Abstract
Auditory feedback plays an important role in phonatory control. When auditory feedback is disrupted, various changes are observed in vocal motor control. Vocal intensity and fundamental frequency (F0) levels tend to increase in response to auditory masking. Because of the close reflexive links between the auditory and phonatory systems, it is likely that phonatory stability may be disrupted when auditory feedback is disrupted or altered. However, studies on phonatory stability under auditory masking condition in adult subjects showed that most of the subjects maintained normal levels of phonatory stability. The authors in the earlier investigations suggested that auditory feedback is not the sole contributor to vocal motor control and phonatory stability, a complex neuromuscular reflex system known as kinaesthetic feedback may play a role in controlling phonatory stability when auditory feedback is disrupted or lacking. This proposes the need to further investigate this phenomenon as to whether children show similar patterns of phonatory stability under auditory masking since their neuromotor systems are still at developmental stage, less mature and are less resistant to altered auditory feedback than adults. A total of 40 normal hearing and speaking children (20 male and 20 female) between the age group of 6 and 8 years participated as subjects. The acoustic parameters such as shimmer, jitter and harmonic-to-noise ratio (HNR) were measures and compared between no masking condition (0 dB ML) and masking condition (90 dB ML). Despite the neuromotor systems being less mature in children and less resistant than adults to altered auditory feedback, most of the children in the study demonstrated increased phonatory stability which was reflected by reduced shimmer, jitter and increased HNR values. This study implicates that most of the children demonstrate well established patterns of kinaesthetic feedback, which might have allowed them to maintain normal levels of vocal motor control even in the presence of disturbed auditory feedback. Hence, it can be concluded that children also exhibit kinaesthetic feedback mechanism to control phonatory stability when auditory feedback is disrupted which in turn highlights the importance of kinaesthetic feedback to be included in the therapeutic/intervention approaches for children with hearing and neurogenic speech deficits.
Keywords: Phonatory stability, Auditory masking, Jitter, Shimmer, Harmonic-to-noise ratio (HNR), Auditory feedback, Kinaesthetic feedback
Introduction
Voice is an acoustic signal generated by the vibrations of the vocal folds and modified by the resonating cavities of the vocal tract. The physical process of exhaling air between adducted vocal folds produces voice, called phonation. In addition to respiration, the intrinsic laryngeal muscles cause the vocal folds to either approximate towards each other, called as adduction or move away from each other, called as abduction. Apart from pre-phonatory tuning, two basic neurological control systems have been described that act on the laryngeal musculature during phonation, namely auditory and kinaesthetic feedback systems [14].
Auditory feedback system plays an important role in phonatory control as well as vocal fold function by regulating respiratory and phonatory physiological processes [7, 10]. When auditory feedback is disrupted, various changes are observed in vocal motor control. A typical example of this is the ‘Lombard effect’ which is the tendency to increase vocal intensity in response to increased background noise [12]. The magnitude of the intensity increase tends to vary among individuals, but it has been found to be significant [1]. In addition to vocal intensity, the vocal fundamental frequency (F0) levels also tend to increase in response to masking noise, although this finding is not as consistent as intensity [3, 11]. Researchers reported that a complex neuromuscular reflex system referred to as kinaesthetic feedback plays crucial role in the control of finer aspects of vocal fundamental frequency (F0) [13].
Phonatory stability refers to the steadiness and periodicity of vocal fold vibration, which is dependent on the laryngeal system maintaining relatively constant levels of muscular force during a sustained phonation [4]. The acoustic dimensions such as jitter, shimmer and harmonic-to-noise ratio (HNR) have been shown to reflect the phonatory stability of vocal fold vibration. Haung et al. [6] investigated the influence of vocal effort level (low, normal and high) under normal auditory feedback condition during sustained vowel production on jitter, shimmer and glottal noise. They observed that the high vocal levels produced relatively low values for jitter, shimmer and glottal noise (i.e. increased phonatory stability). Brockmann et al. [2] investigated the effect of vocal loudness on jitter and shimmer and observed that jitter and shimmer values significantly increased with decreasing vocal loudness (i.e. decreased phonatory stability).
Because of the close reflexive links between the auditory and phonatory systems, it is likely that phonatory stability may be disrupted when auditory feedback is altered. Ferrand et al. [4] studied phonatory stability in adult women subjects under auditory masking condition and found that most of the subjects showed increased phonatory stability when the auditory feedback was disrupted. Kumar et al. [8] studied the effect of auditory masking on phonatory stability in adult male and female subjects and found that most of their subjects, both male and female demonstrated increased phonatory stability. The findings of the above studies reveal that under disrupted auditory feedback, most of the normal adult subjects both male and female maintain relatively better phonatory stability reflected by decreased shimmer, jitter and increased HNR values.
The increased phonatory stability reflected by decreased shimmer and jitter values in the previous studies could be attributed to the high vocal effort and intensity. The explanation for this could be, during high vocal effort the vocal folds are closed for a longer duration of each vocal cycle resulting in increased intensity level than, during normal and low vocal effort levels of phonation [6]. In addition, as intensity increases, the vocalis and cricothyroid muscles exert a more balanced force and create a stabilizing effect by reducing irregular fluctuations in the length and tension of the vocal folds which may lead to increased stability [5]. The balanced muscular force exerted by laryngeal muscles may lead to reduced noise component. This decrease in the noise component of a sound increases the ratio of harmonic to inharmonic components resulting in increased HNR.
These results suggest that although auditory feedback plays an important role in phonatory control, it is not the sole contributor to vocal motor control and phonatory stability. Kinaesthetic feedback, a complex neuromuscular reflex system may also play an important role in vocal motor control and phonatory stability. This intrinsic laryngeal reflex system may be particularly active when auditory feedback is disrupted or lacking, thus engaging the kinaesthetic system more efficiently when auditory–laryngeal control system is prevented from operating efficiently [4].
The review of literature indicates that even when auditory feedback is disrupted normal adult subjects are able to maintain relatively better phonatory stability. A complex neuromuscular reflex system known as kinaesthetic feedback has been reported to be particularly active in controlling phonatory stability when auditory feedback is disrupted [4]. This proposes the need to further investigate this phenomenon as to whether children show similar patterns of phonatory stability under auditory masking since their neuromotor systems which are still at developmental stage, less mature in terms of learned experience and hence would be less resistant than adults to altered auditory feedback.
Aim of the Study
The aim of the present study was to investigate the effect of auditory masking on phonatory stability in normal speaking children in order to find out whether children also exhibit kinaesthetic feedback mechanism for phonatory stability when auditory laryngeal control system is prevented from operating through masking.
Methodology
Subjects
A total of 40 children with normal hearing and no speech deficits were selected as subjects and the subjects were further divided into two groups in the study based on their gender. The age of the subjects ranged from 6 to 8 years. Group I (M)—consisted of 20 male children, with an average age of 7 years. Group II (F)—consisted of 20 female children, with an average age of 7.2 years. All the subjects had undergone thorough medical examination to rule out presence or reported history of voice, hearing, respiratory, or neurological problems and vocal misuse/abuse. None of them was a trained singer.
Procedure
All the participants were subjected to audiological evaluation to ensure the hearing thresholds within normal limits. Voice recording was done for each subject in a sound treated speech and hearing science laboratory. Each subject was seated comfortably during the individual recording session; a professional quality microphone was placed at a distance of 8–10 cm from the subject’s mouth. The subjects were instructed to sustain the vowel /a/ as steady as possible at a comfortable pitch and loudness level and the phonation was recorded. The phonation of /a/ thus elicited was recorded using SONY digital recorder. Same procedure was repeated for the second condition in which subjects were presented with white noise through headphones. A MADSON clinical diagnostic audiometer was used for presenting white noise and audiological evaluation.
Voice Analysis
The recorded sample was fed to a personal computer using line feed and stored on the computer using the software PRAAT (version 4.5.06 Paul and Davis). Each vowel phonation sustained by each subject was displayed on the monitor of the computer, which was further visually inspected to highlight and extract the 2 s of the total stable phonation with the help of selection of the particular segment by moving cursor. In order to minimize any potential initiation and termination effect, the first and last 500 ms of each vowel were discarded and the remaining 1,000 ms portion of the signal were extracted for analysis. Using this procedure each vowel phonation under no masking condition (0 dB ML) and masking condition (90 dB ML) was analyzed. The values for Shimmer, Jitter and HNR were calculated for the production of /a/ vowel for each group (male and female subjects) under two conditions (0 dB ML and 90 dB ML). The magnitude of change in above mentioned parameters from 0 to 90 dB ML condition were also calculated for both male and female child subjects.
Statistical Analysis of Data
Mean and standard deviation values were calculated for each parameter under two conditions for each subject separately. The data was further subjected to paired comparison ttest in order to find out significant difference between two conditions for each parameter within the subjects and independent comparison t-test in order to find out significant difference in terms of the magnitude of change from 0 to 90 dB ML condition between male and female child subjects. The results are discussed for each parameter as follows.
Results
Amplitude Perturbation or Shimmer
As shown in Fig. 1, the mean shimmer value under 0 dB ML condition is 0.4817 dB as compared to the mean shimmer value of 0.2944 dB under 90 dB ML condition for male subjects. The magnitude of change in shimmer between the two conditions averaged 0.1873 dB and it was found to be statistically significant (p < 0.05). Inspection of individual values revealed that 80 % of subjects obtained decreased shimmer values (i.e. increased phonatory stability), whereas only 20 % of subjects (subjects 7, 9, 13 & 15 as shown in Fig. 1) obtained increased shimmer values (i.e. decreased phonatory stability).
Fig. 1.
Mean Shimmer values obtained by male children between two masking conditions (0 and 90 dB ML) Note: Subjects 7, 9, 13 & 15 indicated by solid filled circles obtained increased shimmer values under 90 dB ML depicting decreased phonatory stability
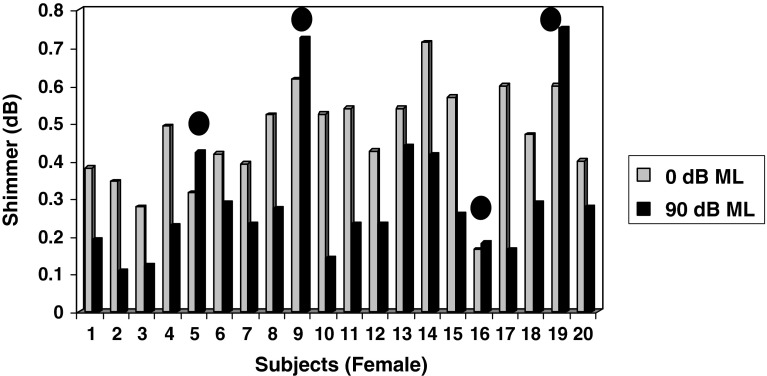
As shown in Fig. 2, the mean shimmer under 0 dB ML condition is 0.4742 dB as compared to the mean shimmer value of 0.2989 dB under 90 dB ML condition for female subjects. The magnitude of change in shimmer between the two conditions averaged 0.1753 dB and was found to be statistically significant (p < 0.05). Inspection of individual values revealed that 80 % of female subjects obtained decreased shimmer values (i.e. increased phonatory stability), whereas only 20 % of female subjects (subjects 5, 9, 16 & 19 as shown in Fig. 2) obtained increased shimmer values (i.e. decreased phonatory stability).
Fig. 2.
Mean Shimmer values obtained by female children between two masking conditions (0 and 90 dB ML) Note: Subjects 5, 9, 16 & 19 indicated by solid filled circles obtained increased shimmer values under 90 dB ML depicting decreased phonatory stability
Pitch Perturbation or Jitter
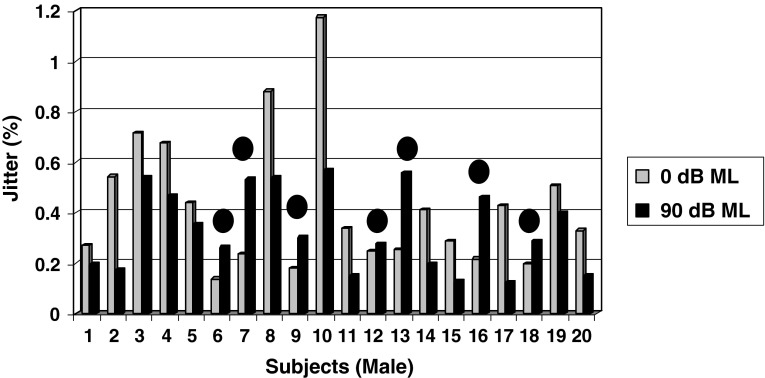
As shown in Fig. 3, the mean jitter value under 0 dB ML condition is 0.4817 % as compared to the mean jitter value of 0.3323 % under 90 dB ML condition for male child subjects and the magnitude of change averaged 0.1494 % and it was found to be statistically significant (p < 0.05). However, inspection of individual values reveals that 65 % of male child subjects decreased jitter levels (i.e. increased phonatory stability), whereas only 35 % of male subjects (subjects 6, 7, 9, 12, 13, 16 & 18 as shown in Fig. 3) obtained increased jitter values (i.e. decreased phonatory stability).
Fig. 3.
Mean Jitter values obtained by male children between two masking conditions (0 and 90 dB ML) Note: Subjects 6, 7, 9, 12, 13, 16 & 18 indicated by solid filled circles obtained increased shimmer values under 90 dB ML depicting decreased phonatory stability
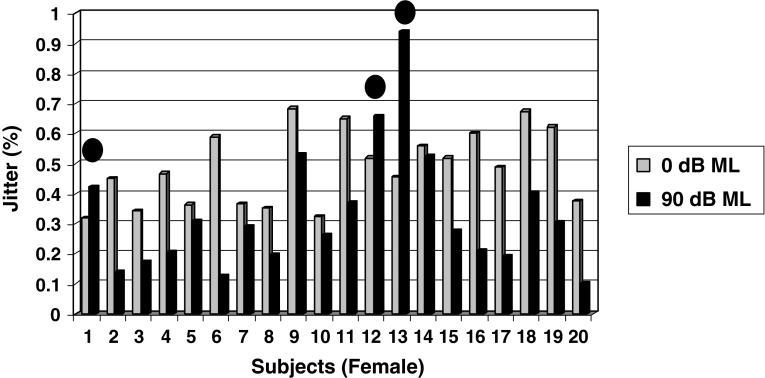
As shown in Fig. 4 the mean jitter value under 0 dB ML condition was 0.4859 % as compared to the mean jitter value of 0.3314 % under 90 dB ML condition for female subjects. The magnitude of change between two conditions averaged 0.1545 % and was found to be statistically significant (p < 0.05). Inspection of individual values revealed that 85 % of female subjects obtained decreased jitter levels (i.e. increased phonatory stability), whereas only 15 % of the female subjects (subjects 1, 12 & 13 as shown in Fig. 4) obtained increased jitter values (i.e. decreased phonatory stability).
Fig. 4.
Mean Jitter values obtained by female children between two masking conditions (0 and 90 dB ML) Note: Subjects 1, 12 & 13 indicated by solid filled circles obtained increased shimmer values under 90 dB ML depicting decreased phonatory stability
Harmonic-to-Noise Ratio (HNR)
As shown in Fig. 5, the mean HNR value under 0 dB ML condition was 13.85 dB as compared to the mean HNR value of 18.52 dB under 90 dB ML condition for male subjects. The magnitude of change in HNR values between two conditions averaged 4.67 dB and was found to be statistically significant (p < 0.05). Inspection of individual values revealed that 90 % of male subjects obtained increased HNR levels (i.e. increased phonatory stability), whereas only 10 % of male subjects (subjects 7 & 13 as shown in Fig. 5) obtained decreased HNR values (i.e. decreased phonatory stability).
Fig. 5.
Mean Harmonic to noise ratio (HNR) Values obtained by Male children between two masking conditions (0 and 90 dB ML) Note: Subjects 7 & 13 indicated by solid filled circles obtained decreased HNR values under 90 dB ML depicting decreased phonatory stability
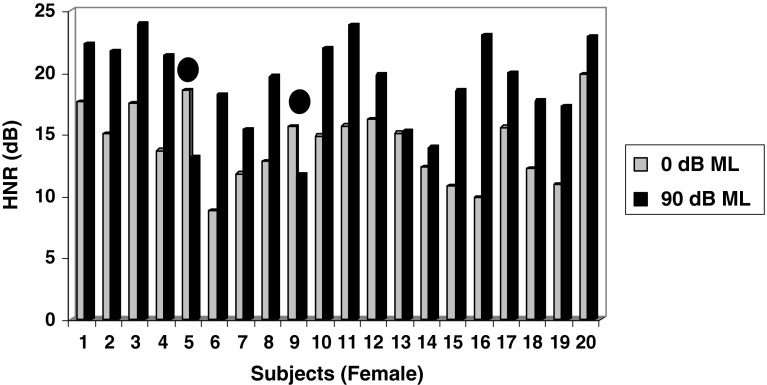
As shown in Fig. 6 above, the mean HNR value under 0 dB ML condition was 14.24 dB as compared to the mean HNR value of 19.07 dB under 90 dB ML condition for female subjects. The magnitude of change in HNR values between two conditions averaged 4.83 dB and was found to be statistically significant (p < 0.05). Inspection of individual values revealed that 90 % of female subject’s increased HNR levels (i.e. increased phonatory stability), whereas only 10 % of female subjects (subjects 5 & 9 as shown in Fig. 6) obtained decreased HNR values (i.e. decreased phonatory stability).
Fig. 6.
Mean Harmonic to noise ratio (HNR) Values obtained by female children between two masking conditions (0 and 90 dB ML) Note: Subjects 5 & 9 indicated by solid filled circles obtained decreased HNR values under 90 dB ML depicting decreased phonatory stability
Overall Findings
As shown in Fig. 7, the children obtained an overall (both male & female) mean shimmer value of 0.477 dB under 0 dB ML as compared to 0.296 dB shimmer under 90 dB ML. The magnitude of change in shimmer value was 0.181 dB and was found to be statistically significant (p < 0.05). Further inspection of individual values revealed that 80 % of children obtained reduced shimmer values (i.e. increased phonatory stability) and only 20 % of children obtained increased shimmer (i.e. decreased phonatory stability). There was no statistically significant difference found between male and female children.
Fig. 7.
Overall mean values of Shimmer, Jitter and HNR between two masking conditions (0 and 90 dB ML)
As shown in Fig. 7, the children obtained an overall (both male & female) mean jitter value of 0.479 % under 0 dB ML as compared to 0.331 % of jitter under 90 dB ML. The magnitude of change in jitter value was 0.148 % and was found to be statistically significant (p < 0.05). Further inspection of individual values revealed that 75 % of children obtained reduced jitter values (i.e. increased phonatory stability) and only 25 % of children obtained increased jitter (i.e. decreased phonatory stability). There was no statistically significant difference found between male and female children.
As shown in Fig. 7, the children obtained an overall (both male & female) mean HNR value of 14.04 dB under 0 dB ML as compared to 18.80 dB of HNR under 90 dB ML. The magnitude of change in HNR value was 4.76 dB and was found to be statistically significant (p < 0.05). Further inspection of individual values revealed that 90 % of children obtained increased HNR values (i.e. increased phonatory stability) and only 10 % of children obtained decreased HNR values (i.e. decreased phonatory stability). There was no statistically significant difference found between male and female children.
Discussion
Given that the phonatory control is highly dependent on the ability in self monitoring of voice through audition [7], most of the children both male and female participated in the present study demonstrated an increased phonatory stability reflected by decreased shimmer, jitter and increased HNR values, is an interesting finding of the current study. The children in the study demonstrated increased phonatory stability like adult subjects as reported in the earlier studies of [4, 8] done on adult population. The increased phonatory stability in children which was reflected by reduced shimmer, jitter and increased HNR values can also be attributed to the increased vocal effort and intensity as a part of compensatory mechanism to improve efficiency of kinaesthetic feedback in regulating phonatory stability under masking condition.
These findings in children could be attributed to the condition that during high vocal effort, the vocal folds are approximated for a longer duration of each cycle resulting in increased intensity level [6] and as intensity increases, the laryngeal muscles exert a more balanced force, stabilizing effect and reduced irregular fluctuations in the length and tension of the vocal folds [5]. This would have led to decreased shimmer, jitter and increased HNR values. However the present results suggest these values remained same even under efficient disruption of auditory feedback. Hence it can be inferred that, though auditory feedback plays an important role in phonatory control, the variability evident in this study may be that auditory feedback is not the sole contributor to vocal motor control and phonatory stability. These results suggest that kinaesthetic feedback may play an important role in maintaining phonatory stability in children as with adults. Kinaesthetic feedback results from the activity of sensory mechanoreceptors in the muscles, joints, and mucosal layers of the larynx, as well in the vocal tract and thoracic cavity [9]. The afferent discharge of the sensory receptors in response to changes in laryngeal and vocal tract conditions is transmitted to the brain stem and then relayed to the motor neurons to the laryngeal muscles. This intrinsic laryngeal reflex system may be particularly active when auditory feedback is disrupted or lacking, thus engaging the kinaesthetic system more efficiently when auditory–laryngeal control system is prevented from operating efficiently [4].
Conclusion
The current study was aimed at investigating the effect of auditory masking on phonatory stability by measuring shimmer, jitter and HNR in children. Despite the neuromotor systems being in the developmental process of maturity in children and less resistant than adults to altered auditory feedback, the children participated in the present study demonstrated increased phonatory stability which was reflected by reduced shimmer, jitter and increased HNR values. It seems most of the children in this study might have compensated for efficient kinaesthetic feedback by increasing vocal level that allowed them to maintain normal levels of phonatory stability even in the presence of disrupted auditory feedback. It can be inferred that children also exhibit preference for kinaesthetic feedback mechanism to control phonatory stability when auditory feedback is disrupted. Hence, this kinaesthetic feedback can be included in the therapeutic/intervention approaches for children with hearing and neurogenic speech deficits.
Acknowledgments
Authors thank all the children participated as subjects in the study. Authors also thank Dr. Prakash Boominathan and Mr. Narasimhan for their valuable suggestions and guidance in completion of this article.
References
- 1.Adams MR, Hutchinson J. The effect of three levels of auditory masking on selected vocal characteristics and frequency of dysfluency of adult stutterers. J Speech Hear Res. 1974;17:671–675. doi: 10.1044/jshr.1704.682. [DOI] [PubMed] [Google Scholar]
- 2.Brockmann M, Storck C, Carding PN, Drinnan MJ. Voice loudness and gender effects on jitter and shimmer in healthy adults. J Speech Lang Hear Res. 2008;51(5):1152–1160. doi: 10.1044/1092-4388(2008/06-0208). [DOI] [PubMed] [Google Scholar]
- 3.Burnett TA, Senner JE, Larson CR. Voice F0 responses to pitch-shifted auditory feedback a preliminary study. J Voice. 1997;11:202–211. doi: 10.1016/S0892-1997(97)80079-3. [DOI] [PubMed] [Google Scholar]
- 4.Ferrand CT. Relationship between masking levels and phonatory stability in normal speaking women. J Voice. 2006;20(2):223–228. doi: 10.1016/j.jvoice.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Gelfer MP. Fundamental frequency, intensity, and vowel selection: Effect on measurements on phonatory stability. J Speech Hear Res. 1995;36:689–699. doi: 10.1044/jshr.3806.1189. [DOI] [PubMed] [Google Scholar]
- 6.Haung DZ, Minifie FD, Kasuya H, Lin SX. Measures of vocal function during changes in vocal effort level. J Voice. 1995;9:429–438. doi: 10.1016/S0892-1997(05)80206-1. [DOI] [PubMed] [Google Scholar]
- 7.Higgins MB, Carney AE, Schulte L. Physiological assessment of speech and voice production of adults with hearing loss. J Speech Hear Res. 1994;37:510–521. doi: 10.1044/jshr.3703.510. [DOI] [PubMed] [Google Scholar]
- 8.Kumar SBR, Mohanty P, Prakash SGR, Anusha K. The effect of auditory masking on phonatory stability in normal speaking young subjects. J Linguistic Soc India. 2010;71:153–172. [Google Scholar]
- 9.Murbe D, Pabst F, Hoffman G, Sundberg J (2002) Significance of auditory and kinaesthetic feedback to singers’ pitch control. J Voice 16:44–51 [DOI] [PubMed]
- 10.Sapir S, McClean MD, Larson CR (1983) Human laryngeal responses to auditory stimulation. J Acoust Soc Am 73:315–321 [DOI] [PubMed]
- 11.Schultz-Coulon HJ (1978) Neuromuscular phonatory control system and vocal function. Acta Otolaryngol 86:142–153 [DOI] [PubMed]
- 12.Silman S (1991) Auditory diagnosis: principles and applications. Academic Press, California, p 142
- 13.Sundberg J, Iwarson J, Billstrom AH. Significance of mechanoreceptors in the sub-glottal mucosa for sub-glottal pressure control in singers. J Voice. 1995;9:20–26. doi: 10.1016/S0892-1997(05)80219-X. [DOI] [PubMed] [Google Scholar]
- 14.Wyke BD. Laryngeal myotatic reflexes and phonation. Foliaphoniatrica. 1974;26:249–264. doi: 10.1159/000263784. [DOI] [PubMed] [Google Scholar]