated primary and memory CD8 + T-cell responses against herpes implex virus type 1. J Virol. 2004;78:13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trandem K, Anghelina D, Zhao J, Perlman S. Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus. J Immunol. 2010;184:4391–4400. doi: 10.4049/jimmunol.0903918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsunemi D, Iwasaki T, Imado T, Higasa S, Kakishita E, Shirasaka T, Sano H. Relationship of CD4 + CD25 + regulatory T cells to immune status in HIV-infected patients. AIDS. 2005;19:879–886. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- 59.van’t Land B, Schijf M, van Esch BC, van Bergenhenegouwen J, Bastiaans J, Schouten B, Boon L, Garssen J. Regulatory T-cells have a prominent role in the immune modulated vaccine response by specific oligosaccharides. Vaccine. 2010;28:5711–5717. doi: 10.1016/j.vaccine.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 60.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4 + T cells. J Immunol. 2006;176:6202–6210. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 61.Veiga-Parga T, Suryawanshi A, Mulik S, Giménez F, Sharma S, Sparwasser T, Rouse BT. On the role of regulatory T cells during viral-induced inflammatory lesions. J Immunol. 2012;189:5924–5933. doi: 10.4049/jimmunol.1202322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vitali C, Mingozzi F, Broggi A, Barresi S, Zolezzi F, Bayry J, Raimondi G, Zanoni I, Granucci F. Migratory, and not lymphoid-resident, dendritic cells maintain peripheral self-tolerance and prevent autoimmunity via induction of iTreg cells. Blood. 2012;120:1237–1245. doi: 10.1182/blood-2011-09-379776. [DOI] [PubMed] [Google Scholar]
- 63.Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–30. [DOI] [PubMed]
- 64.Wang SM, Tsai MH, Lei HY, Wang JR, Liu CC. The regulatory T cells in anti-influenza antibody response post influenza vaccination. Hum Vaccine Immunother. 2012;8:1243–1249. doi: 10.4161/hv.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3 + regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 66.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4 + CD25 + T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]