Introduction
Corticotropin-releasing hormone (CRH), also known as corticotropin-releasing factor (CRF), is a 41-amino acid neuropeptide, expressed abundantly by CRH neurons present in the paraventricular nucleus (PVN) of the hypothalamus, and other parts of the brain (Aguilera and Liu 2012; Vale et al. 1981). CRH is also expressed in adrenal gland, immune cells, placenta, testis, spleen, gut, thymus and skin (Dautzenberg and Hauger 2002; Aguilera and Liu 2012). The activity of the hypothalamic CRH neurons varies in resting and stress conditions. Circadian variations have an effect on CRH neuron activity. On stressful stimuli, sensory information is either transmitted directly to the PVN or integrated by the limbic system and transmitted to the CRH neurons via complex monoaminergic and peptidergic neural pathways. This is dependent on the nature, intensity and duration of the stressor. Systemic and metabolic stressors including blood loss, pain, immune challenge and hypoglycemia, which require immediate response, utilize monosynaptic pathways, whereas, psychogenic stressors utilize complex multisynaptic pathways. These neural pathways trigger signaling in CRH neurons, increasing CRH gene transcription and rapid CRH secretion (Aguilera and Liu 2012). The actions of CRH are transduced through CRH receptors, which belong to the class II/secretin-like family of the G-protein coupled receptor (GPCR) superfamily (Martin et al. 2005). There are three types of CRH receptors – type 1 (CRHR1), type 2 (CRHR2) and type 3 (CRHR3). Among these, CRHR3 has not been identified in mammals. CRHR1 and CRHR2 are encoded by different genes. CRHR1 mRNA has several splice variants encoding different isoforms – R1α, R1β, R1c, R1d, R1e, R1f, R1g and R1h, predominant of which is CRHR1α (Chen et al. 1993; Ross et al. 1994; Grammatopoulos et al. 1999; Pisarchik and Slominski 2001, 2004). CRHR1 is expressed predominantly in the CNS, pituitary, heart (Chen et al. 1993) and also in adrenal gland, ovary, and placenta (Grammatopoulos et al. 1999; Seres et al. 2004; Asakura et al. 1997). The gene encoding the human CRHR2 has three mRNA splice variants which encodes 3 isoforms - R2α, R2β and R2γ and is expressed predominantly in brain and heart (Kostich et al. 1998; Yang et al. 2010). CRH is a high-affinity ligand of CRHR1. It also binds to CRHR2, but with lower affinity (Hsu and Hsueh 2001; Grammatopoulos et al. 1999; Wille et al. 1999). CRH receptors do not have any intrinsic kinase activity and the signal is transduced via the heterotrimeric G-proteins (Freissmuth et al. 1989). Biological activity of CRH is regulated by CRH binding protein (CRHBP), which by binding to the former with high affinity, makes it unavailable for binding to CRH receptors (Potter et al. 1991; Behan et al. 1995; Seasholtz et al. 2002). CRHBP is expressed in high levels in the brain, heart, lungs, and placenta (Potter et al. 1991; Behan et al. 1995; Binder and Nemeroff 2010). CRH exerts its functions through activation of several signaling pathways including adenylate cyclase/protein kinase A (PKA), phospholipase C (PLC)/protein kinase C (PKC), and mitogen-activated protein kinase (MAPK) pathways.
CRH, as a principal mediator of endocrine stress response, activates the HPA axis (Hypothalamic–pituitary–adrenal axis) by binding to the CRHR1 in the anterior pituitary. This, through a cascade of reactions, increases the expression of proopiomelanocortin (POMC) gene and the subsequent release of POMC-derived peptides, adrenocorticotropic hormone (ACTH) and β-endorphin. ACTH, in turn, stimulates the secretion of glucocorticoids from adrenal cortex (Vale et al. 1981). CRH is involved in the etiologies of several stress-related physiological responses and behavioral responses, such as altered blood pressure, increased arousal, enhanced learning, thermogenesis, anxiogenesis, anhendonia, reduced sleep, psychomotor alterations, decreased appetite and libido (Binder and Nemeroff 2010). CRH, upon binding to hippocampal CRHR1, mediates stress-induced enhancement of fear conditioning and learning. In contrast, CRH by binding to the lateral septal CRHR2 mediates stress-induced anxiety and impairs fear conditioning and learning (Radulovic et al. 1999). Pro-inflammatory and anti-inflammatory responses are exerted through peripheral and central CRH, respectively (Karalis et al. 1991; Friedman and Irwin 2001). Binding of peripheral and central CRH to their receptors were implicated in hemodynamic actions (Yang et al. 2010). Endometrial CRH was found to be involved in stromal cell decidualization during estrus cycle (Zoumakis et al. 2000) and also in implantation of blastocyst (Athanassakis et al. 1999). Placental CRH is involved in the maintenance of pregnancy and onset of labor (McLean et al. 1995). Excess secretion of CRH during severe depression and its association with increased levels of cortisol have been observed (Widerlov et al. 1988). CRH was also reported to be involved in anxiety disorders (Roy-Byrne et al. 1986), and anorexia nervosa (Connan et al. 2007). Decrease in cortical CRH content was observed in Alzheimer’s disease (Heilig et al. 1995) and Parkinson’s disease (Suemaru et al. 1995).
Diverse aspects of CRH signaling have been well-studied. Owing to its high biological significance, a detailed documentation of all molecular reactions in a centralized resource and depiction of these reactions into a pathway map is desired in the public domain. Therefore, we have curated each pathway reaction reported downstream to CRH-CRHR interaction and submitted a detailed CRH signaling pathway data to the NetPath (http://www.netpath.org) (Kandasamy et al. 2010). Several such ligand-receptor signaling pathways including Leptin (Nanjappa et al. 2011), TWEAK (Bhattacharjee et al. 2012) and Prolactin (Radhakrishnan et al. 2012), had been developed by our group and submitted to NetPath. Here, we describe the generation of an integrated pathway map of CRH signaling by manual curation.
Materials and methods
A survey of scientific literature pertaining to CRH signaling pathway was carried out using PubMed. The search terms that were used include - ‘corticotropin-releasing hormone signaling pathway NOT Review’ and ‘(CRH OR CRF OR “corticotropin-releasing hormone”) AND (Signaling OR “Signal transduction” OR Pathway OR signal*) NOT Review’. Molecular reactions stimulated by the binding of CRH to CRHR1 and CRHR2 were considered for curation. Reactions induced by the binding of urocortins and non-mammalian CRH-like peptides to CRH receptors were excluded from this study. The molecular reactions that were considered for curation include protein-protein interactions (PPIs), post-translational modifications (PTMs), protein translocation events, activation/inhibition of proteins, and transcriptional regulation of genes and their regulators. The reactions of the signaling pathway were cataloged based on the criteria that have been previously described (Nanjappa et al. 2011; Raju et al. 2011a). PathBuilder (Kandasamy et al. 2009), a pathway data assimilation software previously developed by our group, was used for the annotation of CRH pathway reactions. Specific data pertaining to each of the molecular events has been provided. In addition, information associated with each molecular event including source of the proteins, type of experiment, species of the cells/cell lines used in the experiment, PTM dependence of the event, subcellular localization of the proteins and PubMed identifiers for the research article have been provided. We have also provided reaction comments, with a brief description for each pathway reaction.
We obtained a subset of pathway reactions for CRH signaling by filtering reactions assimilated in NetPath, by applying a set of stringent NetSlim criteria, described previously by our group (Raju et al. 2011b). Based on these confident set of reactions, we developed the pathway map using PathVisio software (van Iersel et al. 2008). The topological arrangement of the molecules in the map was based on the information obtained from, i) inhibitor-based assays; ii) mutation-based assays; iii) knockout studies; iv) canonical pathways; and v) review articles. The CRH pathway map has been provided in the NetSlim resource (http://www.netpath.org/netslim) (Raju et al. 2011b).
Results and discussion
We have screened over 2,000 research articles until December, 2012 and cataloged molecular reactions from 87 articles. Our cataloging effort of CRH signaling pathway from literature has yielded 73 molecules involved in 20 PPIs, 29 PTMs, 25 activation events, 1 inhibition event and 20 protein translocation events. Of the 20 PPIs, 18 were of direct and 2 complex. The upstream enzymes for these 29 PTM events induced by CRH, have not been reported in literature. We could annotate site and residue information for PTMs of 12 proteins. In addition, we have cataloged 25 genes, which are differentially regulated at mRNA level upon CRH signaling in different human cells. The reactions annotated for the CRH pathway have been internally reviewed before being reviewed by a Pathway Authority (SNU, co-author in this manuscript). Pathway data for CRH will be constantly updated in NetPath. Besides, we have submitted 21 reactions to Human Protein Reference Database (HPRD) (Prasad et al. 2009; Goel et al. 2012). CRH signaling pathway is freely available in NetPath (http://www.netpath.org/pathways?path_id=NetPath_129).
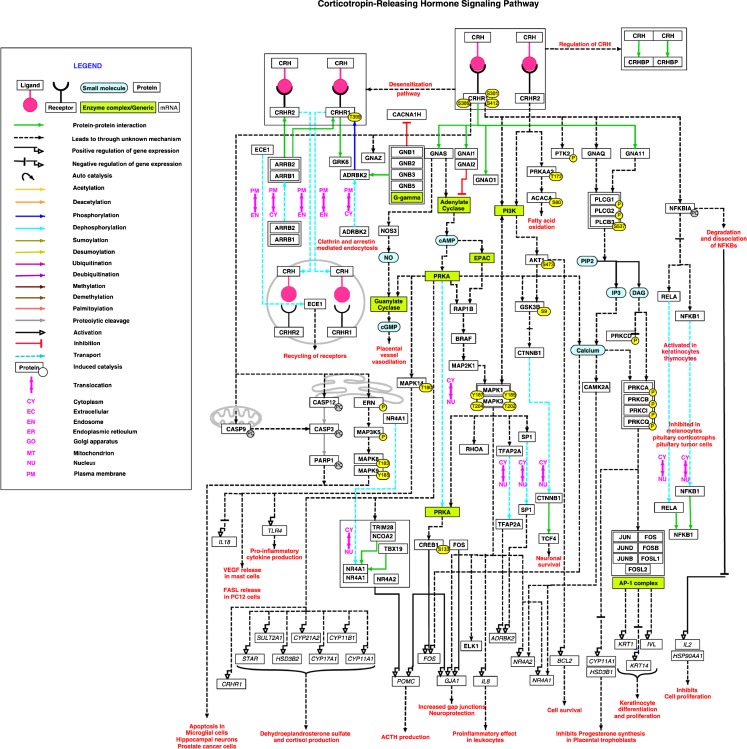
These molecular reactions of CRH signaling pathway, cataloged in NetPath, have been graphically depicted using PathVisio (Fig. 1). On applying the criteria of NetSlim to the CRH signaling pathway data, 64 molecules involved in 84 reactions were obtained. The NetSlim pathway map generated using this data can be accessed from NetSlim at http://www.netpath.org/netslim/CRH_pathway.html. In the pathway map of CRH signaling, each molecule, represented as node, is linked to its corresponding NetPath molecule page. The edge of each reaction is linked to PubMed identifiers. The downstream reactions of the signaling are represented by solid edges for direct reactions and dashed edges for indirect reactions. PPIs, enzyme-catalysis reactions, activation/inhibition reactions and translocation events are depicted in different colors as shown in the legend.
Fig. 1.
Schematic representation of CRH signaling pathway - This map represents the NetPath reactions of CRH signaling pathway. The different types of reactions are distinguished by colors as described in the legend
The CRH pathway page in NetPath provides a short description of the pathway, statistics, molecules involved in the signaling reactions and transcriptionally regulated genes of the pathway. Each molecule in the pathway is linked to its NetPath molecule page, which provides information about that molecule and external links to other databases including HPRD (Prasad et al. 2009; Goel et al. 2012), Entrez gene (Maglott et al. 2011), OMIM (Hamosh et al. 2005), and Swiss-Prot (Boeckmann et al. 2003). The curated reactions of CRH pathway can be downloaded from NetPath at http://www.netpath.org/pathways?path_id=NetPath_129. The CRH pathway data from NetPath and NetSlim can be downloaded in different standard data exchange formats including SBML level 2.1 (Hucka et al. 2003), PSI-MI version 2.5 (Hermjakob et al. 2004), and BioPAX level 3.0 (Demir et al. 2010). In addition, gene regulation information is available in tab-delimited and Microsoft Excel formats. We have also submitted CRH signaling pathway to WikiPathways (http://www.wikipathways.org/index.php/Pathway:WP2355).
The mechanisms involved in stress signaling are yet to be fully understood. CRH signaling pathway, being an important mediator of stress responses, has been studied to a considerable extent. A freely available integrated pathway map of CRH signaling will soon become a part of gene set enrichment analysis, which will allow further molecular discoveries in this signaling pathway. These discoveries will pave way for the identification of candidate biomarkers and therapeutic targets in psychiatric and neurological disorders associated with CRH signaling. We encourage the scientific community to give their valuable suggestions and critical comments to improve the quality and display of information provided in NetPath through http://www.netpath.org/comments
Acknowledgments
We thank the Department of Biotechnology, Government of India for research support to the Institute of Bioinformatics, Bangalore.
Conflict of interest
No potential conflicts of interest were declared.
Abbreviations
- CRH
Corticotropin-releasing hormone
- CRF
Corticotropin-releasing factor
- CRHR
Corticotropin-releasing hormone receptor
- PVN
Paraventricular nucleus
- GPCR
G-protein coupled receptor
- CRHR1
Corticotropin-releasing hormone receptor 1
- CRHR2
Corticotropin-releasing hormone receptor 2
- CRHBP
Corticotropin-releasing hormone binding protein
- POMC
Proopiomelanocortin
- ACTH
Adrenocorticotropic hormone
- PPI
Protein-protein interaction
- PTM
Post-translational modification
- HPRD
Human Protein Reference Database
- SBML
Systems Biology Markup Language
- PSI-MI
Proteomics Standards Initiative for Molecular Interaction
- BioPAX
Biological Pathway Exchange
Contributor Information
Tejaswini Subbannayya, Email: tejaswini@ibioinformatics.org.
Lavanya Balakrishnan, Email: lavanya@ibioinformatics.org.
Granthali Sudarshan, Email: grantha88@gmail.com.
Jayshree Advani, Email: jayshree@ibioinformatics.org.
Santosh Kumar, Email: sk.genesan@gmail.com.
Riaz Mahmood, Email: riaz_sultan@yahoo.com.
Bipin Nair, Email: bipin@amrita.edu.
Ravi Sirdeshmukh, Email: ravisirdeshmukh@gmail.com.
Kanchan K. Mukherjee, Email: kk_mukherjee@hotmail.com
Sudhir N. Umathe, Email: umathesn@hotmail.com
Rajesh Raju, Email: rajesh@ibioinformatics.org.
T. S. Keshava Prasad, Phone: +91-80-28416140, Email: keshav@ibioinformatics.org.
References
- Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H, Zwain IH, Yen SS. Expression of genes encoding corticotropin-releasing factor (CRF), type 1 CRF receptor, and CRF-binding protein and localization of the gene products in the human ovary. J Clin Endocrinol Metab. 1997;82:2720–2725. doi: 10.1210/jc.82.8.2720. [DOI] [PubMed] [Google Scholar]
- Athanassakis I, Farmakiotis V, Aifantis I, Gravanis A, Vassiliadis S. Expression of corticotrophin-releasing hormone in the mouse uterus: participation in embryo implantation. J Endocrinol. 1999;163:221–227. doi: 10.1677/joe.0.1630221. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee M, Raju R, Radhakrishnan A, Nanjappa V, Muthusamy B, Singh K, Kuppusamy D, Lingala BT, Pan A, Mathur PP, Harsha HC, Prasad TSK, Atkins GJ, Pandey A, Chatterjee A. A bioinformatics resource for TWEAK-Fn14 signaling pathway. J Signal Transduct. 2012;2012:376470. doi: 10.1155/2012/376470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O’Donovan C, Phan I, Pilbout S, Schneider M. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003;31:365–370. doi: 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connan F, Lightman SL, Landau S, Wheeler M, Treasure J, Campbell IC. An investigation of hypothalamic-pituitary-adrenal axis hyperactivity in anorexia nervosa: the role of CRH and AVP. J Psychiatr Res. 2007;41:131–143. doi: 10.1016/j.jpsychires.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/S0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, Fukuda K, Lemer C, Vastrik I, Wu G, D’Eustachio P, Schaefer C, Luciano J, Schacherer F, Martinez-Flores I, Hu Z, Jimenez-Jacinto V, Joshi-Tope G, Kandasamy K, Lopez-Fuentes AC, Mi H, Pichler E, Rodchenkov I, Splendiani A, Tkachev S, Zucker J, Gopinath G, Rajasimha H, Ramakrishnan R, Shah I, Syed M, Anwar N, Babur O, Blinov M, Brauner E, Corwin D, Donaldson S, Gibbons F, Goldberg R, Hornbeck P, Luna A, Murray-Rust P, Neumann E, Reubenacker O, Samwald M, van Iersel M, Wimalaratne S, Allen K, Braun B, Whirl-Carrillo M, Cheung KH, Dahlquist K, Finney A, Gillespie M, Glass E, Gong L, Haw R, Honig M, Hubaut O, Kane D, Krupa S, Kutmon M, Leonard J, Marks D, Merberg D, Petri V, Pico A, Ravenscroft D, Ren L, Shah N, Sunshine M, Tang R, Whaley R, Letovksy S, Buetow KH, Rzhetsky A, Schachter V, Sobral BS, Dogrusoz U, McWeeney S, Aladjem M, Birney E, Collado-Vides J, Goto S, Hucka M, Le Novere N, Maltsev N, Pandey A, Thomas P, Wingender E, Karp PD, Sander C, Bader GD. The BioPAX community standard for pathway data sharing. Nat Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M, Casey PJ, Gilman AG. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989;3:2125–2131. [PubMed] [Google Scholar]
- Friedman EM, Irwin M. Central CRH suppresses specific antibody responses: effects of beta-adrenoceptor antagonism and adrenalectomy. Brain Behav Immun. 2001;15:65–77. doi: 10.1006/brbi.2000.0582. [DOI] [PubMed] [Google Scholar]
- Goel R, Harsha HC, Pandey A, Prasad TSK. Human Protein Reference Database and Human Proteinpedia as resources for phosphoproteome analysis. Mol Biosyst. 2012;8:453–463. doi: 10.1039/c1mb05340j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Dai Y, Randeva HS, Levine MA, Karteris E, Easton AJ, Hillhouse EW. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol. 1999;13:2189–2202. doi: 10.1210/me.13.12.2189. [DOI] [PubMed] [Google Scholar]
- Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33:D514–D517. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Sjogren M, Blennow K, Ekman R, Wallin A. Cerebrospinal fluid neuropeptides in Alzheimer’s disease and vascular dementia. Biol Psychiatry. 1995;38:210–216. doi: 10.1016/0006-3223(94)00239-Y. [DOI] [PubMed] [Google Scholar]
- Hermjakob H, Montecchi-Palazzi L, Bader G, Wojcik J, Salwinski L, Ceol A, Moore S, Orchard S, Sarkans U, von Mering C, Roechert B, Poux S, Jung E, Mersch H, Kersey P, Lappe M, Li Y, Zeng R, Rana D, Nikolski M, Husi H, Brun C, Shanker K, Grant SG, Sander C, Bork P, Zhu W, Pandey A, Brazma A, Jacq B, Vidal M, Sherman D, Legrain P, Cesareni G, Xenarios I, Eisenberg D, Steipe B, Hogue C, Apweiler R. The HUPO PSI’s molecular interaction format–a community standard for the representation of protein interaction data. Nat Biotechnol. 2004;22:177–183. doi: 10.1038/nbt926. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, Cornish-Bowden A, Cuellar AA, Dronov S, Gilles ED, Ginkel M, Gor V, Goryanin II, Hedley WJ, Hodgman TC, Hofmeyr JH, Hunter PJ, Juty NS, Kasberger JL, Kremling A, Kummer U, Le Novere N, Loew LM, Lucio D, Mendes P, Minch E, Mjolsness ED, Nakayama Y, Nelson MR, Nielsen PF, Sakurada T, Schaff JC, Shapiro BE, Shimizu TS, Spence HD, Stelling J, Takahashi K, Tomita M, Wagner J, Wang J. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19:524–531. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Keerthikumar S, Raju R, Prasad TSK, Ramachandra YL, Mohan S, Pandey A. PathBuilder–open source software for annotating and developing pathway resources. Bioinformatics. 2009;25:2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, Tattikota SG, Mohan S, Padhukasahasram H, Subbannayya Y, Goel R, Jacob HK, Zhong J, Sekhar R, Nanjappa V, Balakrishnan L, Subbaiah R, Ramachandra YL, Rahiman BA, Prasad TSK, Lin JX, Houtman JC, Desiderio S, Renauld JC, Constantinescu SN, Ohara O, Hirano T, Kubo M, Singh S, Khatri P, Draghici S, Bader GD, Sander C, Leonard WJ, Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol. 1998;12:1077–1085. doi: 10.1210/me.12.8.1077. [DOI] [PubMed] [Google Scholar]
- Maglott D, Ostell J, Pruitt KD, Tatusova T. Entrez Gene: gene-centered information at NCBI. Nucleic Acids Res. 2011;39:D52–D57. doi: 10.1093/nar/gkq1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Lopez de Maturana R, Brenneman R, Walent T, Mattson MP, Maudsley S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromol Med. 2005;7:3–36. doi: 10.1385/NMM:7:1-2:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Nanjappa V, Raju R, Muthusamy B, Sharma J, Thomas JK, Nidhina PAH, Harsha HC, Pandey A, Anilkumar G, Prasad TSK. A comprehensive curated reaction map of leptin signaling pathway. J Proteomics Bioinform. 2011;4:184–189. [Google Scholar]
- Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem. 2004;271:2821–2830. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- Prasad TSK, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey A. Human Protein Reference Database–2009 update. Nucleic Acids Res. 2009;37:D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A, Raju R, Tuladhar N, Subbannayya T, Thomas JK, Goel R, Telikicherla D, Palapetta SM, Rahiman BA, Venkatesh DD, Urmila KK, Harsha HC, Mathur PP, Prasad TSK, Pandey A, Shemanko C, Chatterjee A. A pathway map of prolactin signaling. J Cell Commun Signal. 2012;6:169–173. doi: 10.1007/s12079-012-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R, Balakrishnan L, Nanjappa V, Bhattacharjee M, Getnet D, Muthusamy B, Kurian Thomas J, Sharma J, Rahiman BA, Harsha HC, Shankar S, Prasad TSK, Mohan SS, Bader GD, Wani MR, Pandey A (2011a) A comprehensive manually curated reaction map of RANKL/RANK-signaling pathway. Database (Oxford) 2011:bar021 [DOI] [PMC free article] [PubMed]
- Raju R, Nanjappa V, Balakrishnan L, Radhakrishnan A, Thomas JK, Sharma J, Tian M, Palapetta SM, Subbannayya T, Sekhar NR, Muthusamy B, Goel R, Subbannayya Y, Telikicherla D, Bhattacharjee M, Pinto SM, Syed N, Srikanth MS, Sathe GJ, Ahmad S, Chavan SN, Kumar GS, Marimuthu A, Prasad TSK, Harsha HC, Rahiman BA, Ohara O, Bader GD, Sujatha Mohan S, Schiemann WP, Pandey A (2011b) NetSlim: high-confidence curated signaling maps. Database (Oxford) 2011:bar032 [DOI] [PMC free article] [PubMed]
- Ross PC, Kostas CM, Ramabhadran TV. A variant of the human corticotropin-releasing factor (CRF) receptor: cloning, expression and pharmacology. Biochem Biophys Res Commun. 1994;205:1836–1842. doi: 10.1006/bbrc.1994.2884. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Uhde TW, Post RM, Gallucci W, Chrousos GP, Gold PW. The corticotropin-releasing hormone stimulation test in patients with panic disorder. Am J Psychiatry. 1986;143:896–899. doi: 10.1176/ajp.143.7.896. [DOI] [PubMed] [Google Scholar]
- Seasholtz AF, Valverde RA, Denver RJ. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J Endocrinol. 2002;175:89–97. doi: 10.1677/joe.0.1750089. [DOI] [PubMed] [Google Scholar]
- Seres J, Bornstein SR, Seres P, Willenberg HS, Schulte KM, Scherbaum WA, Ehrhart-Bornstein M. Corticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab. 2004;89:965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- Suemaru S, Suemaru K, Kawai K, Miyata S, Nobukuni K, Ihara Y, Namba R, Urakami K, Hashimoto K. Cerebrospinal fluid corticotropin-releasing hormone in neurodegenerative diseases: reduction in spinocerebellar degeneration. Life Sci. 1995;57:2231–2235. doi: 10.1016/0024-3205(95)02215-5. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C. Presenting and exploring biological pathways with PathVisio. BMC Bioinforma. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widerlov E, Bissette G, Nemeroff CB. Monoamine metabolites, corticotropin releasing factor and somatostatin as CSF markers in depressed patients. J Affect Disord. 1988;14:99–107. doi: 10.1016/0165-0327(88)90051-1. [DOI] [PubMed] [Google Scholar]
- Wille S, Sydow S, Palchaudhuri MR, Spiess J, Dautzenberg FM. Identification of amino acids in the N-terminal domain of corticotropin-releasing factor receptor 1 that are important determinants of high-affinity ligand binding. J Neurochem. 1999;72:388–395. doi: 10.1046/j.1471-4159.1999.0720388.x. [DOI] [PubMed] [Google Scholar]
- Yang LZ, Tovote P, Rayner M, Kockskamper J, Pieske B, Spiess J. Corticotropin-releasing factor receptors and urocortins, links between the brain and the heart. Eur J Pharmacol. 2010;632:1–6. doi: 10.1016/j.ejphar.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Zoumakis E, Margioris AN, Stournaras C, Dermitzaki E, Angelakis E, Makrigiannakis A, Koumantakis E, Gravanis A. Corticotrophin-releasing hormone (CRH) interacts with inflammatory prostaglandins and interleukins and affects the decidualization of human endometrial stroma. Mol Hum Reprod. 2000;6:344–351. doi: 10.1093/molehr/6.4.344. [DOI] [PubMed] [Google Scholar]