Abstract
Biofilm formation is present in the middle-ear mucosa of chronic otitis media (COM) patients and COM is a biofilm-related disease. Biofilms are organized and complex communities in which bacteria communicate to each other and gain tremendous advantages. In this unique structure, bacteria can diffuse nutrients, gain resistance to antimicrobials agents and host defense mechanisms. Recently bacterial biofilms have been proven to be important in infectious diseases of head and neck region. A prospective case–control study was conducted. The study group comprised of patients with chronic otitis media and patients undergoing surgery for cochlear implantation was involved in the control group. Study group also divided to subgroups SSA and SSB according to history of ear discharge within last six months. Direct microscopy (DM) and transmission electron microscopy (TEM) were used to assess presence of biofilms. Totally 19 patients, 10 with ear discharge history within last 6 months and 9 without discharge comprised the study group. Control group comprised of 9 patients undergone cochlear implantation. In all of the patients with ear discharge history and in two of the patients without ear discharge history, biofilm formation was detected by both DM and TEM. All control group members were free of biofilm formation. The differences were statistically significant between study and control groups (p = 0.002) and between study subgroups (p < 0.001); but not significant between study subgroup without ear discharge history and control group (p = 0.470). In the middle ear mucosa of patients with chronic otitis media, biofilm formation is common, especially when ear discharge history is present.
Keywords: Biofilms, Otitis media, Middle ear, Mucosa, Microscopy
Introduction
Bacteria are generally thought as individually living microorganisms, however, most of them organize together in complex communities and attach to surfaces. This is known as biofilm formation and this survival strategy counteract classical strategies which are effective against free-floating bacteria [1, 2]. In biofilms, bacteria are embedded in a slim-like extracellular matrix composed of proteins, polysaccharides and nucleic acids known as extracellular polymeric substances (EPS). In this community, bacteria do not randomly make groups but organize into complex three-dimensional structures. Bacteria communicate to each other by signaling molecules modulating gene expression, deliver nutrients and dispose waste. Defense skills also improve by forming biofilms. They gain protection from environmental changes in temperature, moisture, pH; can escape from phagocytosis and humoral immunity; gain resistance to antibiotics. Beside these, low metabolic activity of bacteria in biofilm make them difficult to cultivate.
We know that biofilm formation is an old prokaryotic strategy for survival in diverse environment and it has been recently shown that biofilms play a major role in chronic infections in human [3]. Also in otolaryngologic chronic infections such as chronic rhinosinusitis, chronic tonsillitis, and device (e.g. voice prosthesis, tympanostomy tubes, tracheostomy tubes) related infections, biofilm formations are increasingly reported [4]. Chronic ear diseases like chronic otitis media with effusion, otitis media with effusion, cholesteatoma, and chronic suppurative otitis media are also thought to be biofilm-related and accumulating evidence is supporting this theory [4, 5]. However, the data is scarce to mention such a cause effect relationship. In this study we aim to investigate the presence of biofilms in the middle-ear mucosa of patients with chronic otitis media patients.
Materials and Methods
The Study and the Control Groups
The study consisted of two groups. Study group comprised of chronic otitis media patients. In the study group, patients were divided into two subgroups: study subgroup A (SSA) with a history of ear discharge within last six months and study subgroup B (SSB) without history of ear discharge within last six months. Patients undergone a cochlear implantation procedure with an intact ear drum and healthy middle ear were involved in the control group. Middle ear mucosa samples were collected from patients in the study and the control groups.
The study was approved by the local ethical committee (Gazi University School of Medicine Ethical Committee) and informed consent was obtained from all participants.
Processing of the Specimens and Microscopy
Tissue samples were divided into small particles with 1 mm3 volume and put into a fixative consisting of 2.5% glutaraldehyde (pH 7.4) in 0.1 M phosphate buffer for two hours at room temperature. After washing with buffer three times, the specimens post-fixed in 1% osmium tetraoxide for one hour and dehydrated in graded solutions of alcohol (50, 60, 70, 80, 90 and 100% ethanol). Tissues were then cleared in propylene oxide and embedded in Araldite. After polymerization in an oven at 56 °C for 48 h, several semi-thin sections were prepared and stained with toluidine blue. Sections were examined with a photomicroscope (BH2 Olympus, Japan). Thin sections (0.5 μm) were stained with uranil-acetate and lead citrate and examined with transmission electron microscope (TEM) (Carl Zeiss EM 900, Germany). All laboratory examinations were carried out blinded.
Statistics
Statistical analysis of the data was performed using MedCalc software version 11.6.1 (Mariakerke, Belgium) for Windows (Microsoft Corporation, Redmond, Washington). Biofilm formation results were compared by using Fisher’s exact test. Data were considered statistically significant when the p value was either equal to or less than 0.05.
Results
In SSA, there were 10 patients ranged in age from 35 to 67 years with a mean age of 44.8 years (6 female, 4 male). SSB comprised of 9 patients with a mean age of 32.3 years, ranged from 12 to 58 years (4 female, 5 male). In control group, there were 9 patients with a mean age of 12.8 years, ranged from 3 to 60 years (4 female, 5 male). One of the patients in SSA had a history of total hearing loss and total facial paralysis related to COM. In none of the patients in all groups, no chronic active infectious upper airway process was present other than COM. All patients in SSA and SSB had mastoidectomy and/or tympanoplasty. In 7 of the patients in SSA (70%) and in 2 of the patients in SSB (22%), cholesteatoma was encountered during operations.
In all SSA patients (100%) and in two of the SSB patients (22%), biofilm formation was demonstrated by DM and TEM (see table 1; Figs. 1, 2). The two biofilm positive SSB patients were the patients having COM with cholesteatoma formation. In none of the control group patients, biofilm formation could be demonstrated. Difference in frequency of biofilm formation was statistically significant between study and control groups (p = 0.002) and between study subgroups (p < 0.001), but not significant between SSB and control group (p = 0.470). When study group patients are regrouped according to presence of cholesteatoma, biofilm formation frequency was found as 100% (9/9) and 30% (3/10) in patients with cholesteatoma and without cholesteatoma, respectively. The difference was not statistically significant (p = 0.158).
Table 1.
Frequency of biofilm formation in study and control groups
| n | Biofilm on DM | Biofilm on TEM | |
|---|---|---|---|
| SSA | 10 | 10 | 10 |
| SSB | 9 | 2 | 2 |
| Control group | 10 | 0 | 0 |
| Total | 29 | 12 | 12 |
SSA study subgroup A, SSB study subgroup B, DM direct microscopy, TEM transmission electron microscopy
Fig. 1.
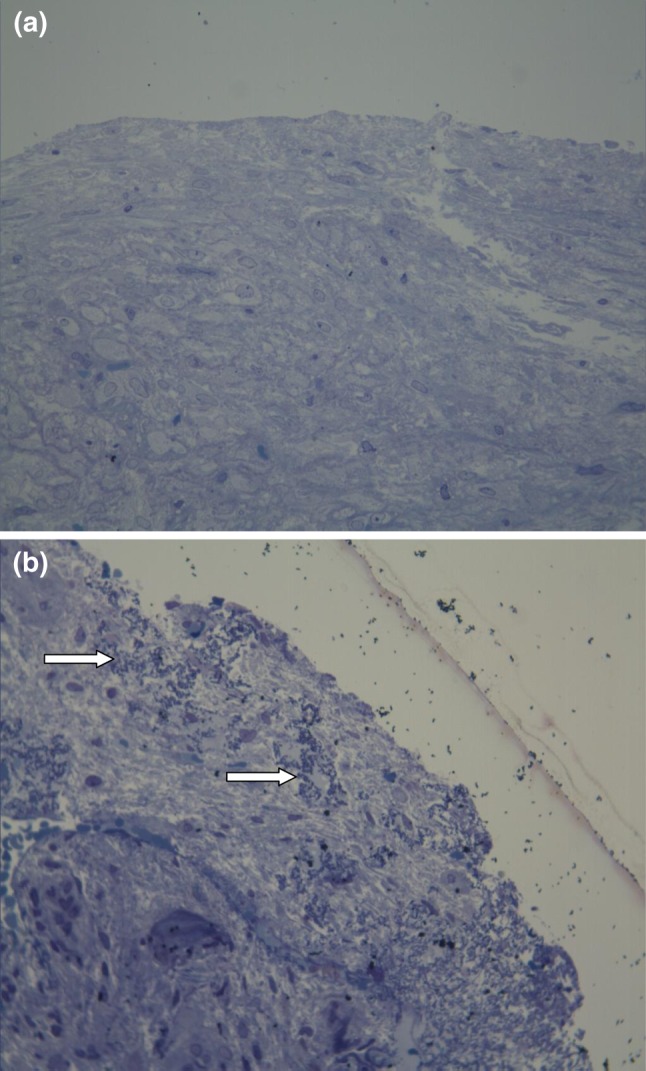
a. View of healthy mucosa on photomicroscopy. (Toluidine blue × 200) b. Photomicroscopy shows biofilm formation. White arrows indicate bacterial colonies embedded in the acellular matrix located on the mucosal surface. (Toluidine blue × 200)
Fig. 2.
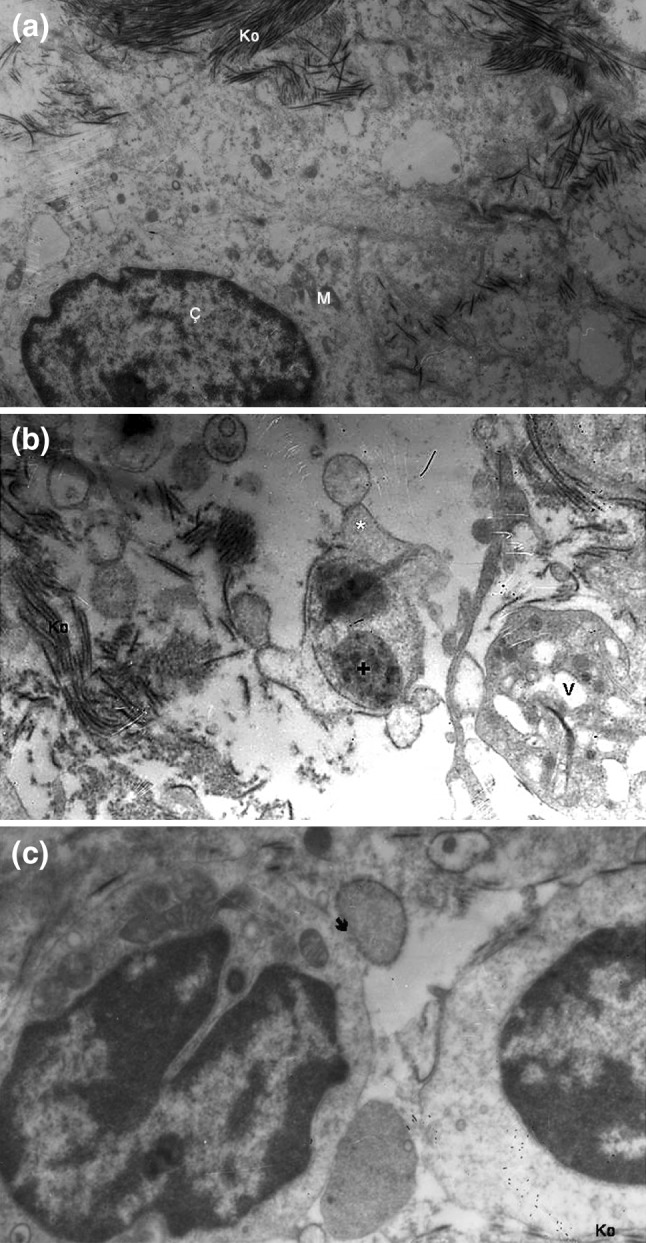
a Transmission electron micrograph of the normal mucosal ultrastructure. Ç nucleus, Ko collagen fibers, M mitochondria (uranil acetate–lead citrate × 10,000). b Appearance of biofilm on transmission electron microscopy. * bleb formation, + bacterial biofilm, Ko collagen fibers, V vacuole. Bleb and vacuole formations are ultrastructural changes related to biofilm. (uranil acetate–lead citrate × 10,000). c Appearance of a biofilm bacterium–cell membrane fusion on transmission electron microscopy. → biofilm–cell membrane fusion, Ko collagen fibers. Another biofilm bacterium is also seen attached to the same cell at the bottom of the figure. (uranil acetate–lead citrate × 10,000)
Discussion
Biofilm formation is known to be important in dental problems and have been recognized as sources of infection on vascular and urinary catheters for at least three decades [6–8]. Recently, it has shown to play role in various infectious human disease processes. Many authorities agree that biofilms have important role in bacterial resistance to antibiotic therapy and chronic infections [9]. However, this issue is controversial. Mladina et al. [10] reported presence of biofilm formation on healthy paranasal sinus mucosa and in their paper, Mladina and Skitarelić [11] suggest that biofilm is just a mucosal blanket. This issue may be debatable for sinonasal tract but not applicable to every mucosal surface especially to middle ear mucosa.
In otolaryngologic diseases, biofilms are being implicated in increasing number. The role of biofilms in chronic mucosal otolaryngological infections was first documented in otitis media by Rayner et al. [12]. Since then, adenoiditis, chronic tonsillitis, chronic rhinosinusitis, cholesteatoma and otolaryngological prosthesis and implants (e.g. voice prosthesis, tympanostomy tubes, cochlear implants) are related with biofilm formation [4, 5]. In clinical samples, detection of biofilm formation is based on microscopic morphological appearance of bacterial aggregates embedded in an extracellular matrix. Both light microscope and electron microscope are used for this purpose in most studies as in our study.
Patients with COM may present with dry perforations or recurrent/chronic ear discharge. In patients with ear discharge, reason of the discharge may be a cholesteatoma formation or an infection resistant to topical and systemic antibiotic treatment. Recurrent infections in COM patients are generally caused by mixed aerobic and anaerobic bacteria. Most commonly, P. aeruginosa and S. aureus are responsible from these chronic infections. Common bacteria detected in COM patients such as P. aeruginosa, E. coli and S. aureus as well as some others responsible from acute otitis media formation (e.g. S. pneumonae and H.influenzae) are documented to form biofilm [1]. In vitro drug concentrations which are lethal to bacteria can be also readily achievable in the middle ear space in vivo. However, if biofilm formation is present, bacteria in this structure gain an enormous resistance to antibiotics and treatment of such an infectious process becomes almost impossible. Thus, when a recurrent infection or an infection resistant to antibiotic treatment is encountered, a biofilm formation may explain failure in eradication of infection.
Relationship of otitis media with biofilm was first demonstrated by Rayner et al. [12]. Most studies in otitis related biofilm formation issue are focusing on otitis media with effusion but studies conducted in COM patients with perforated tympanic membrane are limited yet. The first study in COM with perforated tympanic membrane was conducted in a nonhuman primate model by Dohar et al. [13]. Cynomolgus monkeys underwent perforation of the tympanic membrane and inoculation of the middle ear with a biofilm-forming strain of P. aeruginosa and biofilm formation was successfully demonstrated in this animal model. Chole and Faddis [14] reported biofilm formation frequency in human cholesteatoma specimens and in gerbil experimental cholesteatoma specimens as 67 and 95% respectively. In 2009, Homøe et al. [15] reported biofilm formation in five of the six pediatric COM cases and in nine of the ten adult COM cases. Lee et al. [16] studied 10 COM patients and 10 controls for biofilm formation and found that biofilms were present in 60% of the study group and in 10% of the control group members. In our study we found similar results with the literature. In this study we aimed to evaluate biofilm formation in COM patients with ear discharge history and with dry perforations. Also we have compared biofilm formation in COM patients with and without cholesteatoma. To our results, biofilm formation is not present in all COM cases. We have demonstrated that COM with ear discharge history and COM with cholesteatoma conditions are biofilm related.
Our results may support indication of mastoidectomy in suppurative COM patients. Conventionally, COM cases with purulent discharge or edematous middle ear mucosa refractory to medical treatment are accepted among mastoidectomy indications. In our opinion, resistant bacterial colonies in mastoid cavity may be a factor that influence graft success. As a cleansing procedure, removal of biofilms may be the exact role of mastoidectomy in improving tympanoplasty success. In the future, preoperative middle ear sampling, intraoperative microscopy (like frozen section in oncological surgery), or some other quick tests for detection of biofilms may have role in making decision on mastoidectomy.
Conflict of interest
none.
Footnotes
This material has never been published and is not currently under evaluation in any other peer-reviewed publication.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Veeh R, Shirtliff M, et al. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003;112:1466–1477. doi: 10.1172/JCI20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Post JC, Hiller NL, Nistico L, Stoodley P, Ehrlich GD. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg. 2007;15:347–351. doi: 10.1097/MOO.0b013e3282b97327. [DOI] [PubMed] [Google Scholar]
- 5.Vlastarakos PV, Nikolopoulos TP, Maragoudakis P, Tzagaroulakis A, Ferekidis E. Biofilms in ear, nose, and throat infections: how important are they? Laryngoscope. 2007;117:668–673. doi: 10.1097/MLG.0b013e318030e422. [DOI] [PubMed] [Google Scholar]
- 6.Beikler T, Flemmig TF. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol. 2000;2011(55):87–103. doi: 10.1111/j.1600-0757.2010.00360.x. [DOI] [PubMed] [Google Scholar]
- 7.Nickel JC, Gristina AG, Costerton JW. Electron microscopic study of an infected foley catheter. Can J Surg. 1985;28:50–51. [PubMed] [Google Scholar]
- 8.Marrie TJ, Costerton JW. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol. 1984;19:687–693. doi: 10.1128/jcm.19.5.687-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Mladina R, Skitarelic N, Musić S, Ristić M. A biofilm exists on healthy mucosa of the paranasal sinuses: a prospectively performed, blinded, scanning electron microscope study. Clin Otolaryngol. 2010;35:104–110. doi: 10.1111/j.1749-4486.2010.02097.x. [DOI] [PubMed] [Google Scholar]
- 11.Mladina R, Skitarelić N. Biofilm–the other name for the regular mucosal blanket. Med Hypotheses. 2010;75:391–392. doi: 10.1016/j.mehy.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998;279:296–299. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 13.Dohar JE, Hebda PA, Veeh R, Awad M, Costerton JW, Hayes J, Ehrlich GD. Mucosal biofilm formation on middle-ear mucosa in a nonhuman primate model of chronic suppurative otitis media. Laryngoscope. 2005;115:1469–1472. doi: 10.1097/01.mlg.0000172036.82897.d4. [DOI] [PubMed] [Google Scholar]
- 14.Chole RA, Faddis BT. Evidence for microbial biofilms in cholesteatomas. Arch Otolaryngol Head Neck Surg. 2002;128:1129–1133. doi: 10.1001/archotol.128.10.1129. [DOI] [PubMed] [Google Scholar]
- 15.Homøe P, Bjarnsholt T, Wessman M, Sørensen HC, Johansen HK. Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur Arch Otorhinolaryngol. 2009;266:1533–1538. doi: 10.1007/s00405-009-0940-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee MR, Pawlowski KS, Luong A, Furze AD, Roland PS. Biofilm presence in humans with chronic suppurative otitis media. Otolaryngol Head Neck Surg. 2009;141:567–571. doi: 10.1016/j.otohns.2009.08.010. [DOI] [PubMed] [Google Scholar]


