Abstract
Purpose
Ovarian cancer is the sixth common cancer in women in developed countries. In severe cases, the optimal debulking is necessary. In order to increase optimal debulking and reduce preoperative complications, neoadjuvant chemotherapy followed by debulking surgery, and then chemotherapy again is introduced as substitute for primary surgery. In this study, we aim to evaluate perioperative outcome after neoadjuvant chemotherapy with carboplatin/paclyaxol in comparison with primary cytoreduction in patients with advanced ovarian cancer.
Methods
In this prospective study, 60 patients with advanced ovarian cancer due to the disease severity were assigned into neoadjuvant chemotherapy (n = 30) or control (n = 30) groups. In neoadjuvant chemotherapy group, patients received three cycles of carboplatin (5–6 area under the curve) and paclitaxel (175 mg/m2) preoperatively followed by interval surgery. The control group received primary surgery plus adjuvant chemotherapy. Preoperative outcome was compared between groups.
Results
Neoadjuvant group had significantly lower mean levels of CA 125 (p = 0.01) and less severe bleeding (p = 0.03) than control group. There was no significant difference between surgery time, preoperative complications, residual mass less than 1 cm, and hospital stay between groups. There was no mortality during the study.
Conclusion
Neoadjuvant chemotherapy caused less severe bleeding, but has no effect in decreasing complications after surgery; however, neoadjuvant chemotherapy followed by interval debulking surgery was not superior to primary debulking surgery followed by chemotherapy as a treatment option for patients with advanced ovarian carcinoma in this study.
Keywords: Ovarian cancer, Advanced stage, Neoadjuvant chemotherapy, Primary debulking surgery
Introduction
Ovarian cancer is the leading cause of death among all gynecologic cancers [1]. Most these patients are diagnosed at advanced stage, because of nonspecific signs and symptoms, which results in poor prognosis [2, 3]. The standard therapy of advanced ovarian cancer is primary debulking surgery followed by platinum and taxane-based chemotherapy [4–7]. However, most patients with advanced disease will relapse and eventually die of progressive disease. The goal is optimal cytoreduction to resect as much tumor present within the pelvic and abdominal cavities as possible to increase the survival [4–7].
It was assumed that optimal cytoreductive surgery could be performed in only about 30–60 % of patients with ovarian cancer stages III or IV. Patients with advanced ovarian cancer are usually low candidates for primary surgery due to massive ascites, pleural effusion, and large abdominal tumor. In these patients, sometimes the tumor is unresectable [8–10].
As an alternative to primary debulking surgery followed by chemotherapy and to increase the rate of complete or optimal debulking, some authors have investigated the use of neoadjuvant chemotherapy (NAC) before cytoreductive surgery [11, 12]. Results of these studies showed that NAC may increase the number of patients suitable for interval cytoreductive surgery by reducing tumor burden and reducing preoperative morbidity [12–19]. Thus, because of recent advances in chemotherapy, NAC followed by interval debulking surgery and further chemotherapy has become an alternative treatment for these patients.
In this study, we aim to evaluate surgery and preoperative findings in patients with advanced ovarian cancer undergoing platinum-based neoadjuvant chemotherapy and primary surgery debulking.
Methods
In this prospective cohort study, all patients with advanced ovarian cancer visiting Alzahra University-affiliated Teaching Hospital or referring for treatment to Shahid Tabatabaie Education and Research Hematology and Oncology Center for treatment during 2011 and 2012 were evaluated. Patients with stage III–IV of disease (according to the International Federation of Gynecology and Obstetrics (FIGO) staging) with ascites volume of 500 mL or more diagnosed in sonography or abdominal computed tomography (CT) and positive paracentesis or fine needle aspiration (FNA) report were included. Patients with autoimmune disease and sensitivity to chemotherapy drugs (carboplatine and paxlitaxol) were excluded. All patients provided informed consent, and the protocol was approved by the ethics committee at each participating center. Neoadjuvant chemotherapy was defined as platinum-based chemotherapy administered prior to interval debulking surgery. All chemotherapy regimens were given by intravenous route. In NAC group, patients were treated with three cycles of chemotherapy combination including carboplatin [5–6 of area under the curve (AUC)] plus paclitaxel (175 mg/m2, 3 h infusion). Courses were repeated every 3 weeks. The patients underwent interval debulking surgery and three cycles of consolidation chemotherapy were administered afterward.
Patients with stage III and IV ovarian cancer who received primary surgery represented the conventional treatment group. Conventionally treated patients received a planned minimum of six cycles of platinum-based CT following their initial surgery. Both groups were matched for age and demographic findings.
Our standard surgical treatment for advanced ovarian cancer at the time of primary debulking surgery consists of total abdominal hysterectomy, bilateral salpingo-oophorectomy, infracolic or total omentectomy, and debulking of peritoneal tumor masses with maximum efforts. Patients with no or minimal residual disease (<2 cm in diameter) also underwent systematic retroperitoneal lymphadenectomy, except for patients with severe medical complications, low performance status or long operation time. Retroperitoneal lymphadenectomy included both the pelvic and Para-aortic lymph nodes.
The clinical information such as age, initial serum CA-125 levels, intraoperative blood loss, and the length of hospitalization and treatment complications were recorded and compared between groups.
Data Analysis
Continuous data with normal distribution are given as mean ± standard deviation, otherwise as median. Values were also given as the N and (%). Student t test for testing the significance of mean for independent continuous scale data and Mann–Whitney U test for nonparametric data where appropriate, Chi square or Fisher exact test for testing the significance of percentages were used. A p value of 0.05 or less was considered significant.
Results
Patients’ baseline findings in control and NAC groups are shown in Table 1. There was no difference between groups according to baseline findings. The only significant finding before surgery was higher CA 125 levels in control patients.
Table 1.
Patients’ baseline findings in control and neoadjuvant groups
| Control | Neoadjuvant | p value | |
|---|---|---|---|
| Age (years) | 54.40 ± 15.05 | 5.60 ± 12.77 | 0.91 |
| Marital status | |||
| Married | 28 (93.3 %) | 30 (100 %) | 0.49 |
| Single | 2 (6.7 %) | 0 | |
| Age at marriage (years) | 19.93 ± 5.99 | 20.27 ± 4.92 | 0.47 |
| Occupation | |||
| Urban | 20 (66.7 %) | 21 (70 %) | 0.78 |
| Rural | 10 (33.3 %) | 9 (305) | |
| Previous cancer history | 1 (3.3 %) | 3 (10 %) | 0.61 |
| Familial history of cancer | 4 (13.3 %) | 9 (30 %) | 0.11 |
| Gravida | 4.39 ± 3.08 | 4.20 ± 2.67 | 0.77 |
| Para | 3.75 ± 2.88 | 3.73 ± 2.47 | 0.84 |
| Infertility | 3 (10 %) | 1 (3.3 %) | 0.61 |
| Smoking | 0 | 1 (3.3 %) | 0.9 |
| Disease period | 4.26 ± 1.02 | 5.40 ± 3.65 | 0.1 |
| Hemoglobin (mg/dL) | 12.66 ± 1.41 | 12.38 ± 1.21 | 0.76 |
| CA 125 (U/mL) | 355.65 ± 48.90 | 255.70 ± 55.84 | 0.01* |
* p is two-sided significant
Ovarian cancer type is shown in Table 2. Most cases in both cases were serous and mucinous carcinoma.
Table 2.
Ovarian cancer type
| NAC group n (%) | Control group n (%) | |
|---|---|---|
| Serous carcinoma | 23 (67.7 %) | 22 (73.3 %) |
| Mucinous carcinoma | 4 (13.3 %) | 4 (13.3 %) |
| Endometrioid carcinoma | 2 (6.7 %) | 3 (105) |
| Peritoneal carcinoma | 1 (3.3 %) | 0 |
| Clear cell carcinoma | 0 | 1 (3.3 %) |
n (%) = number (percent)
Intraoperative Findings
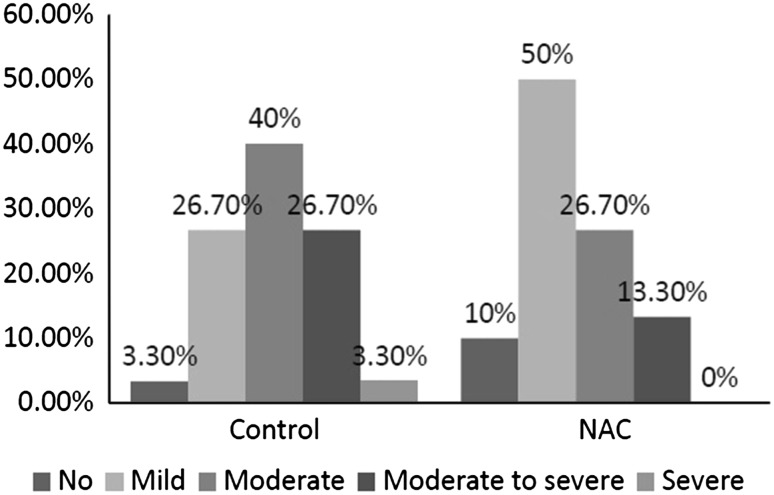
Neoadjuvant chemotherapy group had considerably less severe intraoperative bleeding than controls (Fig. 1). NAC group had significantly less bleeding (no or mild bleeding: 60 % versus 30 %; p = 0.03).
Fig. 1.
Intraoperative bleeding between NAC and controls
Complications due to anesthesia were observed only in one patient (3.3 %) of control group (p = 0.9).
Mean duration of surgery was 3.62 ± 0.07 h in control and 3.68 ± 0.54 h in NAC group. Despite the slightly higher operation time in NAC group, the difference between groups was not significant (p > 0.05).
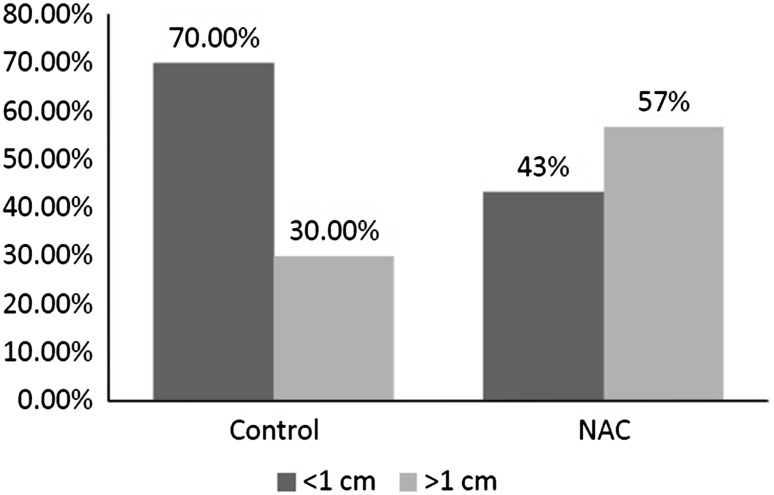
At the end of the surgery, the residual mass <1 cm was more in control groups, but the difference between groups was not significant (Fig. 2, p = 0.06).
Fig. 2.
The size of residual mass between groups
Postoperative Findings
Mean hemoglobin levels after surgery was not significant between control and NAC groups (10.79 ± 1.21 versus 10.78 ± 1.12 mg/dL; p = 0.97).
Postoperative complications are shown in Table 3. Although higher rates of vomiting and ileus were observed in control group, there was no significant difference between groups in the complications. There were also no cases of diarrhea, bowel obstruction, fistulae, or wall rupture, or even death in any patients.
Table 3.
Postoperative complications between groups
| Control n (%) | Neoadjuvant n (%) | p value | |
|---|---|---|---|
| Nausea | 12 (40 %) | 12 (40 %) | |
| Vomiting | 8 (26.7 %) | 4 (13.3 %) | 0.33 |
| Ileus | 7 (23.3 %) | 4 (13.3 %) | 0.31 |
| Wound infection | 1 (3.3 %) | 1 (3.3 %) |
n (%) = number (percent), p value <0.05 considered to be significant
Duration of hospitalization was similar between control and NAC groups (5.73 ± 1.10 versus 5.56 ± 0.97 days; p = 0.34).
Discussion
Ovarian cancer is the sixth common cancer among women in developed countries [7]. In most cases, the cancer is not diagnosed until it has reached an advanced stage. Patients with advanced stages has worse prognosis with 5-year survival rate of 19–33 % due to the possibility of tumor removal and response to chemotherapy [10].
In advanced cancer patients, the aim of treatment is to increase optimal debulking and reduce preoperative complications. NAC followed by interval debulking is a new method which could reduce tumor size and obtain better cytoreduction especially in chemotherapy-sensitive patients [11, 12]. In this prospective study, we evaluated outcome of surgery in patients receiving NAC and interval debulking in comparison to primary cytoreduction surgery.
Patients assigned to NAC in previous studies were usually older than controls. Loizzi and colleagues [17] observed that patients in neoadjuvant group were significantly older and had poor functional state. However, patients in both groups did not differ in age. Similar to our findings, in the study of Vrscaj and Rakar [20], the age was not different between groups. The response to the treatment and treatment outcome could be influenced by the age, the bias of which we tried to delete by age-matching the groups.
It is reported that CA 125 is related to patients’ survival with advanced ovarian cancer [21]. The possible interaction between CA-125 levels and survival benefit of NAC is also reported [22]: patients with persistently elevated CA125 after completing primary treatment had significantly inferior survivals compared with those who normalized CA125 [23]. In our study, we observed significantly lower CA 125 levels before surgery in NAC. In contrast, Burn and colleagues [24] observed higher Ca 125 levels in NAC in comparison with primary surgery patients. The reduced levels of CA 125 in NAC group in our study were associated with the lower intraopertaive bleeding.
As mentioned, patients receiving NAC had significantly less bleeding in comparison with primary surgery group. Similar to our findings, Giannopoulos and colleagues [16] found lower mean blood loss in NAC group. Unlike our findings, Kuhn et al. [25] and Yan and colleagues [26] found no difference in need for transfusion and intraoperative bleeding.
We also observed that NAC group had higher cases with residual tumor < cm. In contrary, Vergote and colleagues [27] observed that tumor size was further reduced to less than 1 cm in patients receiving NAC. Our findings question the ability of NAC in optimal and proper cytoreduction in patients with advanced ovarian cancer.
In previous studies, lower postoperative complications and morbidity is reported for NAC [12]. Overall, postoperative complications were low in both treatment groups and the difference between groups was not significant, although vomiting and ileus was lower in NAC group. In the other study from Iran, Ghaemmaghami and colleagues [28] found no significant difference in complications between groups. The same findings were reported by Kuhn and colleagues [25]. However, Vergote and colleagues [27] found significantly lower postoperative complications in NAC group.
In this study, the duration of the surgery and hospitalization was not significant between groups. Similarly, Kuhn et al. [25] and Yan and colleagues [26] found no significant difference in duration of the operation between NAC and primary surgery groups. However, Giannopoulos and colleagues [16] observed that patients receiving NAC were more likely to be admitted to intensive care units and were discharged earlier.
Kuhn et al. [25] and Inciura et al. [29] found no difference between groups in mortality during surgery and hospital stay. However, Vergote et al. [27] found lower mortality rate in NAC group. In our study, there was no mortality in any patients among groups.
This study had some limitations; although this is a prospective study, our findings need to be confirmed by further randomized clinical trials. These results are also limited to the small sample size of the study. We also did not follow patients later, and the data considering the short-term and long-term outcomes with survival rates are not available, which imposes another limitation to the current study.
Conclusion
In conclusion, in our study neoadjuvant chemotherapy caused less severe bleeding, but has no effect in decreasing complications after surgery; however, neoadjuvant chemotherapy followed by interval debulking surgery was not superior to primary debulking surgery followed by chemotherapy as a treatment option for patients with advanced ovarian carcinoma in this study.
Acknowledgments
This research was financially supported by Vice Chancellor for Research, Tabriz University of Medical Sciences, Iran. The authors are indebted to the Women Reproductive Health Center, Tabriz University of Medical Sciences, Tabriz, Iran for extending its support.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Yeole BB, Kumar AV, Kurkure A, et al. Population based survival from cancers of breast, cervix and ovary in women in Mumbai, India. Asian Pac J Cancer Prev. 2004;5:308–315. [PubMed] [Google Scholar]
- 4.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 5.Du Bois A, Quinn M, Thigpen T, et al. Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann Oncol 2005. 2004;16(Suppl 8):viii7–viii12. doi: 10.1093/annonc/mdi961. [DOI] [PubMed] [Google Scholar]
- 6.Aletti GD, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 7.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Covens AL. A critique of surgical cytoreduction in advanced ovarian cancer. Gynecol Oncol. 2000;78(3 Pt 1):269–274. doi: 10.1006/gyno.2000.5926. [DOI] [PubMed] [Google Scholar]
- 9.Dauplat J, Le Bouedec G, Pomel C, et al. Cytoreductive surgery for advanced stages of ovarian cancer. Semin Surg Oncol. 2000;19:42–48. doi: 10.1002/1098-2388(200007/08)19:1<42::AID-SSU7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 10.Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 6th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 10):S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 11.Baekelandt M. The potential role of neoadjuvant chemotherapy in advanced ovarian cancer. Int J Gynecol Cancer. 2003;13:163–168. doi: 10.1111/j.1525-1438.2003.13354.x. [DOI] [PubMed] [Google Scholar]
- 12.Huober J, Meyer A, Wagner U, et al. The role of neoadjuvant chemotherapy and interval laparotomy in advanced ovarian cancer. J Cancer Res Clin Oncol. 2002;128:153–160. doi: 10.1007/s00432-001-0312-3. [DOI] [PubMed] [Google Scholar]
- 13.Mazzeo F, Berlière M, Kerger J, et al. Neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy in patients with primarily unresectable, advanced-stage ovarian cancer. Gynecol Oncol. 2003;90:163–169. doi: 10.1016/S0090-8258(03)00249-X. [DOI] [PubMed] [Google Scholar]
- 14.Onda T, Kamura T, Ishizuka N, et al. Feasibility study of neoadjuvant chemotherapy followed by interval cytoreductive surgery for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0206. Jpn J Clin Oncol. 2004;34:43–45. doi: 10.1093/jjco/hyh007. [DOI] [PubMed] [Google Scholar]
- 15.Loizzi V, Cormio G, Resta L, et al. Neoadjuvant chemotherapy in advanced ovarian cancer: a case–control study. Int J Gynecol Cancer. 2005;15:217–223. doi: 10.1111/j.1525-1438.2005.15206.x. [DOI] [PubMed] [Google Scholar]
- 16.Giannopoulos T, Butler-Manuel S, Taylor A, et al. Clinical outcomes of neoadjuvant chemotherapy and primary debulking surgery in advanced ovarian carcinoma. Eur J Gynaecol Oncol. 2006;27:25–28. [PubMed] [Google Scholar]
- 17.Hou JY, Kelly MG, Yu H, et al. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol Oncol. 2007;105:211–217. doi: 10.1016/j.ygyno.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Onda T, Matsumoto K, Shibata T, et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol. 2008;38:74–77. doi: 10.1093/jjco/hym145. [DOI] [PubMed] [Google Scholar]
- 19.Onda T, Kobayashi H, Nakanishi T, et al. Feasibility study of neoadjuvant chemotherapy followed by interval debulking surgery for stage III/IV ovarian, tubal, and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0206. Gynecol Oncol. 2009;113:57–62. doi: 10.1016/j.ygyno.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Vrscaj MU, Rakar S. Neoadjuvant chemotherapy for advanced epithelial ovarian carcinoma: a retrospective case-control study. Eur J Gynaecol Oncol. 2002;23(5):405–410. [PubMed] [Google Scholar]
- 21.Scambia G, Benedetti Panici, Foti E, et al. Multiple tumour marker assays in advanced cervical cancer: relationship to chemotherapy response and clinical outcome. Eur J Cancer. 1996;32A(2):259–263. doi: 10.1016/0959-8049(95)00515-3. [DOI] [PubMed] [Google Scholar]
- 22.Kang S, Kim TJ, Seo SS, et al. Interaction between preoperative CA-125 level and survival benefit of neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol Oncol. 2011;120(1):18–22. doi: 10.1016/j.ygyno.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Le T, Faught W, Hopkins L, et al. Importance of CA125 normalization during neoadjuvant chemotherapy followed by planned delayed surgical debulking in patients with epithelial ovarian cancer. J Obstet Gynaecol Can. 2008;30(8):665–670. doi: 10.1016/S1701-2163(16)32914-0. [DOI] [PubMed] [Google Scholar]
- 24.Brun JL, Rouzier R, Selle F, et al. Neoadjuvant chemotherapy or primary surgery for stage III/IV ovarian cancer: contribution of diagnostic laparoscopy. BMC Cancer. 2009;9:171. doi: 10.1186/1471-2407-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn W, Rutke S, Spathe K, et al. Neoadjuvant chemotherapy followed by tumor debulking prolongs survival for patients with poor prognosis in International Federation of Gynecology and Obstetrics Stage IIIC ovarian carcinoma. Cancer. 2001;92:2585–2591. doi: 10.1002/1097-0142(20011115)92:10<2585::AID-CNCR1611>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Yan X, An N, Jiang GQ, et al. [Impact of neoadjuvant chemotherapy on the survival of patients with stage IIIc and IV epithelial ovarian cancer]. [Article in Chinese] Zhonghua Zhong Liu Za Zhi. 2008;30(4):298–301. [PubMed] [Google Scholar]
- 27.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 28.Ghaemmaghami F, Karimi-Zarchi M, Modares-Gilani M, et al. Clinical outcome of Iranian patients with advanced ovarian cancer with neoadjuvant chemotherapy versus primary debulking surgery. Asian Pac J Cancer Prev. 2008;9(4):719–724. [PubMed] [Google Scholar]
- 29.Inciura A, Simavicius A, Juozaityte E, et al. Comparison of adjuvant and neoadjuvant chemotherapy in the management of advanced ovarian cancer: a retrospective study of 574 patients. BMC Cancer. 2006;6:153. doi: 10.1186/1471-2407-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]