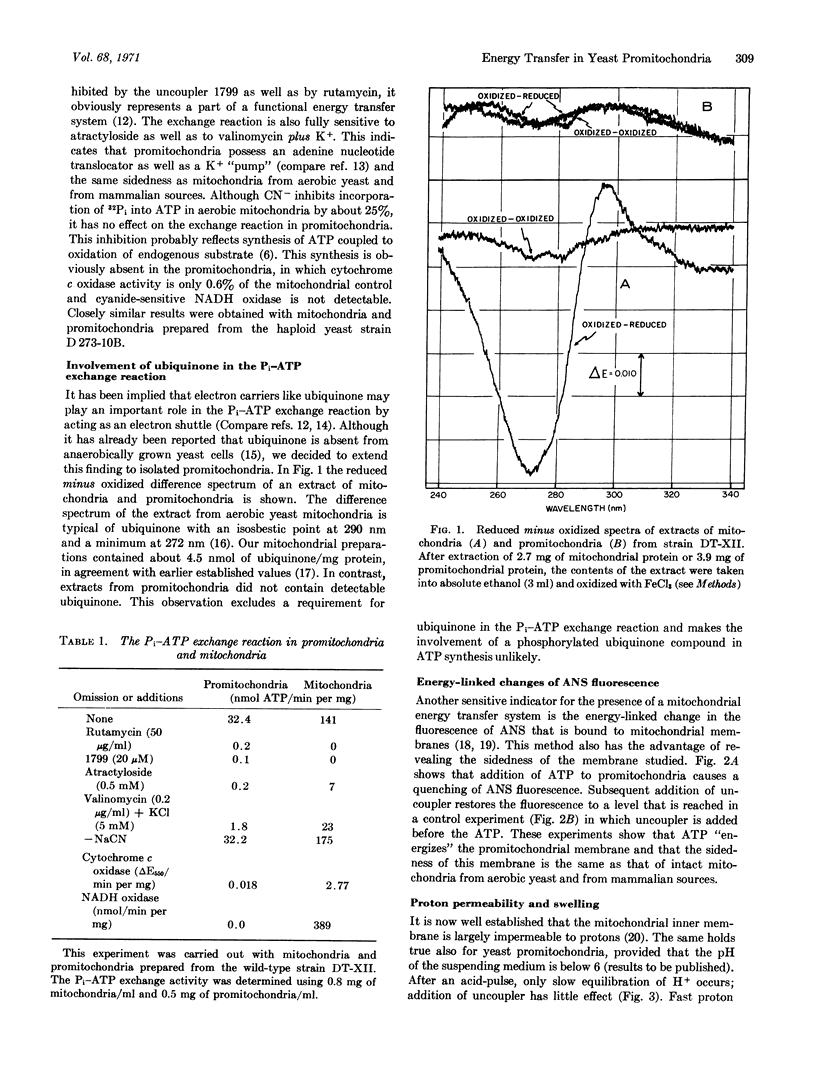
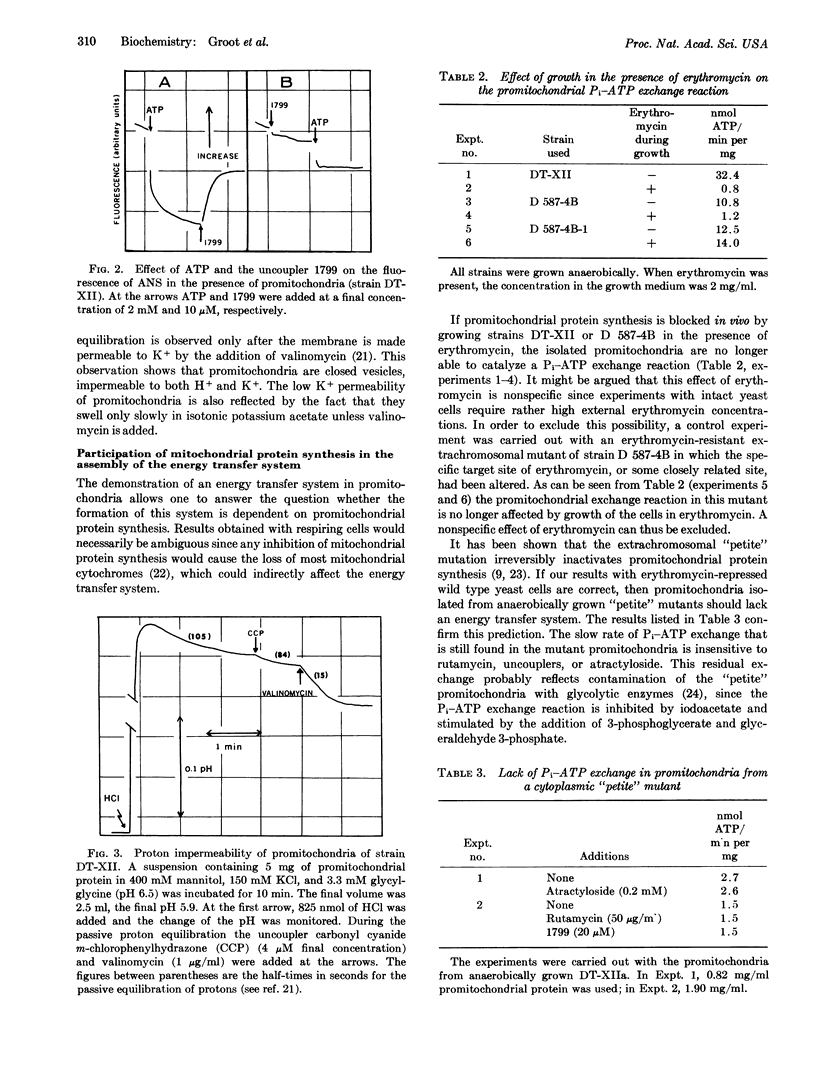
Abstract
Promitochondria of anaerobically grown Saccharomyces cerevisiae lack cytochromes aa3, b, c1, and c, as well as ubiquinone, yet catalyze a Pi-ATP exchange reaction that is sensitive to uncouplers, rutamycin, and atractyloside. The promitochondrial Pi-ATP exchange reaction is abolished by the cytoplasmic “petite” mutation, as well as by growth of the cells in the presence of erythromycin, which indicates a role of mitochondrial protein synthesis in the assembly of the energy transfer system. These observations demonstrate that mitochondrial energy transfer can occur in the absence of a respiratory chain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion W. J., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 23. Preservation of energy coupling in submitochondrial particles lacking cytochrome oxidase. J Biol Chem. 1970 Oct 25;245(20):5186–5194. [PubMed] [Google Scholar]
- Arion W. J., Wright B. J. Preservation of energy coupling in submitochondrial particles during extraction and reinsertion of cytochrome C. Biochem Biophys Res Commun. 1970 Aug 11;40(3):594–599. doi: 10.1016/0006-291x(70)90944-7. [DOI] [PubMed] [Google Scholar]
- Azzi A., Chance B., Radda G. K., Lee C. P. A fluorescence probe of energy-dependent structure changes in fragmented membranes. Proc Natl Acad Sci U S A. 1969 Feb;62(2):612–619. doi: 10.1073/pnas.62.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A. Redistribution of the electrical charge of the mitochondrial membrane during energy conservation. Biochem Biophys Res Commun. 1969 Oct 8;37(2):254–260. doi: 10.1016/0006-291x(69)90727-x. [DOI] [PubMed] [Google Scholar]
- Boyer P. D., Bieber L. L., Mitchell R. A., Szabolcsi G. The apparent independence of the phosphorylation and water formation reactions from the oxidation reactions of oxidative phosphorylation. J Biol Chem. 1966 Nov 25;241(22):5384–5390. [PubMed] [Google Scholar]
- CRANE F. L., DILLEY R. A. DETERMINATION OF COENZYME Q (UBIQUINONE). Methods Biochem Anal. 1963;11:279–306. doi: 10.1002/9780470110294.ch6. [DOI] [PubMed] [Google Scholar]
- Criddle R. S., Schatz G. Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry. 1969 Jan;8(1):322–334. doi: 10.1021/bi00829a045. [DOI] [PubMed] [Google Scholar]
- FALCONE A. B., WITONSKY P. 32P AND 18O EXCHANGE REACTIONS IN SOLUBLE FRACTIONS PREPARED FROM RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Jun;239:1954–1960. [PubMed] [Google Scholar]
- Groot G. S. Comparative studies of the ADP-ATP and the Pi-ATP exchange reactions related to oxidative phosphorylation in rat-liver mitochondria. Biochim Biophys Acta. 1969 Aug 5;180(3):439–444. doi: 10.1016/0005-2728(69)90023-1. [DOI] [PubMed] [Google Scholar]
- Groot G. S., van den Bergh S. G. The role of the ADP-ATP exchange enzyme in oxidative phosphorylation. Biochim Biophys Acta. 1968 Jan 15;153(1):22–31. doi: 10.1016/0005-2728(68)90142-4. [DOI] [PubMed] [Google Scholar]
- Kovác L., Subík J., Russ G., Kollár K. On the relationship between respiratory activity and lipid composition of the yeast cell. Biochim Biophys Acta. 1967 Aug 8;144(1):94–101. doi: 10.1016/0005-2760(67)90080-x. [DOI] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. On the role of ubiquinone in mitochondria. II. Redox reactions of ubiquinone under the control of oxidative phosphorylation. Biochem Z. 1966 Jun 7;344(4):317–336. [PubMed] [Google Scholar]
- Kuzela S., Grecná E. Lack of amino acid incorporation by isolated mitochondria from respiratory-deficient cytoplasmic yeast mutants. Experientia. 1969;25(7):776–777. doi: 10.1007/BF01897625. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi T., Kröger A., Heldt H. W., Pfaff E., Klingenberg M. The response of the respiratory chain and adenine nucleotide system to oxidative phosphorylation in yeast mitochondria. Eur J Biochem. 1967 May;1(3):301–311. doi: 10.1007/978-3-662-25813-2_41. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Rudney H. The possible role of quinone methines in phosphorylation reactions in Rhodospirillum rubrum and liver mitochondria. Biochemistry. 1966 Mar;5(3):1013–1018. doi: 10.1021/bi00867a029. [DOI] [PubMed] [Google Scholar]
- Plattner H., Salpeter M. M., Saltzgaber J., Schatz G. Promitochondria of anaerobically grown yeast. IV. Conversion into respiring mitochondria. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1252–1259. doi: 10.1073/pnas.66.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner H., Schatz G. Promitochondria of anaerobically grown yeast. 3. Morphology. Biochemistry. 1969 Jan;8(1):339–343. doi: 10.1021/bi00829a047. [DOI] [PubMed] [Google Scholar]
- Schatz G. Impaired binding of mitochondrial adenosine triphosphatase in the cytoplasmic "petite" mutant of Saccharomyces cerevisiae. J Biol Chem. 1968 May 10;243(9):2192–2199. [PubMed] [Google Scholar]
- Schatz G., Racker E., Tyler D. D., Gonze J., Estabrook R. W. Studies of the DPNH-cytochrome b segment of the respiratory chain of baker's yeast. Biochem Biophys Res Commun. 1966 Mar 8;22(5):585–590. doi: 10.1016/0006-291x(66)90315-9. [DOI] [PubMed] [Google Scholar]
- Schatz G., Saltzgaber J. Protein synthesis by yeast promitochondria in vivo. Biochem Biophys Res Commun. 1969 Dec 4;37(6):996–1001. doi: 10.1016/0006-291x(69)90230-7. [DOI] [PubMed] [Google Scholar]



