Abstract
Guanosine, a guanine-based purine, is recognized as an extracellular signaling molecule that is released from astrocytes and confers neuroprotective effects in several in vivo and in vitro studies. Astrocytes regulate glucose metabolism, glutamate transport, and defense mechanism against oxidative stress. C6 astroglial cells are widely used as an astrocyte-like cell line to study the astrocytic function and signaling pathways. Our previous studies showed that guanosine modulates the glutamate uptake activity, thus avoiding glutamatergic excitotoxicity and protecting neural cells. The goal of this study was to determine the gliopreventive effects of guanosine against glucose deprivation in vitro in cultured C6 cells. Glucose deprivation induced cytotoxicity, an increase in reactive oxygen and nitrogen species (ROS/RNS) levels and lipid peroxidation as well as affected the metabolism of glutamate, which may impair important astrocytic functions. Guanosine prevented glucose deprivation-induced toxicity in C6 cells by modulating oxidative and nitrosative stress and glial responses, such as the glutamate uptake, the glutamine synthetase activity, and the glutathione levels. Glucose deprivation decreased the level of EAAC1, the main glutamate transporter present in C6 cells. Guanosine also prevented this effect, most likely through PKC, PI3K, p38 MAPK, and ERK signaling pathways. Taken together, these results show that guanosine may represent an important mechanism for protection of glial cells against glucose deprivation. Additionally, this study contributes to a more thorough understanding of the glial- and redox-related protective properties of guanosine in astroglial cells.
Keywords: Guanosine, C6 astroglial cells, Glucose deprivation, Glutamate uptake
Introduction
The neurotransmitter and modulator effects of extracellular purine nucleosides and nucleotides are well established [1–3]. In addition, extracellular purines exert trophic and neuroprotective effects [2–5]. Guanosine, a guanine-based purine, is recognized as an extracellular signaling molecule. Guanosine is released from astrocytes and confers neuroprotective effects in several in vivo and in vitro studies [3, 6–11]. Guanosine can effectively protect cells against hypoxia [12, 13], cytotoxicity induced by the β-amyloid peptide [14], chronic cerebral hypoperfusion [15], ischemic insults [16–18], and other glutamatergic excitotoxic damage, such as seizures that are induced by quinolinic acid [3, 9, 19, 20] and methylmercury-induced oxidative stress [21]. Although there is increasing evidence showing the neuroprotective effects of guanosine on models of neurotoxicity, its mechanisms are not fully understood. However, guanosine may mediate its effects through the modulation of the mitogen-activated protein kinases (MAPKs) and the phosphoinositide 3-kinase (PI3K) signaling pathways [22, 23].
Astrocytes are key cells in the central nervous system (CNS) and serve a wide range of adaptive functions. Specifically, astrocytes regulate the metabolic support of neurons, energy metabolism, neurotransmitter systems, ionic homeostasis, and the defense against oxidative stress. In addition, astrocytes can play a protective role by releasing neurotrophic factors [24–30]. Astrocytes are also the primary cells responsible for glutamate transport into glial cells, maintaining glutamate homeostasis within the brain and contributing to the maintenance of extracellular concentration of glutamate below toxic levels, thus avoiding glutamatergic excitotoxicity [27, 28, 31–33].
Once taken up by astrocytes, glutamate may be converted to glutamine by glutamine synthetase (GS—EC 6.3.1.2) [34–36]. Following this conversion, glutamine returns to neurons and is converted back to glutamate, which is utilized for synaptic transmission [35, 37]. The biosynthesis of glutathione (GSH), a major antioxidant molecule in the brain, is another endpoint of glutamate [34, 38–40].
C6 astroglial cells are widely used as an astrocyte-like cell line to study astrocytic parameters, such as glutamate uptake, GS activity, GSH levels, S100B secretion, and oxidative and inflammatory responses. These cells are an important astrocytic cell model because of their ability to be stained for the presence of glial fibrillary acidic protein and S100B [41–47].
Glucose is the essential energy substrate of the adult brain [25, 48]. Glucose enters into the cells through glucose transporters and astrocytes are the only cells in the brain that have the ability to store glucose as glycogen [25, 48–50]. Astrocytes possess a specific cytoarchitecture that enables them to sense their surroundings and dynamically respond to changes in their microenvironment, such as changes in the glucose levels [25, 51]. Astrocytes take up glucose and characteristically display a high glycolytic rate [52, 53]. C6 astroglial cells metabolize glucose in relation to their sensitivity to the changes in extracellular glucose levels [54–57].
Hypoglycemic encephalopathy, a serious CNS disorder, induces cellular damage, predominantly in the hippocampal and cortical structures [58]. The mechanisms that are responsible for cell death in this pathology include glutamate excitotoxicity and mitochondrial disruption [58]. Furthermore, oxygen and glucose deprivation occurs during cerebral ischemia, which induces an increase in reactive oxygen and nitrogen species (ROS/RNS) levels and changes in the glutamatergic neurotransmission [59–61]. Thus, due to the important role of glucose in CNS metabolism, its homeostatic dysregulation may impair the essential functions of astrocytes.
Our group has reported that guanosine modulates important glial parameters that are involved in brain plasticity [3]. In the present study, we investigated the effect of guanosine on C6 astroglial cells during glucose deprivation in vitro by exchanging DMEM with glucose for glucose-free DMEM. Therefore, using C6 cells that were subjected to glucose deprivation, the following guanosine roles were evaluated: (1) as a glioprotective molecule (by evaluation of the cell membrane integrity and metabolic activity), (2) as an antioxidant molecule (by measuring the ROS/RNS levels and lipid peroxidation), and (3) as a modulator of the glutamatergic system (by measuring the glutamate uptake, GS activity and GSH intracellular levels). Additionally, the putative gliopreventive mechanisms of guanosine were explored.
Materials and Methods
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM) with or without glucose and other materials for cell cultures were purchased from Gibco (Carlsbad, CA, USA). 2′-7′-Dichorofluorescein diacetate, propidium iodide (PI), MTT formazan, γ-glutamylhydroxamate, GSH standard, o-phtaldyaldehyde, BIS II, LY294002, SB203580, PD98059, peroxidase-conjugated anti-rabbit immunoglobulin (IgG), and guanosine were obtained from Sigma-Aldrich (St. Louis, MO, USA). l-[3H]-glutamate, nitrocellulose membrane, and enhanced chemiluminescence (ECL) kit were from Amersham (Buckinghamshire, UK). Anti-EAAC1 was purchased from Alpha Diagnostic (San Antonio, TX, USA). All other chemicals were purchased from common commercial suppliers.
Maintenance of cell culture
The C6 astroglial cells were obtained from the American Type Culture Collection (Rockville, MD, USA) and were maintained essentially according to our previous publication [41]. The cells were seeded in flasks and maintained in culture in DMEM 6 mM glucose (pH 7.4) containing 5 % fetal bovine serum (FBS), 2.5 mg/ml Fungizone®, and 100 U/l gentamicin, at a temperature of 37 °C in an atmosphere of 5 % CO2/95 % air.
Exponentially growing cells were detached from the culture flasks using 0.05 % trypsin/ethylene-diaminetetracetic acid (EDTA) and seeded 10 × 103 cells/cm2 in 96-, 24-, or 6-well plates. The cells were then maintained in DMEM (6 mM glucose and 5 % FBS) at 37 °C in an atmosphere of 5 % CO2/95 % air until reach the confluence (third day in vitro).
Glucose and guanosine treatments
After the cell confluence, the culture medium was removed from well plates and serum-free DMEM 6 mM glucose was added, and cells were preincubated in the absence or presence of 100 μM of guanosine for 1 h. After preincubation, the medium was replaced with serum-free DMEM with 6 mM glucose (control) or glucose- and serum-free DMEM (0 mM) (glucose deprivation), and both groups were maintained for 12 h. Guanosine 100 μM was maintained for this 12 h of treatment (DMEM with or without glucose). To study the signaling pathways involved in the glutamate uptake mechanisms, we coincubated with guanosine: BIS II (1 μM), LY294002 (10 μM), SB203580 (5 μM), and PD98059 (5 μM), the specific inhibitors of PKC, PI3K, p38 MAPK, and ERK, respectively.
Membrane integrity and metabolic activity
For PI incorporation assay (membrane integrity), 7.5 μM PI was added, and cells were incubated for 12 h at 37 °C in an atmosphere of 5 % CO2/95 % air. The optical density of fluorescent nuclei (labeled with PI), used to indicate a loss in membrane integrity, was determined with Optiquant software (Packard Instrument Company). Density values obtained are expressed as a percentage of the control condition.
For MTT reduction assay (metabolic activity), 50 μg/ml MTT was added, and cells were incubated for 30 min at 37 °C in an atmosphere of 5 % CO2/95 % air. Subsequently, the medium was removed and the MTT crystals were dissolved in dimethyl sulfoxide. Absorbance values were measured at 560 and 650 nm. Results are expressed as percentages of the control condition.
DCFH oxidation
Intracellular ROS levels were detected using the nonfluorescent cell-permeating compound, 2′-7′-dichlorofluorescein diacetate (DCFH-DA). It enters the cells and is hydrolyzed by intracellular esterases to dichlorofluorescin (DCFH), which is trapped within the cell. This nonfluorescent molecule is then oxidized into fluorescent dichlorofluorescin (DCF) by the action of cellular oxidants. DCFH-DA (10 μM) was added, and cells were incubated for 30 min at 37 °C. Following DCFH-DA exposure, the cells were scraped into phosphate-buffered saline (PBS) with 0.2 % Triton X-100. The fluorescence was measured in a plate reader (Spectra Max GEMINI XPS, Molecular Devices, USA) with excitation at 485 nm and emission at 520 nm [44]. Results are expressed as percentages of the control condition.
TBARS measurement
Lipid peroxidation can be evaluated by the thiobarbituric acid reactive substances assay. This method evaluates the oxidative stress assayed for malondialdehyde, the last product of lipid breakdown caused by oxidative stress. The assay was performed as previously described [62]. Briefly, cells were lysed in PBS with KCl (140 mM) and 100 μl of lysed cell suspension were added to 200 μl of cold 10 % trichloroacetic acid (TCA) and 300 μl of 0.67 % TBA in 7.1 % sodium sulfate and put in a boiling water bath for 1 h. The mixture was placed in cold water for 3 min. Afterwards, 400 μl of butyl alcohol were added, and samples were centrifuged at 5,000×g for 5 min. Pink-stained thiobarbituric acid reactive substances (TBARS) were determined using resulting supernatants in a spectrophotometric microtiter plate reader at 532 nm. A calibration curve was performed using 1,1,3,3-tetramethoxypropane. Results are expressed as percentages of the control condition.
Nitrite levels
Nitric oxide levels were determined by measuring the amount of nitrite [a stable oxidation product of nitric oxide (NO)], as indicated by the Griess reaction. The Griess reagent was prepared by mixing equal volumes of 1 % sulfanilamide in 0.5 M HCl and 0.1 % N-(1-naphthyl) ethylenediamine in deionized water. The assay was performed as described [63], with modifications. Briefly, cells were cultured on 96-well plate and the Griess reagent was added directly to the cell culture, and the incubation was maintained for 15 min in a dark, room temperature atmosphere. Samples were analyzed at 550 nm on a microplate spectrophotometer. Nitrite concentrations were calculated using a standard curve prepared with sodium nitrite (0–50 μM). Results are expressed as percentages of the control condition.
Glutamate uptake
After the cells reached confluence, the glutamate uptake was performed as previously described [41]. Briefly, C6 cells were incubated at 37 °C in Hank’s balanced salt solution (HBSS) containing the following components (in mM): 137 NaCl, 5.36 KCl, 1.26 CaCl2, 0.41 MgSO4, 0.49 MgCl2, 0.63 Na2HPO4, 0.44 KH2PO4, 4.17 NaHCO3, and 5.6 glucose, adjusted to pH 7.4. The assay was started by the addition of 0.1 mM l-glutamate and 0.33 μCi/ml l-[2,3-3H] glutamate. The cell incubation was stopped after 10 min by removing the medium and rinsing twice with ice-cold HBSS. The cells were then lysed in a solution containing 0.5 M NaOH. Incorporated radioactivity was measured in a scintillation counter. Sodium-independent uptake was determined using N-methyl-d-glucamine instead sodium chloride. Sodium-dependent glutamate uptake, considered specific uptake, was obtained by subtracting the sodium-independent uptake from the total uptake. Results are expressed as percentages of the control condition.
Western blot analysis
Cells were solubilized with lysis solution with 4 % sodium dodecyl sulfate (SDS), 2 mM EDTA, 50 mM Tris–HCl, pH 6.8. Equal amounts of proteins from each sample were boiled in a sample buffer [62.5 mM Tris–HCl, pH 6.8, 2 % (w/v) SDS, 5 % β-mercaptoethanol, 10 % (v/v) glycerol, 0.002 % (w/v) bromophenol blue] and submitted to electrophoresis in 10 % (w/v) SDS-polyacrylamide gel. The separated proteins were blotted onto a nitrocellulose membrane. Equal loading of each sample was confirmed with Ponceau S staining (Sigma). The membrane was incubated with polyclonal antibody anti-EAAC1 (1:1,000), and β-actin was used as loading control. After incubating overnight with the primary antibody at room temperature, membrane was washed and incubated with peroxidase-conjugated antirabbit IgG at a dilution of 1:1,000 for 1 h. The chemiluminescence signal was detected using an ECL, after which the films were scanned and the bands were quantified using the Scion Image software.
Glutamine synthetase activity
The enzymatic assay was performed as previously described [41]. Briefly, 0.1 ml lysed cell suspension solubilized in 140 mM KCl was added to 0.1 ml of the reaction mixture containing (in mM): 10 MgCl2, 50 l-glutamate, 100 imidazole-HCl buffer (pH 7.4), 10 2-mercaptoethanol, 50 hydroxylamine-HCl, and 10 ATP, and incubated for 15 min (37 °C). The reaction was stopped by the addition of 0.4 ml of a solution containing (in mM): 370 ferric chloride, 670 HCl, and 200 TCA. After centrifugation, the absorbance of the supernatant was measured at 530 nm and compared to the absorbance generated using standard quantities of γ-glutamylhydroxamate treated with a ferric chloride reagent. Results are expressed as percentages of the control condition.
GSH levels
GSH levels were assessed as previously described [64]. Lysed cell suspension solubilized in PBS with KCl (140 mM) was diluted in 100 mM sodium phosphate buffer (pH 8.0) containing 5 mM EDTA, and the protein was precipitated with 1.7 % meta-phosphoric acid. The supernatant was assayed with o-phthaldialdehyde (1 mg/ml methanol) at room temperature for 15 min. Fluorescence was measured using excitation and emission wavelengths of 350 and 420 nm, respectively. A calibration curve was performed with standard GSH solutions (0–500 μM). GSH concentrations were calculated as nanomoles per milligram protein. Results are expressed as percentages of the control condition.
Protein assay
Protein content was measured using Lowry’s method with bovine serum albumin as a standard [65].
Statistical analyses
Data were statistically analyzed using one-way analysis of variance (ANOVA), followed by the Tukey’s test. P’s < 0.05 were considered significant. All analyses were performed using the Statistical Package for Social Sciences software version 15.0.
Results
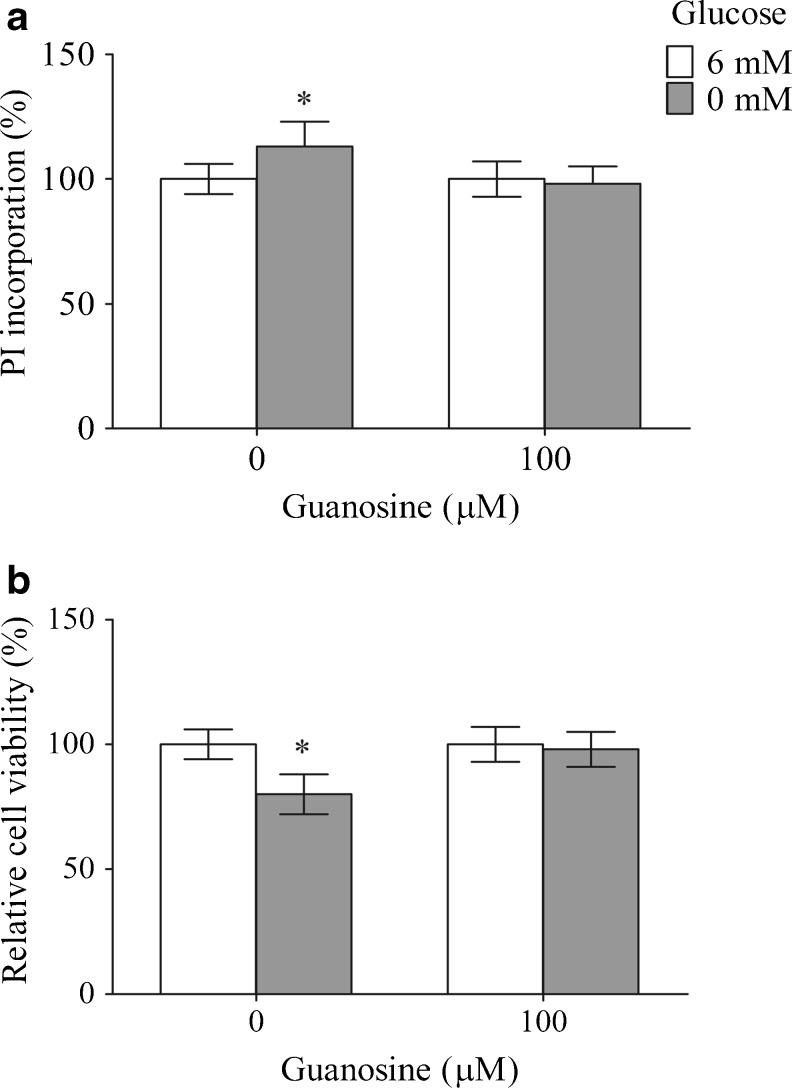
The membrane integrity of C6 astroglial cells after 12 h of glucose deprivation was evaluated by measuring the PI incorporation (Fig. 1a), and the metabolic activity was determined by MTT assay; the results of this assay were expressed as the relative cell viability (Fig. 1b). Glucose deprivation, from 6 to 0 mM, increased the PI incorporation (13 %, P < 0.05) and decreased the cell viability (20 %, P < 0.05) compared to control condition. Guanosine protected the membrane integrity and the cell viability, restoring both parameters to the control value, with no effect in the control condition.
Fig. 1.
The effects of guanosine on membrane integrity and metabolic activity against glucose deprivation-induced cytotoxicity in C6 astroglial cells. Membrane integrity—PI incorporation (a) and metabolic activity—MTT reduction (b), indicated as the relative cell viability, were measured as described in “Materials and Methods.” The data are expressed as a percentage of the control condition and represent the mean ± SEM of three experimental determinations performed in triplicate and analyzed statistically by one-way ANOVA followed by the Tukey’s test. *P < 0.05, significant differences from the control (6 mM glucose)
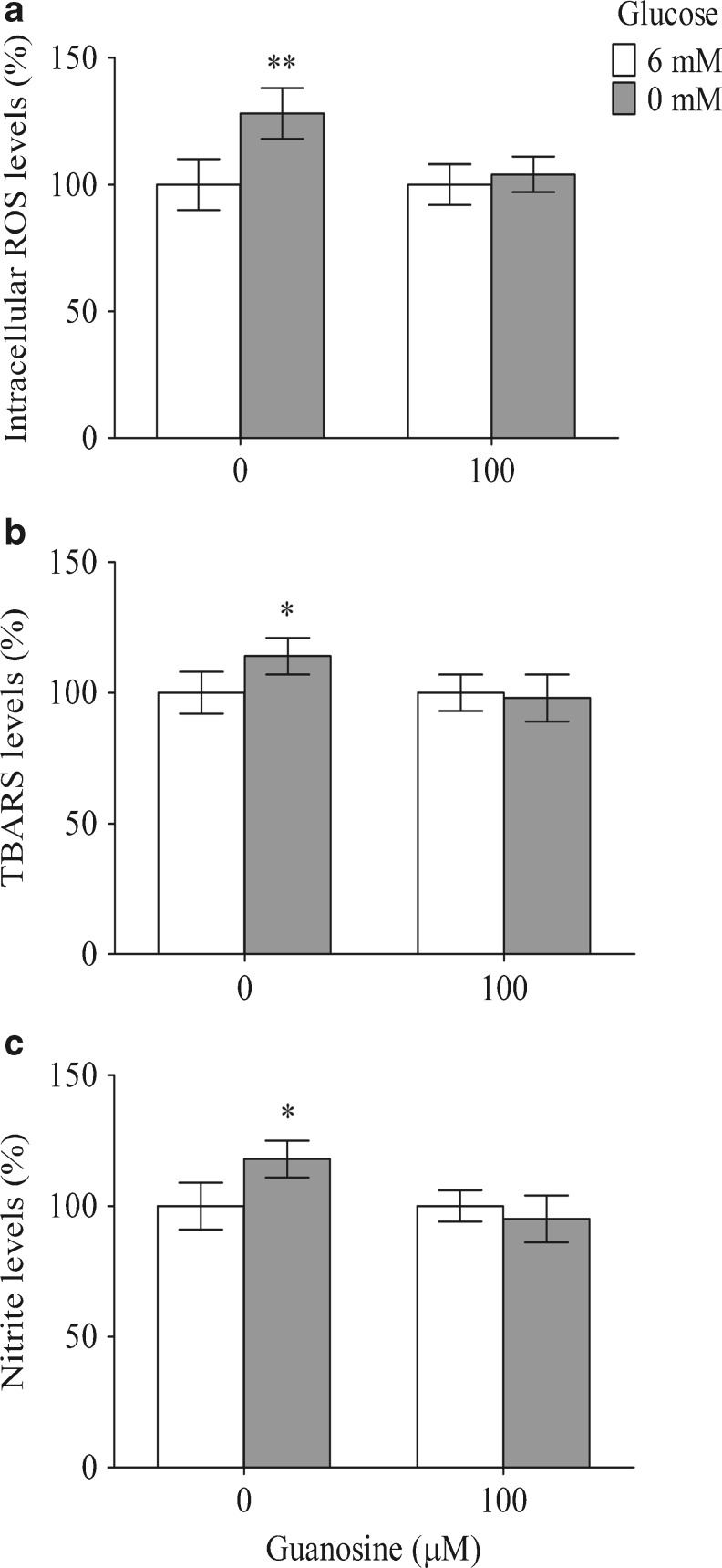
The DCFH oxidation in C6-cultivated cells increased by approximately 28 % following treatment for 12 h with glucose-free DMEM, indicating an increase in the ROS levels (Fig. 2a). Guanosine prevented this effect, dropped the levels from 128 ± 10 % to 104 ± 7 % (P < 0.01), indicating that guanosine may play an antioxidant role. Next, we evaluated the lipid peroxidation using a TBARS assay. Glucose deprivation increased the TBARS levels by about 14 % (P < 0.05) compared with the control condition (Fig. 2b). Guanosine prevented the glucose deprivation-induced cytotoxicity via lipid peroxidation. The production of NO was indirectly measured by the formation of nitrite (Fig. 2c). Glucose deprivation increased the nitrite levels up to 18 % (P < 0.05), whereas guanosine prevented this effect. Guanosine had no effect on these three parameters in the control condition.
Fig. 2.
Guanosine presents antioxidant effect in C6 astroglial cells. Guanosine decreased the ROS levels (a), the lipid peroxidation (b), and the nitrite levels (c) that were induced by glucose deprivation. The assays were performed as described in “Materials and Methods.” The data are expressed as a percentage of the control condition and represent the mean ± SEM of three experimental determinations that were performed in triplicate and analyzed statistically by one-way ANOVA followed by Tukey’s test. *P < 0.05, **P < 0.01, significant differences from the control (6 mM glucose)
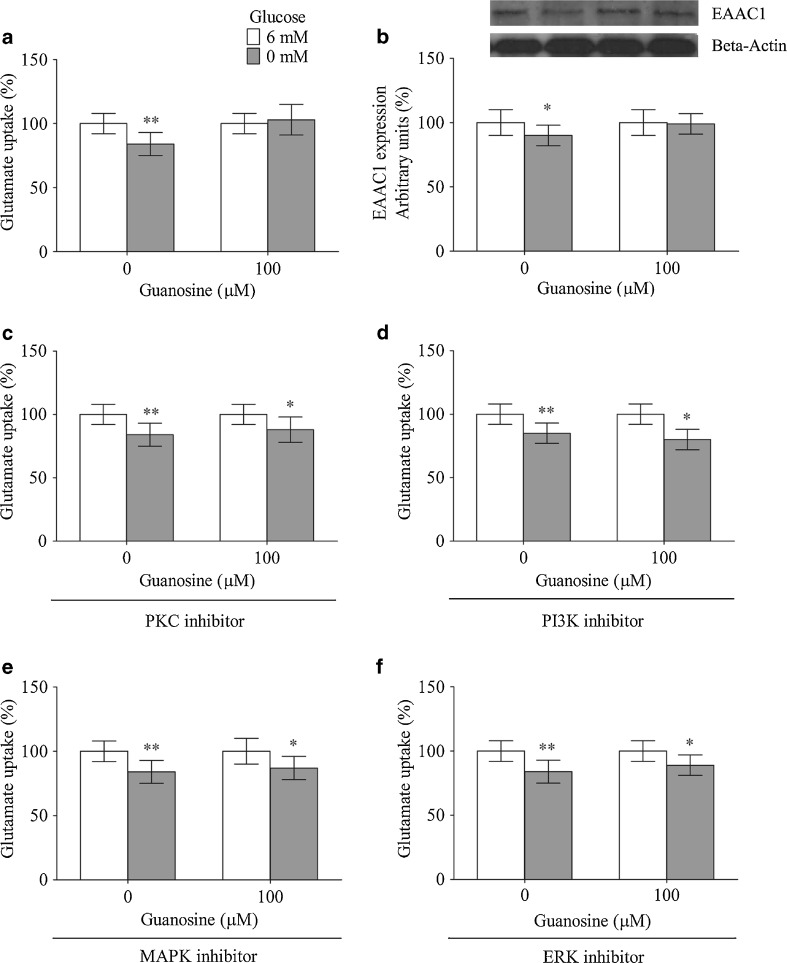
As shown in Fig. 3a, glucose deprivation decreased the glutamate uptake, from 100 ± 8 % to 84 ± 9 % (P < 0.01); guanosine significantly prevented this effect. As expected, the immunocontent of EAAC1, which is the primary glutamate transporter present in C6 cells, was also decreased by glucose deprivation (Fig. 3b). This effect was inhibited by guanosine, and both results indicate that guanosine could effectively modulate the glutamate uptake activity and the glutamate transporter levels under cellular injury. Guanosine had no effect on these two parameters in control condition.
Fig. 3.
Guanosine modulates glutamate uptake. Guanosine prevents glucose deprivation-induced glutamate uptake (a) and the decreased in the EAAC1 levels (b). The signaling pathways involved in the effect of guanosine on glutamate uptake. The following inhibitors were used: BIS II—a PKC inhibitor (c), LY294002—a PI3K inhibitor (d), SB203580—a p38 MAPK inhibitor (e), PD98059—an ERK inhibitor (f). The glutamate uptake and the Western blot analysis were measured as described in “Materials and Methods.” The data are expressed as a percentage of control condition and represent the mean ± SEM of three experimental determinations that were performed in triplicate and analyzed statistically by one-way ANOVA followed by Tukey’s test. The absolute value in the control condition for glutamate uptake was 0.72 ± 0.06 nmol/mg protein/min. *P < 0.05, **P < 0.01, significant differences from control
The regulation of EAAC1 is not fully understood, although signaling pathways, such as protein kinase C (PKC) and PI3K, may be involved in its regulation in C6 cells [66–69]. Therefore, we used the PKC (BIS II, 1 μM) and PI3K (LY294002, 10 μM) inhibitors to investigate the pathways by which guanosine could modulate the glutamate uptake activity. Guanosine coincubated with the PKC (Fig. 3c) and PI3K (Fig. 3d) inhibitors lost the ability to prevent the decreased glutamate uptake activity caused by glucose deprivation. The MAPK signaling pathway has also been described as a modulator of glutamate uptake [70, 71]. Because guanosine modulates this pathway [14], we investigated whether the protective effect of guanosine on glutamate uptake was also dependent of p38 MAPK and ERK. Coincubation with the p38 MAPK inhibitor (SB203580—5 μM, Fig. 3e) and the ERK inhibitor (PD98059—5 μM, Fig. 3f) abolished the preventive effect of guanosine on glucose deprivation-decreased glutamate uptake in C6 cells. Guanosine had no effect in the control condition.
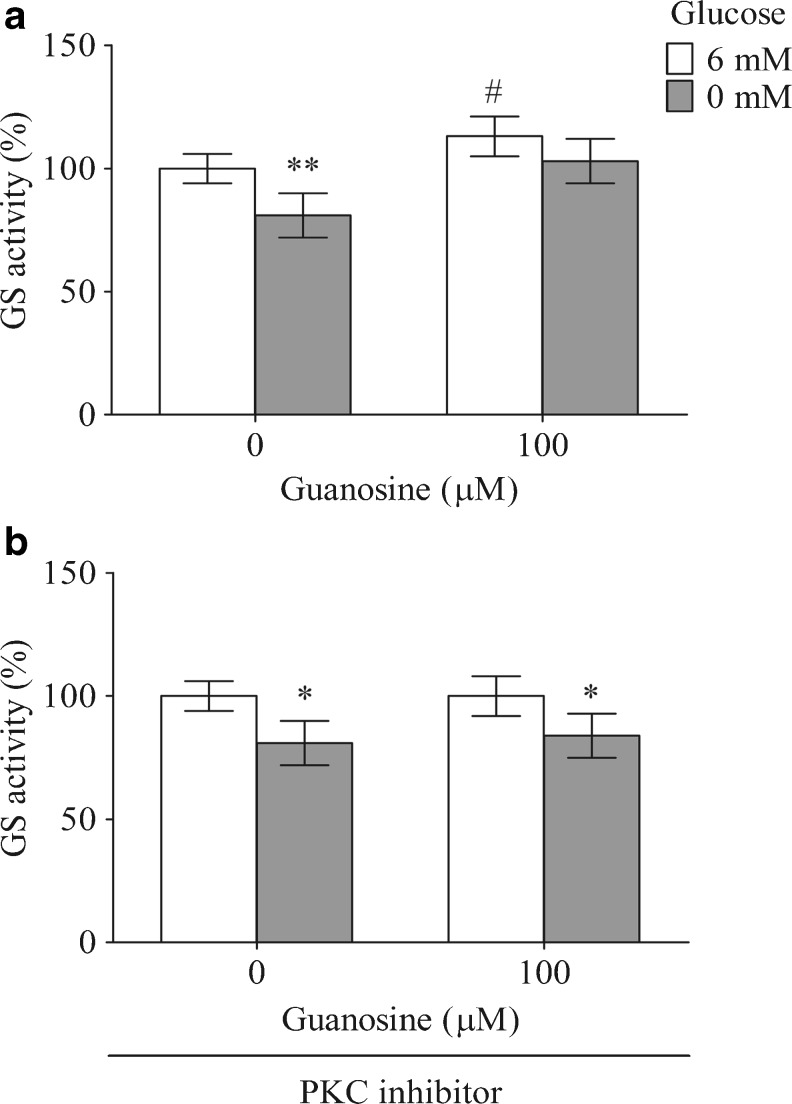
A main destination of glutamate in astroglial cells is its conversion to glutamine by glutamine synthetase. The exposure of C6 astroglial cells to the glucose-free DMEM decreased the GS activity by about 19 %, P < 0.01 (Fig. 4a). Guanosine increased the GS activity in the control conditions (13 %, P < 0.05) and during the glucose deprivation condition (from 81 ± 9 % to 103 ± 9 %). As it was reported that PKC signaling pathway appears to modulate this enzyme [72], we determined whether the effect of guanosine could be dependent of PKC. Coincubation of guanosine with 1 μM BIS II, a PKC inhibitor (Fig. 4b), abolished the guanosine effects.
Fig. 4.
Guanosine increases the GS activity. The basal GS activity (a) and the GS activity with BIS II, a PKC inhibitor (b), were measured as described in “Materials and Methods.” The data are expressed as a percentage of the control condition and represent the mean ± SEM of three experimental determinations that were performed in triplicate and analyzed statistically by one-way ANOVA followed by Tukey’s test. The absolute value was 0.8 ± 0.07 μmol/mg protein/min and 0.95 ± 0.08 μmol/mg protein/min for the control conditions and for the addition of BIS II, respectively. *P < 0.05, **P < 0.01, significant differences from the control; # P < 0.05, significant differences from the condition using 6 mM glucose and guanosine
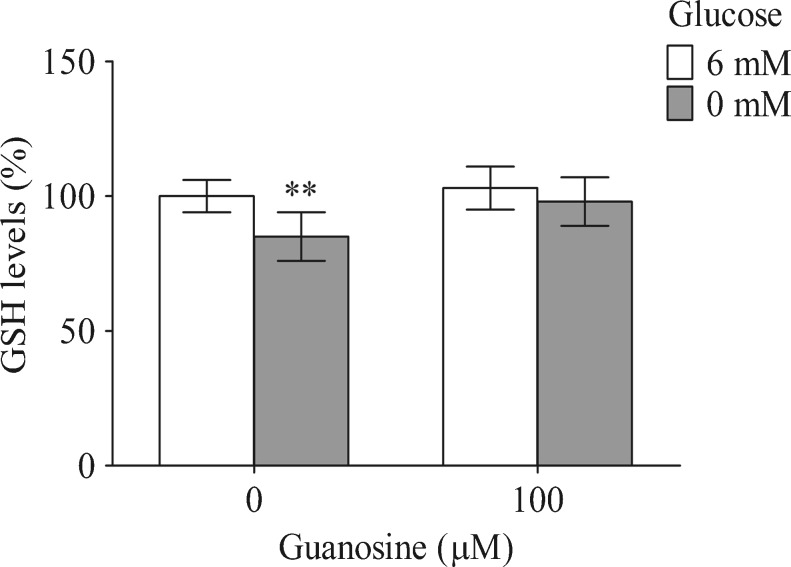
Glutamate is also a precursor of GSH synthesis in glial cells; therefore, the GSH intracellular levels were measured (Fig. 5). Glucose deprivation decreased the GSH intracellular levels by approximately 15 % (P < 0.01). Guanosine prevented this decrease with no effect in the control condition.
Fig. 5.
The effects of guanosine and glucose deprivation on GSH levels. GSH levels were measured as described in “Materials and Methods.” Data are expressed as a percentage of the control condition and represent the mean ± SEM of three experimental determinations that were performed in triplicate and analyzed statistically by one-way ANOVA followed by Tukey’s test. The absolute value obtained in the control conditions for GSH levels was 8.1 ± 0.7 nmol/mg protein. **P < 0.01, significant differences from the control
Discussion
The effects of guanosine on the brain have been studied in a variety of experimental neuropathological protocols [3], including brain trauma [73], brain ischemia [13, 17, 18, 74], and seizures [7, 19]. These conditions may involve glutamatergic excitotoxicity, metabolic changes, and/or oxidative stress. Although the neuroprotective role of guanosine in the CNS is well established [3], the cellular mechanisms underlying the guanosine-induced selective protection of neural cells need to be better elucidated. In this study, we reported that guanosine modulated the important glial functions in C6 cultured cells under in vitro glucose deprivation and the putative mechanisms of its gliopreventive effects.
Astrocytes play a critical role in the regulation of brain metabolic responses, including glucose metabolism [75]. Hypoglycemic conditions induce cellular damage in the brain [76]. C6 astroglial cells under normoglycemic conditions have been used as an astroglial model to study cellular functions and cell signaling [43, 77]. In this study, we investigated the effect of glucose deprivation, as a model of cytotoxicity, on C6 cultured astroglial cells. The decrease in the intracellular glucose levels reduces the amounts of the intermediates that are formed during glucose and glycogen metabolism, inducing failures in the mitochondrial function, a decrease in the NADH levels, an impairment of the redox balance with reduced ATP formation and, consequently, overproduction of the ROS by uncoupling glucose metabolism from the oxidative transport chain activity [51, 76, 78, 79]. Excessive free radicals lead to damages in lipids, proteins and DNA [80–83]. Guanosine prevented the cytotoxicity induced by glucose deprivation. This effect could be related, at least partially, to the release of a guanine-based purine after astrocytic hypoglycemic insults [6], its trophic properties and ability to stimulate astrocyte proliferation [84], its modulatory effects on the glutamatergic system [3], and its antioxidant properties. Additionally, guanosine promotes the activation of the PI3K signaling pathway, upstream of glycogen synthase kinase 3, which may regulate the glucose uptake, ROS levels and, thus, cell survival [13, 85, 86].
The present data show that the damage caused by hypoglycemia (measured by PI and MTT) was attenuated by guanosine (Fig. 1). The putative mechanisms involved in this glioprotection may have been achieved because guanosine abolished an increase in the ROS levels and the lipid peroxidation. Guanosine specifically decreased the NO levels and most likely decreased the levels of peroxynitrite. This effect may arise from an improvement in antioxidant defenses and/or from the inhibition of nitric oxide synthase. Peroxynitrite, which is formed by the reaction between NO and a superoxide anion, is one of the main molecules responsible for cellular damage and neurodegenerative disorders [87]. The oxidative and nitrosative stress may be responsible for cytotoxicity from glucose deprivation. Furthermore, NO is thought to play an important role in the pathophysiology of stroke, involving oxygen and glucose deprivation [88, 89].
Guanosine induces the expression of heme oxygenase 1 (HO1) via the PI3K pathway, thus protecting neural cells [90, 91]. This protein acts as a scavenger of NO and has been associated with adaptive cytoprotection against a wide array of toxic insults by modulating antioxidant defenses, such as GSH system and thioredoxin [92, 93]. Resveratrol is another antioxidant that modulates glial functions [43] and induces HO1 expression, thereby, under experimental conditions, protecting against stroke, which is a condition associated with glucose deprivation [94]. Therefore, guanosine may be effective against glucose deprivation toxicity either directly by its ROS/RNS-scavenging effect and/or by activating HO1.
The antioxidant effect of guanosine was reinforced by the modulation of the GSH homeostasis, the major non-enzymatic antioxidant in the CNS [40]. The increase in GSH levels in glial cells confers protection against neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases [95, 96]. Moreover, the tripeptide GSH constitutes a nonenzymatic scavenger and substrate for glutathione peroxidase [34, 97]. Glucose deprivation induces a decrease in NADPH regeneration, a molecule that is required to maintain glutathione in a reduced form (GSH) via glutathione reductase [79, 98]. Furthermore, Brongholi et al. [99] reported that oxygen-glucose deprivation decreases the GSH levels and glutamate uptake in rat hippocampal slices. As in C6 cells, glutamate and cystine share the same transport system [the cystine/glutamate (xc) antiporter], the elevation of the extracellular glutamate levels may contribute to the impairment of cystine transport and, consequently, the GSH biosynthesis [100]. Note that the depletion of GSH in the glial cells induces cytotoxicity and impairment of the glutamate transporters [96, 101]. In this context, the antioxidant effect of guanosine during glucose deprivation could decrease the oxidation of GSH, providing glioprotection that is associated with a scavenger activity.
The cellular energy failure associated with the glucose-free medium also results in disruption of the ionic gradient and deregulation of the glutamate transporters [102]. The glutamate uptake is performed by a specific system, the excitatory amino acids transporters (EAATs), which are vulnerable to the action of biological oxidants, which reduce their uptake function [32, 103, 104]. In this study, under the deprivation of extracellular glucose, guanosine modulated the EAAC1 (EAAT3) levels. Although this transporter is responsible for the support of de novo GSH synthesis, it is also sensitive to oxidative stress [105]. Typically, the activation of PKC and PI3K pathways regulates the expression of EAAC1 [13, 66]. In addition, the activation of the PI3K pathway increases the surface expression of EAAC1 in the transfected cells, further increasing the glutamate transport activity [68]. Several studies have shown that EAAC1 activity is partially regulated by its traffic between cytosol and plasma membrane [106]. The present data show that the PI3K signaling pathway is involved in the guanosine modulation of the glutamate uptake, which agrees with other studies that demonstrate the ability of guanosine to promote neuroprotection by activation of this pathway [85, 90, 107]. The MAPK signaling pathway has also been described as a modulator of glutamate uptake [70, 71], and guanosine also modulates this pathway [85]. Here, guanosine reversed the decrease in glutamate uptake caused by glucose deprivation; however, this effect was abolished by the p38 MAPK and ERK specific inhibitors. Additionally, the activation of MAPK/ERK is considered one of the major protective pathways in glucose deprivation [108, 109].
Although its mechanism of action remains unknown, our previous results indicate that guanosine may exert neuroprotection by the stimulation of the glutamate uptake activity in astroglial cells, counteracting glutamate excitotoxicity [3]. For over 25 years, our group has studied the effects of guanosine on the CNS. Frizzo et al. and Gottfried et al. [5, 110] showed that guanosine increases the glutamate uptake in putatively basal conditions, without focusing on the mechanism involved in this effect. Herein, we revealed that guanosine prevented the decrease in glutamate uptake under a cytotoxic insult, probably by the activation of EAAC1 glutamate transporter and PKC, PI3K, p38 MAPK, and ERK signaling pathways and/or by antioxidant/scavenger activity.
In astrocytes, glutamate may be oxidized to CO2 and/or may be a precursor of GSH and a precursor of glutamine via the enzyme GS [36], thus entering in the glutamate–glutamine cycle [37, 111]. Guanosine increased the GS activity in the control (normoglycemic) and toxic (deprivation of glucose) conditions, and this activation can represent an important pathway that reinforces the action of guanosine on glutamate metabolism. Moreover, this increase could also indicate that guanosine acts as a neuroprotective endogenous molecule, simultaneously promoting the uptake and clearance of glutamate, avoiding glutamatergic excitotoxicity. Additionally, the activity of GS helps in the maintenance of the GSH production. Importantly, the GS activity decreased under the glucose deprivation condition. This enzyme is highly sensitive to oxidative and nitrosative stress and is dependent of ATP [60]. The PKC isoforms may regulate the GS expression [72], and in this study, we observed that the effect of guanosine on the GS activity is dependent of PKC pathway.
Guanosine is emerging as an important research focus of both in vitro and in vivo studies of neurotoxicity and neuroprotection. The understanding of the influence of guanosine on glial changes is critical for the elucidation of the cellular and molecular mechanisms of the effects of this nucleoside. Our results show that cytotoxicity and oxidative and nitrosative stress that are induced by glucose deprivation were prevented by guanosine. We also found that the effect of guanosine on glutamate metabolism under glucose deprivation was via a mechanism dependent of the PKC, PI3K, and MAPK pathway activation. Thus, the present study contributes to better understanding of the glial- and redox-related protective properties of guanosine in astroglial cells.
Acknowledgments
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Financiadora de Estudos e Projetos (FINEP)—IBN Net (Instituto Brasileiro de Neurociências) 01.06.0842-00, Federal University of Rio Grande do Sul (UFRGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
References
- 1.Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:333–392. doi: 10.1007/978-3-540-79090-7_10. [DOI] [PubMed] [Google Scholar]
- 2.Ciccarelli R, Ballerini P, Sabatino G, Rathbone MP, D'Onofrio M, Caciagli F, Di Iorio P. Involvement of astrocytes in purine-mediated reparative processes in the brain. Int J Dev Neurosci. 2001;19(4):395–414. doi: 10.1016/s0736-5748(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther. 2007;116(3):401–416. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt AP, Tort AB, Silveira PP, Bohmer AE, Hansel G, Knorr L, Schallenberger C, Dalmaz C, Elisabetsky E, Crestana RH, Lara DR, Souza DO. The NMDA antagonist MK-801 induces hyperalgesia and increases CSF excitatory amino acids in rats: reversal by guanosine. Pharmacol Biochem Behav. 2009;91(4):549–553. doi: 10.1016/j.pbb.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Frizzo ME, Lara DR, Dahm KC, Prokopiuk AS, Swanson RA, Souza DO. Activation of glutamate uptake by guanosine in primary astrocyte cultures. Neuroreport. 2001;12(4):879–881. doi: 10.1097/00001756-200103260-00051. [DOI] [PubMed] [Google Scholar]
- 6.Ciccarelli R, Di Iorio P, Giuliani P, D'Alimonte I, Ballerini P, Caciagli F, Rathbone MP. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25(1):93–98. [PubMed] [Google Scholar]
- 7.Lara DR, Schmidt AP, Frizzo ME, Burgos JS, Ramirez G, Souza DO. Effect of orally administered guanosine on seizures and death induced by glutamatergic agents. Brain Res. 2001;912(2):176–180. doi: 10.1016/s0006-8993(01)02734-2. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt AP, Bohmer AE, Schallenberger C, Antunes C, Pereira MS, Leke R, Wofchuk ST, Elisabetsky E, Souza DO. Spinal mechanisms of antinociceptive action caused by guanosine in mice. Eur J Pharmacol. 2009;613(1–3):46–53. doi: 10.1016/j.ejphar.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Vinade ER, Schmidt AP, Frizzo ME, Izquierdo I, Elisabetsky E, Souza DO. Chronically administered guanosine is anticonvulsant, amnesic and anxiolytic in mice. Brain Res. 2003;977(1):97–102. doi: 10.1016/s0006-8993(03)02769-0. [DOI] [PubMed] [Google Scholar]
- 10.Vinade ER, Schmidt AP, Frizzo ME, Portela LV, Soares FA, Schwalm FD, Elisabetsky E, Izquierdo I, Souza DO. Effects of chronic administered guanosine on behavioral parameters and brain glutamate uptake in rats. J Neurosci Res. 2005;79(1–2):248–253. doi: 10.1002/jnr.20327. [DOI] [PubMed] [Google Scholar]
- 11.Chang R, Algird A, Bau C, Rathbone MP, Jiang S. Neuroprotective effects of guanosine on stroke models in vitro and in vivo. Neurosci Lett. 2008;431(2):101–105. doi: 10.1016/j.neulet.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 12.Oleskovicz SP, Martins WC, Leal RB, Tasca CI. Mechanism of guanosine-induced neuroprotection in rat hippocampal slices submitted to oxygen-glucose deprivation. Neurochem Int. 2008;52(3):411–418. doi: 10.1016/j.neuint.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Dal-Cim T, Martins WC, Santos AR, Tasca CI. Guanosine is neuroprotective against oxygen/glucose deprivation in hippocampal slices via large conductance Ca(2)+-activated K+ channels, phosphatidilinositol-3 kinase/protein kinase B pathway activation and glutamate uptake. Neuroscience. 2011;183:212–220. doi: 10.1016/j.neuroscience.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Pettifer KM, Kleywegt S, Bau CJ, Ramsbottom JD, Vertes E, Ciccarelli R, Caciagli F, Werstiuk ES, Rathbone MP. Guanosine protects SH-SY5Y cells against beta-amyloid-induced apoptosis. Neuroreport. 2004;15(5):833–836. doi: 10.1097/00001756-200404090-00019. [DOI] [PubMed] [Google Scholar]
- 15.Ganzella M, de Oliveira ED, Comassetto DD, Cechetti F, Cereser VH, Jr, Moreira JD, Hansel G, Almeida RF, Ramos DB, Figueredo YN, Souza DG, Oses JP, Worm PV, Achaval M, Netto CA, Souza DO. Effects of chronic guanosine treatment on hippocampal damage and cognitive impairment of rats submitted to chronic cerebral hypoperfusion. Neurol Sci. 2012;33(5):985–997. doi: 10.1007/s10072-011-0872-1. [DOI] [PubMed] [Google Scholar]
- 16.Moretto MB, Boff B, Lavinsky D, Netto CA, Rocha JB, Souza DO, Wofchuk ST. Importance of schedule of administration in the therapeutic efficacy of guanosine: early intervention after injury enhances glutamate uptake in model of hypoxia-ischemia. J Mol Neurosci. 2009;38(2):216–219. doi: 10.1007/s12031-008-9154-7. [DOI] [PubMed] [Google Scholar]
- 17.Moretto MB, Arteni NS, Lavinsky D, Netto CA, Rocha JB, Souza DO, Wofchuk S. Hypoxic-ischemic insult decreases glutamate uptake by hippocampal slices from neonatal rats: prevention by guanosine. Exp Neurol. 2005;195(2):400–406. doi: 10.1016/j.expneurol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Rathbone MP, Saleh TM, Connell BJ, Chang R, Su C, Worley B, Kim M, Jiang S. Systemic administration of guanosine promotes functional and histological improvement following an ischemic stroke in rats. Brain Res. 2011;1407:79–89. doi: 10.1016/j.brainres.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt AP, Lara DR, de Faria Maraschin J, da Silveira Perla A, Onofre Souza D. Guanosine and GMP prevent seizures induced by quinolinic acid in mice. Brain Res. 2000;864(1):40–43. doi: 10.1016/s0006-8993(00)02106-5. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt AP, Avila TT, Souza DO. Intracerebroventricular guanine-based purines protect against seizures induced by quinolinic acid in mice. Neurochem Res. 2005;30(1):69–73. doi: 10.1007/s11064-004-9687-2. [DOI] [PubMed] [Google Scholar]
- 21.Roos DH, Puntel RL, Santos MM, Souza DO, Farina M, Nogueira CW, Aschner M, Burger ME, Barbosa NB, Rocha JB. Guanosine and synthetic organoselenium compounds modulate methylmercury-induced oxidative stress in rat brain cortical slices: involvement of oxidative stress and glutamatergic system. Toxicol In Vitro. 2009;23(2):302–307. doi: 10.1016/j.tiv.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Tarozzi A, Merlicco A, Morroni F, Bolondi C, Di Iorio P, Ciccarelli R, Romano S, Giuliani P, Hrelia P. Guanosine protects human neuroblastoma cells from oxidative stress and toxicity induced by Amyloid-beta peptide oligomers. J Biol Regul Homeost Agents. 2010;24(3):297–306. [PubMed] [Google Scholar]
- 23.Molz S, Dal-Cim T, Budni J, Martin-de-Saavedra MD, Egea J, Romero A, del Barrio L, Rodrigues AL, Lopez MG, Tasca CI Neuroprotective effect of guanosine against glutamate-induced cell death in rat hippocampal slices is mediated by the phosphatidylinositol-3 kinase/Akt/ glycogen synthase kinase 3beta pathway activation and inducible nitric oxide synthase inhibition. J Neurosci Res. 2011;89(9):1400–1408. doi: 10.1002/jnr.22681. [DOI] [PubMed] [Google Scholar]
- 24.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86(4):342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26(10):523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Maragakis NJ, Rothstein JD. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2(12):679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 28.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14. [PubMed] [Google Scholar]
- 29.Ransom BR, Ransom CB. Astrocytes: multitalented stars of the central nervous system. Methods Mol Biol. 2012;814:3–7. doi: 10.1007/978-1-61779-452-0_1. [DOI] [PubMed] [Google Scholar]
- 30.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem. 2012;121(1):4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 32.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19(8):328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 33.Coulter DA, Eid T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia. 2012;60(8):1215–1226. doi: 10.1002/glia.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27(12):735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Mates JM, Perez-Gomez C, NunezdeCastro I, Asenjo M, Marquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol. 2002;34(5):439–458. doi: 10.1016/s1357-2725(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 36.McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85(15):3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- 37.Hertz L. Glutamate, a neurotransmitter—and so much more, a synopsis of Wierzba III. Neurochem Int. 2006;48(6–7):416–425. doi: 10.1016/j.neuint.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 38.Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19(2):562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62(6):649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee R, Vitvitsky V, Garg SK. The undertow of sulfur metabolism on glutamatergic neurotransmission. Trends Biochem Sci. 2008;33(9):413–419. doi: 10.1016/j.tibs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos AQ, Nardin P, Funchal C, de Almeida LM, Jacques-Silva MC, Wofchuk ST, Goncalves CA, Gottfried C. Resveratrol increases glutamate uptake and glutamine synthetase activity in C6 glioma cells. Arch Biochem Biophys. 2006;453(2):161–167. doi: 10.1016/j.abb.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 42.Bobermin LD, Quincozes-Santos A, Guerra MC, Leite MC, Souza DO, Goncalves CA, Gottfried C. Resveratrol prevents ammonia toxicity in astroglial cells. PLoS One. 2012;7(12):e52164. doi: 10.1371/journal.pone.0052164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quincozes-Santos A, Gottfried C. Resveratrol modulates astroglial functions: neuroprotective hypothesis. Ann N Y Acad Sci. 2011;1215:72–78. doi: 10.1111/j.1749-6632.2010.05857.x. [DOI] [PubMed] [Google Scholar]
- 44.Quincozes-Santos A, Nardin P, de Souza DF, Gelain DP, Moreira JC, Latini A, Goncalves CA, Gottfried C. The janus face of resveratrol in astroglial cells. Neurotox Res. 2009;16(1):30–41. doi: 10.1007/s12640-009-9042-0. [DOI] [PubMed] [Google Scholar]
- 45.Benda P, Davidson RL. Regulation of specific functions of glial cells in somatic hybrids. I. Control of S100 protein. J Cell Physiol. 1971;78(2):209–216. doi: 10.1002/jcp.1040780207. [DOI] [PubMed] [Google Scholar]
- 46.Benda P, Lightbody J, Sato G, Levine L, Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161(839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- 47.Funchal C, Latini A, Jacques-Silva MC, Dos Santos AQ, Buzin L, Gottfried C, Wajner M, Pessoa-Pureur R. Morphological alterations and induction of oxidative stress in glial cells caused by the branched-chain alpha-keto acids accumulating in maple syrup urine disease. Neurochem Int. 2006;49(7):640–650. doi: 10.1016/j.neuint.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Allaman I, Belanger M, Magistretti PJ. Astrocyte–neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34(2):76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 50.Pellerin L, Bonvento G, Chatton JY, Pierre K, Magistretti PJ. Role of neuron–glia interaction in the regulation of brain glucose utilization. Diabetes Nutr Metab. 2002;15(5):268–273. [PubMed] [Google Scholar]
- 51.Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64(6):654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91(22):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunet JF, Allaman I, Magistretti PJ, Pellerin L. Glycogen metabolism as a marker of astrocyte differentiation. J Cereb Blood Flow Metab. 2010;30(1):51–55. doi: 10.1038/jcbfm.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabernero A, Medina JM, Giaume C. Glucose metabolism and proliferation in glia: role of astrocytic gap junctions. J Neurochem. 2006;99(4):1049–1061. doi: 10.1111/j.1471-4159.2006.04088.x. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Xu N, Cai L, Gao Z, Shen L, Zhang Q, Hou W, Zhong H, Wang Q, Xiong L. NDRG2 is a novel p53-associated regulator of apoptosis in C6-originated astrocytes exposed to oxygen-glucose deprivation. PLoS One. 2013;8(2):e57130. doi: 10.1371/journal.pone.0057130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tramontina AC, Nardin P, Quincozes-Santos A, Tortorelli L, Wartchow KM, Andreazza AC, Braganhol E, de Souza DO, Goncalves CA. High-glucose and S100B stimulate glutamate uptake in C6 glioma cells. Neurochem Res. 2012;37(7):1399–1408. doi: 10.1007/s11064-012-0722-4. [DOI] [PubMed] [Google Scholar]
- 57.Nardin P, Tramontina F, Leite MC, Tramontina AC, Quincozes-Santos A, de Almeida LM, Battastini AM, Gottfried C, Goncalves CA. S100B content and secretion decrease in astrocytes cultured in high-glucose medium. Neurochem Int. 2007;50(5):774–782. doi: 10.1016/j.neuint.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Barros JS, Goncalves CA, Bairos VA. Elastic fibres in the human placenta. Arch Gynecol Obstet. 2003;267(4):208–212. doi: 10.1007/s00404-002-0314-7. [DOI] [PubMed] [Google Scholar]
- 59.Benavides A, Pastor D, Santos P, Tranque P, Calvo S. CHOP plays a pivotal role in the astrocyte death induced by oxygen and glucose deprivation. Glia. 2005;52(4):261–275. doi: 10.1002/glia.20242. [DOI] [PubMed] [Google Scholar]
- 60.Hertz L. Astrocytic amino acid metabolism under control conditions and during oxygen and/or glucose deprivation. Neurochem Res. 2003;28(2):243–258. doi: 10.1023/a:1022377100379. [DOI] [PubMed] [Google Scholar]
- 61.Espinoza-Rojo M, Iturralde-Rodriguez KI, Chanez-Cardenas ME, Ruiz-Tachiquin ME, Aguilera P. Glucose transporters regulation on ischemic brain: possible role as therapeutic target. Cent Nerv Syst Agents Med Chem. 2010;10(4):317–325. doi: 10.2174/187152410793429755. [DOI] [PubMed] [Google Scholar]
- 62.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 63.Hu J, Castets F, Guevara JL, Van Eldik LJ. S100 beta stimulates inducible nitric oxide synthase activity and mRNA levels in rat cortical astrocytes. J Biol Chem. 1996;271(5):2543–2547. doi: 10.1074/jbc.271.5.2543. [DOI] [PubMed] [Google Scholar]
- 64.Browne RW, Armstrong D. Reduced glutathione and glutathione disulfide. Methods Mol Biol. 1998;108:347–352. doi: 10.1385/0-89603-472-0:347. [DOI] [PubMed] [Google Scholar]
- 65.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 66.Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J Neurosci. 1998;18(7):2475–2485. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bianchi MG, Rotoli BM, Dall'Asta V, Gazzola GC, Gatti R, Bussolati O. PKC-dependent stimulation of EAAT3 glutamate transporter does not require the integrity of actin cytoskeleton. Neurochem Int. 2006;48(5):341–349. doi: 10.1016/j.neuint.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 68.Krizman-Genda E, Gonzalez MI, Zelenaia O, Robinson MB. Evidence that Akt mediates platelet-derived growth factor-dependent increases in activity and surface expression of the neuronal glutamate transporter, EAAC1. Neuropharmacology. 2005;49(6):872–882. doi: 10.1016/j.neuropharm.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Lee G, Huang Y, Washington JM, Briggs NW, Zuo Z. Carbamazepine enhances the activity of glutamate transporter type 3 via phosphatidylinositol 3-kinase. Epilepsy Res. 2005;66(1–3):145–153. doi: 10.1016/j.eplepsyres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Zhou H, Chen Q, Kong DL, Guo J, Wang Q, Yu SY. Effect of resveratrol on gliotransmitter levels and p38 activities in cultured astrocytes. Neurochem Res. 2011;36(1):17–26. doi: 10.1007/s11064-010-0254-8. [DOI] [PubMed] [Google Scholar]
- 71.Lee E, Sidoryk-Wegrzynowicz M, Wang N, Webb A, Son DS, Lee K, Aschner M. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem. 2012 doi: 10.1074/jbc.M112.341867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kronfeld I, Zsukerman A, Kazimirsky G, Brodie C. Staurosporine induces astrocytic phenotypes and differential expression of specific PKC isoforms in C6 glial cells. J Neurochem. 1995;65(4):1505–1514. doi: 10.1046/j.1471-4159.1995.65041505.x. [DOI] [PubMed] [Google Scholar]
- 73.Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog Neurobiol. 1999;59(6):663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 74.Connell BJ, Di Iorio P, Sayeed I, Ballerini P, Saleh MC, Giuliani P, Saleh TM, Rathbone MP, Su C, Jiang S. Guanosine protects against reperfusion injury in rat brains after ischemic stroke. J Neurosci Res. 2013;91(2):262–272. doi: 10.1002/jnr.23156. [DOI] [PubMed] [Google Scholar]
- 75.Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55(12):1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 76.Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*, S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmet hyl)propyl]-1H-indole-2-carboxamide) J Pharmacol Exp Ther. 2007;321(1):45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- 77.Obara Y, Nemoto W, Kohno S, Murata T, Kaneda N, Nakahata N. Basic fibroblast growth factor promotes glial cell-derived neurotrophic factor gene expression mediated by activation of ERK5 in rat C6 glioma cells. Cell Signal. 2011;23(4):666–672. doi: 10.1016/j.cellsig.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 78.Swanson RA, Choi DW. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J Cereb Blood Flow Metab. 1993;13(1):162–169. doi: 10.1038/jcbfm.1993.19. [DOI] [PubMed] [Google Scholar]
- 79.Druz A, Betenbaugh M, Shiloach J. Glucose depletion activates mmu-miR-466h-5p expression through oxidative stress and inhibition of histone deacetylation. Nucleic Acids Res. 2012;40(15):7291–7302. doi: 10.1093/nar/gks452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quincozes-Santos A, Andreazza AC, Goncalves CA, Gottfried C. Actions of redox-active compound resveratrol under hydrogen peroxide insult in C6 astroglial cells. Toxicol In Vitro. 2010;24(3):916–920. doi: 10.1016/j.tiv.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 81.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18(9):685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 82.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141(2):312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35(Pt 5):1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 84.Ciccarelli R, Di Iorio P, D'Alimonte I, Giuliani P, Florio T, Caciagli F, Middlemiss PJ, Rathbone MP. Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the co-presence of microglia. Glia. 2000;29(3):202–211. [PubMed] [Google Scholar]
- 85.Di Iorio P, Ballerini P, Traversa U, Nicoletti F, D'Alimonte I, Kleywegt S, Werstiuk ES, Rathbone MP, Caciagli F, Ciccarelli R. The antiapoptotic effect of guanosine is mediated by the activation of the PI 3-kinase/AKT/PKB pathway in cultured rat astrocytes. Glia. 2004;46(4):356–368. doi: 10.1002/glia.20002. [DOI] [PubMed] [Google Scholar]
- 86.Fukui M, Choi HJ, Zhu BT. Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic Biol Med. 2010;49(5):800–813. doi: 10.1016/j.freeradbiomed.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 88.Garnier P, Ying W, Swanson RA. Ischemic preconditioning by caspase cleavage of poly(ADP-ribose) polymerase-1. J Neurosci. 2003;23(22):7967–7973. doi: 10.1523/JNEUROSCI.23-22-07967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang M, Ning GM, Hong DH, Yang Y, Kutor J, Zheng XX. The influence of oxygen-glucose deprivation on nitric oxide and intracellular Ca(2+) in cultured hippocampal neurons. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35(6):561–566. [PubMed] [Google Scholar]
- 90.Dal-Cim T, Molz S, Egea J, Parada E, Romero A, Budni J, Martin de Saavedra MD, del Barrio L, Tasca CI, Lopez MG. Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3beta pathway. Neurochem Int. 2012;61(3):397–404. doi: 10.1016/j.neuint.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 91.Bau C, Middlemiss PJ, Hindley S, Jiang S, Ciccarelli R, Caciagli F, Diiorio P, Werstiuk ES, Rathbone MP. Guanosine stimulates neurite outgrowth in PC12 cells via activation of heme oxygenase and cyclic GMP. Purinergic Signal. 2005;1(2):161–172. doi: 10.1007/s11302-005-6214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bastianetto S, Quirion R. Heme oxygenase 1: another possible target to explain the neuroprotective action of resveratrol, a multifaceted nutrient-based molecule. Exp Neurol. 2010;225(2):237–239. doi: 10.1016/j.expneurol.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 93.Li MH, Jang JH, Na HK, Cha YN, Surh YJ. Carbon monoxide produced by heme oxygenase-1 in response to nitrosative stress induces expression of glutamate-cysteine ligase in PC12 cells via activation of phosphatidylinositol 3-kinase and Nrf2 signaling. J Biol Chem. 2007;282(39):28577–28586. doi: 10.1074/jbc.M701916200. [DOI] [PubMed] [Google Scholar]
- 94.Sakata Y, Zhuang H, Kwansa H, Koehler RC, Dore S. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol. 2010;224(1):325–329. doi: 10.1016/j.expneurol.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 96.Lee M, Cho T, Jantaratnotai N, Wang YT, McGeer E, McGeer PL. Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. FASEB J. 2010;24(7):2533–2545. doi: 10.1096/fj.09-149997. [DOI] [PubMed] [Google Scholar]
- 97.Pope SA, Milton R, Heales SJ. Astrocytes protect against copper-catalysed loss of extracellular glutathione. Neurochem Res. 2008;33(7):1410–1418. doi: 10.1007/s11064-008-9602-3. [DOI] [PubMed] [Google Scholar]
- 98.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 99.Brongholi K, Souza DG, Bainy AC, Dafre AL, Tasca CI. Oxygen-glucose deprivation decreases glutathione levels and glutamate uptake in rat hippocampal slices. Brain Res. 2006;1083(1):211–218. doi: 10.1016/j.brainres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 100.Lewerenz J, Klein M, Methner A. Cooperative action of glutamate transporters and cystine/glutamate antiporter system Xc- protects from oxidative glutamate toxicity. J Neurochem. 2006;98(3):916–925. doi: 10.1111/j.1471-4159.2006.03921.x. [DOI] [PubMed] [Google Scholar]
- 101.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267(16):4904––4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 102.Camacho A, Massieu L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch Med Res. 2006;37(1):11–18. doi: 10.1016/j.arcmed.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 103.Volterra A, Trotti D, Floridi S, Racagni G. Reactive oxygen species inhibit high-affinity glutamate uptake: molecular mechanism and neuropathological implications. Ann N Y Acad Sci. 1994;738:153–162. doi: 10.1111/j.1749-6632.1994.tb21800.x. [DOI] [PubMed] [Google Scholar]
- 104.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14(5 Pt 1):2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Escartin C, Won SJ, Malgorn C, Auregan G, Berman AE, Chen PC, Deglon N, Johnson JA, Suh SW, Swanson RA. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J Neurosci. 2011;31(20):7392–7401. doi: 10.1523/JNEUROSCI.6577-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robinson MB. Acute regulation of sodium-dependent glutamate transporters: a focus on constitutive and regulated trafficking. Handb Exp Pharmacol. 2006;175:251–275. doi: 10.1007/3-540-29784-7_13. [DOI] [PubMed] [Google Scholar]
- 107.Bettio LE, Cunha MP, Budni J, Pazini FL, Oliveira A, Colla AR, Rodrigues AL. Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav Brain Res. 2012;234(2):137–148. doi: 10.1016/j.bbr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 108.Chen XQ, Lau LT, Fung YW, Yu AC. Inactivation of bad by site-specific phosphorylation: the checkpoint for ischemic astrocytes to initiate or resist apoptosis. J Neurosci Res. 2005;79(6):798–808. doi: 10.1002/jnr.20396. [DOI] [PubMed] [Google Scholar]
- 109.Ciccarelli R, D'Alimonte I, Ballerini P, D'Auro M, Nargi E, Buccella S, Di Iorio P, Bruno V, Nicoletti F, Caciagli F. Molecular signalling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Mol Pharmacol. 2007;71(5):1369–1380. doi: 10.1124/mol.106.031617. [DOI] [PubMed] [Google Scholar]
- 110.Gottfried C, Tramontina F, Goncalves D, Goncalves CA, Moriguchi E, Dias RD, Wofchuk ST, Souza DO. Glutamate uptake in cultured astrocytes depends on age: a study about the effect of guanosine and the sensitivity to oxidative stress induced by H(2)O(2) Mech Ageing Dev. 2002;123(10):1333–1340. doi: 10.1016/s0047-6374(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 111.Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Dev Neurosci. 1995;17(4):203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]