Abstract
Introduction
Psoriasis is a common, long-term skin condition associated with high levels of psychological distress and considerable life impact. The impact of psoriasis, beyond the skin, is often not recognised and under-treated.
Methods
This paper explores the relationship between psychological distress and psoriasis including reference to the ‘brain–skin access’. The life impact of psoriasis is discussed and pharmacological interventions which affect distress associated with psoriasis and psychological interventions are reviewed. Evidence from peer-reviewed journals and controlled trials inform the text.
Results
Psoriasis has a profound impact on mental health and well-being which is under-recognised by clinicians. The sympathetic adrenal medullary axis and hypothalamic pituitary adrenal axis are likely to be involved in the onset of psoriasis and there may also be an effect from inflammation in the skin on the central release of corticotrophin-releasing hormone. Psoriasis can be stigmatising and may affect all aspects of life including relationships, employment, social life and leisure activities. There is some evidence for psychological interventions being effective in the management of distress associated with psoriasis and psoriasis itself. Studies, however, have used disparate outcomes and methods and largely involve low numbers of patients. There is very limited access to psychological support for the patients with psoriasis despite evidence of high levels of psychological distress and considerable life impact.
Conclusions
Psoriasis is a long-term skin condition associated with high levels of distress and considerable life impact, both of which are under-recognised. Routine screening for distress with access to effective treatment is required. There is a need for high-quality studies to assess the effect of psychological intervention in patients with psoriasis both to inform guidance and facilitate the provision of effective psychological support services.
Keywords: Brain–skin axis, Psoriasis, Psycho-dermatology, Psychological distress, Psychological impact, Psychological intervention, Psycho-neuroimmunology, Stress
Introduction
Psoriasis affects approximately 1.2 million people in the UK [1]. The profound impact of psoriasis on mental health, well-being and quality of life is increasingly recognised [2–4]. The burden of living with psoriasis is equivalent or greater than that of other long-term conditions such as congestive cardiac failure and chronic lung disease [5], but tends to be under-recognised [6]. There is limited evidence regarding the management of stress and distress associated with psoriasis [7] and even less scope within our present health system for psychological intervention [8].
This article reviews the relationship between distress and psoriasis, exploring the life impact of psoriasis and the current evidence for pharmacological and psychological interventions in this population. Papers from peer-reviewed journals have been used to inform the text and all interventional studies cited are from controlled trials.
Psychological Impact of Psoriasis
Stress and Psoriasis
Distress, in the form of excessive worrying, impairs clearance of psoriasis during phototherapy [9]. Retrospective studies have demonstrated that 37–88% of patients believe that their psoriasis is caused or exacerbated by stress [10–12]. In prospective studies, Verhoeven et al. [13] confirmed that daily stressors trigger increased itch and psoriasis severity, and that patients with high levels of worrying are most vulnerable to the impact of stressors [14].
Psychoneuroimmunology: The Brain–Skin Axis (Fig. 1)
Fig. 1.
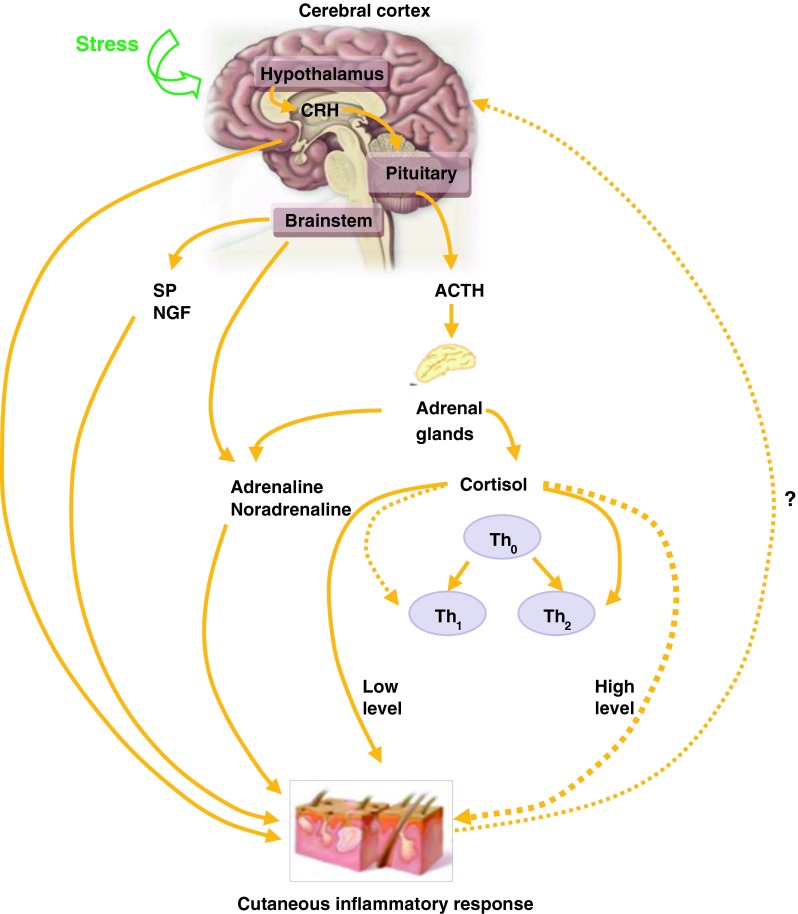
The brain–skin axis. ACTH adrenocorticotropic hormone, NGP nerve growth factor, SP substance P, Th 0-2 Thelper 0-2 cells, ? up-regulation of CRH by the cutaneous inflammatory response
The stress response is an evolutionary mechanism enabling both a rapid (fight or flight) response to an acute stressor (e.g. avoiding a predator) and a response to chronic stress by saving energy [15]. Modern day stressors trigger established pathophysiological pathways, which can act as exacerbating or causative factors in inflammatory disease [16].
Hypothalamic–Pituitary–Adrenal Axis
Activation of neuro-hormones by stress is predominantly via the hypothalamic–pituitary–adrenal (HPA) axis with up-regulation of corticotrophin-releasing hormone (CRH), adrenocorticotrophic hormone and glucocorticoids [17], alongside neuropeptide stress response mediators [18]. The immune system is profoundly altered: glucocorticoids inhibit interleukin (IL)-12, interferon-gamma and tumour necrosis factor (TNF) via antigen-presenting cells and T-helper (Th)1 cells. IL-4, IL-10 and IL-13 are up-regulated via Th2 cells. Cortisol exerts an immunosuppressive effect shifting Th1 to Th2-mediated immunity [19]. CRH also directly affects localised inflammatory responses [20]. Skin has a fully functional peripheral HPA axis that translates and co-ordinates the peripheral stress response from the central HPA axis, creating its own cutaneous homeostasis [21]. Neuropeptides, substance P and nerve growth factor facilitate communication between neuronal and immune cells, and promote migration of macrophages and monocytes through vascular endothelium. [22].
Very interestingly, the cutaneous immune system may also regulate the central nervous system. Mast-cell histamine increases the expression of CRH messenger RNA in the hypothalamus of dogs [23], and IL-1 and IL-6 (elevated in psoriasis) trigger CRH release [24]. Furthermore, pro-inflammatory cytokines can induce sickness behaviour and depressive symptoms [25]. This raises the question of whether psoriasis triggers stress or stress triggers psoriasis, or both.
Low cortisol levels in people with psoriasis who describe themselves as stress responders and who have a blunted HPA axis response to acute social stress in psoriasis [26] may result in hypocortisolism and partly explains the immune overactivity involved in psoriatic flares.
Sympathetic–Adrenal–Medullary Axis
While the HPA axis tends to be involved in slow and sustained responses to stressors [27], the sympathetic–adrenal–medullary axis is activated within seconds of a stressful stimulus. Catecholamines are released, activating CD4+ lymphocytes and trafficking of lymphocytes into the skin; an event that precedes the development of psoriatic plaques [28].
Psychiatric Co-Morbidity
The prevalence of depression in psoriasis is estimated to be up to 30% [29]. Fortune et al. [30] identified 38% of 140 patients with psoriasis as pathological worriers, while Gupta and Gupta [31] found that 5.5% of patients with psoriasis had active suicidal ideation and that 9.5% expressed a death wish.
A UK population-based cohort study of 146,042 patients [4] demonstrated an increased incidence of diagnoses of depression, anxiety and suicidality in psoriasis; the authors estimated that over 10,400 diagnoses of depression, 7,100 diagnoses of anxiety, and 350 diagnoses of suicidality were attributable to psoriasis each year.
Accurate epidemiology of psychiatric co-morbidity is limited by disparities in methodologies, with some studies using symptom questionnaires to diagnose depression and others employing clinical diagnosis alone. Most studies lack a control group and are cross-sectional in nature, preventing determination of causal relationships.
A recent study suggests that maladaptive schema-level cognitions may underlie the psychological distress experienced by people with psoriasis [32]. Mizara et al. [32] investigated the relationship between maladaptive schemas and psychological distress in patients with psoriasis. Three schemas—vulnerability to harm, defectiveness and social isolation—predicted anxiety and depression.
Despite evidence for psychiatric morbidity, most guidelines regarding psoriasis do not include screening for anxiety and depression; hence patients may not receive appropriate intervention.
Disease Severity and Psychological Impact
Psychological and psychiatric symptoms are generally in keeping with disease severity [33, 34], but not universally [33, 35]. In a prospective study of 72 patients undergoing phototherapy, Fortune et al. [36] demonstrated significant improvement in psoriasis, without change in anxiety, depression or worrying. In another prospective study, Gupta et al. [35] found that 16 of 98 patients receiving phototherapy had persistent or worsening psoriasis disability index scores despite an improvement in psoriasis. Sampogna et al. [33] reported persistent psychiatric disorder with clearance of psoriasis in one-third of subjects.
The lack of correlation between psychological impact and physical severity in some patients may be due to the cumulative impact of living with psoriasis [2], maintenance of distress by established maladaptive schemas and coping responses [32], or by psoriasis affecting ‘high impact’ sites [37]. Lower levels of distress have been reported with longer disease duration [38, 39], which may indicate psychological acceptance, or reflect long-term adaptation [40] and avoidance of activities that trigger distress. The latter hypothesis is supported by Kleyn et al. [41] in a study using functional magnetic resonance imaging, which demonstrated reduced signal responses to disgusted faces in patients with psoriasis compared with controls, suggesting coping mechanisms among those with psoriasis to prevent distress when seeing disgusted facial expressions.
Recognition of Distress by Clinicians
Clinicians and patients may differ in their assessment of disease severity and health-related quality of life (HRQoL). In a study comparing a checklist completed by both dermatologists and patients [42], patients ranked “embarrassment over one’s appearance” as the most severe feature of psoriasis, whereas dermatologists ranked it the lowest feature. Patient-, rather than clinician-rated severity is the strongest predictor of HRQoL impairment [2, 33, 43]. Richards et al. [44] assessed 43 outpatient consultations where self-report questionnaires indicated that 37% and 12% of patients had clinical anxiety and depression, respectively. The dermatologist discussed psychological difficulties in only 39% of consultations with significantly distressed patients. There was poor correlation between the patient and clinician regarding the presence of anxiety and depression.
The discrepancy between patients and clinicians is probably multi-factorial. Patients’ own underlying perceptions of illness, coping strategies [43–46], personality traits [32], and body shame contribute to distress and are factors often unexplored by clinicians. Richards et al. [47] found that 33% of 300 patients with psoriasis had alexithymia (impaired ability to build mental representations of emotions and express emotions). This may limit a patient’s ability to communicate emotions effectively and lead to the misinterpretation of emotional arousal as physical illness. People with psoriasis may be reluctant to disclose personal information through fear of rejection and previous experience of clinicians’ lack of empathy [3, 45].
Social Impact of Psoriasis
Stigmatisation
Psoriasis may attract attention, and cause avoidance, public rejection and reactions of disgust. Ginsburg and Link [48] identified six main areas associated with stigma: anticipation of rejection; feelings of being flawed; sensitivity to others’ attitudes; guilt and shame; secretiveness; and positive attitudes. The main predictors of stigma were: younger age at onset; extent of bleeding; employment status; duration of disease; and rejection experience. Hrehorów et al. [49] repeated the study in 2012 and found that over 78% of patients had high levels of stigmatisation. Stigmatising experiences appear to be very common. Ginsburg et al. [50] found that 99 of 100 patients described experiencing stigmatising events and Gupta et al. [51] reported that 26% of patients had experienced public rejection. Recently, Sampogna et al. [3] found that 37% of patients experienced humiliation often or all the time, and that shame was one of the most frequently reported emotions.
Feelings of shame and stigmatisation can lead to avoidance of sporting activity, social opportunities, intimacy and public places. It may also affect access to health care, with patients reluctant to expose their skin even to a doctor.
Relationships
Eghlileb et al. [52] interviewed family members of patients with psoriasis; 57% described psychological distress, 55% social disruption, 44% limitations to leisure activities and 37% deterioration of close relationships. The patient’s level of distress was more closely related to the family members’ level of distress and social disruption than the severity of psoriasis. In a multicentre, case-controlled study, Poot et al. [53] found severe family dysfunction to be 16-fold more likely in patients with psoriasis than in families without a skin condition.
Psoriasis also affects sexual functioning. In Gupta and Gupta’s cross-sectional survey of 120 inpatients, 40% reported a decline in sexual activity since the onset of psoriasis. This decline was associated with joint pains, but not psoriasis severity [54]. Sampogna et al. [55] confirmed the impaired sexual life of 936 inpatients with psoriasis. Using individual questions relating to sexual functioning from dermatology- and psoriasis-specific questionnaires, they found that 35.571.3% of patients reported sexual problems. In contrast to the Gupta and Gupta findings, psoriasis severity and psychological problems were associated with sexual functioning. In neither study was the presence of genital lesions determined.
In a cross-sectional study of 92 male patients with psoriasis, Goulding et al. [56] found an increased prevalence of erectile dysfunction (ED) (58%) compared with controls with other skin conditions (49%). Psoriasis was not an independent risk factor for ED. The link between psoriasis, ED and atherosclerosis was suggested as a mechanism for sexual dysfunction.
Difficulties in relationships are unsurprising in a condition with high levels of distress and stigma. Despite this, the quality of relationships and sexual functioning are not routinely included in consultations or as outcomes of studies.
Employment and Financial Burden
The choice of employment or career, and therefore income, is affected by psoriasis [57–59]. In one study, 40% of patients reported experiencing major difficulties at work [52] and in another, 2% stopped work due to psoriasis [57]. There is an inverse relationship between psoriasis severity, employment and income [59–62]. In a study of 601 patients by Horn et al., 31.2% of patients with severe psoriasis had a low income (less than US$30,000) compared with 18.1% of patients with mild psoriasis [59]. Approximately 20% were unemployed. Significantly more patients with severe psoriasis (17%) reported their psoriasis as the reason for unemployment compared with patients with mild psoriasis (6%). Though the results were consistent with other studies [60–62], its cross-sectional design prevents determination of causal relationships.
Recently, biological intervention studies have incorporated broader ‘life impairment’ outcomes into their study designs. Psoriasis-related work productivity and activity impairment (WPAI) are significantly improved by treatment with adalimumab [61]. Treatment with ustekinumab is linked with an improvement in productivity and reduced absenteeism [60]. Both studies show strong correlations between disease severity and work disability. Kimball et al. [61] reported that a reduction in Dermatology Life Quality Index (DLQI) was more highly correlated with WPAI outcomes than an improvement in Psoriasis Area Severity Index (PASI) score, and Reich et al. [60] reported a greater improvement in WPAI outcomes in patients with higher DLQI scores and Hospital Anxiety and Depression Score at baseline, suggesting that a reduction in work disability may only be partially attributable to an improvement in disease severity.
Alcohol and Smoking Misuse
Alcohol and cigarette misuse are common among patients with psoriasis [63, 64]. Kirby et al. [65] reported excess alcohol consumption to be up to 50%. Mills et al. [66] found that twice as many patients with psoriasis smoked compared with controls. Alcohol exacerbates psoriasis severity and pruritus, and impairs treatment response [67]. Poikolainen et al. [68] demonstrated an increased standardised mortality ratio in all parameters of alcohol- and smoking-related causes in people with psoriasis. Naldi et al. [69] found that the risk of psoriasis was higher (but not significantly) for current smokers and drinkers, and a trend for increased risk of psoriasis with increased usage of cigarettes and alcohol [70].
Treatment of Psychological Distress in Psoriasis
Pharmacological Interventions
Biological Agents
TNF-α antagonists are safe and effective in improving both the physical severity of psoriasis and HRQoL [71–73]. Patients with depression also have increased levels of TNF-α [74]. It is speculated that TNF is related to fatigue and sleepiness, and may account for the coexistence of depression with fatigue [75, 76]. TNF-α antagonists may reverse depressive symptoms and fatigue in patients with psoriasis [61, 74, 77]. Reductions in anxiety and depression scores have also been demonstrated with ustekinumab (an anti-IL-12/23 agent used to treat psoriasis) [78].
Psychotropic Medications
Few studies have investigated the role of psychotropic medications in psoriasis. A double-blind, placebo-controlled study by Alpsoy et al. [79] compared treatment with moclobemide (a monoamine oxidase inhibitor antidepressant) plus topical corticosteroid to treatment with topical corticosteroid alone in 60 patients. Improvements in PASI, depression and anxiety scores were significantly greater in the moclobemide group at 6 weeks compared with topical corticosteroid alone. Baseline PASI scores, however, were low (<5) in both groups, making it difficult to evaluate changes in scores. A recent retrospective open study of 38 patients with moderate/severe psoriasis and depressive and/or anxiety disorders who recently started anti-TNFα therapy compared treatment with escitalopram (a serotonin selective reuptake inhibitor) plus psychotherapeutic support with psychotherapeutic support alone [80]. Reduced depression and anxiety scores, and pruritus and PASI scores were seen in the escitalopram plus psychotherapeutic support group compared with psychotherapeutic support only. No details of the ‘psychotherapeutic support’ were given and randomisation was by patient preference.
The evidence for psychotropic medications and psoriasis is conflicting, with several case-reports of fluoxetine-induced or -exacerbated psoriasis [81–83], while the atypical antidepressant, bupropion, has been linked to exacerbations of psoriasis [84]. In addition, the mood stabiliser, lithium, commonly precipitates or aggravates psoriasis [85].
Psychological Interventions
Despite the association of distress with psoriasis, evidence for a ‘brain–skin axis’ and the effect of stress on immune functioning and disease severity, there have been few high-quality studies investigating the effectiveness of psychological interventions. A recent meta-analysis of psychological interventions designed to improve skin condition outcomes identified only eight studies involving psoriasis that fulfilled the criteria of having a control group, being published in a peer-reviewed journal and written in English [7]. The majority of these studies had small sample sizes and non-randomised study designs. A diverse range of psychological interventions with differing outcomes were included, making comparisons impossible.
Psychological interventions can be divided into those addressing cognitive and behavioural processes—cognitive behaviour therapy (CBT)—and those targeting a reduction in stress (arousal reduction).
Arousal Reduction
A prospective, randomised, single-blinded study by Kabat-Zinn et al. [86] introduced a mindfulness meditation-based stress reduction audio-tape intervention as an adjunct to phototherapy and compared it to phototherapy alone. A significant improvement in the rate of psoriasis clearance and a non-significant reduction in stress levels were found. The duration of remission was not assessed.
Gaston et al. [87] performed a single-blinded, randomised, controlled trial comparing 20 weeks of meditation or meditation plus imagery with a waiting list control group or treatment-free group. A significant improvement in psoriasis severity (P ≤ 0.01) was seen in the meditation intervention groups, with no additional benefit of imagery. Psychological outcomes were not evaluated and patient numbers were small.
Vedhara et al. [88] evaluated the effects of an emotional disclosure programme. Patients with psoriasis were asked to spend 20 min writing or talking about their thoughts or the most upsetting times of their lives on four consecutive days. A control group wrote a factual (non-emotional) account of the previous day. They found no difference in psoriasis severity between the two groups; however, changes in mood predicted psoriasis severity in the intervention group only. Assessments were at 12 weeks after the intervention. Paradisi et al. [89] compared the efficacy of two different written emotional disclosure techniques performed on three consecutive days with a control group in patients undergoing phototherapy. The results were difficult to interpret, but appeared to demonstrate a slightly longer duration of remission in one of the emotional disclosure groups. Both of these studies had a very short duration of intervention. The inclusion of emotional disclosure techniques on a more regular basis may warrant further investigation.
Hypnosis
A potential role for hypnosis has been demonstrated in two studies [90–92]. Tausk and Whitmore [91] randomised highly or moderately hypnotisable patients with psoriasis to either hypnosis with active suggestions of improvement or neutral hypnosis. No difference in PASI scores was found between the two groups, although there was a suggestion that highly hypnotisable patients in both groups demonstrated a greater percentage reduction in PASI score than moderately hypnotisable patients (P = 0.01). Patient numbers, however, were very low and there was no non-intervention control group. Price et al. [92] employed self-hypnosis, together with relaxation and support group discussions over eight weekly sessions in a matched-controlled trial. A significant reduction in anxiety and neuroticism, and an increase in self-esteem, maintained at 6 months, were demonstrated. No significant improvement in psoriasis severity was found. Both studies are limited by small group size and the improvement seen in the study by Price et al. cannot be concluded as the sole benefit of self-hypnosis training. Hypnosis may, however, be a therapeutic modality that warrants further testing.
Cognitive Behaviour Therapy
Individual coping strategies, illness perceptions, beliefs about control and curability of illness, and individual personality traits are closely linked with health outcomes and psychological distress in psoriasis [46, 93–95]. Only two studies, however, have investigated CBT as an adjunctive treatment for psoriasis. A high-quality, case-controlled study by Fortune et al. [96] involving 40 patients with psoriasis receiving adjunctive group CBT demonstrated a significant reduction in PASI scores at 6 weeks and 6 months follow-up, with 64% of patients achieving >75% psoriasis clearance at 6-month follow-up compared with 23% of the control group. The CBT group also showed reductions in anxiety and depression scores, and almost double the reduction in self-reported disability and life stress scores at 6-week and 6-month follow-ups.
In a randomised, controlled trial, Zacharie et al. [97] compared the effect of seven individual psychotherapy sessions—which included imagery, stress management and relaxation training over a 12-week period—with a control therapy in 51 patients with psoriasis. Slight, but significant, reductions in reference lesion severity and a non-significant trend for overall reduction in psoriasis severity were observed in the psychotherapy test group. Perceived stress remained unchanged. This study, however, combined arousal reduction with a cognitive intervention. Further studies investigating the effectiveness of CBT, including work- and relationship-related outcomes, are warranted.
Discussion
Psoriasis is a common, visible, long-term condition with a considerable psychological and life impact, potentially affecting every aspect of an individual’s existence including work, recreation, relationships, sexual functioning, family and social life.
Increasing awareness of the life impact of psoriasis has led to clinical guidelines that include the evaluation of patients’ HRQoL in addition to physical severity [98–100]. European evidence-based (S3) clinical guidelines propose a DLQI of 0 or 1 as a treatment goal [99], and Scottish Intercollegiate Guidelines Network guidance states that the “assessment of patients with psoriasis or psoriatic arthritis should include psychosocial measures, with referral to mental health services as appropriate”, and recommends at least annual screening for depressive symptoms [100].
Despite high levels of psychological distress, the provision of psychology services in the UK for patients with skin disease is, at best, limited. In spite of clear demand, only 4% of dermatology units in the UK provide a dedicated counselling service [101]. Despite attempts to increase awareness and the provision of resources by groups such as the British Association of Dermatologists and Psoriasis Association, psychology services provision has, in fact, deteriorated since 2004 [101].
Good-quality evidence to support the instigation of dedicated psychology services for patients with psoriasis and other skin conditions is presently limited. Existing data for psychological interventions mainly come from studies that lack consistency in design and outcome measurements. The effect of psychological intervention on work, relationships and the need for systemic therapies, which would make these services cost-effective, has not been investigated adequately. The degree of heterogeneity in the studies prevents further extrapolation of results. There is a need for well-designed, adequately powered and well-controlled interventional studies to investigate the optimal psychological interventions for people with psoriasis. These data are required not only to inform future provision of psychological services for this population of patients who are presently under-supported, but also to provide hard evidence for the efficacy of such interventions as part of the successful management of psoriasis.
However, there is cause for optimism. Routinely measuring PASI and DLQI and asking about the life impact of psoriasis in clinics is a manageable start, even without dedicated psychology resources. Screening for psychological distress would detect un-diagnosed cases of anxiety and depression, enabling patients to be directed towards services that may offer treatment. Increased recognition of the psychological and life impact of psoriasis among clinicians is the first step towards improving care.
An understanding of the links between physical and psychological health is increasing. Government initiatives, such as ‘No Health Without Mental Health’ may help transform understanding into service improvement [102]. The future management of psoriasis may routinely involve multi-disciplinary teams to manage physical, psychological and social aspects of the condition in line with other systemic long-term conditions.
Acknowledgments
We are grateful for a grant from Dermatrust for funding the work of Dr Alexandra Mizara.
No funding was received in relation to this paper.
Dr. McBride is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Dr Sandy McBride has received funding from Abbott and Janssen for lecturing services and is a member of ‘See Psoriasis: Look Deeper’ and SPARK—both advisory groups funded by Abbott. Attendance at conferences has been funded by Abbott and Pfizer. Dr Alexandra Mizara received funding from Dermatrust for her research investigating schemas and psoriasis. Dr Hee-Sun Moon has no conflict of interest to declare.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 2.Kimball AB, Gieler U, Linder D, et al. Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol. 2010;24:989–1004. doi: 10.1111/j.1468-3083.2010.03705.x. [DOI] [PubMed] [Google Scholar]
- 3.Sampogna F, Tabolli S, Abeni D, et al. Living with psoriasis: prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Derm Venereol. 2012;92:299–303. doi: 10.2340/00015555-1273. [DOI] [PubMed] [Google Scholar]
- 4.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–407. doi: 10.1016/S0190-9622(99)70112-X. [DOI] [PubMed] [Google Scholar]
- 6.Russo PAJ, Ilchef R, Cooper AJ. Psychiatric morbidity in psoriasis: a review. Australas J Dermatol. 2004;45:155–161. doi: 10.1111/j.1440-0960.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 7.Lavda AC, Webb TL, Thompson AR. A meta-analysis of the effectiveness of psychological interventions for adults with skin conditions. Br J Dermatol. 2012;167:970–979. doi: 10.1111/j.1365-2133.2012.11183.x. [DOI] [PubMed] [Google Scholar]
- 8.British Association of Dermatologists: National Survey on Psychodermatology services. http://www.bad.org.uk//site/1464/default.aspx. (Accessed May 27, 2013).
- 9.Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752–756. doi: 10.1001/archderm.139.6.752. [DOI] [PubMed] [Google Scholar]
- 10.Gupta MA, Gupta AK, Kirkby S, Schork NJ. A psychocutaneous profile or psoriasis patients who are stress reactors: a study of 127 patients. Gen Hosp Psychiatry. 1989;11:166–173. doi: 10.1016/0163-8343(89)90036-4. [DOI] [PubMed] [Google Scholar]
- 11.Al’Abadie MS, Kent GG, Gawkrodger DJ. The relationship between stress and the onset and exacerbation of psoriasis and other skin conditions. Br J Dermatol. 1994;130:199–203. doi: 10.1111/j.1365-2133.1994.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 12.Fortune DG, Richards HL, Main CJ, Griffiths CEM. What patients with psoriasis believe about their condition. J Am Acad Dermatol. 1998;39:196–201. doi: 10.1016/S0190-9622(98)70074-X. [DOI] [PubMed] [Google Scholar]
- 13.Verhoeven EW, Kraaimaat FW, de Jong EM, et al. Effect of daily stressors on psoriasis: a prospective study. J Invest Dermatol. 2009;129:2075–2077. doi: 10.1038/jid.2008.460. [DOI] [PubMed] [Google Scholar]
- 14.Verhoeven EW, Kraaimaat FW, de Jong EM, et al. Individual differences in the effect of daily stressors on psoriasis: a prospective study. Br J Dermatol. 2009;161:295–299. doi: 10.1111/j.1365-2133.2009.09194.x. [DOI] [PubMed] [Google Scholar]
- 15.Cannon WB. The emergency function of the adrenal medulla in pain and the major emotions. Am J Physiol. 1914;33:356–372. [Google Scholar]
- 16.Qiu BS, Vallance BA, Blennerhassett PA, et al. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med. 1999;5:1178–1182. doi: 10.1038/13503. [DOI] [PubMed] [Google Scholar]
- 17.Cacioppo JT, Berntson GG, Malarkey WB, et al. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann N Y Acad Sci. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- 18.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 19.Elenkov IJ, Webster EL, Torpy DJ, Chrousos GP. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann NY Acad Sci. 1999;876:1–11. doi: 10.1111/j.1749-6632.1999.tb07618.x. [DOI] [PubMed] [Google Scholar]
- 20.McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP. Corticotropin-releasing hormone signalling in synovial tissue from patients with early inflammatory arthritis is mediated by the type 1a corticotrophin-releasing hormone receptor. Arthritis Rheum. 2001;44:1761–1767. doi: 10.1002/1529-0131(200108)44:8<1761::AID-ART311>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Ito N, Ito T, Kromminha A, et al. Human hair follicles display a functional equivalent of the hypothalamic–pituitary–adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 22.Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto I, Inoue Y, Shimada T, et al. Brain mast cells act as an immune gate to the hypothalamic–pituitary–adrenal axis in dogs. J Exp Med. 2001;194:71–78. doi: 10.1084/jem.194.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotrophin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci USA. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Richards HI, Ray DW, Kirby B, et al. Response of the hypothalamic–pituitary–adrenal axis to psychological stress in patients with psoriasis. Br J Dermatol. 2005;153:1114–1120. doi: 10.1111/j.1365-2133.2005.06817.x. [DOI] [PubMed] [Google Scholar]
- 27.Bear MF, Connors BW, Paradiso MA. Neuroscience exploring the brain. 2. Baltimore: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 28.Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- 29.Weiss SC, Kimball AB, Liewehr DJ, et al. Quantifying the harmful effect of psoriasis on health-related quality of life. J Am Acad Dermatol. 2002;47:512–518. doi: 10.1067/mjd.2002.122755. [DOI] [PubMed] [Google Scholar]
- 30.Fortune DG, Richards HL, Main CJ, Griffiths CEM. Pathological worrying, illness perceptions and disease severity in patients with psoriasis. Br J Health Psychol. 2000;5:71–82. doi: 10.1348/135910700168775. [DOI] [Google Scholar]
- 31.Gupta MA, Schork NJ, Gupta AK, et al. Suicidal ideation in psoriasis. Int J Dermatol. 1993;32:188–190. doi: 10.1111/j.1365-4362.1993.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 32.Mizara A, Papadopoulos L, McBride SR. Core beliefs and psychological distress in patients with psoriasis and atopic eczema attending secondary care: the role of schemas in chronic skin disease. Br J Dermatol. 2012;166:986–993. doi: 10.1111/j.1365-2133.2011.10799.x. [DOI] [PubMed] [Google Scholar]
- 33.Sampogna F, Tabolli S, Abeni D. The impact of changes in clinical severity of psychiatric morbidity in patients with psoriasis: a follow-up study. Br J Dermatol. 2007;157:508–513. doi: 10.1111/j.1365-2133.2007.08071.x. [DOI] [PubMed] [Google Scholar]
- 34.Choi J, Koo JYM. Quality of life issues in psoriasis. J Am Acad Dermatol. 2003;49:S57–S61. doi: 10.1016/S0190-9622(03)01136-8. [DOI] [PubMed] [Google Scholar]
- 35.Gupta G, Long J, Tillman DM. The efficacy of narrowband ultraviolet B phototherapy in psoriasis using objective and subjective outcome measures. Br J Dermatol. 1999;140:887–890. doi: 10.1046/j.1365-2133.1999.02820.x. [DOI] [PubMed] [Google Scholar]
- 36.Fortune DG, Richards HL, Kirby B, et al. Successful treatment of psoriasis improves psoriasis-specific but not more general aspects of patients’ well-being. Br J Dermatol. 2004;151:1219–1226. doi: 10.1111/j.1365-2133.2004.06222.x. [DOI] [PubMed] [Google Scholar]
- 37.Fortune DG, Main CJ, O’Sullivan TM, Griffiths CE. Quality of life in patients with psoriasis: the contribution of clinical variables and psoriasis-specific stress. Br J Dermatol. 1997;137:755–760. doi: 10.1111/j.1365-2133.1997.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 38.Wahl A, Moum T, Hanestad BR, Wiklund I. The relationship between demographic and clinical variables, and quality of life aspects in patients with psoriasis. Qual Life Res. 1999;8:319–326. doi: 10.1023/A:1008935921866. [DOI] [PubMed] [Google Scholar]
- 39.Devrimci-Ozguven H, Kundakci TN, Kumbasar H, Boyvat A. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. J Eur Acad Dermatol Venereol. 2000;14:267–271. doi: 10.1046/j.1468-3083.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 40.Vladut CI, Kallay È. Psychosocial implications of psoriasis—theoretical review. Cogn Brain Behav. 2010;14:23–35. [Google Scholar]
- 41.Kleyn CE, McKie S, Ross AR, et al. Diminished neural and cognitive responses to facial expressions of disgust in patients with psoriasis: a functional magnetic resonance imaging study. J Invest Dermatol. 2009;129:2613–2619. doi: 10.1038/jid.2009.152. [DOI] [PubMed] [Google Scholar]
- 42.Baughman RD, Sobel R. Psoriasis: a measure of severity. Arch Dermatol. 1970;101:390–395. doi: 10.1001/archderm.1970.04000040012004. [DOI] [PubMed] [Google Scholar]
- 43.Zachariae R, Zachariae H, Blomqvis K, et al. Quality of life in 6497 Nordic patients with psoriasis. Br J Dermatol. 2002;146:1006–1016. doi: 10.1046/j.1365-2133.2002.04742.x. [DOI] [PubMed] [Google Scholar]
- 44.Richards HL, Fortune DG, Weidmann A, Sweeney SK, Griffiths CE. Detection of psychological distress in patients with psoriasis: low consensus between dermatologist and patient. Br J Dermatol. 2004;151:1227–1233. doi: 10.1111/j.1365-2133.2004.06221.x. [DOI] [PubMed] [Google Scholar]
- 45.Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cognit Ther Res. 1992;16:143–163. doi: 10.1007/BF01173486. [DOI] [Google Scholar]
- 46.Fortune DG, Richards HL, Griffiths CE, Main CJ. Psychological stress, distress and disability in patients with psoriasis: consensus and variation in the contribution of illness perceptions, coping and alexithymia. Br J Clin Psychol. 2002;41:157–174. doi: 10.1348/014466502163949. [DOI] [PubMed] [Google Scholar]
- 47.Richards HL, Fortune DG, Griffiths CE, Main CJ. Alexithymia in patients with psoriasis: clinical correlates and psychometric properties of the Toronto Alexithymia Scale-20. J Psychosom Res. 2005;58:89–96. doi: 10.1016/j.jpsychores.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Ginsburg IH, Link BG. Feelings of stigmatization in patients with psoriasis. J Am Acad Dermatol. 1989;20:53–63. doi: 10.1016/S0190-9622(89)70007-4. [DOI] [PubMed] [Google Scholar]
- 49.Hrehorów E, Salomon J, Matusiak L, Reich A, Szepietowski JC. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67–72. doi: 10.2340/00015555-1193. [DOI] [PubMed] [Google Scholar]
- 50.Ginsburg IH, Link BG. Psychosocial consequence of rejection and stigma feelings in psoriatic patients. Int J Dermatol. 1993;32:587–591. doi: 10.1111/j.1365-4362.1993.tb05031.x. [DOI] [PubMed] [Google Scholar]
- 51.Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experienced in dermatologic disorders. Cutis. 1998;61:339–342. [PubMed] [Google Scholar]
- 52.Eghlileb AM, Davies EEG, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245–1250. doi: 10.1111/j.1365-2133.2007.07881.x. [DOI] [PubMed] [Google Scholar]
- 53.Poot F, Antoine E, Gravellier M, et al. A case-control study on family dysfunction in patients with alopecia areata, psoriasis and atopic dermatitis. Acta Derm Venereol. 2011;91:415–421. doi: 10.2340/00015555-1074. [DOI] [PubMed] [Google Scholar]
- 54.Gupta MA, Gupta AK. Psoriasis and sex: a study of moderately to severely affected patients. Int J Dermatol. 1997;36:259–262. doi: 10.1046/j.1365-4362.1997.00032.x. [DOI] [PubMed] [Google Scholar]
- 55.Sampogna F, Gisondi P, Tabolli S, Abeni D, IDI Multipurpose Psoriasis Research on Vital Experiences Investigators Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144–150. doi: 10.1159/000098574. [DOI] [PubMed] [Google Scholar]
- 56.Goulding JM, Price CL, Defty CL, Hulangamuwa CS, Bader E, Ahmed I. Erectile dysfunction in patients with psoriasis: increased prevalence, an unmet need, and a chance to intervene. Br J Dermatol. 2011;154:103–109. doi: 10.1111/j.1365-2133.2010.10077.x. [DOI] [PubMed] [Google Scholar]
- 57.Hughes JE, Barraclough BM, Hamblin LG, White JE. Psychiatric symptoms in dermatology patients. Br J Psychol. 1983;143:51–54. doi: 10.1192/bjp.143.1.51. [DOI] [PubMed] [Google Scholar]
- 58.Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772–780. doi: 10.1016/j.jaad.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 59.Horn EJ, Fox KM, Patel V, et al. Association of patient-reported psoriasis severity with income and employment. J Am Acad Dermatol. 2007;57:963–971. doi: 10.1016/j.jaad.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 60.Reich K, Schenkel B, Zhao N, et al. Ustekinumab decreases work limitations, improves work productivity, and reduces work days missed in patients with moderate-to-severe psoriasis: results from PHOENIX 2. J Dermatol Treat. 2011;22:337–347. doi: 10.3109/09546634.2010.499931. [DOI] [PubMed] [Google Scholar]
- 61.Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67–e76. doi: 10.1016/j.jaad.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 62.Pearce DJ, Singh S, Balkrishnan R, et al. The negative impact of psoriasis on the workplace. J Dermatolog Treat. 2006;17:24–28. doi: 10.1080/09546630500482886. [DOI] [PubMed] [Google Scholar]
- 63.Higgins EM, Peters TJ, du Vivier AW. Smoking, drinking and psoriasis. Br J Dermatol. 1993;129:749–750. doi: 10.1111/j.1365-2133.1993.tb03349.x. [DOI] [PubMed] [Google Scholar]
- 64.McAleer MA, Mason DL, Cunningham S, et al. Alcohol misuse in patients with psoriasis: identification and relationship to disease severity and psychological distress. Br J Dermatol. 2011;164:1256–1261. doi: 10.1111/j.1365-2133.2011.10345.x. [DOI] [PubMed] [Google Scholar]
- 65.Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138–140. doi: 10.1111/j.1365-2133.2007.08299.x. [DOI] [PubMed] [Google Scholar]
- 66.Mills CM, Srivastava ED, Harvey IM. Smoking habits in psoriasis: a case controlled study. Br J Dermatol. 1992;127:18–21. doi: 10.1111/j.1365-2133.1992.tb14818.x. [DOI] [PubMed] [Google Scholar]
- 67.Gupta MA, Schork NJ, Gupta AK, Ellis CN. Alcohol intake and treatment responsiveness of psoriasis: a prospective study. J Am Acad Dermatol. 1993;28:730–732. doi: 10.1016/0190-9622(93)70101-X. [DOI] [PubMed] [Google Scholar]
- 68.Poikolainen K, Karvonen J, Pukkala E. Excess mortality related to alcohol and smoking among hospital-treated patients with psoriasis. Arch Dermatol. 1999;135:1490–1493. doi: 10.1001/archderm.135.12.1490. [DOI] [PubMed] [Google Scholar]
- 69.Naldi L, Parazzini P, Brevi A, et al. Family history, smoking habits, alcohol consumption and risk of psoriasis. Br J Dermatol. 1992;127:212–217. doi: 10.1111/j.1365-2133.1992.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 70.Naldi L. Cigarette smoking and psoriasis. Clin Dermatol. 1998;16:571–574. doi: 10.1016/S0738-081X(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 71.Gordon KB, Langley RG, Lenardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55:598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 72.Shikiar R, Heffernan M, Langley RG, et al. Adalimumab treatment is associated with improvement in health-related quality of life in psoriasis: patient-reported outcomes from a phase II randomized controlled trial. J Dermatol Treat. 2007;18:25–31. doi: 10.1080/09546630601121060. [DOI] [PubMed] [Google Scholar]
- 73.Revicki DA, Menter A, Feldman S, et al. Adalimumab improves health-related quality of life in patients with moderate to severe plaque psoriasis compared with the United States general population norms: results from a randomized, controlled Phase III study. Health Qual Life Outcomes. 2008;6:75. doi: 10.1186/1477-7525-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Himmerich H, Fulda S, Linseisen J, et al. Depression, comorbidities and the TNF-alpha system. Eur Psychiatry. 2008;23:421–429. doi: 10.1016/j.eurpsy.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Patarca R, Klimas NG, Lugtendorf S, Antoni M, Fletcher MA. Dysregulated expression of tumor necrosis factor in chronic fatigue syndrome: interrelations with cellular sources and patterns of soluble immune mediator expression. Clin Infect Dis. 1994;18(Suppl 1):S147–S153. doi: 10.1093/clinids/18.Supplement_1.S147. [DOI] [PubMed] [Google Scholar]
- 76.Illman J, Corringham R, Robinson D, Jr, et al. Are inflammatory cytokines the common link between cancer-associated cachexia and depression? J Support Oncol. 2005;3:37–50. [PubMed] [Google Scholar]
- 77.Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomized phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 78.Langley RG, Feldman SR, Han C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63:457–465. doi: 10.1016/j.jaad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 79.Alpsoy E, Ozcan E, Cetin L, et al. Is the efficacy of topical corticosteroid therapy for psoriasis vulgaris enhanced by concurrent moclobemide therapy? A double-blind, placebo-controlled study. J Am Acad Dermatol. 1998;38:197–200. doi: 10.1016/S0190-9622(98)70240-3. [DOI] [PubMed] [Google Scholar]
- 80.D’Erme AM, Zanieri F, Campolmi E, et al. Therapeutic implications of adding the psychotropic drug escitalopram in the treatment of patients suffering from moderate–severe psoriasis and psychiatric comorbidity: a retrospective study. J Eur Acad Dermatol Venereol. 2012 (Epub ahead of print). [DOI] [PubMed]
- 81.Hemlock C, Rosenthal JS, Winston A. Fluoxetine-induced psoriasis. Ann Pharmacother. 1992;26:211–212. doi: 10.1177/106002809202600215. [DOI] [PubMed] [Google Scholar]
- 82.Tan Pei Lin L, Kwek SK. Onset of psoriasis during therapy with fluoxetine. Gen Hosp Psychiatry. 2010;32:446.e9–10. [DOI] [PubMed]
- 83.Tamer E, Gur G, Polat M, Alli N. Flare-up of pustular psoriasis with fluoxetine: possibility of a serotoninergic influence? J Dermatol Treat. 2009;20:137–140. doi: 10.1080/09546630802449096. [DOI] [PubMed] [Google Scholar]
- 84.Cox NH, Gordon PM, Dodd H. Generalized pustular and erythrodermic psoriasis associated with bupropion treatment. Br J Dermatol. 2002;146:1061–1063. doi: 10.1046/j.1365-2133.2002.04679.x. [DOI] [PubMed] [Google Scholar]
- 85.Basavaraj KH, Ashok NM, Rashmi R, Praveen TK. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351–1361. doi: 10.1111/j.1365-4632.2010.04570.x. [DOI] [PubMed] [Google Scholar]
- 86.Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA) Psychosom Med. 1998;60:625–632. doi: 10.1097/00006842-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 87.Gaston L, Crombez JC, Joly J, et al. Efficacy of imagery and meditation techniques in treating psoriasis. Imagin Cogn Pers. 1988;8:25–38. doi: 10.2190/PQKE-CMAM-T4MT-QVBL. [DOI] [Google Scholar]
- 88.Vedhara K, Morris RM, Booth R, et al. Changes in mood predict disease activity and quality of life in patients with psoriasis following emotional disclosure. J Psychosom Res. 2007;62:611–619. doi: 10.1016/j.jpsychores.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 89.Paradisi A, Abeni D, Finore E, et al. Effect of written emotional disclosure interventions in persons with psoriasis undergoing narrow band ultraviolet B phototherapy. Eur J Dermatol. 2010;20:599–605. doi: 10.1684/ejd.2010.1018. [DOI] [PubMed] [Google Scholar]
- 90.Papadopoulos L. Psychological therapies for dermatological problems. In: Walker C, Papadopoulos L, editors. Psychodermatology: the psychological impact of skin disorders. Cambridge: Cambridge University Press; 2005. pp. 29–43. [Google Scholar]
- 91.Tausk F, Whitmore S. A pilot study of hypnosis in the treatment of patients with psoriasis. Psychother Psychosom. 1999;68:221–225. doi: 10.1159/000012336. [DOI] [PubMed] [Google Scholar]
- 92.Price ML, Mottahedin I, Mayo PR. Can psychotherapy help patients with psoriasis? Clin Exp Dermatol. 1991;16:114–117. doi: 10.1111/j.1365-2230.1991.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 93.Rapp SR, Cottrell CA, Leary MR. Social coping strategies associated with quality of life decrements among psoriasis patients. Br J Dermatol. 2001;145:610–616. doi: 10.1046/j.1365-2133.2001.04444.x. [DOI] [PubMed] [Google Scholar]
- 94.Wahl A, Hanestad BR, Wiklund I, Moum T. Coping and quality of life in patients with psoriasis. Qual Life Res. 1999;8:427–433. doi: 10.1023/A:1008944108101. [DOI] [PubMed] [Google Scholar]
- 95.Scharloo M, Kaptein AA, Weinman J, et al. Patients’ illness perceptions and coping as predictors of functional status in psoriasis: a 1-year follow-up. Br J Dermatol. 2000;142:899–907. doi: 10.1046/j.1365-2133.2000.03469.x. [DOI] [PubMed] [Google Scholar]
- 96.Fortune DG, Richards HL, Kirby B, et al. A cognitive-behavioural symptom management programme as an adjunct in psoriasis therapy. Br J Dermatol. 2002;146:458–465. doi: 10.1046/j.1365-2133.2002.04622.x. [DOI] [PubMed] [Google Scholar]
- 97.Zacharie R, Oster H, Bjerring P, Kragballe K. Effects of psychologic intervention on psoriasis: a preliminary report. J Am Acad Dermatol. 1996;34:1008–1015. doi: 10.1016/S0190-9622(96)90280-7. [DOI] [PubMed] [Google Scholar]
- 98.National Institute for Clinical Excellence (NICE) clinical guideline 153 (Oct 2012). Psoriasis. The assessment and management of psoriasis. http://www.nice.org.uk/nicemedia/live/13938/61192/61192.pdf. (Accessed June 21, 2013).
- 99.Pathirana D, Ormerod AD, Saiag P, et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris. http://www.abw-verlag.com/db_picture.php?120;BOOKS_BookList;Leseprobe. (Accessed Oct 28, 2012). [DOI] [PubMed]
- 100.Scottish Intercollegiate Guidelines Network (2010). Diagnosis and management of psoriasis and psoriatic arthritis in adults. Edinburgh: SIGN (SIGN publication no. 121). http://www.sign.ac.uk/guidelines/fulltext/121/contents.html. (Accessed Oct 28, 2012).
- 101.Bewley AP, Fleming C, Taylor R. Psychocutaneous medicine and its provision in the UK. Poster presentation at the Annual Meeting of the British Association of Dermatologists, 2012. Abstract number P32 Br J Dermatol. 2012;167(Suppl.1):36.
- 102.Department of Health (2011). No Health Without Mental Health: A cross-government mental health outcomes strategy for people of all ages. http://www.dh.gov.uk/health/2011/07/mental-health-strategy. (Accessed June 21, 2013).


