Abstract
Extracellular adenosine triphosphate (eATP) transduces purinergic signal and plays an important regulatory role in many biological processes, including tumor cell growth and cell death. A large amount of eATP exists in the fast-growing tumor center and inflammatory tumor microenvironment. Tumor cells could acquire anoikis resistance and anchorage independence in tumor microenvironment and further cause metastatic lesion. Whether such a high amount of eATP has any effect on the anchored and non-anchored tumor cells in tumor microenvironment has not been elucidated and is investigated in this study. Our data showed that autophagy helped hepatoma cells to maintain survival under the treatment of no more than 1 mM of eATP. Only when eATP concentration reached a relatively high level (2.5 mM), cell organelle could not be further maintained by autophagy, and apoptosis and cell death occurred. In hepatoma cells under treatment of 2.5 mM of eATP, an AMP-activated protein kinase (AMPK) pathway was dramatically activated while mTOR signaling pathway was suppressed in coordination with apoptosis. Further investigation showed that the AMPK/mTOR axis played a key role in tipping the balance between autophagy-mediated cell survival and apoptosis-induced cell death under the treatment of eATP. This work provides evidence to explain how hepatoma cells escape from eATP-induced cytotoxicity as well as offers an important clue to consider effective manipulation of cancer.
Keywords: Apoptosis, Autophagy, Anoikis, Extracellular ATP, AMPK, mTOR
Introduction
Adenosine triphosphate (ATP) is a vital molecule used by living organisms as a universal source of energy required to drive the cogwheels of intracellular biochemical reactions necessary for growth and development. In response to tissue damage and cellular stress, intracellular ATP is actively released into the extracellular environment and acts as an important signaling molecule [1]. Extracellular ATP (eATP) exerts many functions in physiological and pathological conditions, and its effect on cells is mediated by ionotropic (P2X) and metabotropic (P2Y) receptors [2–4]. Extracellular ATP could be hydrolyzed by ectonucleotidases; thus, its concentration is maintained at very low level in physiological conditions [5]. However, in some pathological conditions, such as tumor microenvironment and fast-growing tumor center which are less vascularized or have lots of cell death, a large amount of intracellular ATP is released from damaged cells, and the concentration of eATP in pericellular space is maintained at a relatively high level [4, 6]. Whether such a high concentration of eATP has any beneficial or detrimental effect on hepatoma cells in tumor microenvironment remains elusive.
It is well known that when epithelial cells are deprived of anchorage, they undergo detachment-induced apoptosis, also known as anoikis. However, many cancer cells acquire anoikis resistance and maintain survival without adhering strongly to the substratum [7, 8]. Our previous work showed that anchorage-deprived hepatoma cells maintained anchorage-independent survival by a synoikis-like survival style, characterized by multicellular aggregation formation, cell cycle arrest, non-proliferation, non-apoptosis, and insensitivity to extracellular stimuli [9]. These properties confer anchorage-independent cells more opportunities to escape from immune surveillance and traditional cancer therapy, maintain survival in the circulation, and lodge to a secondary place as metastatic lesions. The high concentration of extracellular ATP in local tumor microenvironment is a harsh stress and the first challenge that detached hepatoma cells encounter. Whether anchorage-independent hepatoma cells could escape from this stress and acquire more opportunities to maintain survival than anchorage-dependent cells is not known and is of great significance to be extensively clarified.
It is not surprising that demise of a cell is a complex well-controlled process. Apoptosis has been comprehensively studied, and its contribution to the pathogenesis of a disease has been well documented. However, apoptosis does not take exclusive control of cell fate. More recently, autophagy, which is characterized by self-eating process of consuming cellular components, has been shown to be engaged in a complex interplay with apoptosis [10–12]. In some cellular settings, it serves as a cell survival pathway by suppressing apoptosis, whereas in other situations, it leads to cell death by collaboration with apoptosis [13–15]. Whether apoptosis and autophagy have any contribution to the effect of eATP on anchorage-dependent and anchorage-independent hepatoma cells has not been defined. Our data in this study showed that a delicate balance was maintained in hepatoma cells under the treatment of eATP, which acted as a critical switch from protective autophagy-mediated cell survival to apoptosis-induced cell death.
Materials and methods
Materials and reagents
Antibodies used for Western blot were as follows: caspase 3, LC3, phosphor-mTOR (Ser2448), phosphor-S6K1 (Thr389), and phosphor-AMPKα (Thr172) antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA), and β-actin antibody was from Abcam (Cambridge, MA, USA). All antibodies were used in a dilution of 1:1,000, except for β-actin (1:10,000). ATP, 3-MA (3-methyladenine), poly-HEMA (poly-2-hydroxyethyl methacrylate), apyrase, KN-62, BzATP, and compound C were obtained from Sigma (St. Louis, MO, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan). Cyto-IDTM Autophagy Detection Kit was a product of Enzo Life Sciences (Farmingdale, NY, USA). MitoCaptureTM Mitochondrial Apoptosis Detection Kit was from BioVision (Mountain View, CA, USA).
Cell culture and establishment of anchorage-dependent and anchorage-independent hepatoma models
Human BEL7402, SMMC7721, and HepG2 hepatoma cells were maintained in RPMI 1640 medium (Gibco by Invitrogen, Carlsbad, CA, USA), supplemented with 10 % FBS at 37 °C and 5 % CO2 conditions. Poly-HEMA solution was prepared as described before [9, 16]. Six-well plates and 96-well plates were coated with 1.5 and 0.1 mL of poly-HEMA solution, respectively. Coated plates were allowed to air dry under sterile conditions in a laminar flow hood. Before use, poly-HEMA-coated dishes were washed with PBS three times. Hepatoma cells grown on normal plates and poly-HEMA-coated plates were established as anchorage-dependent and anchorage-independent models, respectively.
Response of hepatoma cells to eATP
In tumor microenvironment, tumor cells may encounter eATP separately or as multicellular aggregates. To clarify effect of eATP in these situations, we treated cells separately with eATP to mimic the situation when cells meet eATP individually during the formation process of anchored or non-anchored models, or treated cells with eATP as an integrative unit when the anchorage-dependent and anchorage-independent models were already formed.
BEL7402 hepatoma cells were plated in 96-well plates with or without poly-HEMA at a density of 3 × 105 cells/mL as described before [9, 16]. For the condition that cells meet eATP separately, hepatoma cells were treated with different concentrations of ATP (0, 0.05, 0.1, 0.5, 1, and 2.5 mM) and maintained in plates without or with poly-HEMA as anchorage-dependent or anchorage-independent model for 24 h before analysis. For the condition that cells meet eATP as an integrative unit, hepatoma cells were allowed to grow on normal plates or poly-HEMA-coated plates for 24 h as anchorage-dependent or anchorage-independent model, followed by treatment with different concentrations of eATP for 24 h before Western blot analysis. KN-62 (a potent P2X7 receptor antagonist) and apyrase (a soluble NTPDase) were used to further clarify the purinergic signaling transduced from eATP.
Cell viability and apoptosis assay
Cell viabilities were analyzed by CCK-8 kit according to the manufacturer’s instruction. For apoptosis assay, one of the earliest events inside apoptotic cells is reduction of mitochondrial membrane potential which can be measured by a fluorescent cationic dye named MitoCaptureTM (BioVision, Mountain View, CA, USA). Hepatoma cells in 96-well plates were treated with eATP (0, 1, and 2.5 mM) for 24 h before apoptosis analysis by fluorescent microscopy according to the manufacturer’s protocol. In healthy cells, MitoCapture was aggregated in the mitochondria and presented red fluorescence. In apoptotic cells, MitoCapture remained as monomers in the cytoplasm and exerted green fluorescence.
Western blot
Total proteins were extracted from hepatoma cells and lyzed with RIPA lysis buffer (Beyotime, Hayman, China). Cell extract was centrifuged at 12,000 rpm at 4 °C for 25 min. Total proteins (60 μg) were fractionated on 12 % SDS-PAGE and transferred to polyvinylidene difluoride membranes for blotting. Western blot analysis was performed by using specific monoclonal antibodies to detect caspase 3, LC3, phosphor-mTOR (Ser2448), phosphor-S6K1 (Thr389), and phosphor-AMPKα (Thr172). Expression of β-actin was assessed as an internal control.
Autophagy detection
Hepatoma cells were plated in 96-well plates and incubated with eATP for 24 h, and then, autophagy analysis was performed by Cyto-IDTM Autophagy Detection Kit according to the manufacturer’s protocol. To confirm the effect of autophagy under treatment of eATP, hepatoma cells were pretreated with 1 mM of 3-MA (autophagy inhibitor) for 0.5 h, incubated with eATP for 24 h, followed by autophagy and cell viability analysis.
Statistical analysis
Data were expressed as means ± standard deviation (SD) and were representative data from at least three independent experiments. Statistical significance was examined by two-tailed Student’s t-test and one-way ANOVA analysis. In all of the analyses, P < 0.05 was considered statistically significant.
Results
eATP-induced cell death of anchorage-dependent and anchorage-independent hepatoma cells
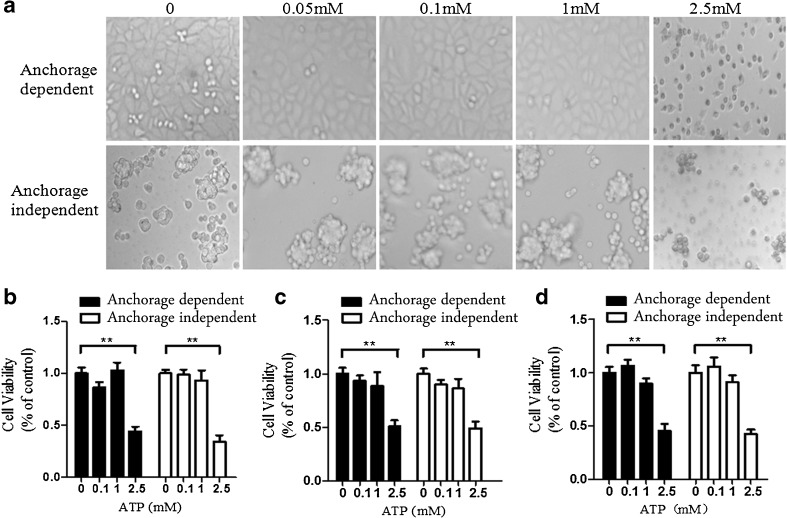
Anchorage-dependent and anchorage-independent hepatoma cells were treated with different concentrations of eATP, and morphology and cell viabilities were investigated thereafter. Morphology investigation showed that when hepatoma cells were treated with 1 mM or lower concentrations of eATP, both types of cells did not have any significant morphological changes. However, when cells were treated with 2.5 mM of eATP, the morphology of both types of cells changed significantly, which indicated dramatic cytotoxicities induced by eATP in cells (Fig. 1a). Anchorage-dependent and anchorage-independent BEL7402, SMMC7721, and HepG2 cells were treated with different concentrations of eATP (0–2.5 mM), and cell viabilities were detected by CCK-8 assay. When hepatoma cells were treated with a high concentration of eATP, significantly decreased cell viability was detected in both types of cells (Fig. 1b–d).
Fig. 1.
Cytotoxicity of eATP on anchorage-dependent and anchorage-independent hepatoma cells. BEL7402, SMMC7721, and HepG2 hepatoma cells were plated into 96-well plates without or with poly-HEMA as anchorage-dependent and anchorage-independent hepatoma models. a BEL7402 cells were treated with increasing concentrations of eATP (0–2.5 mM) and further cultured for another 24 h. Morphology changes were monitored and photographed for these models. b–d Anchorage-dependent and anchorage-independent BEL7402 cells (b), SMMC7721 cells (c), and HepG2 cells (d) were treated with different concentrations of eATP for 24 h, and cell viabilities were detected by CCK-8 analysis. All figures presented are representative data from at least three independent triplicate experiments. **P < 0.01
Effect of eATP on the anchorage-dependent hepatoma cells
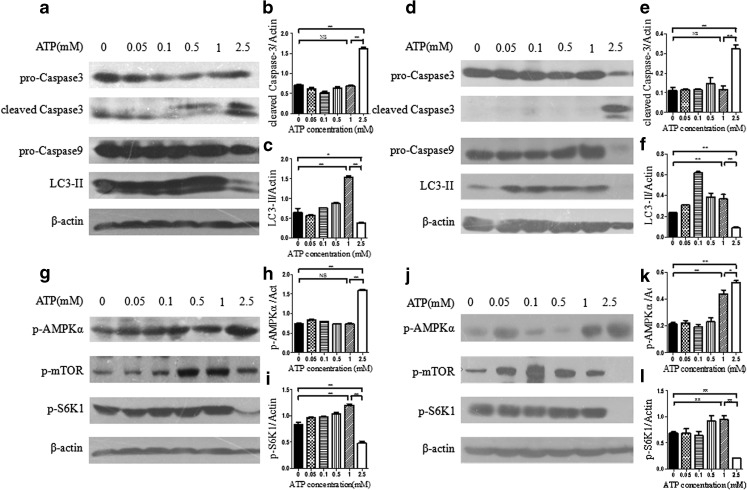
To further determine whether autophagy and apoptosis were involved in eATP-induced cell death, caspase activation and autophagy marker LC3-II were analyzed by Western blot. Caspase 3 is an executive caspase, and caspase 9 is a representative molecule involved in the mitochondria apoptosis pathway. Activation of caspase 3 and caspase 9 was analyzed by Western blot to detect the apoptotic level in eATP-treated hepatoma cells. Microtubule-associated protein 1 light chain 3 (LC3) is a major constituent of the autophagosome. When autophagy occurs, the cytoplasmic form LC3-I is processed and recruited to autophagosomes to generate LC3-II. Therefore, LC3-II is considered as a sensitive specific autophagy marker [16, 17], and its expression was detected to measure the autophagy level in eATP-treated cells.
Hepatoma cells were treated with eATP during or after the formation of anchorage-dependent model. Both apoptosis and autophagy markers were analyzed by Western blot. As shown in Fig. 2a, d, regardless of whether cells were treated with eATP during or after the anchorage-dependent model was formed, both caspase activation and LC3 procession displayed dose-dependent changes in hepatoma cells. When eATP concentration was no more than 1 mM, there was relatively low caspase 3 and caspase 9 activation. When eATP concentration reached 2.5 mM, both caspase 3 and caspase 9 were dramatically activated, while LC3-II level was significantly decreased.
Fig. 2.
Effect and signaling transduction of eATP on anchorage-dependent hepatoma cells. a–f BEL7402 hepatoma cells were plated in normal six-well plates, and different concentrations of eATP (0–2.5 mM) were added to the cells simultaneously (a–c) or after incubation for 24 h (d–f). Cells were incubated for another 12 h, and activation of caspase 3, caspase 9, and LC3-II was detected by Western blot (a and d). β-actin was used as an internal control. Quantitation of caspase 3 (b and e) and LC3-II (c and f) band intensities was analyzed densitometrically using Image J software and normalized to β-actin. g–l In these two anchorage-dependent hepatoma models, activation of mTOR and AMPK signaling pathway was analyzed by Western blot, and β-actin was used as a loading control (g and j). p-AMPKα (h and k) and p-S6K1 (i and j) bands were quantified by densitometric analysis and normalized to β-actin. Presented figures are representative data from three independent experiments. *P < 0.05; **P < 0.01. NS not statistically significant
Adenosine monophosphate-activated protein kinase (AMPK) is a known physiological cellular energy sensor, which is phosphorylated at Thr172 in response to changes of cellular ATP level [17]. Activated AMPK is regarded as an efficient growth inhibitor and apoptosis inducer, even though the exact signaling transduced from AMPK has not been elucidated [18, 19]. To determine whether the AMPK pathway was involved in the effect of eATP on anchorage-dependent hepatoma cells, we analyzed the activation of the AMPK pathway by using a specific antibody against the phosphorylated form of AMPK (Thr172). As shown in Fig. 2g, j, regardless of during (Fig. 2g) or after (Fig. 2j) the anchorage-dependent model was formed, treatment with eATP induced AMPK activation in a dose-dependent manner in hepatoma cells. Under treatment of 2.5 mM of eATP, AMPK activation was significantly increased compared with cells treated with lower dose of eATP. mTOR is another defined energy sensor as well as a downstream molecule of the AMPK pathway. To define whether the mTOR pathway was involved in eATP-induced signaling, the phosphorylated form of mTOR (Ser2448) and its downstream molecule S6K1 (Thr389) were detected by Western blot. Since an mTOR molecule sometimes has a spontaneous and non-specific phosphorylation, activation of its downstream substrate S6K1 is regarded as an effective readout of the mTOR pathway [20–22], which was detected, analyzed, and presented (Fig. 2i, l). When the eATP concentration reached a relatively high level (2.5 mM), mTOR pathway activation was significantly decreased in coordination with dramatically increased activation of the AMPK pathway (Fig. 2g, j).
Effect of eATP on the anchorage-independent hepatoma cells
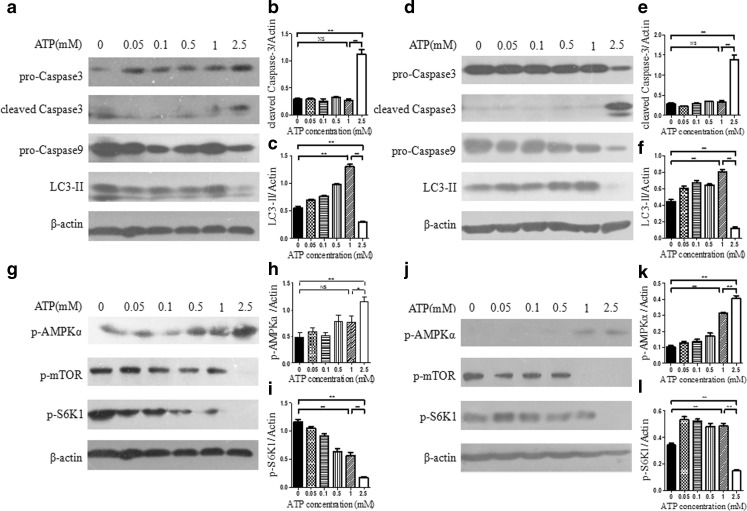
An anchorage-independent hepatoma model was established by putting BEL7402 hepatoma cells in poly-HEMA-coated plates as described before [9, 16]. The effect of eATP on BEL7402 cells was investigated during and after the anchorage-independent model was formed. Caspase cascade and autophagy activation were analyzed by Western blot in the anchorage-independent model. As shown in Fig. 3a, d, activation of caspase 3 and caspase 9 as well as autophagy marker LC3-II was maintained at a stable level under treatment of eATP with concentrations of no more than 1 mM, while caspase 3 and caspase 9 activation was dramatically increased in coordination with significantly decreased LC3-II level under treatment of 2.5 mM of eATP. These data indicated that autophagy helped cells maintain survival under low dose of eATP, whereas autophagy was significantly inhibited, and apoptosis was dramatically activated in hepatoma cells under treatment of high dose of eATP (2.5 mM).
Fig. 3.
Effect and signaling transduction of eATP on anchorage-independent hepatoma cells. a–f BEL7402 hepatoma cells were seeded into poly-HEMA-coated six-well plates as anchorage-independent hepatoma model, and increasing concentrations of eATP (0–2.5 mM) were added to cells simultaneously (a–c) or after incubation for 24 h (d–f). Cells were incubated for another 12 h, and activation of caspase 3, caspase 9, and LC3-II was analyzed by Western blot (a and d). Caspase 3 (b and e) and LC3-II (c and f) bands were quantified densitometrically using Image J software. g–l In these two anchorage-independent models, activation of mTOR and AMPK signaling pathways was analyzed by Western blot, and β-actin was used as a loading control (g and j). Relative levels of p-AMPKα (h and k) and p-S6K1 (i and j) were quantified by densitometric analysis and normalized to β-actin. Presented figures are representative data from three independent experiments. *P < 0.05; **P < 0.01. NS not statistically significant
To further elucidate the mechanism involved in the switch between caspase 3 activation and autophagy, both AMPK and mTOR pathways were analyzed. As shown in Fig. 3g, j, when anchorage-independent hepatoma cells were treated with 2.5 mM of eATP, the AMPK pathway was dramatically activated, while the mTOR pathway was significantly inhibited.
High dose of eATP-induced apoptosis by counteracting autophagy in hepatoma cells
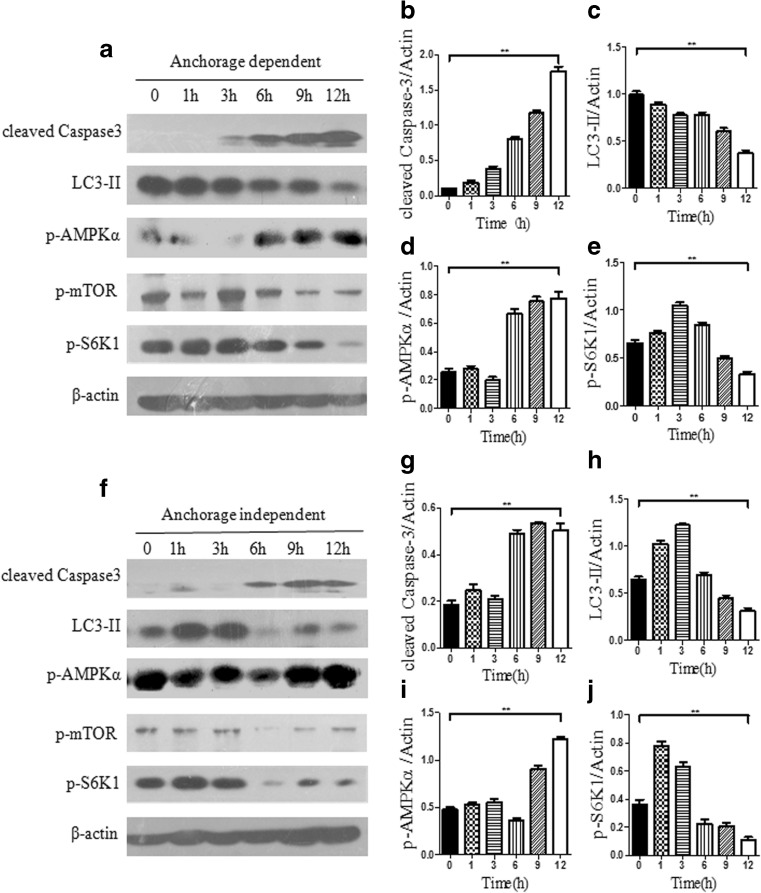
As previous data indicated that 2.5 mM of eATP efficiently induced apoptosis in both anchorage-dependent and anchorage-independent hepatoma cells, we further treated hepatoma cells with 2.5 mM of eATP and analyzed the apoptotic caspase cascade and autophagy marker LC3-II at different time points. As shown in Fig. 4a, f, in both anchorage-dependent and anchorage-independent models, caspase 3 was effectively activated by 2.5 mM of eATP in a time-dependent manner, while LC3-II level was significantly decreased. This indicated that 2.5 mM of eATP could effectively induce caspase-dependent apoptosis while suppressing autophagy.
Fig. 4.
Effect of 2.5 mM of eATP on anchorage-dependent and anchorage-independent hepatoma cells. BEL7402 hepatoma cells were cultured in six-well plates without or with poly-HEMA for 24 h as anchorage-dependent and anchorage-independent hepatoma models. Then, cells were treated with 2.5 mM of eATP and harvested for Western blot analysis at 0, 1, 3, 6, 9, and 12 h after eATP treatment. a Western blot assay of caspase 3 activation, LC3-II procession, and AMPK and mTOR pathway activation in the anchorage-dependent hepatoma model. b–e Densitometric analysis of Western blot bands was calculated using Image J software and normalized to the internal control (β-actin). f Western blot analysis of activation of caspase 3, LC3-II, and phosphorylation of AMPK and mTOR pathways in the anchorage-independent hepatoma model. g–j Densitometric analysis of Western blot bands was calculated using Image J software and normalized to β-actin. Presented figures are representative data from three independent experiments. **P < 0.01
Effect of eATP on hepatoma cells was further confirmed by non-hydrolysable ATP analogue, BzATP
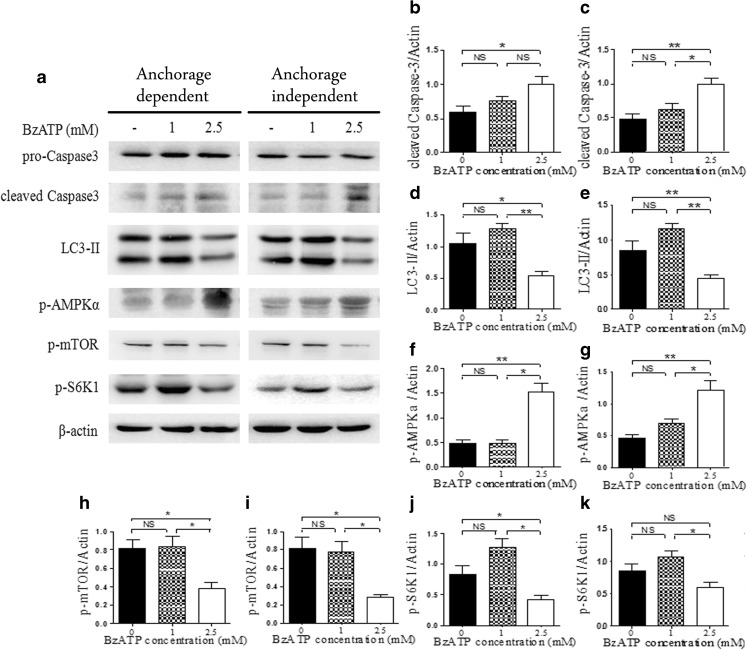
eATP could be degraded to AMP and further adenosine at the presence of ectonucleotidase [5]. To confirm that eATP was responsible for this apoptosis and autophagy switch in anchorage-dependent and anchorage-independent hepatoma cells, BzATP (a non-hydrolysable form of eATP) was used, followed by detection of apoptosis marker caspase 3, autophagy marker LC3-II, AMPK, and mTOR pathway activation. As shown in Fig. 5, in both types of cells, the AMPK pathway was activated, while the mTOR pathway was significantly inhibited in accordance with caspase 3 activation and autophagy inhibition under the treatment of 2.5 mM of BzATP. While at the concentration of 1 mM of BzATP, autophagy was activated, and apoptosis was not induced, which indicated that protective autophagy helped to maintain survival of hepatoma cells. This further confirmed our data obtained from eATP-treated anchorage-dependent and anchorage-independent hepatoma cells.
Fig. 5.
Treatment of anchorage-dependent and anchorage-independent hepatoma cells with BzATP. a Anchorage-dependent and anchorage-independent BEL7402 cells were treated with 1 or 2.5 mM of BzATP and cultured for 24 h. Activation of caspase 3, LC3-II, and phosphorylation of AMPK and mTOR pathways were detected by Western blot. b, d, f, h, j Caspase 3 (b), LC3-II (d), p-AMPK (f), p-mTOR (h), and p-S6K1 (j) in anchorage-dependent cells were quantified by densitometric analysis and normalized to β-actin. c, e, g, i, k Caspase 3 (c), LC3-II (e), p-AMPK (g), p-mTOR (i), and p-S6K1 (k) in anchorage-independent cells were quantified by densitometric analysis and normalized to β-actin. Presented figures are representative data from three independent experiments. *P < 0.05; **P < 0.01. NS not statistically significant
Autophagy inhibition increased the sensitivity of hepatoma cells to eATP-induced apoptosis
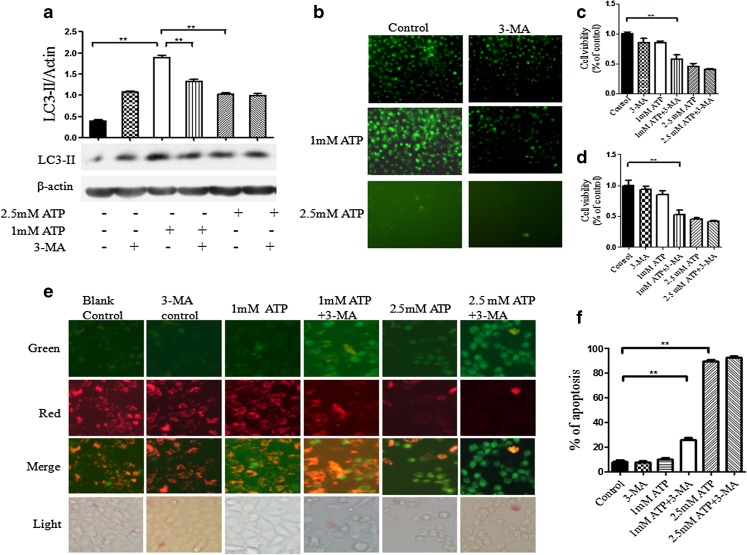
To further confirm the involvement of protective autophagy in eATP-induced stress, BEL7402 hepatoma cells were pretreated with autophagy inhibitor 3-MA before treatment with 1 mM of eATP. Effective blockade of autophagy by 3-MA was confirmed by analysis of autophagy marker by Western blot (Fig. 6a) and fluorescence microcopy (Fig. 6b). Cell viability assay showed that when autophagy in the hepatoma cells was effectively blocked, the number of live cells under treatment of 1 mM of eATP was significantly decreased compared with that in 1 mM of eATP-treated cells without 3-MA pretreatment (P < 0.05, Fig. 6c, d). It indicated that blockade of autophagy effectively rescued eATP-induced cytotoxicity in hepatoma cells.
Fig. 6.
Effect of eATP on 3-MA pretreated hepatoma cells. a, b BEL7402 hepatoma cells were pretreated with 3-MA for 0.5 h before treatment with 1 or 2.5 mM of eATP for 24 h. Autophagy levels in hepatoma cells were detected by Western blot analysis (a) and fluorescence microscopy (b). c, d Cell viabilities of anchorage-dependent (c) and anchorage-independent (d) hepatoma cells were detected by CCK-8 analysis. e Apoptosis of anchorage-dependent hepatoma cells under eATP treatment was stained with MitoCaptureTM apoptosis assay kit and detected by fluorescent microscope. Red fluorescence stained non-apoptotic healthy cells, while green fluorescence represented apoptotic cells. f Histogram of apoptotic rate in 3-MA and eATP treated anchorage-dependent hepatoma cells. Presented figures are representative data from three independent experiments. **P < 0.01
To further determine whether eATP-induced cytotoxicity after autophagy inhibition was caused by increased apoptosis, we detected apoptosis level in hepatoma cells under the treatment of 3-MA and eATP. It showed that inhibition of autophagy significantly increased the apoptosis rate induced by 1 mM of eATP, a dose that failed to induce significant apoptosis while working alone (P < 0.05, Fig. 6e, f). This further confirmed the protective role of autophagy in eATP-induced cell death.
Blockade of eATP-induced purinergic signaling abrogated its cytotoxic effect
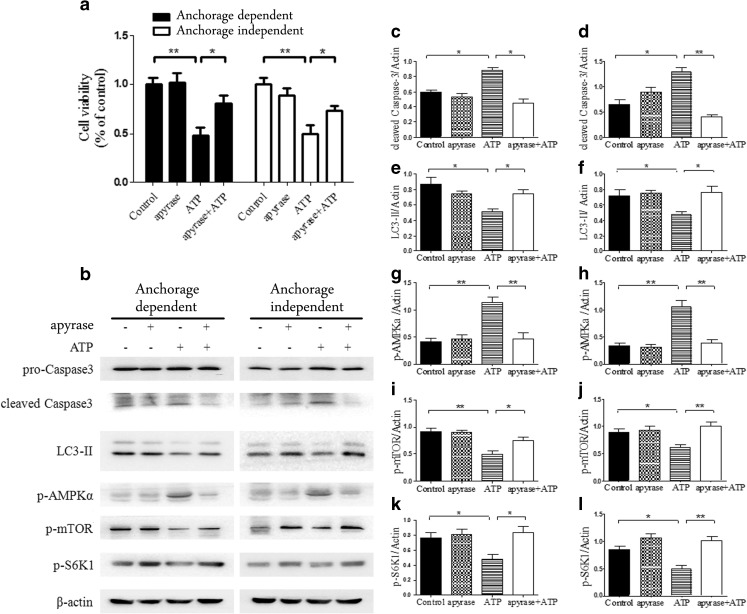
To further assess the possible involvement of high dose of eATP in apoptosis induction and autophagy suppression in anchorage-dependent and anchorage-independent hepatoma cells, both types of BEL7402 cells were pretreated with soluble NTPDase, apyrase (1 U/mL) for 0.5 h before treatment with 2.5 mM of eATP for 24 h. CCK-8 analysis indicated that eATP-induced cytotoxicity was reversed after eATP was degraded by apyrase (Fig. 7a). Western blot analysis showed that when cells were treated with apyrase and then 2.5 mM of eATP, AMPKα phosphorylation was dramatically reduced in accordance with down-regulation of caspase 3 activation, and the phosphorylation level of mTOR and S6K1 was up-regulated, while LC3-II was down-regulated (Fig. 7b–l).
Fig. 7.
Treatment of anchorage-dependent and anchorage-independent hepatoma cells with apyrase and eATP. a Anchorage-dependent and anchorage-independent BEL7402 cells were treated with 2.5 mM of eATP in the absence or presence of apyrase (1 U/mL). Cell viabilities were measured by CCK-8 assay after incubation for 24 h. b Anchorage-dependent and anchorage-independent BEL7402 cells were pretreated with apyrase for 0.5 h, followed by 2.5 mM of eATP treatment for 24 h before harvested for Western blot analysis. c–l Caspase 3 (c, d), LC3-II (e, f), p-AMPKα (g, h), p-mTOR (i, j), and p-S6K1 (k, l) in two types of cells were quantified by densitometric analysis and normalized to β-actin (c, e, g, i, and k for anchorage-dependent model and d, f, h, j, and l for anchorage-independent model). Presented figures are representative data from three independent experiments. *P < 0.05; **P < 0.01
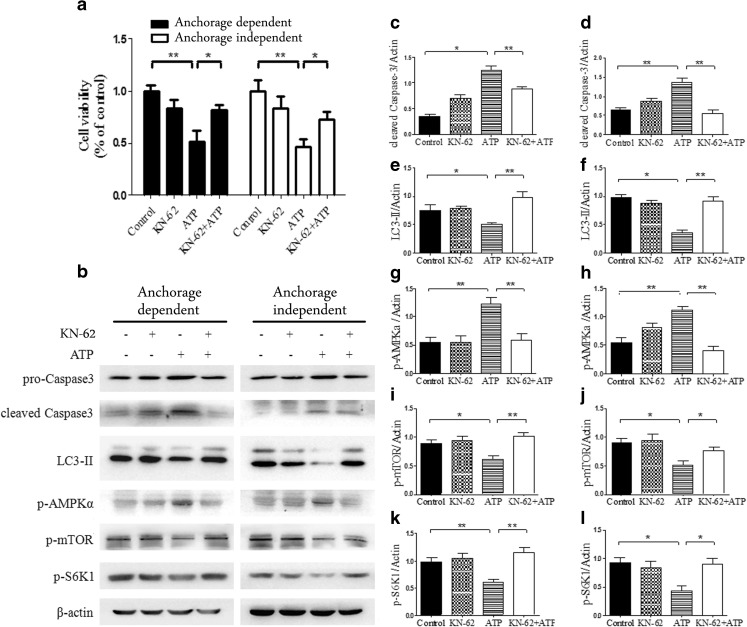
P2X7 receptor was responsible for high dose of eATP-induced cytotoxicity in a variety of cells; whether it was involved in the effect of eATP on anchored and non-anchored hepatoma cells was not known and was investigated in this study. Two types of cells were treated with a potent P2X7 receptor antagonist KN-62 before treatment with 2.5 mM of eATP to test whether eATP-induced cytotoxicity was mediated by P2X7 receptor. CCK-8 analysis showed that KN-62 pretreatment could effectively rescue hepatoma cells from 2.5 mM of eATP-induced cytotoxicity (Fig. 8a). Western blot analysis showed that blockade of P2X7 receptor significantly inhibited high dose of eATP-induced purinergic signaling and abrogated its apoptosis-inducing effect (Fig. 8b–l). These data indicated that P2X7 receptor was responsible for, at least to a great degree, eATP-induced cytotoxicity in hepatoma cells.
Fig. 8.
Treatment of anchorage-dependent and anchorage-independent hepatoma cells with KN-62 and eATP. Anchorage-dependent and anchorage-independent BEL7402 cells were preincubated with KN-62 (1 μM) and then treated with 2.5 mM of eATP for 24 h. a Cell viabilities were tested by CCK-8 analysis. b Cells were harvested for Western blot analysis. c–l Caspase 3 (c, d), LC3-II (e, f), p-AMPKα (g, h), p-mTOR (i, j), and p-S6K1 (k, l) bands were quantified densitometrically by using Image J software. Caspase 3, LC3-II, p-AMPKα, p-mTOR, and p-S6K1 in two types of cells were quantified by densitometric analysis and normalized to β-actin (c, e, g, i, and k for anchorage-dependent model and d, f, h, j, and l for anchorage-independent model). Presented figures are representative data from three independent experiments. *P < 0.05; **P < 0.01
High dose of eATP-induced cytotoxicity was mediated through the AMPK/mTOR pathway
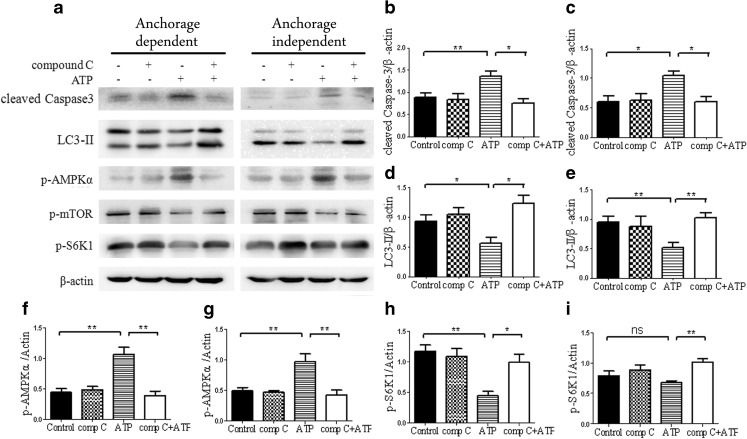
Based on these data, we further sought to determine whether eATP-induced cytotoxicity in two types of hepatoma cells was mediated by the AMPK/mTOR pathway or by two independent pathways of AMPK and mTOR. We used compound C, an AMPK inhibitor, to block AMPK signaling and analyzed activation of the mTOR pathway. As shown in Fig. 9, compound C and 2.5 mM of eATP treatment led to attenuated activation of AMPK and up-regulated mTOR pathway, in coordination with decreased activation of caspase 3 activation and up-regulation of LC3-II. These data indicated that AMPK pathway activation was responsible for mTOR pathway inhibition, which further confirmed that the AMPK/mTOR pathway was responsible for, at least to a great degree, the high dose of eATP-induced effect on anchorage-dependent and anchorage-independent hepatoma cells (Fig. 10).
Fig. 9.
High dose of eATP-induced cytotoxicity was mediated through the AMPK/mTOR pathway. Anchorage-dependent and anchorage-independent BEL7402 cells were treated with 30 μM of compound C for 0.5 h and treated with 2.5 mM of eATP for 24 h. a–i Western blot analysis was performed (a), and densitometric analysis of bands was calculated using Image J software and normalized to β-actin (b, d, f and h for anchorage-dependent hepatoma cells and c, e, g and i for anchorage-independent cells). Presented figures are representative data from three independent experiments. *P < 0.05; **P < 0.01
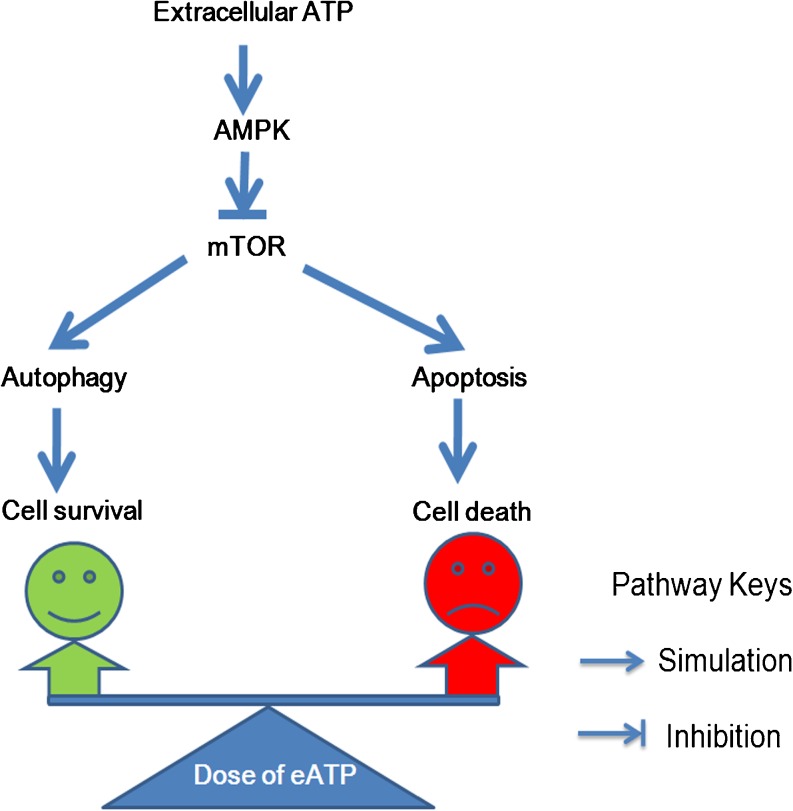
Fig. 10.
Schematic figure of the effect of extracellular ATP on anchorage-dependent and anchorage-independent hepatoma cells. When the eATP concentration was low, the AMPK/mTOR pathway was not adequately activated, and mTOR inhibition was dominant, which led to autophagy-mediated cell survival; thus, hepatoma cells escaped from low dose of eATP-induced cytotoxicity; when the eATP concentration was high, the AMPK/mTOR pathway was adequately activated, and AMPK activation was dominant, which led to caspase cascade activation-mediated cell death. Altogether, different concentrations of eATP delicately tipped the balance between autophagy-mediated cell survival and apoptosis-induced cell death through tuning the activation of the AMPK/mTOR pathway. Cell fate under the effect of different doses of eATP was jointly determined by the balance maintained by these intracellular molecular events
Discussion
Though purinergic signaling of extracellular ATP is well recognized, its influence on malignant cells is still controversial. The effect of extracellular ATP on target tumor cells may have dosage dependence and tissue specificity, which constitutes its functional variation [6, 23–26]. One of the most important aspects of this field is the participation of eATP in determination of cell fate and its possible involvement in promoting, preventing, and treating malignancies [3, 27]. Whether eATP has any functional role in determination of cell fate of anchorage-dependent and anchorage-independent hepatoma cells has not been clarified and is investigated in this study.
Anoikis (or anchorage-deprival-induced apoptosis) is a self-defense strategy that organisms use to eliminate “misplaced” cells. Acquiring anoikis resistance and anchorage independence are hallmarks of cancer, which allow tumor cells to invade adjacent tissues, disseminate through the body, and give rise to metastasis. Thus, anoikis resistance and anchorage independence not only confer cells with ability to survive without attachment to extracellular cell matrix, but also provide molecular signatures to secure their successful migration, invasion, and metastasis to distant sites. Our previous study showed that anchorage-independent hepatoma cells escaped from chemotherapy and maintained survival in circulation [9, 16]. However, our data in this study showed that they were susceptible to cytotoxicity induced by high dose of extracellular ATP through tipping the balance between autophagy and apoptosis. When hepatoma cells were treated with lower concentration of eATP (no more than 1 mM), autophagy helped cells go through stress by providing basal energy for survival. At a situation when hepatoma cells could not withstand the overwhelming effect of high dose of eATP, cell fate was altered accordingly from autophagy-mediated cell survival to apoptosis-induced cell death. Our data showed similar susceptibility of anchorage-dependent and anchorage-independent hepatoma cells to eATP-induced cytotoxicity, which was quite different from our previous data showing that anchorage-independent hepatoma cells were resistant to extracellular stimuli. Our data in this study may open a new fascinating research area with substantial therapeutic potential of eATP, because targeting of both primary and metastatic tumor cells endows it with considerable therapeutic promise.
Crosstalk between apoptosis and autophagy manifests itself in several layers. A full understanding of this multifaceted relationship is critical for final control of cancer. Based on the essential nature of ATP as “energy currency,” it is plausible to predict that deregulation of AMPK and mTOR, two recognized energy sensors, may be involved in the critical signaling transduction network in cells under eATP-induced stress. Our data showed that this significant switch from autophagy to apoptosis under high dose of eATP treatment was coordinated with AMPK activation and mTOR antagonization. mTOR is a validated downstream target of AMPK, and the AMPK/mTOR axis not only participates in regulation of intracellular metabolism, but also is involved in signaling network controlling proliferation, survival, and cell death [28–31]. However, this is the first report to show their effect on eATP-induced signaling transduction in anchorage-dependent and anchorage-independent hepatoma cells.
In tumor microenvironment, hepatoma cells may encounter eATP as detached single cell or as cellular aggregates. To clarify the effect of eATP in these situations, we treated separate cell or integrated cell clusters with eATP, respectively. Our data showed that in all of these situations that we tested, intricate balance between autophagy and apoptosis was defined, which indicated that this delicate control of cell death and survival was a constant common mechanism involved in eATP-induced cytotoxicity.
The effect of eATP on cancer progression is complex and controversial, and its roles in both cancer initiation [32] and suppression have been reported [6]. It has been recognized that low concentration of eATP (at micromolar range) could promote proliferation of hepatocytes and ultimately lead to malignant transformation in ectonucleotidase cd39 null mice [32]. However, in this study, a dominant cytotoxic effect of high dose of eATP (around 2.5 mM) with no apparent pro-growth effect of low dose of eATP was observed in the already malignantly transformed hepatoma cells. This discrepancy may be due to the different and complex biological rationales of premalignant hepatocytes and already malignantly transformed hepatoma cells. Another alternative explanation is that eATP may play discrepant roles in different development stages of cancer, which is necessary to be considered in manipulating different stages of malignancies.
Based on our work and studies from other researchers, resistance to apoptosis is ascribed not only to their inability to activate apoptotic death program, but also to the protection exerted by autophagy [33, 34]. Only when this dominant protection of autophagy is removed or overtaken, low dose of eATP could effectively exert its cytotoxicity on hepatoma cells. It is confirmed by our study showing that blockade of autophagy achieves efficient cytotoxic effect on hepatoma cells treated with lower concentrations of eATP.
Extracellular ATP could be hydrolyzed by CD39/ENTPD1 (ectonucleoside triphosphate diphosphohydrolase-1) to AMP, and the latter could further be hydrolyzed by CD73 to adenosine [5]. Several types of cancer cells express CD39, which can hydrolyze eATP to adenosine directly [35]. To further verify that eATP, not its derivates, was responsible for these effects on hepatoma cells, we used a non-hydrolysable ATP analogue, BzATP, to investigate its effect on hepatoma cells and confirmed that these effects were mediated by eATP. Though both literature [36] and our data showed that hepatoma cells had no detectable CD39 expression (data not shown), another two ectonucleotidases, NTPDase2 and NTPDase8, were expressed on hepatoma cells [36, 37], which might cause degradation of eATP. Furthermore, CD39/ENTPD1 expressed on non-parenchymal cells, including sinusoidal immune and endothelial cells, could hydrolyze eATP in a paracrine manner. These ectonucleotidases expressed in liver may jointly hydrolyze eATP and substantially attenuate cytotoxicity of eATP. However, the final derivate hydrolysable product adenosine is also recognized to have tumoricidal effect, which had a compensatory role for attenuated effect of eATP. Also, our data in this study showed that autophagy inhibitor in conjugation with eATP achieved efficient anti-tumor effect at lower concentrations of eATP, which opened a new avenue for the efficient manipulation of cancer.
In conclusion, our study indicated that both autophagy and apoptosis were involved in eATP-induced hepatoma cell death, and these two biological processes jointly dictated the fate of hepatoma cells in response to changes of eATP levels in tumor microenvironment. This work provides evidence to explain how hepatoma cells escape from eATP-induced cytotoxicity and offers an important clue to consider effective manipulation of cancer. Elucidation of these questions provides foundation for administration and control of eATP in favor of management of hepatoma.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (No. 81172352, No. 30700357), Science and Technology Development Project of Shandong Province (2011GGE27020), and Project for Outstanding Young Scientist in Jinan City (No.20080211 ).
Conflict of interest
The authors declare no conflict of interests.
Abbreviations
- ATP
Adenosine triphosphate
- eATP
Extracellular adenosine triphosphate
- PCR
Polymerase chain reaction
- RT-PCR
Reverse transcriptase polymerase chain reaction
- 3-MA
3-Methyladenine
- LC3
Microtubule-associated protein light chain 3
- CCK-8
Cell Counting Kit-8
- Min
Minutes
- H
Hours
- mTOR
Mammalian target of rapamycin
- S6K1
Ribosomal S6 kinase 1
- PBS
Phosphate-buffered saline
- FBS
Fetal bovine serum
References
- 1.Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Di Virgilio F. P2 receptors and extracellular ATP: a novel homeostatic pathway in inflammation. Front Biosci (Schol Ed) 2011;3:1443–1456. doi: 10.2741/235. [DOI] [PubMed] [Google Scholar]
- 2.Apolloni S, Montilli C, Finocchi P, Amadio S. Membrane compartments and purinergic signalling: P2X receptors in neurodegenerative and neuroinflammatory events. FEBS J. 2009;276(2):354–364. doi: 10.1111/j.1742-4658.2008.06796.x. [DOI] [PubMed] [Google Scholar]
- 3.Deli T, Csernoch L. Extracellular ATP and cancer: an overview with special reference to P2 purinergic receptors. Pathol Oncol Res. 2008;14(3):219–231. doi: 10.1007/s12253-008-9071-7. [DOI] [PubMed] [Google Scholar]
- 4.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2(2):409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13(3):206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2011 doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 8.Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226(2):380–393. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Cao L, Li J, Liang X, Liu Y, Liu H, Du J, Qu Z, Cui M, Liu S, Gao L, Ma C, Zhang L, Han L, Sun W. Acquisition of anoikis resistance reveals a synoikis-like survival style in BEL7402 hepatoma cells. Cancer Lett. 2008;267(1):106–115. doi: 10.1016/j.canlet.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Giansanti V, Torriglia A, Scovassi AI. Conversation between apoptosis and autophagy: "is it your turn or mine?". Apoptosis. 2011;16(4):321–333. doi: 10.1007/s10495-011-0589-x. [DOI] [PubMed] [Google Scholar]
- 11.Thorburn A. Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis. 2008;13(1):1–9. doi: 10.1007/s10495-007-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16(7):966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 13.Corcelle EA, Puustinen P, Jaattela M. Apoptosis and autophagy: targeting autophagy signalling in cancer cells—‘trick or treats’? FEBS J. 2009;276(21):6084–6096. doi: 10.1111/j.1742-4658.2009.07332.x. [DOI] [PubMed] [Google Scholar]
- 14.Mora R, Regnier-Vigouroux A. Autophagy-driven cell fate decision maker: activated microglia induce specific death of glioma cells by a blockade of basal autophagic flux and secondary apoptosis/necrosis. Autophagy. 2009;5(3):419–421. doi: 10.4161/auto.5.3.7881. [DOI] [PubMed] [Google Scholar]
- 15.Guillon-Munos A, van Bemmelen MX, Clarke PG. Autophagy can be a killer even in apoptosis-competent cells. Autophagy. 2006;2(2):140–142. doi: 10.4161/auto.2.2.2443. [DOI] [PubMed] [Google Scholar]
- 16.Cao L, Han L, Zhang Z, Li J, Qu Z, Du J, Liang X, Liu Y, Liu H, Shi Y, Liu S, Gao L, Sun W. Involvement of anoikis-resistance in the metastasis of hepatoma cells. Exp Cell Res. 2009;315(7):1148–1156. doi: 10.1016/j.yexcr.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of osteoarthritis within an experimental model. Apoptosis. 2010;15(5):631–638. doi: 10.1007/s10495-010-0458-z. [DOI] [PubMed] [Google Scholar]
- 18.Kim YM, Hwang JT, Kwak DW, Lee YK, Park OJ. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann N Y Acad Sci. 2007;1095:496–503. doi: 10.1196/annals.1397.053. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Pan X, Song J. AMP-activated protein kinase is required for induction of apoptosis and epithelial-to-mesenchymal transition. Cell Signal. 2010;22(11):1790–1797. doi: 10.1016/j.cellsig.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Ikenoue T, Hong S, Inoki K. Monitoring mammalian target of rapamycin (mTOR) activity. Methods Enzymol. 2009;452:165–180. doi: 10.1016/S0076-6879(08)03611-2. [DOI] [PubMed] [Google Scholar]
- 21.Villalonga P, Fernandez de Mattos S, Ridley AJ. RhoE inhibits 4E-BP1 phosphorylation and eIF4E function impairing cap-dependent translation. J Biol Chem. 2009;284(51):35287–35296. doi: 10.1074/jbc.M109.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moody CA, Scott RS, Amirghahari N, Nathan CO, Young LS, Dawson CW, Sixbey JW. Modulation of the cell growth regulator mTOR by Epstein-Barr virus-encoded LMP2A. J Virol. 2005;79(9):5499–5506. doi: 10.1128/JVI.79.9.5499-5506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shabbir M, Burnstock G. Purinergic receptor-mediated effects of adenosine 5'-triphosphate in urological malignant diseases. Int J Urol. 2009;16(2):143–150. doi: 10.1111/j.1442-2042.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 24.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29(39):5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Gong LH, Zhang HQ, Du Q, You JF, Tian XX, Fang WG. Extracellular ATP enhances in vitro invasion of prostate cancer cells by activating Rho GTPase and upregulating MMPs expression. Cancer Lett. 2010;293(2):189–197. doi: 10.1016/j.canlet.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Yaguchi T, Saito M, Yasuda Y, Kanno T, Nakano T, Nishizaki T. Higher concentrations of extracellular ATP suppress proliferation of Caco-2 human colonic cancer cells via an unknown receptor involving PKC inhibition. Cell Physiol Biochem. 2010;26(2):125–134. doi: 10.1159/000320518. [DOI] [PubMed] [Google Scholar]
- 27.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27(4):211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, Trajkovic V. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52(1):524–531. doi: 10.1016/j.bone.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Yuan H, Niu Y, Niu W, Fu L. The role of AMPK/mTOR/S6K1 signaling axis in mediating the physiological process of exercise-induced insulin sensitization in skeletal muscle of C57BL/6 mice. Biochim Biophys Acta. 2012;1822(11):1716–1726. doi: 10.1016/j.bbadis.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Green AS, Chapuis N, Lacombe C, Mayeux P, Bouscary D, Tamburini J. LKB1/AMPK/mTOR signaling pathway in hematological malignancies: from metabolism to cancer cell biology. Cell Cycle. 2011;10(13):2115–2120. doi: 10.4161/cc.10.13.16244. [DOI] [PubMed] [Google Scholar]
- 31.Bian S, Sun X, Bai A, Zhang C, Li L, Enjyoji K, Junger WG, Robson SC, Wu Y. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS One. 2013;8(4):e60184. doi: 10.1371/journal.pone.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Han L, Seth P, Bian S, Li L, Csizmadia E, Junger WG, Schmelzle M, Usheva A, Tapper EB, Baffy G, Sukhatme VP, Wu Y, Robson SC. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology. 2013;57(1):205–216. doi: 10.1002/hep.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grander D, Panaretakis T. Autophagy: cancer therapy's friend or foe? Future Med Chem. 2010;2(2):285–297. doi: 10.4155/fmc.09.155. [DOI] [PubMed] [Google Scholar]
- 34.Moretti L, Yang ES, Kim KW, Lu B. Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resist Updat. 2007;10(4–5):135–143. doi: 10.1016/j.drup.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187(2):676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 36.Wood E, Broekman MJ, Kirley TL, Diani-Moore S, Tickner M, Drosopoulos JH, Islam N, Park JI, Marcus AJ, Rifkind AB. Cell-type specificity of ectonucleotidase expression and upregulation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Biochem Biophys. 2002;407(1):49–62. doi: 10.1016/S0003-9861(02)00465-4. [DOI] [PubMed] [Google Scholar]
- 37.Fausther M, Lecka J, Kukulski F, Levesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sevigny J. Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G785–G795. doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]