Abstract
The objectives are to evaluate role of magnetic resonance imaging (MRI) in diagnosis of cholesteatoma and correlate imaging findings with intraoperative findings, and to emphasize of role of imaging in the follow-up of postoperative patients for differentiating residual/recurrent cholesteatoma from granulation/inflammatory tissue. In this prospective study, 31 patients were evaluated with a specific MRI protocol and high resolution computed tomography of the temporal bones. These included patients with a strong suspicion of having a cholesteatoma on clinical examination and postoperative cases on clinical follow up. Based on specific MRI findings, presence of cholesteatoma was reported in 17 out of 31 patients. All 31 patients underwent surgery and 19 patients had confirmed intraoperative cholesteatoma. This study shows high sensitivity of a specific sequence based MRI examination in detection of cholesteatoma and in differentiating cholesteatoma from postoperative inflammatory/granulation tissue. To the best of the author’s knowledge, this is the first such study performed in the Indian Asian population.
Keywords: Cholesteatoma, Magnetic resonance imaging (MRI), Diffusion weighted sequence, Delayed contrast imaging
Introduction
Cholesteatoma is a lesion lined with keratin producing squamous epithelium and filled with desquamation debris. It can be located in the external auditory canal, mastoid process of temporal bone, middle ear cavity, or within the petrous apex [1]. Due to it’s locally aggressive nature and insidious clinical course cholesteatomas are potentially dangerous, surgery being the primary universally accepted treatment for this condition.
High resolution computed tomography (HRCT) is a useful imaging modality for cholesteatomas. It depicts the bony and soft tissue involvement of the mastoid–middle ear complex, associated ossicular/facial canal erosions and integrity of the tegmen tympani. However it cannot differentiate between granulation/scar tissue and cholesteatomatous tissue [2, 3].
Magnetic resonance imaging (MRI) plays an important role in the preoperative and postoperative diagnosis of cholesteatomas. By using an MRI protocol of specific sequences it is possible to characterize an indeterminate soft tissue abnormality identified on HRCT of the temporal bone. These MRI sequences, which include diffusion weighted and contrast enhanced MR have the potential to differentiate cholesteatoma from granulation tissue and inflammatory tissue [4]. MRI is also particularly useful for evaluating intracranial complications like dural invasion, subdural or epidural abscess formation, and sigmoid sinus thrombosis [5].
Materials and Methods
Study Design
This prospective study i.e. MRI examinations of the temporal bone, was performed after submission and approval from the local ethics committee of our institution.
HRCT of the temporal bone was performed additionally in every case for better anatomical details and especially to look for ossicular status/bony involvement and to assess status of the facial nerve canal.
Inclusion Criteria
Patients with clinical and otoscopically suspected cholesteatoma referred from the Otorhinolaryngology Departments of Grant Medical Foundation and KEM Hospital, Pune.
Patients who had undergone previous surgery (intact canal wall technique) for middle ear cholesteatoma.
Exclusion Criteria
Claustrophobic patients or patients with MRI incompatible metallic implants/prosthesis in the head and neck region.
Imaging Technique
MRI with special reference to temporal bone was performed on a 1.5 Tesla superconductive Nova Dual Philips MRI Scanner using SENSE 8
Flex S dual element surface coil.
The following MRI examination protocol is used in our centre:
Precontrast T1 weighted (T1W) coronal images: Repetition time [TR]: 25 ms; time to echo [TE]: 5.2 ms; field of view [FOV]: 180 mm; slice thickness: 4 mm.
T2 weighted (T2W) coronal images: TR: 3029 ms; TE: 100 ms; FOV: 230 mm; slice thickness: 2 mm.
Single shot (non-echoplanar; non-EP) turbo spin echo diffusion weighted (SS TSE DW) coronal images: TR: 2,250 ms; TE: 63 ms; FOV: 200 mm; bfactor 0 and 800 mm2/s.
Delayed postcontrast-enhanced (CEMR) T1W coronal fat suppressed images: Images were acquired 45 minutes after intravenous contrast injection of 0.1 mmol/kg of body weight of gadoterate meglumine (Dotarem). Coronal plane was preferred over axial plane as there were fewer artefacts.
All patients also underwent HRCT of the temporal bone on a Multidetector CT scanner (40 slice scanner with 40 × 0.625 detector configuration, pitch factor of 0.426, reconstruction slice thickness of 0.67 mm with reconstruction interval of 0.3 mm and reconstruction algorithm of 360°, rotation time of 0.5 s and an image matrix of 768 × 768).
Results
31 patients (male: 20 and female: 11; age range 5–56 years, with mean age of 27 years) were evaluated between February 2009 and December 2010. Out of 31 patients, 11 patients had undergone cholesteatoma surgery using intact canal wall technique and were being followed up clinically.
All four coronal MRI sequences were evaluated simultaneously and SS TSE DW images were considered positive for cholesteatoma if a focal hyperintense lesion (focal area of diffusion restriction) was observed. This lesion was correlated with other MRI sequences and coronal HRCT images.
Cholesteatomatous tissue showed an intermediate to hypointense signal on T1W images and was hyperintense on the T2W images. It showed either no corresponding enhancement or a faint peripheral rim enhancement on delayed post gadolinium T1W images obtained 45 minutes after the intravenous contrast enhancement. The cholesteatoma showed a non-specific soft tissue density appearance on coronal HRCT images. Figure 1 illustrates the typical HRCT and MRI findings in a case of acquired cholesteatoma.
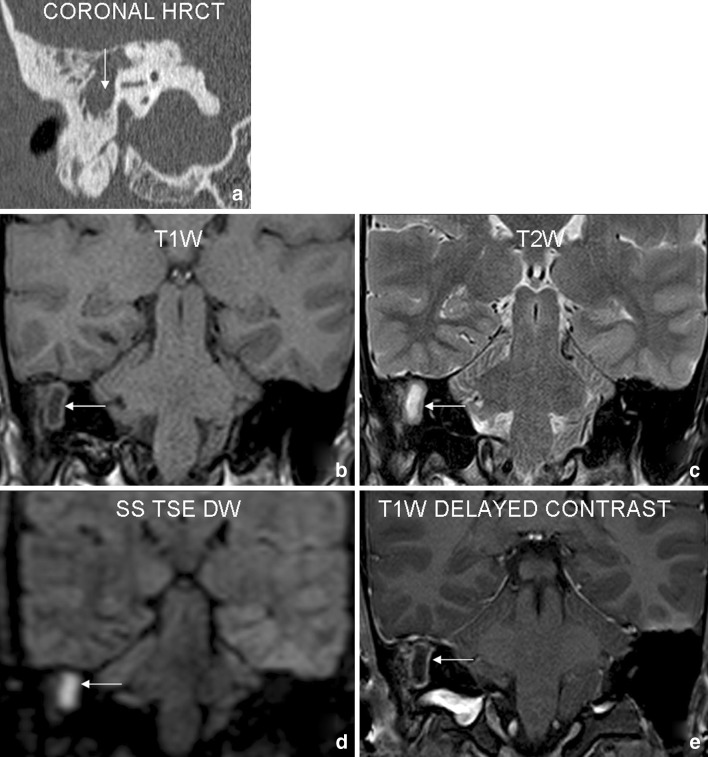
Fig. 1.
Case 1: 23 year old male patient with history of right sided conductive hearing loss and purulent discharge. Coronal HRCT (a) shows a soft tissue density lesion (arrow) involving right mastoid–middle ear complex with irregular bony destruction. Focal area of signal abnormality in right mastoid (arrows) on T1W (b) and T2W (c) coronal images shows diffusion restriction (appears bright) on SS TSE DW (d) sequence. Delayed postcontrast (e) images reveal non-enhancing nature of the lesion with mild peripheral rim enhancement. The findings were interpreted as positive for cholesteatoma. Surgery revealed right sided cholesteatoma
In postoperative and/or inflammatory tissue no hyperintensity or diffusion restriction was seen on the SS TSE DW images, and a brighter hyperintense signal on T2W images was observed. Delayed post gadolinium T1W images showed homogenous enhancement of the tissue. These lesions also showed a non-specific soft tissue density appearance on coronal HRCT images.
Figure 2 illustrates MRI findings in a postoperative case suspected to have recurrent cholesteatoma. The MRI was negative for cholesteatoma and intraoperatively the soft tissue abnormality was found to be due to granulation tissue.
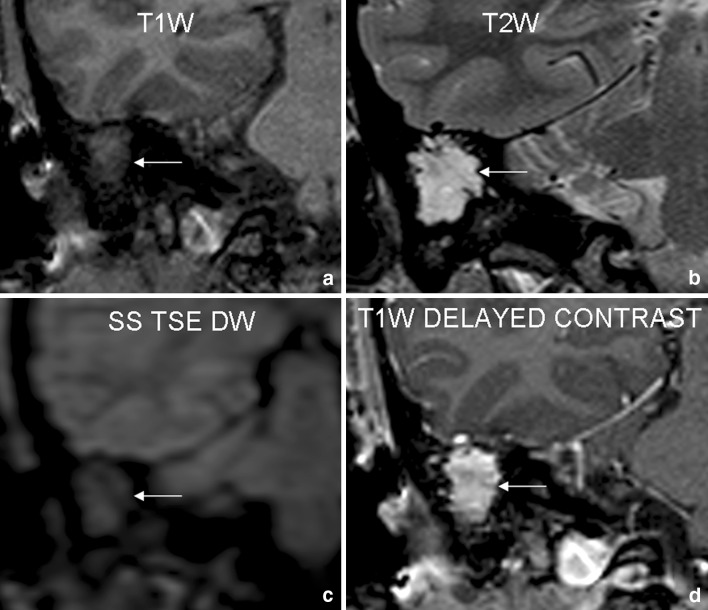
Fig. 2.
Case 2: 30 year old female patient was operated for right sided cholesteatoma 1 year back. She presented with symptoms of headache, persistent right ear discharge and conductive hearing loss on right side. Coronal T1W (a) and T2W (b) images reveal large area of signal abnormality in right mastoid–middle ear complex (arrows). No focal area of diffusion restriction is noted on SS TSE DW (c) sequence. Delayed postcontrast (d) images reveal homogenous enhancement within the lesion. This scan was interpreted as negative for cholesteatoma. Surgery revealed presence of granulation tissue
All examinations were classified as either positive or negative for cholesteatoma. The greatest diameter of the cholesteatoma was measured. Associated findings of surgical relevance ie involvement of ossicles, tegmen tympani, facial nerve canal, middle ear recesses, oto-labyrinthine communication were commented on the HRCT study and MRI images were also evaluated for status of the facial nerve and presence of any intracranial complications.
The sensitivity, specificity, negative predictive values and positive predictive values were calculated and compared to recent existing studies in medical literature. Of the 31 patients, SS TSE DW imaging showed hyperintense signal in 17 patients. However, cholesteatoma was found in 19 cases intraoperatively. The two false negative cases on TSE DW imaging included a 3 mm recurrent epitympanic cholesteatoma in a young child misinterpreted due to movement artefacts and a very small 2 mm primary acquired cholesteatoma misinterpreted due to use of slice thickness of 4 mm. No false positive case was noted in our study.
Sensitivity and specificity of SS TSE DW imaging for detection of acquired cholesteatoma were 92% and 100% respectively. Positive and negative predictive values were 100% and 85% respectively. These figures were compared to earlier existing comparable studies conducted by different groups in the Western literature (Table 1).
Table 1.
Sensitivity and specificity of DW imaging for acquired cholesteatoma: comparison of our study with other groups
| Author | Cases | Technique of DW | Sensitivity | Specificity | NPV | PPV |
|---|---|---|---|---|---|---|
| Vercruysse et al. [26] | 55 | EPI DW | 81 | 100 | 100 | 40 |
| Khan et al. [28] | 15 | EPI DW | 83 | 100 | – | |
| Foer et al. [25] | 57 | TSE DW | 82.6 | 87.2 | 96 | 56.5 |
| Our study | 20 | TSE DW | 92.86 | 100 | 100 | 85 |
PPV positive predictive value, NPV negative predictive value
In our study we carried out SS TSE DW imaging in 11 postoperative patients with clinical suspicion of residual/recurrent cholesteatoma. In 4 cases the images showed clear hyperintense focal area of diffusion restriction consistent with cholesteatoma. These findings were correlated with intraoperative results. Imaging was negative for cholesteatoma in 6 cases. In all these cases, intraoperative findings showed the mastoidectomy cavity to be completely or partially filled with postoperative/inflammatory changes. There were no false positive cases.
Sensitivity and specificity were 80% and 100% respectively in postoperative clinical setting. Positive and negative predictive values were 100% and 85% respectively. These figures were also compared to earlier existing comparable studies conducted by different groups in the Western literature (Table 2).
Table 2.
Sensitivity and specificity of DW imaging for postoperative recurrent cholesteatoma: comparison of our study with other groups
| Author | Case | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Aikele et al. [29] | 22 | 77 | 100 | 100 | 75 |
| Stasolla et al. [30] | 18 | 86 | 100 | 100 | 92 |
| Vercruysse et al. [26] | 45 | 12.5 | 100 | 100 | 72 |
| Dubruelle et al. [10] | 24 | 100 | 91 | 93 | 100 |
| De Foer et al. [25] | 32 | 90 | 100 | 100 | 96 |
| Our study | 11 | 80 | 100 | 100 | 85.71 |
PPV positive predictive value, NPV negative predictive value
Discussion
HRCT of the temporal bone is a useful imaging modality for diagnosis, description of extension and possible complications of middle ear cholesteatoma. This modality, however, cannot differentiate between inflammatory/granulomatous tissue and cholesteatoma. It is also difficult on HRCT to diagnose recurrent or residual cholesteatoma after surgery. Hence this issue remains a constant dilemma for the ENT surgeon and the imaging specialist and can, at times, leads to an unnecessary second-look surgery.
MRI plays an important role in the preoperative and postoperative diagnosis of cholesteatomas by characterizing the soft tissue abnormality identified on HRCT. MR has potential to differentiate cholesteatoma from granulation tissue and inflammatory tissue. Diffusion weighted (DW) imaging and delayed contrast enhanced MR play important roles in diagnosing residual and recurrent cholesteatoma [4].
Fitzek et al. [6] first described the use of echo-planar diffusion-weighted (EP DW) MRI to demonstrate acquired middle ear cholesteatoma and to differentiate it from middle ear inflammation.
DW imaging is a non-contrast enhanced MR sequence based on the measurement of random movement of water molecules in tissues. Different signals (ie hyperintense/bright or hypointense/dark) can be recorded in various tissue types by determining their diffusion coefficient in reference to the diffusion coefficient of water [7]. In comparison with free water molecules, bound water molecules in lesions such as epidermoid cysts or cholesteatomas produce an increased (bright) signal in DW images [8, 9]. DW imaging can be performed by two techniques, echo-planar (EP) DW sequence and the non-echo-planar single-shot (SS) turbo spin-echo (TSE) DW sequence. Two reports in recent medical literature have compared results of EP DW imaging and SS TSE DW imaging in the evaluation of middle ear cholesteatoma [10, 11].
The SS TSE DW sequence has fewer susceptibility artefacts, thus reducing artefactual hyperintensities at the bone/brain/air interface which may be erroneously interpreted as cholesteatoma. This sequence also has a higher spatial/contrast resolution than the EP DW sequence and hence can reliably pick up cholesteatomas as small as 2.5 mm [12]. Hence all our patients underwent DW imaging using the non-echoplanar technique.
DW MRI is particularly sensitive to cholesteatoma tissue [6, 9, 13]. Numerous reports abound in medical literature establishing the role of DW Imaging in diagnosis of intracranial and extracranial epidermoid cysts [14–20]. As middle ear cholesteatomas have similar histopathological characteristics to epidermoid cysts [21–23] this MR sequence forms the mainstay of imaging work up in these conditions.
Ayache et al. [24] described the use of delayed postcontrast T1W sequences in demonstrating postoperative residual cholesteatoma. Performing a T1W sequence 45 minutes after intravenous gadolinium (a paramagnetic MRI contrast agent), allows a distinction to be made between avascular, non enhancing cholesteatoma and delayed homogenous enhancement seen in inflammatory and/or scar tissue.
In our study, cholesteatomas showed either no enhancement or a faint peripheral rim enhancement on delayed post gadolinium T1W images obtained 45 minutes after the intravenous contrast enhancement. Postoperative inflammatory/granulomatous tissue, however, showed homogenous enhancement on delayed post gadolinium T1W images.
Magnetic resonance imaging also has an important role to play in evaluating associated intracranial complications [11]. De Foer et al. [25] showed the value of MRI for the assessment of possible complications such as erosion of the lateral semicircular canal, invasion of the membranous labyrinth, and invasion of the middle cranial fossa through an eroded tegmen.
Cholesteatomatous tissue shows an intermediate to hypointense signal on T1W images and appears hyperintense on the corresponding T2W images. This hyperintensity is, however, significantly less as compared to that seen in inflammatory lesions. These specific MRI findings differentiating cholesteatoma and inflammatory/granulomatous lesions are summarized in Table 3.
Table 3.
MRI findings on four sequences differentiating cholesteatoma from inflammatory/granulomatous lesions
| MRI sequence | Cholesteatoma | Inflammatory tissue |
|---|---|---|
| T1W coronal | Iso-heterointense | Iso-hypointense |
| T2W coronal | Iso-hyperintense | Hyperintense |
| TSE diffusion coronal | Diffusion restriction | No diffusion restriction |
| Delayed postcontrast T1W coronal | No enhancement/mild rim enhancement | Homogenous enhancement |
Though the middle ear cavity is the most common site for presence of cholesteatoma, these lesions have also been described in the external auditory canal, mastoid and petrous apex [1]. Figure 3 demonstrates a large right petrous apex cholesteatoma with extensive bony changes showing typical MRI findings.
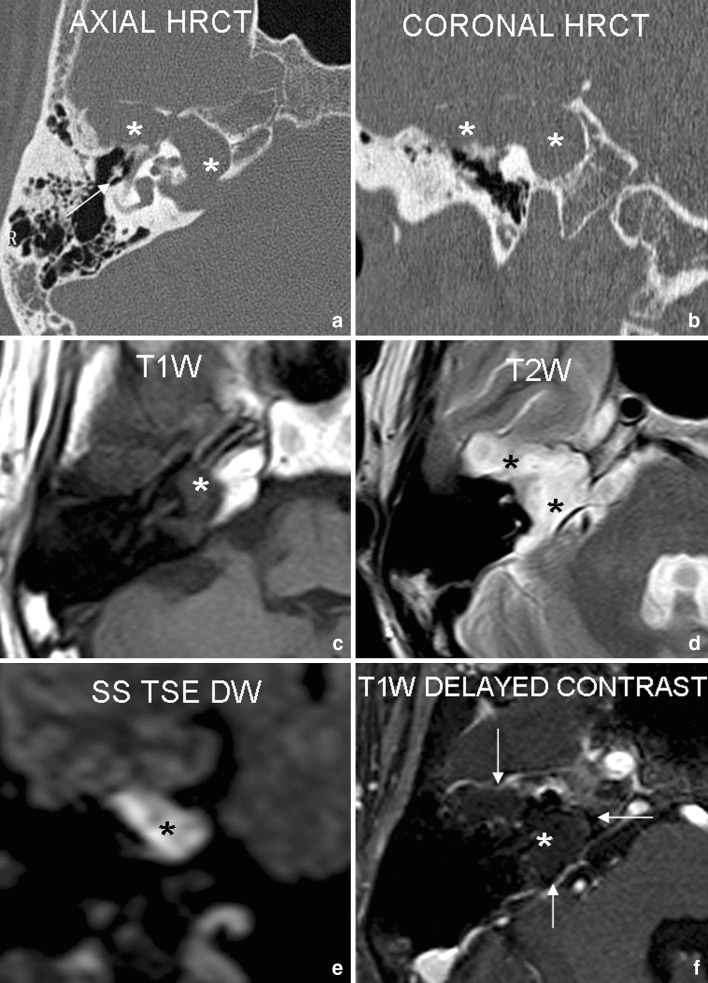
Fig. 3.
Case 3: 37 year old male patient with history of right sided mixed hearing loss and right lower motor neuron facial nerve palsy. Axial and coronal HRCT (a, b) show a large well-defined expansile osteolytic lesion (asterisk) involving the right petrous apex, internal auditory canal (IAC) and geniculate fossa with associated soft tissue density lesion. Associated ossicular chain destruction was also noted (arrow). Large corresponding area of focal signal abnormality is seen on T1W (c) and T2W (d) axial images. The lesion shows diffusion restriction (appears bright) on coronal SS TSE DW sequence (e). Delayed postcontrast axial images (f) reveal non-enhancing nature of the lesion with thin marginal rim enhancement (arrows). These findings are highly suggestive of a petrous apex cholesteatoma. Surgery revealed a large petrous apex cholesteatoma involving the right geniculate fossa and extending into the right IAC
Drawbacks of MRI
The lack of clear visualization of the anatomical landmarks of the temporal bone on SS TSE DW images can be regarded as one of the major drawbacks of the sequence. Ossicular erosion, the hallmark of cholesteatoma, cannot be identified on MRI.
Accumulated keratin (responsible for the hyperintensity on DW images) in the cholesteatoma sac can evacuate into the external auditory canal and can cause false negative finding on DW imaging. The possibility of missing small (<5 mm) retraction pockets or evacuated retraction pockets on DW images has been reported previously [6, 26].
Silastic sheet material has been reported to cause false positive hyperintense signal on DW imaging [10, 27] due to it’s magnetic characteristics and circular shape in the mastoid leading to a wrong diagnosis of residual cholesteatomas in postoperative cases. These materials are also not readily identifiable by HRCT scan of the temporal bone as they appear as an intermediate density lesion similar to that of soft tissue. Radiologists should, therefore, elicit the specific history or peruse operative notes meticulously for mention of any foreign material to avoid an incorrect diagnosis of residual cholesteatoma.
Delayed postcontrast imaging is a time consuming examination and comes with an additional cost to the patient. In very young children general anaesthesia is required to obtain optimal diagnostic images which also adds to the cost of the study.
In the author’s opinion it is mandatory to perform, in addition to the MRI, a non-contrast enhanced HRCT of the temporal bone for accurate localization & extension of the cholesteatoma, for evaluating integrity of the ossicular chain, identifying early erosions of the tegmen tympani/sinus plate and confirming presence of oto-labyrinthine fistula [2, 3, 5].
Conclusion
Cholesteatoma is a potentially dangerous, life threatening and eminently treatable condition. The management and prognosis depends on the early diagnosis and appropriate surgical line of treatment. The utilization of HRCT scans of the temporal bone has significantly improved the assessment of cholesteatoma preoperatively. However HRCT cannot differentiate between different soft tissue pathologies of the mastoid–middle ear complex.
Magnetic resonance imaging plays a valuable role in pre and postoperative diagnosis of cholesteatoma. Combination of specific sequences (DW imaging, routine T1W & T2W images and delayed post gadolinium T1W images) used at the authors centre is very useful for the diagnosis of acquired cholesteatoma and to detect residual/recurrent postoperative cholesteatomas.
The authors advocate use of this limited sequence MR examination in conjunction with HRCT of the temporal bone as a working protocol for this pathology. However, as the sensitivity of MRI examination is comparatively low for very small postoperative residual cholesteatoma, and due to the inherent cost of the examination, the authors recommend establishing protocols for scheduling follow-up imaging studies to ensure early and accurate diagnosis of residual/recurrent disease.
References
- 1.Balogh K. The head and neck. In: Rubin E, Farber JL, editors. Pathology. 3. Philadelphia: Lippincott-Raven; 1998. pp. 1300–1334. [Google Scholar]
- 2.Mafee MF, Levin BC, et al. Cholesteatoma of middle ear and mastoid: a comparison of CT scan and operative findings. Otolarngol Clin North Am. 1998;21:265–293. [PubMed] [Google Scholar]
- 3.Johnson DW, Voorhees RL, et al. Cholesteatoma of temporal bone: role of computed tomography. Radiology. 1983;148:733–737. doi: 10.1148/radiology.148.3.6878694. [DOI] [PubMed] [Google Scholar]
- 4.Martin N, Sterkers O, Nahum M. Chronic inflammatory disease of the middle ear cavities: Gd-DTPA-enhanced MR imaging. Radiology. 1990;176:399–405. doi: 10.1148/radiology.176.2.2367654. [DOI] [PubMed] [Google Scholar]
- 5.Joel D, Swartz H, Harnsberger R. Imaging of temporal bone. Stuttgart: Thieme Medical Publishers; 1997. [Google Scholar]
- 6.Fitzek C, Meves T, Fitzek S, Mentzel HJ, Hunsche S, Stoeter P. Diffusion-weighted MRI of cholesteatomas of the petrous bone. J Magn Reson Imaging. 2002;15:636–641. doi: 10.1002/jmri.10118. [DOI] [PubMed] [Google Scholar]
- 7.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D, Turner R. Intravoxel incoherent motion imaging using spin echoes. Magn Reson Med. 1991;19:221–227. doi: 10.1002/mrm.1910190206. [DOI] [PubMed] [Google Scholar]
- 9.Bergui M, Zhong J, Bradac GB, Sales S. Diffusion-weighted images of intracranial cyst-like lesions. Neuroradiology. 2001;43:824–829. doi: 10.1007/s002340100595. [DOI] [PubMed] [Google Scholar]
- 10.Dubrulle F, Souillard R, Chechin D, Vaneeclo FM, Desaulty A, Vincent C. Diffusion-weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology. 2006;238:604–610. doi: 10.1148/radiol.2381041649. [DOI] [PubMed] [Google Scholar]
- 11.Lemmerling M, De Foer B. Radiology of the petrous bone. In: Lemmerling M, Kollias SS, editors. Imaging of cholesteatomatous and non-cholesteatomatous middle ear disease. Berlin: Springer; 2004. pp. 31–47. [Google Scholar]
- 12.Williams MT, Ayache D, Alberti C, Heran F, Lafitte F, Elmalech-Berges M, Piekarski JD. Detection of postoperative residual cholesteatoma with delayed contrast-enhanced MR imaging: initial findings. Eur Radiol. 2003;13:169–174. doi: 10.1007/s00330-002-1423-1. [DOI] [PubMed] [Google Scholar]
- 13.Fitzek CM, Fitzek S, Meves T, Mann W, Stoeter P. Ultrafast MRI examination of cholesteatomas of the petrous bone. Eur Radiol. 2000;10(suppl):295. [Google Scholar]
- 14.Osborne AG. Diagnostic neuroradiology. St. Louis: Mosby-Year Book, Inc; 1994. [Google Scholar]
- 15.Atlas SW. Magnetic resonance imaging of the brain and spine. 3. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 16.Ikushima I, Korogi Y, Hirai T. MR of epidermoids with a variety of pulse sequences. Am J Neuroradiol. 1997;18(7):1359–1363. [PMC free article] [PubMed] [Google Scholar]
- 17.Kallmes DF, Provenzale JM, Cloft HJ. Typical and atypical MR imaging features of intracranial epidermoid tumors. Am J Roentgenol. 1997;169(3):883–887. doi: 10.2214/ajr.169.3.9275916. [DOI] [PubMed] [Google Scholar]
- 18.Jolapara M, Kesavadas C, Radhakrishnan VV, Saini J, Patro SN, Gupta AK, et al. Diffusion tensor mode in imaging of epidermoid cysts: one step ahead of fractional anisotropy. Neuroradiology. 2009;51(2):123–129. doi: 10.1007/s00234-008-0464-9. [DOI] [PubMed] [Google Scholar]
- 19.Hu XY, Hu CH, Fang XM, Cui L, Zhang QH. Intraparenchymal epidermoid cysts in the brain: diagnostic value of MR diffusion-weighted imaging. Clin Radiol. 2008;63(7):813–818. doi: 10.1016/j.crad.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Gao PY, Osborn AG, Smirniotopoulos JG. Radiologic-pathologic correlation. Epidermoid tumor of the cerebellopontine angle. Am J Neuroradiol. 1992;13(3):863–872. [PMC free article] [PubMed] [Google Scholar]
- 21.Sadé J. Retraction pockets and attic cholesteatomas. Acta Otorhinolaryngol Belg. 1980;34(1):62–84. [PubMed] [Google Scholar]
- 22.Mafee MF, Kumar A, Heffner DK. Epidermoid cyst (cholesteatoma) and cholesterol granuloma of the temporal bone and epidermoid cysts affecting the brain. Neuroimaging Clin N Am. 1994;4(3):561–578. [PubMed] [Google Scholar]
- 23.Ferlito A. A review of the definition, terminology and pathology of an aural cholesteatoma. J Laryngol Otol. 2007;107(6):483–488. doi: 10.1017/S0022215100123539. [DOI] [PubMed] [Google Scholar]
- 24.Ayache D, Williams MT, Lejeune D, et al. Usefulness of delayed postcontrast magnetic resonance imaging in the detection of residual cholesteatoma after canal wall-up tympanoplasty. Laryngoscope. 2005;115:607–610. doi: 10.1097/01.mlg.0000161360.66191.29. [DOI] [PubMed] [Google Scholar]
- 25.De Foer B, Vercruysse JP, Pilet B, et al. Technical report: single-shot turbo spin echo diffusion-weighted mr imaging versus spin echo planar diffusion-weighted mr imaging in the detection of acquired middle ear cholesteatoma: case report. Am J Neuroradiol. 2006;27:1480–1482. [PMC free article] [PubMed] [Google Scholar]
- 26.Vercruysse JP, De Foer B, Pouillon M, et al. The value of diffusion weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients. Eur Radiol. 2006;16:1461Y7. doi: 10.1007/s00330-006-0160-2. [DOI] [PubMed] [Google Scholar]
- 27.Venail F, Bonafec A, Poirrierc V, Mondaina M, Uziel A. Comparison of echo-planar diffusion-weighted imaging and delayed postcontrast t1-weighted MR imaging for the detection of residual cholesteatoma. Am J Neuroradiol. 2008;29:1363–1368. doi: 10.3174/ajnr.A1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan S, Rowlands RG, Benjamin E, Abramovich S (2008) Accuracy of diffusion-weighted magnetic resonance imaging in the diagnosis of cholesteatoma. Clin Otolarngol 33(6):643
- 29.Aikele P, Kittner T, Offergeld C et al (2003) Diffusion-weighted MR imaging of cholesteatoma in pediatric and adult patients who have undergone middle ear surgery. Am J Roentgenol 181:261–265 [DOI] [PubMed]
- 30.Stasolla A, Magliulo G, Parrotto D et al (2004) Detection of postoperative relapsing/residual cholesteatoma with diffusion-weighted echo-planar magnetic resonance imaging. Otol Neurotol 25:879–884 [DOI] [PubMed]