Introduction
Neurotrophic factors are growth factors, which play an essential role in the development and maintenance of nervous system. These include members of the nerve growth factor (NGF) family, known as neurotrophins. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), neurotrophin 4 (NT4/5) and neurotrophin 6 (NT-6) constitute the family of neurotrophins (Barbacid 1995). The identification of neurotrophic factors began from the search for target-derived molecules, which act on neurons and promote their survival (Lindsay 1996). BDNF is one such essential survival factor.
BDNF was first isolated and purified from pig brain (Barde et al. 1982). BDNF, encoded by the BDNF gene, is the second member of the neurotrophin family after NGF (Timmusk et al. 1993). BDNF, like other neurotrophins, exists as a homodimer. It is synthesized as a precursor molecule, proBDNF (32 kDa), which is cleaved to form the mature and biologically active form of BDNF (∼13 kDa) (Negro et al. 1994). It is expressed throughout the central nervous system (Leibrock et al. 1989) and also in peripheral tissues including muscle, liver, pancreas, colon, duodenum, lung, kidney and bladder (Lommatzsch et al. 1999).
The dimerized BDNF binds to the tyrosine kinase receptor, tropomyosin-related kinase B (TrkB), leading to activation of various signaling modules. Trk was first identified as an oncoprotein in human tumors (Martin-Zanca et al. 1986). The Trk family of receptors includes TrkA, which binds to NGF (Kaplan et al. 1991); TrkB, which is the receptor for BDNF and NT-4 (Klein et al. 1989); and TrkC, the receptor for NT-3 (Lamballe et al. 1991). TrkB is an 821 amino acid long glycoprotein, encoded by the NTRK2 gene. It comprises of a signal peptide followed by an extracellular domain, a transmembrane domain and a cytoplasmic region consisting of the tyrosine kinase domain (Nakagawara et al. 1995). TrkB is expressed in the central and peripheral nervous system (Allen et al. 1994). Like other neurotrophins, BDNF also binds to nerve growth factor receptor (NGFR), also known as p75 neurotrophin receptor (p75NTR), albeit with a low-affinity (Chao et al. 1986). Binding of BDNF to TrkB results in the dimerization of the receptor and activation of its cytoplasmic kinase domain (Ohira et al. 2001; Wu et al. 1996), which results in the recruitment of adaptor proteins to the receptor, which in turn leads to the activation of intracellular signaling cascades including RAS/ERK pathway, PI3K/AKT pathway, PLC/PKC pathway and NFκB pathway.
BDNF/TrkB signaling is essential for growth, differentiation and survival of neurons. It is also important for neuronal morphogenesis and synaptic plasticity (Binder and Scharfman 2004). BDNF also plays a major role in other processes such as energy metabolism (Matthews et al. 2009), behavior, learning, memory (Hall et al. 2000), pain (Pezet et al. 2002) and apoptosis (Yeiser et al. 2004). BDNF is implicated in Alzheimer’s disease (Ferrer et al. 1999), Huntington’s disease (Zuccato et al. 2001), epilepsy (Takahashi et al. 1999) and bipolar disorder (Neves-Pereira et al. 2002). However, despite its biological significance, molecular events induced by either BDNF/TrkB or BDNF/p75NTR interactions, were not organized into a signaling network. As a part of the ongoing NetPath project, which aims at the development of a centralized resource for human signaling pathways (Kandasamy et al. 2010; Raju et al. 2011b), we have documented molecular reactions induced by several signaling systems by systematically reviewing published reports and assembled them in the form of signaling pathways. We have previously published molecular networks of signaling induced by prolactin (Radhakrishnan et al. 2012), oncostatin M (Dey et al. 2012); corticotropin releasing hormone (Subbannayya et al. 2013) and advanced glycation end-products (Soman et al. 2013). Similarly, as BDNF signaling pathway is of immense biomedical interest, we analyzed literature pertaining to BDNF-induced signaling events and developed a graphical network map of BDNF/TrkB and BDNF/p75NTR signaling pathways.
Methods
Literature search pertaining to BDNF was carried out using search terms ‘BDNF’ and ‘Brain-derived neurotrophic factor’ in PubMed and Google Scholar. Articles were screened for information related to BDNF signaling such as molecular interactions, post-translational modifications (PTMs), activation and inhibition processes, translocation events and transcriptional gene regulation. The molecular reactions from the articles were documented using PathBuilder, a software utility developed by our group for annotation of signaling pathways (Kandasamy et al. 2009).
We have largely followed the annotation criteria that have been previously described in the generation of RANKL (Raju et al. 2011a), Leptin (Nanjappa et al. 2011), FSH (Telikicherla et al. 2011) and TSH (Goel et al. 2012b) signaling pathways. Briefly, protein–protein interactions (PPIs) were categorized as either direct or complex interactions. For every PPI, data such as the gene identifiers, species of the interacting proteins, host organism, interaction location and the PubMed identifier of the article from which the reaction was taken were gathered. A brief comment on involvement of any PTM, domain or motif for every PPI was also included. Enzyme catalysis reactions were documented in the same way as PPIs. However, we mapped PTM site and residue to the longest isoform sequence of corresponding protein as provided by RefSeq before annotating it in PathBuilder. Transport of molecules from one subcellular compartment to the other upon stimulation with BDNF was entered under translocation events. The list of proteins, which were either activated or inhibited under influence of BDNF, was also provided. We have also cataloged genes, which were up- or down-regulated upon BDNF binding to its receptor. In addition, we documented known transcriptional regulators of genes controlled by BDNF. In addition to internal review system, each pathway reaction was also reviewed by the pathway authority, who is an expert in the field (RC, co-author in this article). BDNF signaling pathway information is made publicly available through NetPath (http://www.netpath.org) . The pathway map of BDNF signaling was drawn based on the NetSlim criteria, by selecting high confidence reactions from the gathered data. The molecules in the map were arranged using information from inhibition assays, mutation studies and review articles. The pathway map was designed using the visualization tool, PathVisio (van Iersel et al. 2008).
Results and discussion
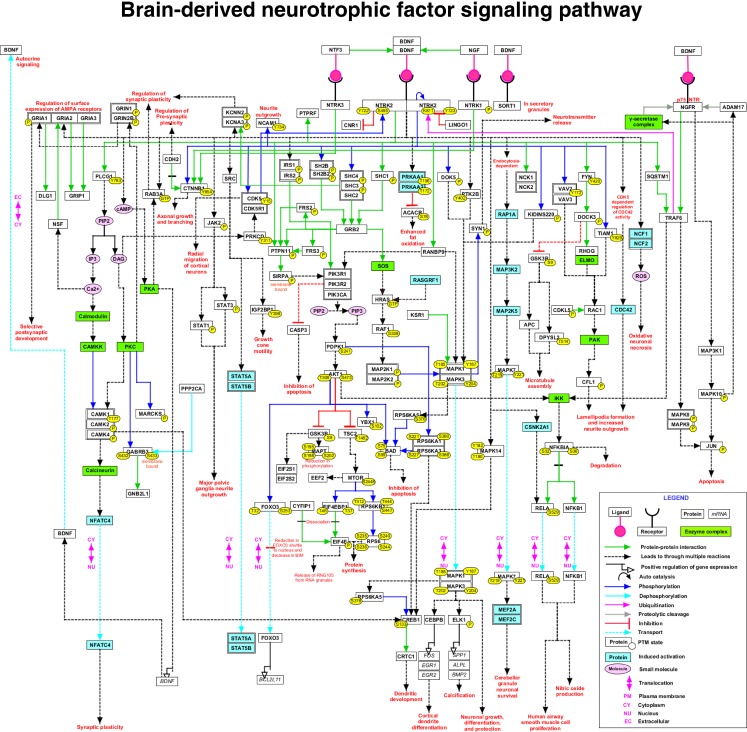
We have developed a pathway resource for molecular reactions that occur upon stimulation of cells by BDNF. By manually reviewing 140 articles, we documented 56 molecular associations, 93 enzyme-substrate reactions, 15 translocation events and 23 activation/inhibition events, which occur upon stimulation of TrkB and p75NTR with BDNF. We identified 261 differentially regulated genes upon BDNF treatment. We have depicted these pathway reactions as a signaling network (Fig. 1). The BDNF pathway is accessible at http://www.netpath.org/pathways?path_id=NetPath_76.
Fig. 1.
Schematic representation of the BDNF signaling network. The BDNF signaling pathway map contains 129 molecules for which the PPIs, PTMs, translocation and activation/inhibition reactions are experimentally proven in BDNF signaling. The major signaling modules activated by BDNF include PI3K/AKT, RAS/ERK, PLC/PKC, AMPK/ACC and JAK/STAT pathways. These signaling events in neurons lead to various context-specific processes such as growth, cell proliferation, differentiation, maintenance of synaptic plasticity, microtubule assembly, fat metabolism, protection, survival, calcification, production of nitric oxide and apoptosis. This pathway map is made publicly available at (http://www.wikipathways.org/index.php/Pathway:WP2380). A high confidence version of this map is available at http://www.netpath.org/netslim/BDNF_pathway.html
The pathway map is also generated based on NetSlim criteria as mentioned earlier. We have included only 72 molecules in NetSlim map out of 129 molecules described in NetPath. NetSlim pathway diagram can be accessed at http://www.netpath.org/netslim/BDNF_pathway.html. The reactions in the map are provided with citations linked to the research articles in PubMed. The molecules are linked to their respective molecule pages in NetPath and HPRD (Goel et al. 2012a) and the genes to their corresponding gene pages in NCBI.
BDNF signaling is triggered when it binds to the tyrosine kinase receptor TrkB (Klein et al. 1991). Subsequently, the tyrosine residues in the kinase domain of TrkB undergo autophosphorylation (Wu et al. 1996), which leads to the recruitment of molecules such as SHP2 (PTPN11) (Yamada et al. 1999), SHC (SHC1) and PLC-gamma (PLCG1) (Yamada et al. 2002). These molecules further interact with their downstream targets and lead to activation of various signaling modules such as PI3K/AKT pathway (Araki et al. 2000), RAS/ERK pathway (Ou and Gean 2006), PLC/PKC pathway (Groth and Mermelstein 2003), AMPK/ACC (Matthews et al. 2009) and NFκB pathway (Burke and Bothwell 2003). BDNF stimulation of PI3K/AKT signaling cascade is essential for proliferation, protection and survival of neuronal cells (Yamada et al. 2001). BDNF also leads to neuronal survival through ERK5/MEF pathway (Shalizi et al. 2003). Activation of PI3K/AKT further activates mTOR pathway and subsequently protein synthesis (Takei et al. 2004). BDNF through activation of ERK1/2 (MAPK3/MAPK1) plays major role in various cellular processes including growth (Sugimoto et al. 2001), differentiation (Yin et al. 2010), cell invasion (Zhang et al. 2010), calcification (Kajiya et al. 2008), protection of neuronal cells (Szatmari et al. 2007) and release of neurotransmitters (Jovanovic et al. 2000). Other than activation of PKC, PLC also leads to release of intracellular calcium and phosphorylation of CREB (CREB1) (Finkbeiner et al. 1997), neuronal migration (Zhao et al. 2009) and maintenance of synaptic plasticity (Groth and Mermelstein 2003). BDNF also maintains synaptic plasticity through cAMP/PKA signaling (Thakker-Varia et al. 2001). cAMP/PKA module is also involved in BDNF induced secretion of BDNF in an autocrine manner (Cheng et al. 2011). BDNF regulates axonal growth and branching through phosphorylation of catenin-beta (CTNNB1) (David et al. 2008).
BDNF induces neurite outgrowth through activation of JAK/STAT (Lin et al. 2006), RAC (RAC1) and cell division cycle 42 (CDC42) pathways (Miyamoto et al. 2006). It enhances oxidation of fat through AMPK-dependent inhibition of ACC (ACACB) (Matthews et al. 2009). It also mediates microtubule assembly through inhibition of GSK3-beta (GSK3B) (Namekata et al. 2012). BDNF leads to oxidative neuronal necrosis through activation of p47-PHOX (NCF1) and p67-PHOX (NCF2) (Kim et al. 2002). BDNF also regulates the surface expression of AMPA and NMDA receptors (Wu et al. 2004). Further, BDNF regulates the expression of genes leading to processes such as differentiation of dendrites and calcification of cementoblast-like cells (Kajiya et al. 2008). BDNF induces ubiquitination of TrkB receptor through TRAF6 (TRAF6) (Jadhav et al. 2008). TRAF6 also mediates phosphorylation of c-Jun (JUN) through activation of JNK, which leads to apoptosis (Yeiser et al. 2004). BDNF/p75NTR signaling through NFκB pathway leads to production of nitric oxide (Burke and Bothwell 2003). Activation of JNK3 (MAPK10) by BDNF leads to proteolytic cleavage of p75NTR (Kenchappa et al. 2010).
The pathway data is freely available in various data exchange formats such as PSI-MI version 2.5 (Hermjakob et al. 2004), BioPAX version 3.0 (Demir et al. 2010) and SBML version 2.1 (Hucka et al. 2003). The NetSlim version can be downloaded in .gpml, .png and .pdf formats. The pathway is also made available through WikiPathways (http://www.wikipathways.org/index.php/Pathway:WP2380) to the scientific community. We will constantly update the BDNF pathway as and when more published literature becomes available.
Conclusions
BDNF is an essential neurotrophin involved in neuroprotection and survival. BDNF signaling has significant clinical implications in many neurological disorders. We have gathered experimental data related to BDNF signaling from the published literature and integrated into a bioinformatics resource, which will facilitate future bioinformatics analyses and scientific investigations using high-throughput experiments. Freely available BDNF signaling pathway will find its way into gene set enrichment analysis software utilities. Such a development will increase the chances of identifying the role of BDNF signaling in normal and disease physiology in humans.
Acknowledgements
We thank the Department of Biotechnology, Government of India for research support to the Institute of Bioinformatics, Bangalore. Varot K. Sandhya is a recipient of Inspire Fellowship from the Department of Science and Technology (DST), Government of India. Renu Verma is a recipient of Junior Research Fellowship from the University Grants Commission (UGC), Government of India. Rakesh Sharma is a Research Associate supported by the Department of Biotechnology (DBT), Government of India. Aneesha Radhakrishnan is a recipient of Senior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India. We acknowledge Dr. Renu Goel for her assistance in curation.
Conflict of interests
The author(s) declared no conflicts of interests.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- TRK
Tropomyosin-related kinase
- NGF
Nerve growth factor
- NT
Neurotrophin
- p75NTR
p75 neurotrophin receptor
- PPIs
Protein–protein interactions
- PTMs
Post-translational modifications
- BioPAX
Biological PAthway eXchange
- SBML
Systems Biology Markup Language
- PSI-MI
Proteomics Standards Initiative for Molecular Interaction
Footnotes
Varot K. Sandhya and Rajesh Raju contributed equally to this paper.
Contributor Information
Varot K. Sandhya, Email: sandhya@ibioinformatics.org
Rajesh Raju, Email: rajesh@ibioinformatics.org.
Renu Verma, Email: renuverma@ibioinformatics.org.
Jayshree Advani, Email: jayshree@ibioinformatics.org.
Rakesh Sharma, Email: rakesh@ibioinformatics.org.
Aneesha Radhakrishnan, Email: aneesha@ibioinformatics.org.
Vishalakshi Nanjappa, Email: vishalakshi@ibioinformatics.org.
Jayasuryan Narayana, Email: jayasuryanmn@gmail.com.
B. L. Somani, Email: blsomani@gmail.com
Kanchan K. Mukherjee, Email: kk_mukherjee@hotmail.com
Akhilesh Pandey, Email: pandey@jhmi.edu.
Rita Christopher, Email: rita@nimhans.kar.nic.in.
T. S. Keshava Prasad, Phone: +91-80-28416140, FAX: +91-80-28416132, Email: keshav@ibioinformatics.org.
References
- Allen SJ, Dawbarn D, Eckford SD, Wilcock GK, Ashcroft M, Colebrook SM, Feeney R, MacGowan SH. Cloning of a non-catalytic form of human trkB and distribution of messenger RNA for trkB in human brain. Neuroscience. 1994;60:825–834. doi: 10.1016/0306-4522(94)90507-X. [DOI] [PubMed] [Google Scholar]
- Araki T, Yamada M, Ohnishi H, Sano SI, Hatanaka H. BIT/SHPS-1 enhances brain-derived neurotrophic factor-promoted neuronal survival in cultured cerebral cortical neurons. J Neurochem. 2000;75:1502–1510. doi: 10.1046/j.1471-4159.2000.0751502.x. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MA, Bothwell M. p75 neurotrophin receptor mediates neurotrophin activation of NF-kappa B and induction of iNOS expression in P19 neurons. J Neurobiol. 2003;55:191–203. doi: 10.1002/neu.10174. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell MA, Ross AH, Koprowski H, Lanahan AA, Buck CR, Sehgal A. Gene transfer and molecular cloning of the human NGF receptor. Science. 1986;232:518–521. doi: 10.1126/science.3008331. [DOI] [PubMed] [Google Scholar]
- Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci U S A. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David MD, Yeramian A, Dunach M, Llovera M, Canti C, de Herreros AG, Comella JX, Herreros J. Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential beta-catenin phosphorylation. J Cell Sci. 2008;121:2718–2730. doi: 10.1242/jcs.029660. [DOI] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, Fukuda K, Lemer C, Vastrik I, Wu G, D’Eustachio P, Schaefer C, et al. The BioPAX community standard for pathway data sharing. Nat Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey G, Radhakrishnan A, Syed N, Thomas JK, Nadig A, Srikumar K, Mathur PP, Pandey A, Lin SK et al (2012) Signaling network of Oncostatin M pathway. J Cell Commun Signal. doi:10.1007/s12079-012-0186-y [DOI] [PMC free article] [PubMed]
- Ferrer I, Marin C, Rey MJ, Ribalta T, Goutan E, Blanco R, Tolosa E, Marti E. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/S0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Goel R, Harsha HC, Pandey A, Prasad TSK. Human Protein Reference Database and Human Proteinpedia as resources for phosphoproteome analysis. Mol Biosyst. 2012;8:453–463. doi: 10.1039/c1mb05340j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R, Raju R, Maharudraiah J, Kumar GSS, Ghosh K, Kumar A, Lashmi PT, Sharma J, Sharma R, et al. A signaling network of thyroid-stimulating hormone. J Proteomics Bioinforma. 2012;4:238–241. doi: 10.4172/jpb.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth RD, Mermelstein PG. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J Neurosci. 2003;23:8125–8134. doi: 10.1523/JNEUROSCI.23-22-08125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hermjakob H, Montecchi-Palazzi L, Bader G, Wojcik J, Salwinski L, Ceol A, Moore S, Orchard S, Sarkans U, et al. The HUPO PSI’s molecular interaction format—a community standard for the representation of protein interaction data. Nat Biotechnol. 2004;22:177–183. doi: 10.1038/nbt926. [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, et al. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19:524–531. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- Jadhav T, Geetha T, Jiang J, Wooten MW (2008) Identification of a consensus site for TRAF6/p62 polyubiquitination. Biochem Biophys Res Commun 371:521–524 [DOI] [PMC free article] [PubMed]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kajiya M, Shiba H, Fujita T, Ouhara K, Takeda K, Mizuno N, Kawaguchi H, Kitagawa M, Takata T, et al. Brain-derived neurotrophic factor stimulates bone/cementum-related protein gene expression in cementoblasts. J Biol Chem. 2008;283:16259–16267. doi: 10.1074/jbc.M800668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Keerthikumar S, Raju R, Prasad TSK, Ramachandra YL, Mohan S, Pandey A. PathBuilder—open source software for annotating and developing pathway resources. Bioinformatics. 2009;25:2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, et al. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kenchappa RS, Tep C, Korade Z, Urra S, Bronfman FC, Yoon SO, Carter BD. p75 neurotrophin receptor-mediated apoptosis in sympathetic neurons involves a biphasic activation of JNK and up-regulation of tumor necrosis factor-alpha-converting enzyme/ADAM17. J Biol Chem. 2010;285:20358–20368. doi: 10.1074/jbc.M109.082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Sohn S, Kwon HJ, Lee JY, Park JH, Gwag BJ. Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase. J Cell Biol. 2002;159:821–831. doi: 10.1083/jcb.200112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Parada LF, Coulier F, Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989;8:3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, et al. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66:967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde YA. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Lin G, Bella AJ, Lue TF, Lin CS. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J Sex Med. 2006;3:821–827. doi: 10.1111/j.1743-6109.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond B Biol Sci. 1996;351:365–373. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am J Pathol. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, Akerstrom T, Yfanti C, Broholm C, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A. 2006;103:10444–10449. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A, Liu XG, Ikegaki N, White PS, Yamashiro DJ, Nycum LM, Biegel JA, Brodeur GM. Cloning and chromosomal localization of the human TRK-B tyrosine kinase receptor gene (NTRK2) Genomics. 1995;25:538–546. doi: 10.1016/0888-7543(95)80055-Q. [DOI] [PubMed] [Google Scholar]
- Namekata K, Harada C, Guo X, Kimura A, Kittaka D, Watanabe H, Harada T. Dock3 stimulates axonal outgrowth via GSK-3beta-mediated microtubule assembly. J Neurosci. 2012;32:264–274. doi: 10.1523/JNEUROSCI.4884-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa V, Raju R, Muthusamy B, Sharma J, Thomas JK, Nidhina PAH, Harsha HC, Pandey A, Anilkumar G, et al. A comprehensive curated reaction map of leptin signaling pathway. J Proteomics Bioinforma. 2011;4:184–189. [Google Scholar]
- Negro A, Tavella A, Grandi C, Skaper SD. Production and characterization of recombinant rat brain-derived neurotrophic factor and neurotrophin-3 from insect cells. J Neurochem. 1994;62:471–478. doi: 10.1046/j.1471-4159.1994.62020471.x. [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Shimizu K, Hayashi M. TrkB dimerization during development of the prefrontal cortex of the macaque. J Neurosci Res. 2001;65:463–469. doi: 10.1002/jnr.1175. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31:287–296. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Pezet S, Malcangio M, Lever IJ, Perkinton MS, Thompson SW, Williams RJ, McMahon SB. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol Cell Neurosci. 2002;21:684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Raju R, Tuladhar N, Subbannayya T, Thomas JK, Goel R, Telikicherla D, Palapetta SM, Rahiman BA, et al. A pathway map of prolactin signaling. J Cell Commun Signal. 2012;6:169–173. doi: 10.1007/s12079-012-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R, Balakrishnan L, Nanjappa V, Bhattacharjee M, Getnet D, Muthusamy B, Kurian Thomas J, Sharma J, Rahiman BA et al (2011a) A comprehensive manually curated reaction map of RANKL/RANK-signaling pathway. Database (Oxford) 2011:bar021 [DOI] [PMC free article] [PubMed]
- Raju R, Nanjappa V, Balakrishnan L, Radhakrishnan A, Thomas JK, Sharma J, Tian M, Palapetta SM, Subbannayya T et al (2011b) NetSlim: high-confidence curated signaling maps. Database (Oxford) 2011:bar032 [DOI] [PMC free article] [PubMed]
- Shalizi A, Lehtinen M, Gaudilliere B, Donovan N, Han J, Konishi Y, Bonni A. Characterization of a neurotrophin signaling mechanism that mediates neuron survival in a temporally specific pattern. J Neurosci. 2003;23:7326–7336. doi: 10.1523/JNEUROSCI.23-19-07326.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soman S, Raju R, Sandhya VK, Advani J, Khan AA, Harsha HC, Prasad TSK, Sudhakaran PR, Pandey A, et al. A multicellular signal transduction network of AGE/RAGE signaling. J Cell Commun Signal. 2013;7:19–23. doi: 10.1007/s12079-012-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbannayya T, Balakrishnan L, Sudarshan G, Advani J, Kumar S, Mahmood R, Nair B, Sirdeshmukh R, Mukherjee KK et al (2013) An integrated map of corticotropin-releasing hormone signaling pathway. J Cell Commun Signal. doi:10.1007/s12079-013-0197-3 [DOI] [PMC free article] [PubMed]
- Sugimoto T, Kuroda H, Horii Y, Moritake H, Tanaka T, Hattori S. Signal transduction pathways through TRK-A and TRK-B receptors in human neuroblastoma cells. Jpn J Cancer Res. 2001;92:152–160. doi: 10.1111/j.1349-7006.2001.tb01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari E, Kalita KB, Kharebava G, Hetman M. Role of kinase suppressor of Ras-1 in neuronal survival signaling by extracellular signal-regulated kinase 1/2. J Neurosci. 2007;27:11389–11400. doi: 10.1523/JNEUROSCI.3473-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Hayashi S, Kakita A, Wakabayashi K, Fukuda M, Kameyama S, Tanaka R, Takahashi H, Nawa H. Patients with temporal lobe epilepsy show an increase in brain-derived neurotrophic factor protein and its correlation with neuropeptide Y. Brain Res. 1999;818:579–582. doi: 10.1016/S0006-8993(98)01355-9. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telikicherla D, Ambekar A, Palapetta SM, Dwivedi SB, Raju R, Sharma J, Prasad TSK, Ramachandra Y, Mohan SS, et al. A comprehensive curated resource for follicle stimulating hormone signaling. BMC Res Notes. 2011;4:408. doi: 10.1186/1756-0500-4-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S, Alder J, Crozier RA, Plummer MR, Black IB. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci. 2001;21:6782–6790. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-O. [DOI] [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C. Presenting and exploring biological pathways with PathVisio. BMC Bioinforma. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Xu JL, Suen PC, Levine E, Huang YY, Mount HT, Lin SY, Black IB. Functional trkB neurotrophin receptors are intrinsic components of the adult brain postsynaptic density. Brain Res Mol Brain Res. 1996;43:286–290. doi: 10.1016/S0169-328X(96)00211-2. [DOI] [PubMed] [Google Scholar]
- Wu K, Len GW, McAuliffe G, Ma C, Tai JP, Xu F, Black IB. Brain-derived neurotrophic factor acutely enhances tyrosine phosphorylation of the AMPA receptor subunit GluR1 via NMDA receptor-dependent mechanisms. Brain Res Mol Brain Res. 2004;130:178–186. doi: 10.1016/j.molbrainres.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohnishi H, Sano S, Araki T, Nakatani A, Ikeuchi T, Hatanaka H. Brain-derived neurotrophic factor stimulates interactions of Shp2 with phosphatidylinositol 3-kinase and Grb2 in cultured cerebral cortical neurons. J Neurochem. 1999;73:41–49. doi: 10.1046/j.1471-4159.1999.0730041.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tanabe K, Wada K, Shimoke K, Ishikawa Y, Ikeuchi T, Koizumi S, Hatanaka H. Differences in survival-promoting effects and intracellular signaling properties of BDNF and IGF-1 in cultured cerebral cortical neurons. J Neurochem. 2001;78:940–951. doi: 10.1046/j.1471-4159.2001.00497.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Numakawa T, Koshimizu H, Tanabe K, Wada K, Koizumi S, Hatanaka H. Distinct usages of phospholipase C gamma and Shc in intracellular signaling stimulated by neurotrophins. Brain Res. 2002;955:183–190. doi: 10.1016/S0006-8993(02)03432-7. [DOI] [PubMed] [Google Scholar]
- Yeiser EC, Rutkoski NJ, Naito A, Inoue J, Carter BD. Neurotrophin signaling through the p75 receptor is deficient in traf6-/- mice. J Neurosci. 2004;24:10521–10529. doi: 10.1523/JNEUROSCI.1390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin YX, Sun ZP, Huang SH, Zhao L, Geng Z, Chen ZY. RanBPM contributes to TrkB signaling and regulates brain-derived neurotrophic factor-induced neuronal morphogenesis and survival. J Neurochem. 2010;114:110–121. doi: 10.1111/j.1471-4159.2010.06745.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Guo D, Luo W, Zhang Q, Zhang Y, Li C, Lu Y, Cui Z, Qiu X. TrkB is highly expressed in NSCLC and mediates BDNF-induced the activation of Pyk2 signaling and the invasion of A549 cells. BMC Cancer. 2010;10:43. doi: 10.1186/1471-2407-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CT, Li K, Li JT, Zheng W, Liang XJ, Geng AQ, Li N, Yuan XB. PKCdelta regulates cortical radial migration by stabilizing the Cdk5 activator p35. Proc Natl Acad Sci U S A. 2009;106:21353–21358. doi: 10.1073/pnas.0812872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]