Abstract
Background
A combination of hepatitis B immunoglobulin and nucleos(t)ide analogues is the current standard of care for controlling hepatitis B recurrence after orthotopic liver transplantation (OLT). However, frequent immunoglobulin treatment is expensive and inconvenient. This study investigated the efficacy of hepatitis B virus (HBV) vaccination in preventing the recurrence of hepatitis B after living donor OLT.
Methods
Twenty-seven patients who had undergone living donor OLT participated in the study; five had acute HBV infected liver failure (ALF-OLT) and 22 had HBV related liver cirrhosis (LC-OLT). Hepatitis B surface antigen (HBsAg)-containing vaccine was administered to them for at least 1 year after transplantation and continued once monthly for up to 36 months post-OLT. Patients who had anti-HBs antibody titers above 100 mIU/mL for a minimum of 6 months without immunoglobulin administration were defined as good responders; the others were defined as poor responders. Interferon-γ enzyme-linked immunospot assays against HBs and HBc antigens were used to assay cellular immune responses.
Results
All five of the ALF-OLT patients had good responses after a median of four (range 2.5–5) vaccinations. Nine of the 22 LC-OLT patients had good responses after a median of 19 (range 11.5–30) vaccinations. Among the LC-OLT group, those with livers donated by relatively higher-aged, marital and high-titer anti-HBs antibody donors were good responders. LC-OLT patients classed as good responders showed interferon-γ responses comparable to those of the ALF-OLT patients.
Conclusions
The ALF-OLT and LC-OLT patients who received livers from relatively higher-aged, marital, high-titer anti-HBs antibody donors were the best candidates for HBV vaccine administration. Boosting donors before transplantation may facilitate later vaccine response of the recipients.
Keywords: Vaccination, Living donor liver transplantation, Hepatitis B immunoglobulin, Marital donor, Immune response
Introduction
Prior to the introduction of effective post-transplantation antiviral prophylaxis, liver transplantation for hepatitis B virus (HBV)-related disease was usually followed by immediate HBV reinfection of the allograft, resulting in a fatal hepatitis B recurrence [1–3]. Recent studies have found that treatment with a combination of hepatitis B immunoglobulin (HBIg) and nucleos(t)ide analogues decreases the risk of hepatitis B recurrence, and achieves a higher rate of graft survival [4–8]. However, long-term administration of HBIg is associated with several unresolved issues, including limited availability and extremely high cost, so several protocols for treatment with low-dose HBIg in combination with nucleos(t)ide analogue have been reported [9–12]. Previously, we reported that treatment with high-dose HBIg in the early period post-transplantation followed by low-dose HBIg with nucleos(t)ide analogues offers reliable, cost-effective control of hepatitis B recurrence [13]. However, even with such a simplified protocol, patients would still need to receive a drip infusion or intramuscular injection of hundreds to thousands of units of HBIg every 2–3 months.
Active immunization of post-orthotopic liver transplantation (OLT) recipients with HBV vaccine is a recently emerging approach. However, most studies report low response rates, even with double concentration of vaccines or prolonged vaccination regimens [14, 15]. Patients who had not been HBV carriers [e.g., acute liver failure (ALF) patients following sexual transmission of HBV as an adult; or non-chronic HBV carrier patients who received hepatitis B core antibody (HBcAb)-positive livers] are accepted as good candidates for vaccine administration [15, 16]. Vaccination in patients who have been HBV carriers or liver cirrhosis (LC) patients typically yields disappointing results [14, 15]. Understanding how different cohorts respond to HBV vaccination is critical to the design of safe, cost-saving, and custom-designed prophylaxis protocols.
It remains unclear to what extent cellular immune responses may contribute to protection from HBV reinfection. Since non-carrier patients respond well to the HBV vaccination, immune tolerance is expected to play a large role in this process. Yet only a few reports have mentioned T cell immune reaction after HBV-related OLT [14].
In this report, we assessed a monthly, long-term vaccination protocol starting 1 year after OLT, to investigate those characteristics that could discriminate between the vaccine-responsive and non-responsive patients. In addition to anti-hepatitis B surface (anti-HBs) antibody titer due to a humoral immune response, CD4 T cell immune responses to hepatitis B surface antigen (HBsAg) were used to assess the cellular immune response to vaccination in immunocompetent patients.
Methods
Patients
From October 1996 to June 2011, OLT was performed in 264 adults at Okayama University Hospital. Of these, ten patients had ALF due to acute HBV infection. Thirty-seven patients had end-stage LC due to chronic life-long HBV infection. Five-year survival rates were 88 and 87 % for HBV-related ALF patients and for HBV-related LC patients, respectively.
The HBV vaccine was administered to five ALF patients (ALF-OLT) and 22 LC patients (LC-OLT). The general characteristics of the patients included in this study are summarized in Table 1. All of them received living donor liver transplantation (LDLT). The numerical data are expressed as median and interquartile range values, and categorical data are presented as positive counts or percentages in all tables.
Table 1.
Patient characteristics
| N | ALF | LC |
|---|---|---|
| 5 | 22 | |
| Recipient related factors | ||
| Age at OLT | 29 (27–46) | 53 (47–56) |
| Age at start of vaccine | 36 (30–51) | 56 (49–59) |
| Sex (M) | 1 (20 %) | 19 (86 %) |
| HBsAg at OLT | 0.7 (0–1) | 2000 (100–2000) |
| HBV DNA at OLT (≥3.7) | 0 (0 %) | 8 (36 %) |
| MELD at OLT | 21 [19–21] | 15 [9–18] |
| HCC at OLT (+) | 0 (0 %) | 15 (68 %) |
| Donor related factors | ||
| Age at OLT | 32 (27–44) | 46 (31–49) |
| Sex (M) | 4 (80 %) | 9 (40 %) |
| ABO (identical) | 4 (80 %) | 12 (54 %) |
| Blood relation (no) | 0 (0 %) | 8 (36 %) |
| Anti-HBs antibody (>100) | 1 (20 %) | 9 (40 %) |
| Anti-HBc antibody (+) | 1 (20 %) | 11 (50 %) |
| Anti-HBc(+)/anti-HBs(+) | 1 (20 %) | 10 (45 %) |
| Anti-HBc(+)/anti-HBs(−) | 0 (0 %) | 1 (4 %) |
| Anti-HBc(−)/anti-HBs(+) | 0 (0 %) | 0 (0 %) |
ALF acute liver failure, LC liver cirrhosis, OLT orthotopic liver transplantation, MELD Model for End-stage Liver Disease, HCC hepatocellular carcinoma
For analysis of the HBV-specific cellular immune response (Table 2), the study enrolled all five ALF-OLT patients, along with 15 of the 22 LC-OLT patients. Additionally, 11 healthy volunteers who had received the HBV vaccine and developed a successful anti-HBs antibody response (termed ‘Healthy vaccine’), ten patients with chronic hepatitis B (termed ‘Chronic hepatitis’), and five patients who recovered from acute hepatitis B (termed ‘Self-limited’) were enrolled as controls. The five patients who recovered from acute hepatitis B had a history of acute hepatitis B diagnosed with high-titer IgM-HBc antibody response, and presented as HBsAg negative, anti-HBs antibody positive, anti-HBc antibody positive at the time of the study. The chronic hepatitis B patients were followed for several years at our hospital and all were HBsAg positive with a median HBV-DNA titer of 2.5 (interquartile range 2.1–4.2) logcopies/mL. The healthy volunteers had no HBsAg and anti-HBc antibodies, and the median anti-HBs antibody level was 240 (interquartile range 100–797) mIU/mL.
Table 2.
Characteristics of the cases for HBV antigen-specific T cell response
| N | Healthy vaccine | Chronic hepatitis | Self-limited | ALF-OLT | LC-OLT-good | LC-OLT-poor |
|---|---|---|---|---|---|---|
| 11 | 10 | 5 | 4 | 8 | 7 | |
| Age | 29 (28–31) | 53 (42.5–61) | 67 (58.5–77) | 41.5 (37.2–47.2) | 60 (53–62) | 55 (40–58) |
| Sex [M (%)] | 10 (91) | 7 (70) | 2 (40) | 0 (0) | 8 (100) | 7 (100) |
| HBs Ag (+) | 0 | 10 [titer 2000 (1893–2000)] | 0 | 0 | 0 | 0 |
| HBs Ab (IU/l) (>100/≤100) | 8/3 | 0/10 | 2/3 | 2/2 | 4/4 | 1/6 |
LC-OLT-poor patients received HBIG within 3 months
Age and HBsAg were shown as median (interquartile range)
ALF-OLT acute liver failure patients who received OLT, LC-OLT-good liver cirhosis patients who received OLT and had a good vaccine response, LC-OLT-poor liver cirrhosis patients who received OLT and had a poor vaccine response
Informed consent was obtained from each patient included in the study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in the approval by the Ethics Committee at the Okayama University Hospital.
Antiviral prophylaxis
Our HBV prophylaxis protocol was as follows. We administered HBIg at 200 IU/kg intraoperatively. Recipients were administered another 2000 IU/week HBIg for an additional 1 week post-operatively. HBIg (2000 IU) was administered thereafter only when anti-HBs antibody titers fell below 100 mIU/mL. After 6 months, HBIg was administered only to maintain anti-HBs antibody titers at >10 mIU/mL. We measured levels of HBsAg and anti-HBs antibody and/or HBV-DNA every month for 6 months after LDLT, and every 2–3 months thereafter. Three of the ALF-OLT patients were anti-HBs antibody positive at the time of OLT, these patients were not administered nucleos(t)ide analogues. The remaining two ALF-OLT patients, and all of the LC patients were given nucleos(t)ide analogues. The two ALF-OLT patients were given lamivudine (LAM), and of the 22 LC-OLT patients, 14 received LAM, six were given LAM + adefovir dipivoxyl (ADV), and two received entecavir (ETV). Administration of nucleos(t)ide analogues was started a minimum of 1 month pre-operatively, when possible.
Post-OLT re-activation of HBV was defined as continuous positivity for serum HBsAg and/or serum HBV-DNA.
HBV vaccine protocol
HBV vaccine administration was initiated at least 1 year after OLT, and when patients showed no active infection or rejection episode in the preceding month. The vaccine consisted of recombinant purified HBsAg (Bimmugen; Kaketsuken, Kumamoto, Japan). Ten micrograms were administered every 1–2 months. Based on the effect of the vaccine, patients were classified as “good responders; LC-OLT good” or “poor responders; LC-OLT poor”. Patients who showed anti-HBs antibody titers above 100 mIU/mL without HBIg for a minimum of 6 months were defined as good responders, since all of these patients did not need HBIg administration for an additional 2 years (median) of follow-up. All other patients were defined as poor responders. Patients who showed a good response within 36 months were given additional vaccinations when their anti-HBs antibody titer decreased, whereas vaccination was stopped in patients who showed no good response after 36 months.
Immune suppression
Patients were treated using a standard immunosuppressive regimen (tacrolimus or cyclosporine A with steroids and/or mycophenolate mofetil). One patient was free from calcineurin inhibitors at the time of vaccine administration.
Routine laboratory tests and serum HBV-DNA assay
Hepatitis B surface antigen, anti-HBs antibody, hepatitis Be antigen (HBeAg), and anti-HBe antibody (HBeAb) levels were measured routinely using a commercially available chemiluminescent enzyme immunoassay system (Lumipulse System; Fujirebio, Tokyo, Japan). HBV-DNA levels were measured using a transcription-mediated amplification assay (TMA) (SRL, Tokyo, Japan), a polymerase chain reaction (PCR) assay (Amplicor HBV Monitor assay; Roche Diagnostics, Tokyo, Japan), or a real-time PCR assay (COBAS TaqMan HBV Test; Roche Diagnostics).
HBV recombinant proteins for cellular immune response analysis
Hepatitis B virus recombinant protein HBsAg was purchased from Advanced ImmunoChemical, Inc. (Long Beach, CA). Recombinant protein hepatitis B core antigen (HBcAg) was purchased from the Institute of Immunology (Tokyo, Japan). These proteins were used as stimulating antigens at 1 μg/mL for the enzyme-linked immunospot (ELISPOT) assay.
CD14-positive monocyte isolation and myeloid DC generation
Mononuclear cells were separated from peripheral blood by centrifugation on the Ficoll-Hypaque density gradient (Amersham Pharmacia, Uppsala, Sweden), as previously described. CD14-positive monocytes were purified using microbeads (Miltenyi Biotec, Auburn, CA) in accordance with the protocols of the manufacturer. Subsequently, CD4-positive T cells (T4) were positively sorted in the same way. T4 cells were frozen immediately. CD14-positive cells were cultured at 1 × 106/mL in RPMI containing 5 % heat-inactivated human AB serum (ICN Biomedicals; Aurora, OH) supplemented with 100 ng/mL of granulocyte macrophage colony-stimulating factor (kindly provided by Kirin Pharma, Tokyo, Japan) and 50 ng/mL of interleukin-4 (kindly provided by Ono Pharmaceuticals, Osaka, Japan) at 37 °C in 5 % CO2 for 5 days. Cells were confirmed to be CD11c-positive myeloid immature dendritic cells (DC).
Interferon-γ (IFNγ) ELISPOT assay with myeloid DC and CD4-positive T-cells
The immature DC cultures were exposed to recombinant HBsAg and HBcAg (1 μg/mL each) for 1 day. To mature the DCs, 1 ng/mL of lipopolysaccharide (LPS) (Sigma, St. Louis, MO) was added to the culture 1 day after HBV protein addition. On the same day, mouse anti-human interferon-γ antibody (MABTECH, Sweden) was diluted to 5 μg/mL with ELISPOT buffer (0.159 % Na2CO3, 0.293 % NaHCO3) and coated overnight at 4 °C onto 96-well filtration plates (Millipore, Billerica, MA) at 100 μL per well. The coated plate was washed with phosphate-buffered saline (PBS) and blocked with 10 % fetal calf serum in RPMI1640 medium for 1–2 h. Myeloid DCs were counted and seeded at 5 × 103/well. Cryopreserved T4 cells were thawed, counted, and seeded at 2 × 105/well. On the next day, the plate was washed six times with PBS. Wells were coated with rabbit anti-interferon-γ serum (diluted to 1/800 in PBS), and the plate was incubated at 37 °C for 2 h. The plate was washed six times with PBS and coated with goat anti-rabbit immunoglobulin G-alkaline phosphatase (IgG-AP; Southern Biotech, Birmingham, AL) diluted to 1/2000 with PBS. After a 1 h incubation at 37 °C, the plate was washed six times with water and spots were developed using 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt and nitroblue tetrazolium chloride (BCIP/NBT) as a substrate. Spot development was stopped after 10 min by washing with distilled water. The spots were viewed and counted under a microscope.
Statistical analysis
Statistical comparisons were performed using JMP version 9 (SAS Institute, Cary, NC, USA). The Wilcoxon rank-sum test was used to compare the continuous data and the Chi-square test was used to compare categorical data. For multivariate analysis, logistic regression analysis was used. The Steel–Dwass test was used for multiple group analysis. A p value of <0.05 was considered significant.
Results
The effects of HBV vaccination
None of the patients in the ALF-OLT group showed reactivation of the virus. One patient of the LC-OLT group showed transient positive responses for HBsAg and HBV DNA, however, these became negative again with frequent HBIg administration. At the final observation point, no patients showed HBsAg or HBV DNA-positive response. All five ALF-OLT patients had good responses to vaccination (Table 3). A median of four (range 2.5–5) vaccinations were sufficient to induce a good response. In contrast, LC-OLT patients were less responsive, with only nine of 22 displaying a good response. Additionally, these nine good responders required a median of 19 (range 11.5–30) vaccinations before these patients could be weaned from HBIg administration (Fig. 1).
Table 3.
Results of HBV vaccination
| N | ALF | LC |
|---|---|---|
| 5 | 22 | |
| Response to vaccination (good/poor responders) | 5/0 | 9/13 |
| Number of vaccinations require before ceasing HBIg treatment | 4 (2.5–5) | 19 (11.5–30) |
HBIg Hepatitis B immunoglobulin
Fig. 1.
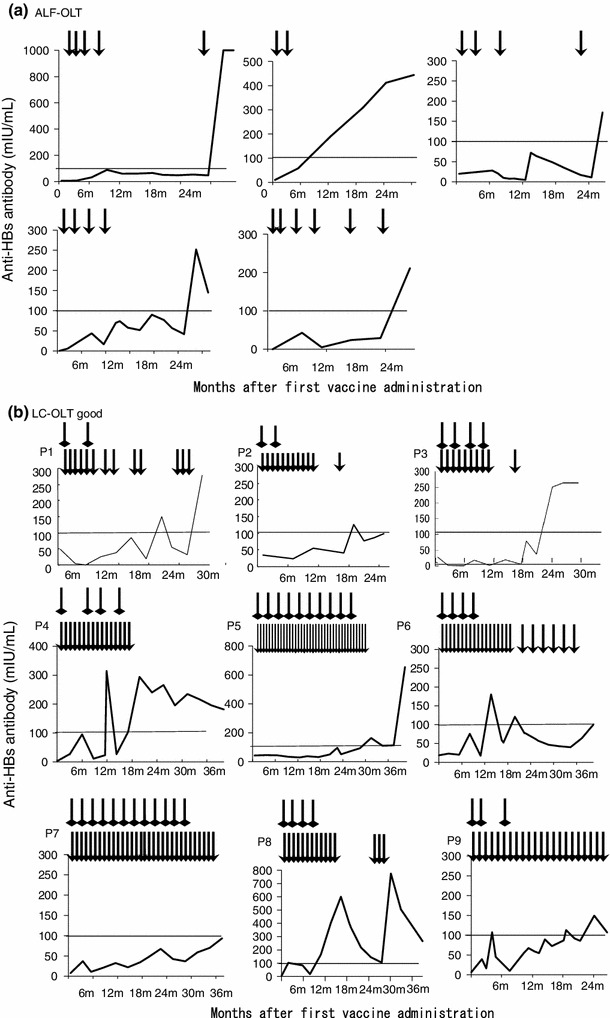
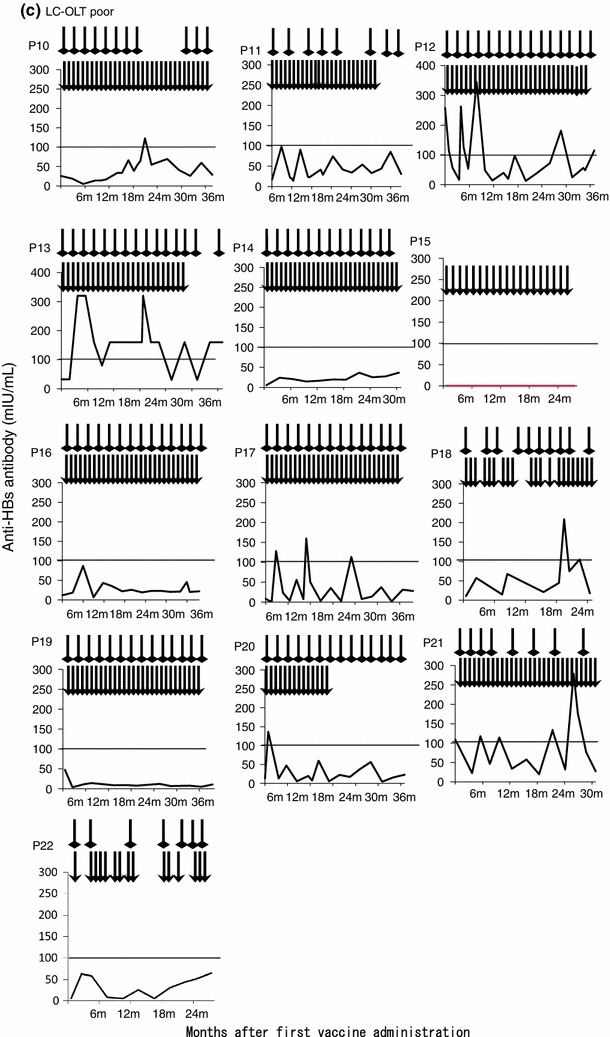
Individual patients’ timecourse of anti-HBs antibody titer after vaccine administration. The timecourse of the anti-HBs antibody titer after the first vaccine administration is shown. The arrowhead indicates a vaccine administration point, and the square head indicates an HBIg administration point. a Patients who received orthotopic liver transplantation (OLT) due to hepatitis B-related acute liver failure (ALF-OLT). All patients had a good response to vaccination. b Patients who received OLT due to liver cirrhosis with a good response to vaccination (LC-OLT good). c LC-OLT patients with a poor response to vaccination (LC-OLT poor)
Vaccine safety
None of the patients showed any adverse reactions as judged by their general condition, or by laboratory examination. One patient reported itchiness after injection of the eighth vaccination dose, although the symptom subsequently stopped.
The characteristics of vaccine responsiveness in LC-OLT patients
To determine the characteristics for defining a good response in LC-OLT patients, clinical data from recipients and donors were investigated (Table 4). The background data of the recipients, including HBV-DNA levels, HBeAg positive reactions, HBsAg levels at the time of OLT, and the anti-HBs antibody titer at the time of the initial vaccination did not differ between the good and poor responder groups (Table 5). However, the donor-related factors did differ. Notably, the good responders’ donors were relatively high in age (p = 0.019) and not blood relatives of the recipients (p < 0.001). These donors (to good responders) showed high anti-HBs antibody titers at the time of OLT (p = 0.038). Since all of the patients in this study received LDLT, non-blood-related donors all corresponded to spouses of the OLT recipients. Multivariate logistic regression analysis was carried out with the following variables: donor age at OLT ≥47, non-blood-related donor, donor anti-HBs antibody titer >100 mIU/mL (Table 6). A status of non-blood-related donor was identified as a significant independent predictor of a good response to vaccination. Since the donor anti-HBs antibody was one of the factors associated with a good response, we asked whether the donors had received vaccination, and found that none of them had ever received an HBV vaccine. As shown in Table 4, none of the donors showed the anti-HBc antibody-negative, anti-HBs antibody-positive condition which indicates vaccine-induced seropositivity to the HBs antigen.
Table 4.
LC patient characteristics
| Characteristics of recipients | Characteristics of donors | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient’s number | Response to vaccine | Age (year) at OLT | Sex | HBsAg (mIU/mL) at OLT | HBsAb at OLT | HBeAg/HBeAb at OLT | HBV DNA (logcopies/mL) at OLT | MELD at OLT | HCC at OLT | Time of vaccinatio n (months post-OLT) | HBsAb (mIU/mL) at vaccine | NA at vaccine | Age at OLT | Sex | Blood relation | ABO compatibility | HBcAb | HBsAb | HBsAb (mIU/mL) |
| 1 | Good | 56 | M | 100 | – | −/+ | <3.7 | 17 | + | 51 | 49 | LAM | 52 | F | – | Compatible | – | – | <0.1 |
| 2 | Good | 48 | M | >2000 | – | +/+ | 3.5 | 20 | + | 24 | 23 | LAM | 46 | F | – | Compatible | + | + | 134 |
| 3 | Good | 44 | M | 100 | – | +/− | <3.7 | 12 | – | 55 | 1 | LAM | 48 | F | + | Identical | + | + | 189 |
| 4 | Good | 50 | M | >2000 | – | +/− | 3.4 | 9 | + | 42 | 25 | LAM + ADV | 48 | F | – | Compatible | + | + | 627 |
| 5 | Good | 54 | M | >2000 | – | −/+ | 3.8 | 15 | – | 40 | 43 | LAM + ADV | 48 | F | – | Compatible | – | – | <0.1 |
| 6 | Good | 57 | M | >2000 | – | −/+ | 2.7 | 15 | + | 45 | 18 | LAM | 53 | F | – | Identical | – | – | <0.1 |
| 7 | Good | 48 | M | 642 | – | +/− | 4.8 | 17 | – | 29 | 7 | LAM | 44 | F | – | Compatible | + | + | 179 |
| 8 | Good | 47 | F | >2000 | – | +/− | 4.5 | 12 | – | 19 | 6 | LAM | 50 | M | – | Compatible | + | + | 1000 |
| 9 | Good | 55 | M | >2000 | – | +/− | 6.1 | 21 | + | 49 | 6 | LAM + ADV | 48 | M | + | Identical | + | + | 133 |
| 10 | Poor | 52 | M | >2000 | – | +/− | 5.3 | 8 | + | 25 | 4 | LAM | 21 | M | + | Compatible | + | + | 1000 |
| 11 | Poor | 62 | M | >2000 | – | −/+ | <2.6 | 8 | + | 13 | 17 | LAM + ADV | 36 | M | + | Identical | – | – | <0.1 |
| 12 | Poor | 39 | M | >2000 | – | +/− | <2.6 | 7 | – | 30 | 169 | LAM | 35 | F | + | Identical | – | – | <0.1 |
| 13 | Poor | 49 | M | 100 | – | −/+ | 4.0 | 21 | + | 107 | 32 | LAM | 22 | F | + | Identical | – | – | <0.1 |
| 14 | Poor | 26 | M | 100 | – | +/− | 5.5 | 20 | + | 75 | 30 | LAM | 53 | M | + | Identical | + | + | 397 |
| 15 | Poor | 54 | F | 100 | – | +/− | 4.6 | 22 | + | 55 | 1 | LAM | 28 | M | + | Identical | – | – | <0.1 |
| 16 | Poor | 50 | M | 160 | – | −/+ | 2.7 | 18 | + | 38 | 6 | LAM | 25 | M | + | Compatible | + | – | <0.1 |
| 17 | Poor | 44 | M | >2000 | – | −/+ | <2.6 | 15 | – | 32 | 14 | LAM | 47 | F | + | Compatible | – | – | <0.1 |
| 18 | Poor | 55 | F | >2000 | – | +/− | 2.8 | 10 | + | 19 | 10 | LAM + ADV | 51 | F | + | Identical | + | + | 44 |
| 19 | Poor | 54 | M | >2000 | – | −/− | <2.6 | 8 | + | 18 | 47 | ETV | 49 | F | – | Compatible | + | + | 1000 |
| 20 | Poor | 63 | M | 1740 | – | −/+ | <2.6 | 12 | – | 17 | 42 | LAM + ADV | 36 | M | + | Identical | – | – | 0.2 |
| 21 | Poor | 58 | M | 35 | – | −/+ | <2.6 | 16 | – | 16 | 19 | ETV | 33 | F | + | Identical | – | – | 0.3 |
| 22 | Poor | 61 | M | >2000 | – | −/+ | 2.9 | 15 | + | 68 | 5 | LAM | 26 | M | + | Identical | – | – | <0.1 |
NA nucleos(t)ide analogue, LAM lamivudine, ADV adefovir dipivoxyl, ETV entecavir, HBcAb anti-HBc antibody, HBsAb anti-HBs antibody
Table 5.
Patient characteristics according to vaccine responsiveness in LC (univariate analysis)
| N | Good responders | Poor responders | p value |
|---|---|---|---|
| 9 | 13 | ||
| Recipient related factors | |||
| Age at OLT | 50 (47–55) | 54 (46–59) | 0.546 |
| Sex (male) | 8 (88 %) | 11 (84 %) | 0.774 |
| Time of vaccination (months after OLT) | 42 (26–50) | 30 (17–61) | 0.442 |
| HBsAg at OLT (≥1500 IU/l) | 6 (66 %) | 8 (61 %) | 0.805 |
| HBeAg positive at OLT | 6 (66 %) | 5 (38 %) | 0.190 |
| HBV DNA at OLT (≥3.7 logcopies/mL) | 4 (44 %) | 4 (30 %) | 0.513 |
| MELD at OLT | 15 [12–18] | 15 [8–19] | 0.480 |
| Child-Pugh score at OLT | 10 [8–10] | 9 [6–11] | 0.845 |
| HCC at OLT (+) | 6 (66 %) | 9 (69 %) | 0.899 |
| Anti-HBs antibody titer at the start of vaccination | 18.6 (6.4–34.6) | 17.4 (5.9–37.1) | 0.920 |
| Nucleos(t)ide analogue (LAM/LAM + ADV/ETV) | 6/3/0 | 8/3/2 | 0.312 |
| Tacrolimus/cyclosporinA | 6/3 | 11/1# | 0.148 |
| Tacrolimus level (ng/mL) | 4.7 (3.0–5.6) | 3.8 (2.9–5.8) | 0.744 |
| Donor-related factors | |||
| Age at OLT | 48 (47–51) | 33 (25–48) | 0.019* |
| Sex (M) | 2 (22 %) | 7 (53 %) | 0.138 |
| ABO (identical) | 3 (33 %) | 9 (69 %) | 0.093 |
| Blood relation (no) | 7 (77 %) | 1 (7 %) | <0.001* |
| Anti-HBs antibody titer (>100) | 6 (66 %) | 3 (23 %) | 0.038* |
| Anti-HBc antibody (+) | 6 (66 %) | 5 (38 %) | 0.190 |
| Anti-HBc(+)/anti-HBs(+) | 6 (66 %) | 4 (30 %) | 0.093 |
| Anti-HBc(+)/anti-HBs(−) | 0 (0 %) | 1 (7 %) | 0.297 |
| Anti-HBc(−)/anti-HBs(+) | 0 (0 %) | 0 (0 %) | – |
MELD Model for End-stage Liver Disease, HCC hepatocellular carcinoma, LAM lamivudine, ADV adefovir dipivoxyl, ETV entecavir
#One patient received no calcineurin inhibitor
Table 6.
Multiple logistic analysis of factors associated with good responses to HBV vaccine in LC
| N | Odds ratio | 95 % CI | p value |
|---|---|---|---|
| Age at OLT (>47) | 5.4 | 0.300–214.000 | 0.244 |
| Blood relation (no) | 29.4 | 2.551–984.110 | 0.005* |
| Anti-HBs antibody titer (>100) | 5.0 | 0.343–149.947 | 0.233 |
Note: Variables significant at p < 0.05
HBV antigen-specific immune responses
To determine the effectiveness of vaccine-induced cellular immune responses in post-OLT patients, we used the IFN-γ ELISPOT assay. First of all, we analyzed the clinical characteristics of those patients showing strong HBsAg-specific T cell immune responses when compared with those of non-transplanted patients, and vaccine-induced anti-HBs antibody-positive, healthy volunteers (Fig. 2). The patients with stronger HBsAg-specific CD4 T cell IFN-γ responses (equal or more than the median; 7 spots) showed lower levels of HBV DNA, lower HBsAg, higher anti-HBs antibody titer, and higher HBcAg-specific immune responses. The HBsAg and HBcAg-specific CD4 T cell immune response under different clinical conditions is shown (Fig. 3). Volunteer controls who were positive for anti-HBs antibodies (as a result of previous vaccine administration) showed numerous HBsAg-specific IFNγ spots. Spot numbers were reduced in control chronic hepatitis B patients, but remained high (against both HBsAg and HBcAg) in acute resolved hepatitis B patients. The ALF-OLT and LC-OLT good responders had relatively higher HBsAg-specific T-cell immune responses than LC-OLT poor responders. The LC-OLT patients with successful vaccine-induced humoral immune responses also showed higher cellular immune responses than control chronic hepatitis B patients. The LC-OLT patients with poor vaccine responses also had low cellular responses, similar to those seen in chronic hepatitis B patients.
Fig. 2.
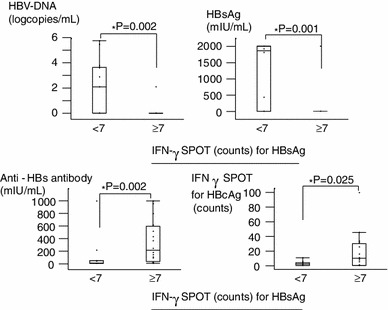
The clinical characteristics of the non-OLT patients with strong HBsAg-specific T cell interferon-γ response. The clinical characteristics of the non-OLT patients showing strong HBsAg-specific T cell immune responses by enzyme-linked immunospot (ELISPOT) assay are shown. Those patients with stronger HBsAg-specific CD4 T cell IFN-γ response (equal or more than the median; 7 spots) showed lower HBV DNA, lower HBsAg, higher anti-HBs antibody titer, and higher HBcAg-specific immune responses
Fig. 3.
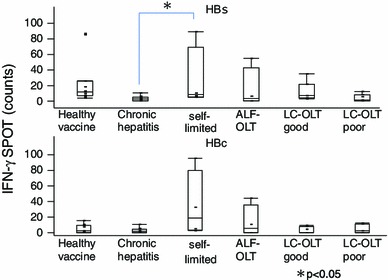
Cellular immune responses against HBsAg including OLT patients. The number of spots due to interferon-γ response in the ELISPOT assay for HBsAg (upper figure) and HBcAg (lower figure) is shown. 1 Healthy vaccine: healthy controls who were positive for anti-HBs antibodies with HBV vaccine (n = 11). 2 Chronic hepatitis: chronic hepatitis B patients (n = 10). 3 Self-limited: self-limited acute hepatitis B patients who showed serum anti-HBs antibody-positive/HBcAb-positive with no HBsAg or HBV-DNA (n = 5). 4 ALF-OLT: post-OLT acute liver failure patients (n = 4). 5 LC-OLT good: post-OLT liver cirrhosis patients who showed good response to vaccine (n = 8). 6 LC-OLT poor: post-OLT liver cirrhosis patients who showed poor response to vaccine (n = 7). Values are plotted as median (range)
Discussion
In this study we found that HBV vaccination was effective in OLT patients whose donors were relatively high in age, marital (non-blood-related), with high-titer anti-HBs antibodies. The multivariate analysis revealed that a marital (non-blood-related) donor was the only factor that associated strongly with a good response to vaccine. Among these OLT recipients, a good response to vaccination included effective responses in both the humoral and cellular arms of the immune system.
Controlling HBV reactivation after OLT is critical. In the absence of prophylaxis, hepatitis B recurs very frequently and results in early graft failure. The prophylaxis protocols have progressed from HBIg immunoprophylaxis in the early 1990s, to lamivudine in the late 1990s, to the more recent application of HBIg combined with nucleos(t)ide analogues. In 1991, Muller et al. [17] reported the first use of long-term HBIg immunoprophylaxis, reducing the HBV recurrence rate to 25 % after 6 months of OLT and 18 % after 12 months. A multicenter study revealed that the three-year risk of HBV recurrence was 75 ± 6 % without HBIg, 74 ± 5 % with short-term (2-month) HBIg, and 36 ± 4 % with long-term (>6-month) HBIg treatment [18]. Patients who were positive for HBeAg or HBV-DNA displayed the greatest risk of recurrence (83 %); patients with acute fulminant liver failure showed the lowest risk (16 %).
In 1996, Grellier et al. [19] reported a trial of LAM as a prophylactic treatment, achieving 18 % recurrence of HBV at 6 months after OLT. However, the long-term recurrence rate at 3 years after OLT progressed to 41 %, indicating that LAM monotherapy is not recommendable for post-transplantation prophylaxis.
Although monotherapy with HBIg or LAM resulted in a high rate of recurrence, a combination of these agents has been administered with reasonable success. In 1998, Markowitz et al. [20] reported no recurrences after 1 year of combination therapy. Since HBIg is very expensive, several reports have described modified combination therapies. We previously have shown that long-term LAM with short-term, high-dose HBIg followed by low-dose HBIg (sufficient to maintain an anti-HBs antibody titer of >10 mIU/mL) is cost-effective and powerful enough to control HBV recurrence after LDLT [13]. With this cost-saving method, no clinical evidence of HBV recurrence has been seen.
In 2000, Sanchez-Fueyo et al. [21] reported an 82 % response to HBV vaccination after OLT. These researchers used three cycles of double-dose recombinant HBsAg vaccine for immunization over 6 months, with a target antibody titer of >10 mIU/mL. The cohort included six acute infected patients and 11 chronic carriers. However, recent reports show that chronic HBV carrier recipients did not respond well, with response rates ranging from 7.7 to 12.5 % [22, 23]. Acute HBV-infected patients who underwent OLT were often positive for the anti-HBs antibody even before OLT, with strong immune responses. Such patients might be expected to respond well to vaccination, since these individuals (unlike chronic carriers) have not developed a tolerance to HBV. In our patients, five acute infected patients showed good responses to vaccination, responding after a median of only four vaccinations. These results indicate that while acute HBV-infected patients are good candidates for HBV vaccination post-OLT; chronic HBV carriers are poorer candidates for this protocol. However, as some HBV carriers did respond to vaccination; further studies should be performed to clarify the differences between the good and poor responders.
Several reports have identified the differences between good responders and poor responders in non-HBV-infected patients who received HBcAb-positive donor livers. Lacking previous HBV exposure, these recipients should not have developed tolerance to the virus and so should have been good responders. Of these, good responses were seen in pediatric cases where the recipients had higher anti-HBs antibody titers at the time of OLT and lower tacrolimus levels at the time of vaccination [24]. The present study revealed that repeated vaccine administration resulted in successful immunization in 40 % of the LC-OLT recipients. For these recipients, the strength of the response did not correlate with recipient characteristics, not even with age, one of the most important factors for successful immunization [25]. In contrast, the characteristics of the donor were important. The good responders’ donors were relatively high in age, non-blood-related and had high anti-HBs antibody titers before donation. Note that, in our trial, the term “non-blood-related donor” indicates the spouse of the recipient, since deceased donor liver transplantation is not widely accepted in Japan [26]. The donors with high-titer anti-HBs antibody probably were infected with HBV by the recipients after their marriage, resulting in the anti-HBs antibody boost. These donors’ immune systems should not have developed tolerance to the virus. This elevated immunity might be the reason why our patients had relatively better outcomes following vaccination than those of previous reports [27]. Adoptive immune transfer of HBV-specific immune response could be possible [28]. For successful transfer of immune memory to the recipients, the anti-HBs antibody titer of the donors should be high, and vaccine-induced anti-HBs antibody might be less effective than antibodies produced in a previous self-limited infection. Luo et al. [29] have shown that a particularly high anti-HBs antibody titer (>1000 IU/L) in the donor is essential for adoptive immune transfer. The results of the present study suggest that HBV vaccination of non-blood-related living donor candidates having a lower anti-HBs antibody titer (<100 mIU/mL) might facilitate improved vaccine response post-OLT in LC recipients.
The present study of HBV vaccine efficacy in ALF-OLT and LC-OLT patients revealed that the vaccine response depended on the immune tolerance to the virus in both recipients and donors. The liver is the biggest immune organ in the abdomen and so can play a critical role in immune responses. Multiple populations of non-hematopoietic liver cells, including sinusoidal endothelial cells, stellate cells located in the subendothelial space, and liver parenchymal cells, take on the roles of antigen-presenting cells [30]. The viral-specific immune competence of the grafted liver might overcome the general immunotolerance to the virus in chronic HBV carriers.
In conclusion, patients who received OLT due to acute infection of HBV were good candidates for HBV vaccination. The chronic HBV carrier recipients who received livers from donors who were non-blood-related (i.e, the recipient’s spouse) and who harbored high anti-HBs antibody titers were the best candidates for HBV vaccine administration. Vaccine-induced, HBV-specific immune responses were strong enough to induce not only humoral but also cellular responses in vitro.
Acknowledgments
We thank Taiko Kameyama, Asuka Maeda, Chizuru Mori, and Mayumi Honda for carrying out the ELISPOT assay experiments at our institute. Toshie Ishii assisted in the collection of the clinical data and assembly of the data files.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991;13(4):619–626. [PMC free article] [PubMed] [Google Scholar]
- 2.Davies SE, Portmann BC, O’Grady JG, Aldis PM, Chaggar K, Alexander GJ, et al. Hepatic histological findings after transplantation for chronic hepatitis B virus infection, including a unique pattern of fibrosing cholestatic hepatitis. Hepatology. 1991;13(1):150–157. doi: 10.1002/hep.1840130122. [DOI] [PubMed] [Google Scholar]
- 3.O’Grady JG, Smith HM, Davies SE, Daniels HM, Donaldson PT, Tan KC, et al. Hepatitis B virus reinfection after orthotopic liver transplantation. Serological and clinical implications. J Hepatol. 1992;14(1):104–111. doi: 10.1016/0168-8278(92)90138-F. [DOI] [PubMed] [Google Scholar]
- 4.Bartholomew MM, Jansen RW, Jeffers LJ, Reddy KR, Johnson LC, Bunzendahl H, et al. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet. 1997;349(9044):20–22. doi: 10.1016/S0140-6736(96)02266-0. [DOI] [PubMed] [Google Scholar]
- 5.Fontana RJ, Hann HW, Wright T, Everson G, Baker A, Schiff ER, et al. A multicenter study of lamivudine treatment in 33 patients with hepatitis B after liver transplantation. Liver Transpl. 2001;7(6):504–510. doi: 10.1053/jlts.2001.24896. [DOI] [PubMed] [Google Scholar]
- 6.Papatheodoridis GV, Sevastianos V, Burroughs AK. Prevention of and treatment for hepatitis B virus infection after liver transplantation in the nucleoside analogues era. Am J Transplant. 2003;3(3):250–258. doi: 10.1034/j.1600-6143.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H, Kato T, Levi DM, Regev A, Madariaga JR, Nishida S, et al. Lamivudine monoprophylaxis for liver transplant recipients with non-replicating hepatitis B virus infection. Clin Transplant. 2007;21(2):166–171. doi: 10.1111/j.1399-0012.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferretti G, Merli M, Ginanni Corradini S, Callejon V, Tanzilli P, Masini A, et al. Low-dose intramuscular hepatitis B immune globulin and lamivudine for long-term prophylaxis of hepatitis B recurrence after liver transplantation. Transplant Proc. 2004;36(3):535–8. [DOI] [PubMed]
- 9.Roche B, Samuel D. Evolving strategies to prevent HBV recurrence. Liver Transpl. 2004;10(10 Suppl 2):S74–S85. doi: 10.1002/lt.20258. [DOI] [PubMed] [Google Scholar]
- 10.Buti M, Mas A, Prieto M, Casafont F, Gonzalez A, Miras M, et al. A randomized study comparing lamivudine monotherapy after a short course of hepatitis B immune globulin (HBIg) and lamivudine with long-term lamivudine plus HBIg in the prevention of hepatitis B virus recurrence after liver transplantation. J Hepatol. 2003;38(6):811–817. doi: 10.1016/S0168-8278(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 11.Di Paolo D, Tisone G, Piccolo P, Lenci I, Zazza S, Angelico M. Low-dose hepatitis B immunoglobulin given “on demand” in combination with lamivudine: a highly cost-effective approach to prevent recurrent hepatitis B virus infection in the long-term follow-up after liver transplantation. Transplantation. 2004;77(8):1203–1208. doi: 10.1097/01.TP.0000118904.63669.EB. [DOI] [PubMed] [Google Scholar]
- 12.Karasu Z, Ozacar T, Akyildiz M, Demirbas T, Arikan C, Kobat A, et al. Low-dose hepatitis B immune globulin and higher-dose lamivudine combination to prevent hepatitis B virus recurrence after liver transplantation. Antivir Ther. 2004;9(6):921–927. [PubMed] [Google Scholar]
- 13.Takaki A, Yagi T, Iwasaki Y, Sadamori H, Matsukawa H, Matsuda H, et al. Short-term high-dose followed by long-term low-dose hepatitis B immunoglobulin and lamivudine therapy prevented recurrent hepatitis B after liver transplantation. Transplantation. 2007;83(2):231–233. doi: 10.1097/01.tp.0000246310.75638.86. [DOI] [PubMed] [Google Scholar]
- 14.Rosenau J, Hooman N, Rifai K, Solga T, Tillmann HL, Grzegowski E, et al. Hepatitis B virus immunization with an adjuvant containing vaccine after liver transplantation for hepatitis B-related disease: failure of humoral and cellular immune response. Transpl Int. 2006;19(10):828–833. doi: 10.1111/j.1432-2277.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 15.Ishigami M, Kamei H, Nakamura T, Katano Y, Ando H, Kiuchi T, et al. Different effect of HBV vaccine after liver transplantation between chronic HBV carriers and non-HBV patients who received HBcAb-positive grafts. J Gastroenterol. 2011;46(3):367–377. doi: 10.1007/s00535-010-0313-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Zhou L, Zheng SS. Clinical management of hepatitis B virus infection correlated with liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9(1):15–21. [PubMed] [Google Scholar]
- 17.Muller R, Gubernatis G, Farle M, Niehoff G, Klein H, Wittekind C, et al. Liver transplantation in HBs antigen (HBsAg) carriers. Prevention of hepatitis B virus (HBV) recurrence by passive immunization. J Hepatol. 1991;13(1):90–96. doi: 10.1016/0168-8278(91)90869-D. [DOI] [PubMed] [Google Scholar]
- 18.Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329(25):1842–1847. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 19.Grellier L, Mutimer D, Ahmed M, Brown D, Burroughs AK, Rolles K, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348(9036):1212–1215. doi: 10.1016/S0140-6736(96)04444-3. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz JS, Martin P, Conrad AJ, Markmann JF, Seu P, Yersiz H, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28(2):585–589. doi: 10.1002/hep.510280241. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Fueyo A, Rimola A, Grande L, Costa J, Mas A, Navasa M, et al. Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination: a new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation. Hepatology. 2000;31(2):496–501. doi: 10.1002/hep.510310233. [DOI] [PubMed] [Google Scholar]
- 22.Rosenau J, Hooman N, Hadem J, Rifai K, Bahr MJ, Philipp G, et al. Failure of hepatitis B vaccination with conventional HBsAg vaccine in patients with continuous HBIG prophylaxis after liver transplantation. Liver Transpl. 2007;13(3):367–373. doi: 10.1002/lt.21003. [DOI] [PubMed] [Google Scholar]
- 23.Lo CM, Liu CL, Chan SC, Lau GK, Fan ST. Failure of hepatitis B vaccination in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B. J Hepatol. 2005;43(2):283–287. doi: 10.1016/j.jhep.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Kwon CH, Suh KS, Yi NJ, Chang SH, Cho YB, Cho JY, et al. Long-term protection against hepatitis B in pediatric liver recipients can be achieved effectively with vaccination after transplantation. Pediatr Transplant. 2006;10(4):479–486. doi: 10.1111/j.1399-3046.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- 25.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura N, Okajima H, Ushigome H, Sakamoto S, Fujiki M, Okamoto M. Current status of organ transplantation in Japan and worldwide. Surg Today. 2010;40(6):514–525. doi: 10.1007/s00595-009-4214-3. [DOI] [PubMed] [Google Scholar]
- 27.Wursthorn K, Wedemeyer H, Manns MP. Managing HBV in patients with impaired immunity. Gut. 2010;59(10):1430–1445. doi: 10.1136/gut.2009.195834. [DOI] [PubMed] [Google Scholar]
- 28.Schumann A, Lindemann M, Valentin-Gamazo C, Lu M, Elmaagacli A, Dahmen U, et al. Adoptive immune transfer of hepatitis B virus specific immunity from immunized living liver donors to liver recipients. Transplantation. 2009;87(1):103–111. doi: 10.1097/TP.0b013e31818bfc85. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Lo CM, Cheung CK, Lau GK, Fan ST, Wong J. Identification of hepatitis B virus-specific lymphocytes in human liver grafts from HBV-immune donors. Liver Transpl. 2007;13(1):71–79. doi: 10.1002/lt.20887. [DOI] [PubMed] [Google Scholar]
- 30.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]


