Abstract
Extracellular nucleotides acting via P2 receptors play important roles in cardiovascular physiology/pathophysiology. Pyrimidine nucleotides activate four G protein-coupled P2Y receptors (P2YRs): P2Y2 and P2Y4 (UTP-activated), P2Y6, and P2Y14. Previously, we showed that uridine 5′-triphosphate (UTP) activating P2Y2R reduced infarct size and improved mouse heart function after myocardial infarct (MI). Here, we examined the cardioprotective role of P2Y2R in vitro and in vivo following MI using uridine-5′-tetraphosphate δ-phenyl ester tetrasodium salt (MRS2768), a selective and more stable P2Y2R agonist. Cultured rat cardiomyocytes pretreated with MRS2768 displayed protection from hypoxia [as revealed by lactate dehydrogenase (LDH) release and propidium iodide (PI) binding], which was reduced by P2Y2R antagonist, AR-C118925 (5-((5-(2,8-dimethyl-5H-dibenzo[a,d][7]annulen-5-yl)-2-oxo-4-thioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)-N-(1H-tetrazol-5-yl)furan-2-carboxamide). In vivo, echocardiography and infarct size staining of triphenyltetrazolium chloride (TTC) in 3 groups of mice 24 h post-MI: sham, MI, and MI+MRS2768 indicated protection. Fractional shortening (FS) was higher in MRS2768-treated mice than in MI alone (40.0 ± 3.1 % vs. 33.4 ± 2.7 %, p < 0.001). Troponin T and tumor necrosis factor-α (TNF-α) measurements demonstrated that MRS2768 pretreatment reduced myocardial damage (p < 0.05) and c-Jun phosphorylation increased. Thus, P2Y2R activation protects cardiomyocytes from hypoxia in vitro and reduces post-ischemic myocardial damage in vivo.
Keywords: Cardioprotection, Heart, Ischemia/hypoxia, P2Y2 receptors
Introduction
Ischemic heart disease and consequent heart failure remain the leading cause of morbidity and mortality worldwide [1]. Considerable effort has been devoted to improving functional recovery and reducing the extent of infarction after ischemic episodes. A powerful tool of protection against myocardial infarction (MI) is ischemic preconditioning (PC). PC refers to the ability of short periods of ischemia to make the myocardium more resistant to a subsequent prolonged ischemic insult [2]. This PC effect can be mimicked by several pharmacological agents, including adenosine, a purine metabolite [3, 4]. In our previous study, we have shown that uridine 5′-triphosphate (UTP) provides cardioprotection, in vivo and in vitro, against ischemia via activation of P2Y2 receptors [5, 6].
P2 receptors are expressed on the surface of almost all cells. The physiological effects of the purinergic signaling system are dependent on the release of extracellular nucleotides, their degradation by ectonucleotidases, the type of P2 receptors expressed on the cells, their desensitization rates, and their second messengers [7]. Although nucleotides (such as ATP and UTP) are mainly intracellular, they can be released into the extracellular fluids by various mechanisms. One possible mechanism is cell damage: both necrotic and apoptotic cells release ATP and other nucleotides that thus constitute “danger signals” [8, 9]. Once in the extracellular fluid, nucleotides can activate two families of receptors: metabotropic P2Y receptors (P2YRs) that are coupled to G proteins and fast P2X ion channels. The P2X receptors are more structurally restrictive than P2YRs in agonist selectivity. P2X receptors respond principally to ATP as the active ligand, while the P2YRs are activated by a group of five or more naturally occurring nucleotides, including ATP, UTP, ADP, UDP, and UDP-glucose. The P2YR family is composed of eight members encoded by distinct genes [10]. The signal-transduction pathway used by the subset of P2Y1, P2Y2, P2Y4, and P2Y6Rs involves G-protein activation that stimulates phospholipase C, inositol trisphosphate formation, and Ca2+ release [11]. These P2YRs are expressed in a variety of tissues and can be differentiated pharmacologically on the basis of their selectivity for adenine (ATP, ADP) and uracil (UTP, UDP) nucleotides [12].
Evidence is accumulating to suggest an important role for the purinergic system in cardiovascular regulation [7, 13–18]. It stimulates vasoconstriction and vasodilatation [13], the growth of vascular smooth muscle cells and endothelial cells [16], angiogenesis, vascular remodeling [15] and platelet aggregation, and it regulates coagulation [18], inflammation [17] and several aspects of cardiac function [14]. The purine- and pyrimidine-sensitive P2YRs belong to the large group of G protein-coupled receptors (GPCRs) that are the target of approximately one third of the pharmaceutical drugs used in the clinic today. It is therefore not unexpected that the P2YRs could be useful targets for drug development [19].
In our previous work using P2Y2 receptor-knockout mice, this receptor was found to mediate the anti-ischemic cardioprotection induced by UTP [5, 6]. In the absence of the P2Y2 receptor, the protective effect of UTP was abolished. Uridine-5′-tetraphosphate δ-phenyl ester (MRS2768) is a selective and moderately potent P2Y2 receptor agonist with enhanced stability against the action of ectonucleotidases [20]. In the present work, we provide evidence that the P2Y2 receptor agonist MRS2768 has cardioprotective effects both in vitro and in vivo.
Materials and methods
Cell culture
Sprague Dawley rat hearts (2–3 days old) were removed under sterile conditions and washed three times in phosphate-buffered saline (PBS) to remove excess blood cells. The hearts were minced and then gently agitated in RDB, a solution of proteolytic enzymes [Life Science Research Inst., Nes Ziona, Israel], prepared from a fig tree extract [21]. The RDB was diluted 1:100 in Ca2+ and Mg2+-free PBS at 25 °C for a few cycles of 10 min each, as previously described [22].
Hypoxic conditions
Cultured cardiomyocytes (5–7 days old) were washed twice from the medium with glucose-free PBS containing 5 mmol/l HEPES at pH 7.4 before exposing the cardiomyocytes to the hypoxic conditions at 37 °C. The hypoxic conditions lasted for 120 min in a hypoxic incubator in which the atmosphere was replaced by the inert gas argon (100 %) in glucose-free media [5]. The hypoxic damage was characterized at the end of the hypoxic period through biochemical and morphological evaluations.
Release of lactate dehydrogenase (LDH)
LDH activity in the medium was determined as previously described [23]. Briefly, 25 μl of supernatant was transferred into a 96-well plate, and the LDH activity was determined with an LDH-L kit (Sigma, St. Louis, MO). The product of the enzyme was measured spectrometrically at 30 °C at a wavelength of 340 nm. The results were expressed relative to the control untreated cardiomyocytes, which was set at 100 %.
Propidium iodide (PI)
The assay is based on binding of PI (5 μmol/l) to nuclei of cells whose plasma membranes have become permeable because of hypoxic damage. The assay was performed as previously described [5]. For measurement of the amount of total cells (damaged and undamaged), the cells were treated with 300 μmol/l digitonin. After 1 h, the cells were washed and stained again with PI to evaluate the total number of affected cells. Evaluation of PI was done using a SpectraFluorPlus plate reader (TECAN, Männedorf, Switzerland) at excitation and emission wavelengths of 540 and 630 nm, respectively.
Animals and experimental protocol
Male wild-type mice (C57BL) were purchased from Harlan (Jerusalem, Israel). All experiments were carried out in accordance with the guidelines of the Animal Care and Use Committee of Tel Aviv University, with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Three experimental groups were used:
sham without ligation of the left anterior descending coronary artery (LAD)
myocardial infarction (MI) = LAD ligation
animals injected with MRS2768 (4.44 μg/kg i.v.) 1 h before MI
MI model—LAD ligation
Mice were anesthetized (with a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine), intubated, and ventilated with air. Ischemia was achieved by LAD ligation as we have previously described [24]. Mice in the sham group were operated without LAD ligation. The mice were ventilated until spontaneous breathing commenced.
Two-dimensional guided M-mode echocardiography
Animals were lightly anesthetized by inhaling isoflurane. Two-dimensional guided M-mode echocardiography was performed using an echocardiogram (Siemens 512, Sequoia, CA, USA) equipped with a 15-MHz linear transducer. The heart was imaged in the two-dimensional mode in the parasternal long-axis view. From this view, an M-mode cursor was positioned perpendicular to the intraventricular septum and posterior wall of the left ventricle (LV) at the level of the papillary muscles. An M-mode image was obtained at a sweep speed of 100 mm/s. Diastolic and systolic left-ventricular wall thickness, left-ventricular end-diastolic dimensions (LVDD), and left-ventricular end-systolic chamber dimensions (LVSD) were measured. The percentage of left-ventricular fractional shortening (FS) was calculated as [(LVDD − LVSD)/LVDD] × 100 [25].
Assessment of infarct size
At 24 h of coronary ligation, the mice were sacrificed, and their hearts were removed. Midventricular heart sections 0.8 mm thick were placed in a 1 % solution of 2,3,5-triphenyl tetrazolium chloride (TTC) in phosphate buffer for 30 min at 37 °C. TTC stained the viable tissue red, while the necrotic tissue remained uncolored. Sections were fixed overnight in 4 % formaldehyde for enhancement of contrast between stained and unstained tissue. The sections were then placed between two cover slips and digitally photographed using a Coolpix 5000 camera (Nikon Instruments Europe B.V., Amsterdam, The Netherlands), with a resolution of 1,400 × 960 pixels and quantified by IMAGE J 5.1 software (NIH, Bethesda, MD, USA). The area of irreversible injury (TTC-negative) is presented as a percentage of the entire area of the section [26].
Biochemical and biochemical analysis
Levels of Troponin T were determined in the serum using Cardiac T 2017423 kit (Roche, Indianapolis, IN, USA). Levels of LDH were determined in the serum using commercial Olympus OSR6126 (Center Valley, PA, USA). MRS2768 was synthesized as discribed [20] and dissolved in PBS. 5-((5-(2,8-dimethyl-5H-dibenzo[a,d][7]annulen-5-yl)-2-oxo-4-thioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)-N-(1H-tetrazol-5-yl)furan-2-carboxamide (AR-C118925) obtained from Christa E. Müller, Bonn, Germany, was dissolved in DMSO.
Histopathology
Hearts were removed and fixed in 4 % formalin, then embedded in paraffin. Transverse sections were stained with hematoxylin and eosin (H&E; Pioneer Research, Colchester, UK) to assess leukocyte infiltration. In addition, to assess leukocyte infiltration immunohistochemistry staining using rat anti-mouse neutrophil antibody MCA771GA (1:3,000, AbD Serotec, Kidlington, UK) was performed. Secondary antibodies for neutrophil staining consisted of biotinylated donkey anti-rat (1:500) followed by incubation with peroxidase-conjugated streptavidin (1:1000) and development with 3,3′-diaminobenzidine substrate (reagents from Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Histological analysis was performed on hearts from 5 mice (10 fields each) using ImagePro PLUS software (Media Cybernetics, Bethesda, MD, USA).
Western blotting
Heart tissue samples (20 mg) were homogenized in lysis buffer and quantified for protein levels using a commercial assay (Bio-Rad, Haifa, Israel). Proteins (60 μg/sample) were separated using sodium dodecyl sulfate (SDS) polyacrylamide gel (10 %) under denaturing conditions and electrotransferred onto nitrocellulose (Bio-Rad) for 1 h at 100 V. Membranes were blocked with 5 % nonfat milk in Tris-buffered saline with 0.1 % Tween 20 (TBST) for 1 h at room temperature. Primary antibodies were used at a 1:1,000 concentration in TBST with 5 % nonfat milk overnight at 4 °C. Mouse monoclonal antibodies directed against the dually phosphorylated c-Jun NH2-terminal kinase. The p-c-Jun antibody was obtained from Santa Cruz Biotechnology (sc-822). Rabbit anti-IκB (the inhibitory protein of NF-κB) and actin (used as loading control) polyclonal antibody were purchased from Santa-Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). IRDye 800 secondary antibodies were added at a concentration of 1:15,000 for 1 h at room temperature, and detection was carried out with LI-COR Odyssey imaging system (both from LI-COR, Lincoln, NE). Quantification of signals was carried out with the Odyssey program.
Statistical analysis
Results were expressed as mean ± standard error (SE). Values during the stabilization period were defined as 100 %. A statistical difference between the groups was assessed by analysis of variance (ANOVA) with repeated measurements. If differences were observed, values were compared using Student’s t test. p < 0.05 was considered significant.
Results
Cardiomyocyte culture study
Protective effect of MRS2768 on cardiomyocytes subjected to hypoxia
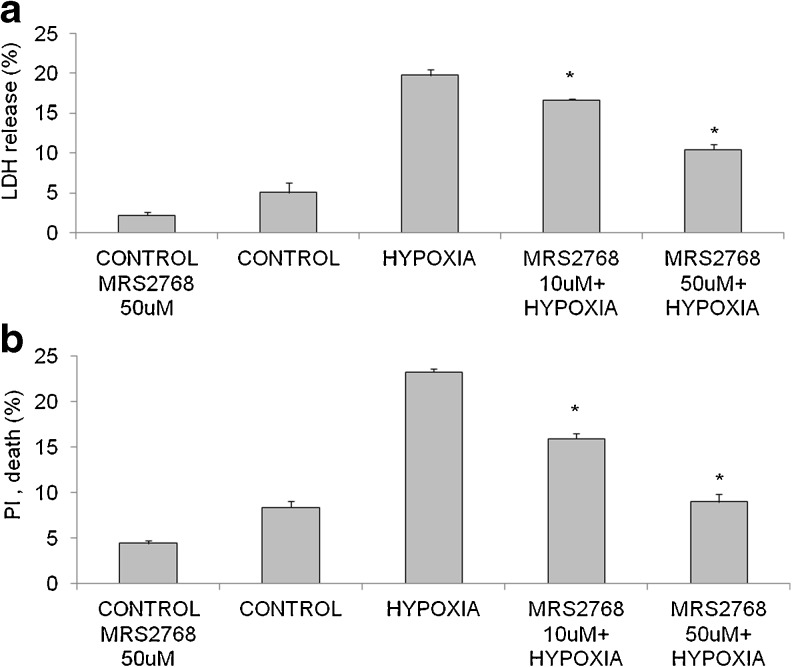
To investigate the role of MRS2768 in attenuation of myocyte injury during prolonged hypoxia of 120 min, cultured cardiomyocytes were incubated with two different concentrations of MRS2768 (10 μM, 50 μM) for 1 h prior to hypoxia. To evaluate cell damage, LDH levels in the medium were measured immediately after hypoxia. The results presented in Fig. 1a show that hypoxic untreated cells were significantly damaged, as revealed by LDH released relative to the normoxic cells (19.7 ± 1.1 % vs. 5.0 ± 1.1 %). Treatment with MRS2768 at both concentrations (10 and 50 μM) brought about a significant attenuation of the cellular injury produced by hypoxia (16.6 ± 0.2 % and 10.4 ± 1.2 % LDH release, p < 0.05 and p < 0.001, respectively). Thus, this effect was concentration-dependent, and similar results were obtained with PI staining as presented in Fig. 1b. It was demonstrated that 8.3 ± 1.2 % of the cells was dead in the control group, while 23.8 ± 1.0 % of the cells subjected to hypoxia was dead. However, treatment with 10 μM of MRS2768 reduced cell death in hypoxia to 16.9 ± 1.8 %, and treatment with 50 μM MRS2768 in hypoxia gave 8.9 ± 1.7 % death, i.e. similar to the result with untreated control cells (Fig. 1b, p < 0.0001).
Fig. 1.
Effect of MRS2768 on cardiomyocytes subjected to hypoxia. a Cultured cardiomyocytes, 5 days in vitro, were washed twice with glucose-free PBS and incubated with two concentrations of MRS2768 (10 and 50 μM) for 1 h prior to hypoxic stress at 37 °C. (*p < 0.001 vs. hypoxia). b Viability of MRS2768-treated cardiomyocytes subjected to hypoxia as tested with PI staining. (*p < 0.001 vs hypoxia). Values are means ± SE. n = 5 in each group
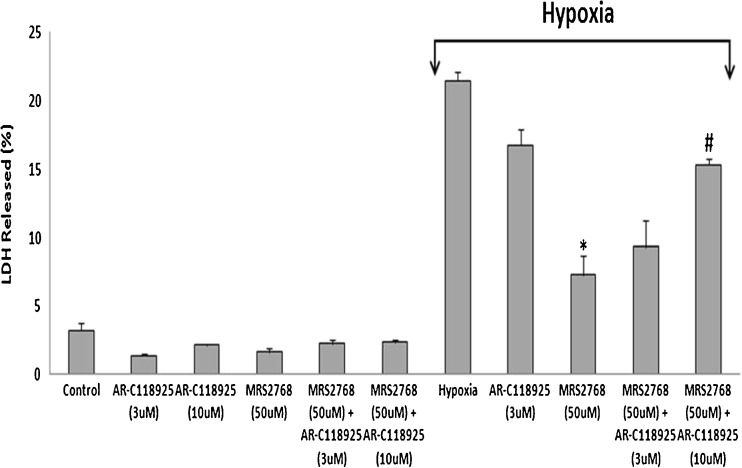
To investigate whether MRS2768 exerts its protection via activating P2Y2R, a selective antagonist AR-C118925 was applied to the cardiac cells 2 h before the hypoxia. The same cells also received MRS2768 1 h before the hypoxia. It was found that the antagonist at 10 μM reduced the protection induced by MRS2768, as revealed by LDH release [from 21.5 ± 0.6 % in hypoxia, to 7.3 ± 1.4 % in hypoxia+agonist (*p < 0.001), and to 15.3 ± 0.5 in hypoxia+agonist+antagonist #p < 0.01 vs. hypoxia+MRS2768], indicating that MRS2768 exerted its protection via P2Y2R (Fig. 2).
Fig. 2.
Effect of AR-C118925 and MRS2768 on cardiomyocytes subjected to hypoxia. Cultured cardiomyocytes, 5 days in vitro, were washed twice with glucose-free PBS and incubated with two concentrations of AR-C118925 (3 and 10 μM). After 1 h, MRS2768 (50 μM) was included to the same dishes for 1 h prior to hypoxic stress at 37 °C. (*p < 0.001 vs. hypoxia, # p < 0.01 vs hypoxia+MRS2768+AR-C118925). Values are means ± SE. n = 5 in each group
In vivo study
Assessment of LV function post-MI using echocardiography
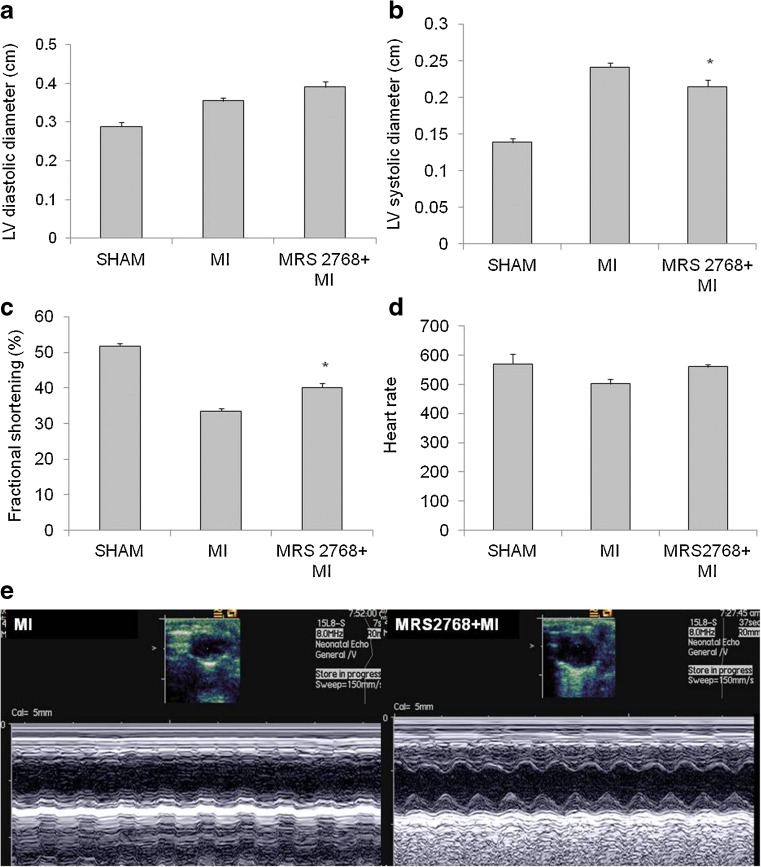
There were no significant differences between groups in the echocardiographic measurements of cardiac structure or function at baseline. Induction of MI resulted in a more pronounced increase of left ventricular systolic diameter compared with the MRS2768-pretreated mice (p < 0.05, Fig. 3a, b). The infarcted mice also demonstrated a significantly reduced FS (33.4 ± 2.7 %) compared to MRS2768-treated mice (40.0 ± 3.13 %), or sham mice (51.8 ± 1.7 %, Fig. 3c, p < 0.001). Representative M mode pictures of the echo measurements are presented in Fig. 3e. Ischemic damage was measured using several markers as follows.
Fig. 3.
Echocardiographic studies. Echocardiographic studies 24 h after MI demonstrated left ventricular (LV) end systolic diameter (LVSD) and LV end diastolic diameter (LVDD) of sham and of infarcted hearts compared with the MRS2768-pretreated mice (a, b). In addition, FS of sham and of infarcted hearts were compared with MRS2768-pretreated mice (c). Heart rate at sham and post-MI of control (saline treated) and MRS2768-treated (d). Representative M mode images are presented in Fig. 2e.Values represent means ± SE, n = 7 in each group. *p < 0.05 vs MI
Biochemical marker of ischemic damage
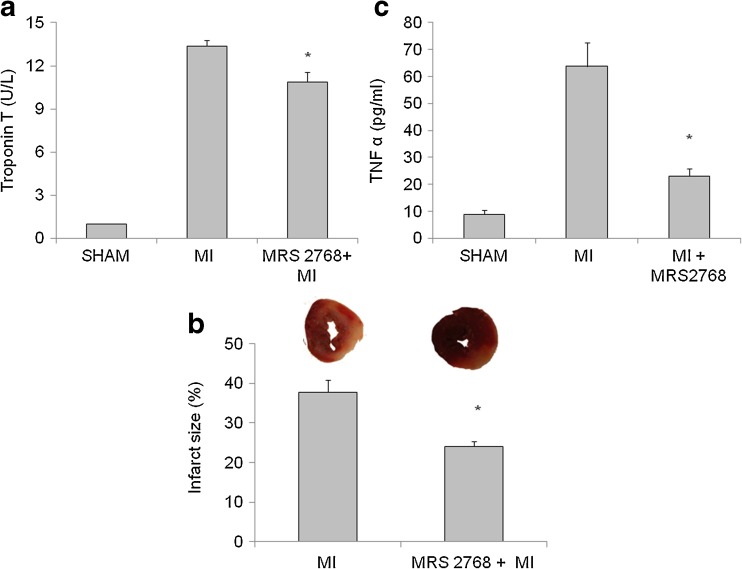
Troponin T: The level of Troponin T, a specific marker denoting damage in the heart muscle, was significantly elevated in mice post-MI (13.4 ± 1.4 ng/ml) compared with the sham group. MRS2768 pretreatment significantly lowered Troponin T levels in the serum (10.6 ± 0.8 ng/ml, p < 0.05, Fig. 4a).
Fig. 4.
Measurements of Troponin T, infarct size, and TNF-α. a The release of Troponin T to the serum 24 h post-LAD ligation. Values are means ± SE. n = 12 hearts in each group. *p < 0.05 vs. MI. b The percent of irreversible injury was determined by scanning the images of mice hearts ventricular sections with triphenyl tetrazolium chloride (TTC). Representative images of the two different groups, revealing different degrees of myocardial ischemia (white to red zones after TTC staining). Hearts were subjected to 24 h of LAD ligation. A significant size of damaged tissue was noted in the myocardium of MI group. Mice pretreated with MRS2768 had significantly smaller infarct size. This figure represents the percent of irreversible injury from the total area of the section at 24 h post-LAD ligation. Values represent means ± SE. n = 7 hearts in each group. c The levels of TNF-α in the serum 24 h post-LAD ligation. Values are means ± SE. n = 4 in each group. *p < 0.05 vs MI
Irreversible ischemic damage measured by TTC
To investigate the effect of MRS2768 on infarct size following LAD ligation, the mice received the agonist 30 min before MI. Infarct size was analyzed 24 h later. It was found that the damage was significantly smaller in mice pretreated with MRS2768 compare to the untreated mice, subjected to MI (25.6 ± 4.5 % vs. 39.2 ± 6.3 %, p < 0.001, Fig. 4b).
Circulating levels of tumor necrosis factor-α (TNF-α)
TNF-α levels in the serum 24 h post-MI were greatly elevated in MI mice compared with the sham group (63.8 ± 14.9 vs. 8.9 ± 3.1 pg/ml, p < 0.05). Mice pretreated with MRS2768 had significantly lower levels of TNF-α (23.0 ± 3.1 pg/ml, p < 0.05, Fig. 4c).
Acute inflammatory cells infiltrated the myocardium
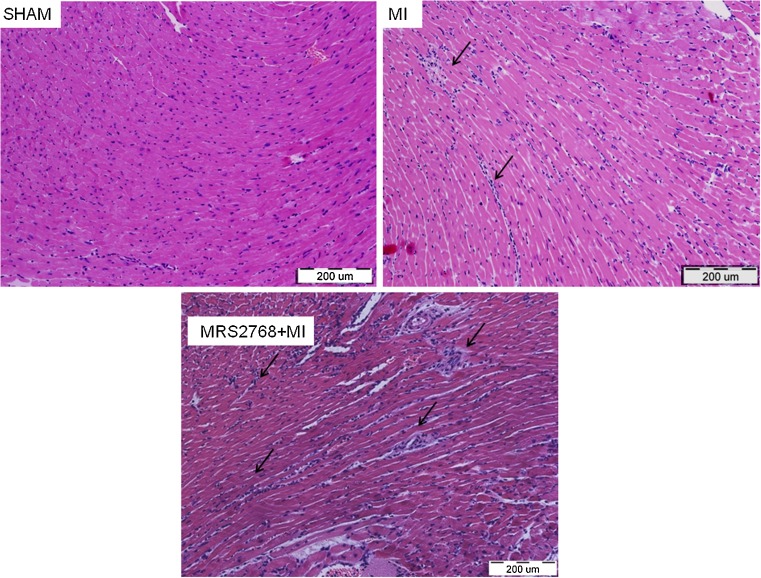
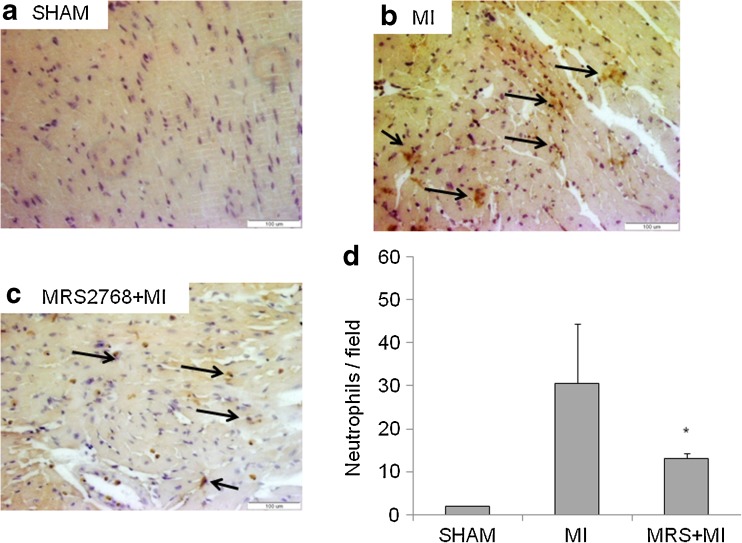
Twenty-four hours after ischemia the infarcted myocardium lost its regular structure, and there was a significant infiltration of inflammatory cells. To determine the extent of immune cell infiltration into the heart, the tissues were excised 24 h post-MI and evaluated by H&E histological staining (Fig. 5). To assess leukocyte infiltration, immunohistochemical staining using an anti-neutrophil antibody was performed. It was found that in the sham group neutrophils did not infiltrate the hearts. At 24 h post-MI, neutrophils were observed in infarcted tissues. A significant reduction (p < 0.05) of neutrophil infiltration was obtained in MI+MRS2768 vs MI-treated hearts (Fig. 6).
Fig. 5.
Histological study of hearts 24 h post-MI: Hematoxylin and eosin (H&E) staining showed neutrophil infiltration to the hearts 24 h post-MI. There was a significant infiltration of neutrophils to the heart tissue in both MI and MI pretreated with MRS2768 mice, n = 4/group (a–c)
Fig. 6.
Histological study of hearts 24 h post-MI using neutrophil marker: Immunostaining showed the infiltration of neutrophils to the infarcted area 24 h post-MI. There was a significant infiltration of neutrophils to the heart tissue compared to sham control (a) in both MI (b) and MI pretreated with MRS2768 (c) mice (n = 4/group). Quantification of immunostaining for neutrophils was done by counting 10 fields per slide. Data are means ± SE, n = 4/group. A significant difference was observed between MI and MI+MRS2768 in neutrophil infiltration (d), p < 0.05
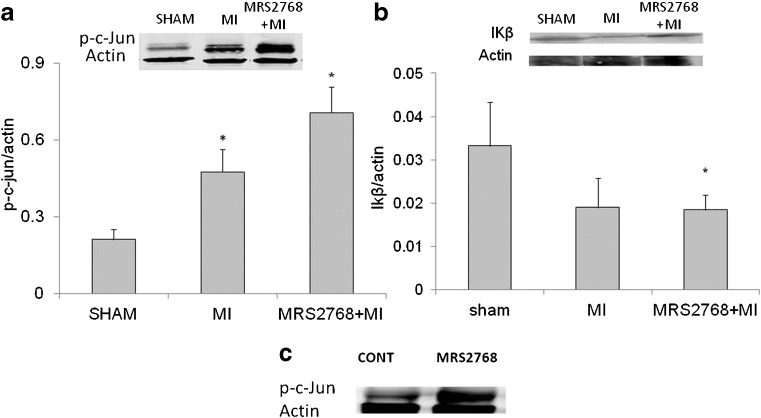
Phospho-c-Jun protein and IκB expression
To study the signal transduction pathway following MRS2768 treatment, phospho-c-Jun protein expression was determined via western blotting. Low levels of p-c-Jun expression were observed in the hearts of sham group. A significant elevation in the expression of p-c-Jun as a result of MI or MRS2768 was noted (p < 0.03). There was also a significant difference in p-c-Jun expression between mice pretreated with MRS2768 before MI vs. MI mice, p < 0.03 (Fig. 7a, c). The activation of IκB in the ischemic cardiac tissue was similar in MI and MRS2768-treated groups (Fig. 7b).
Fig. 7.
Western blot analyses of phosphorylated c-Jun (p-c-Jun) and IκB. Cardiac ischemic extracts were subjected to immunoblotting with antibodies to c-Jun phosphorylated at Ser63. a MI caused elevation in the expression of p-c-Jun. MRS2768-pretreated mice before MI also showed elevation in the expression of p-c-Jun. These figures are representative of western blot analyses of three similar separate experiments in each group. b The activation of IκB in the ischemic cardiac tissue was similar in MI and MRS2768-treated groups
Discussion
We have previously shown that UTP can protect the heart from ischemic damage, both in vivo and in vitro, via activation of P2Y2 receptors [5, 6, 27]. In the present study, we extend this observation and show that MRS2768, a moderately potent P2Y2 receptor agonist, has a protective effect on cardiomyocytes from ischemic damage in vivo and in vitro. This agonist was shown to be P2Y2 receptor-selective at 10 μM (one of the concentrations that was cardioprotective), but the selectivity at higher concentrations has not been characterized. It was significantly more stable than UTP in the presence of mammalian cell membranes and in simulated gastric fluid [28]. MRS2768 was shown to fully activate the human P2Y2 receptor expressed in astrocytoma cells (EC50 = 1.89 μM), without effect at human P2Y4 or P2Y6 receptors at 10 μM [20]. Thus, this agonist is selective, but not highly potent. The greater stability of this agonist is because the terminal δ-phosphate is blocked as a phosphoester, so it is not subject to cleavage by ectonucleoside triphosphate diphosphohydrolase-1 (E-NTPDase1)/CD39 [20].
Prior treatment with MRS2768 reduced the ischemic damage to cardiomyocytes. The protection in cardiomyocytes with MRS2768 (50 μM) was inhibited by coadministration of the P2Y2R antagonist AR-C118925 [29]. Thus, activation of P2Y2 receptors appears to be involved in the in vitro protection by MRS2768 in newborn cardiomyocytes, as indicated by significantly reduced LDH release and cell death caused by hypoxia.
In vivo myocardial functions, as well as biochemical parameters of damage, were improved by prior administration of MRS2768. Mice pretreated with MRS2768 before MI had a smaller infarct size together with improved cardiac function compared to the MI operated animals. Troponin T, a biochemical marker of myocardial damage, demonstrated the same protective effect. Moreover, TNF-α in the serum was significantly reduced in mice treated with MRS2768 before MI. A significant elevation in the expression of cardiac p-c-Jun as a result of MI and MRS2768 was noted. Collectively, MRS2768 has a potent protective effect on the heart against ischemic injury.
In response to a wide variety of infectious or inflammatory stimuli, transcription and translation of TNF-α precursor is increased, and large amounts of mature protein are rapidly released into the circulation. The increase in circulating TNF-α in patients with congestive heart failure was associated with a substantial decrease in myocardial TNF-α receptors and an increase in soluble TNF-α receptors. Perhaps of greatest importance was the demonstration by Mann and colleagues [30], that the nonfailing human heart does not express TNF-α, while at the end stage failing human hearts express robust amounts of the protein. Furthermore, there is a direct relationship between the level of TNF-α expression and the severity of the disease. In addition, both in vivo and in vitro studies demonstrate that TNF-α affects cellular and biochemical changes that mirror those seen in patients with congestive heart failure [31]. Furthermore, in animal models, the development of the heart failure phenotype can be abrogated at least in part by anticytokine therapy [31]. Indeed, TNF-α was significantly reduced in mice pretreated with MRS2768 compared with MI mice (Fig. 6).
Neutrophil infiltration to the heart tissue was considered. Surprisingly, activation of P2Y2 receptors with MRS2768 did not reduce the activation of c-Jun, but lowered the neutrophil infiltration into the heart tissue. Neutrophils are recruited very early following cardiac injury. A critical step in neutrophil recruitment to sites of infection and/or inflammation is transendothelial migration (TEM). TEM is a step-wise process that involves complex neutrophil–endothelium interactions leading to neutrophil rolling, firm adhesion, and ultimately exit from blood vessels [32, 33]. These steps are regulated by many factors including inducible adhesion molecules and chemokines produced by these cell types [33, 34]. Previous studies showed that P2Y2 receptors are involved in neutrophil migration: The work of Kukulski et al. [35] demonstrates that an endothelial P2Y2 receptor regulates LPS-induced neutrophil TEM in vitro. Chen et al. [36] found that disruption of this purinergic signaling system by inhibiting or silencing P2Y2 receptors blocked neutrophil activation and impaired innate host responses to bacterial infection [36]. Furthermore, P2Y2 receptors expressed in neutrophils are also important for neutrophil migration in vitro [37, 38]. Interestingly, P2Y2 receptor-knockout mice showed reduced neutrophil migration that has been speculated to be due to a P2Y2 deficiency in neutrophils [39]. In the light of the data presented here, we propose that the activation of P2Y2 receptors on neutrophils in MI mice by the addition of MRS2768 reduced the neutrophil infiltration into the infarcted area in the heart. In vitro experiments suggested that the mechanism of neutrophil–cardiomyocyte adhesion is dependent on CD18 integrin activation on neutrophils and on expression of ICAM-1, one of the primary ligands for the CD18 integrins [40], by injured cardiomyocytes. Integrin-related strategies have been utilized to mitigate post-reperfusion inflammation in various experimental models. Inhibition of CD11/CD18 integrin resulted in a significant reduction of infarct size in animal models of experimental myocardial infarction [41–44]. However, despite the promising results of the experimental studies, inhibition of leukocyte integrin in clinical studies has led to disappointment [45]. It has been suggested that, in addition to clearing the wound from dead cells and debris, the neutrophils may play a direct role in wound healing by secreting cytokines and growth factors [46]. Attempts to mitigate inflammatory injury in clinical practice in general have been unsuccessful. The catastrophic experience of the methylprednisolone trial [47] emphasized the need for a better understanding of the cellular and molecular events associated with the inflammatory response in order to achieve effective suppression of injurious processes without interfering with healing and cardiac repair [48]. The present results support the hypothesis that inflammatory cell recruitment after MI is modified in mice pretreated with MRS2768, a P2Y2 receptor agonist.
Since no differences in IκB levels were observed between the ischemic groups, we can assume that NFκB degradation is not part of the cardioprotective mechanism achieved by MRS2768. With respect to activation of c-Jun N-terminal kinases (JNKs), there are conflicting results. Several studies suggested that activation of JNK occurs specifically on reperfusion [49]. We and others found that ischemia alone can activate this pathway [6, 50]. Moreover, the role of JNK in myocardial ischemia/reperfusion (I/R) remains controversial, since equally robust studies have reported dichotomous results suggesting both cardioprotective [51–53] and detrimental effects [6, 49, 54]. In contrast to our previous report, we have found a significant reduction in the expression of phospho-c-Jun in wild type (WT) mice pretreated with UTP compared to MI alone (p < 0.05). This difference might be the result of a difference in in vivo half-life, i.e., the rapidly degraded UTP compared to MRS2768. However, the in vivo pharmacokinetics and pharmacodynamics of MRS2768 remain to be determined. It seems that cardioprotective effects of MRS2768 are related in part to the phosphorylation of c-Jun.
In conclusion, we have demonstrated that activation of the P2Y2 receptor with MRS2768 protects the heart from ischemia, minimizes infarct size and improves cardiac function post-MI. Although the precise mechanism for the inhibition of myocardial ischemic injury remains unclear, therapeutic targeting of P2YRs for protection against ischemic myocardial damage is very promising and justifies further exploration.
Acknowledgments
Research support from the Intramural Research Program of the NIH, NIDDK is acknowledged.
This work was supported in part by Israel Science Foundation grant no. 1352/09. The sponsors were not involved in any way in the making of this paper or the decision to submit it for publication.
Conflicts of interest
None.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Downey JM, Liu GS, Thornton JD. Adenosine and the anti-infarct effects of preconditioning. Cardiovasc Res. 1993;27(1):3–8. doi: 10.1093/cvr/27.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Miura T, Tsuchida A. Adenosine and preconditioning revisited. Clin Exp Pharmacol Physiol. 1999;26:92–99. doi: 10.1046/j.1440-1681.1999.03003.x. [DOI] [PubMed] [Google Scholar]
- 5.Golan O, Issan Y, Isak A, Leipziger J, Robaye B, Shainberg A. Extracellular nucleotide derivatives protect cardiomyocytes against hypoxic stress. Biochem Pharmacol. 2011;81(10):1219–1227. doi: 10.1016/j.bcp.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Cohen R, Shainberg A, Hochhauser E, Cheporko Y, Tobar A, Birk E, Pinhas L, Leipziger J, Don J, Porat E. UTP reduces infarct size and improves mice heart function after myocardial infarct via P2Y2 receptor. Biochem Pharmacol. 2011;82(9):1126–1133. doi: 10.1016/j.bcp.2011.07.094. [DOI] [PubMed] [Google Scholar]
- 7.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4(1):1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MAM, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13(8):913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 9.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–U165. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 12.Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci. 1997;18(3):79–82. doi: 10.1016/S0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnstock G, Kennedy C. A dual function for adenosine 5′-triphosphate in the regulation of vascular tone—excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986;58(3):319–330. doi: 10.1161/01.RES.58.3.319. [DOI] [PubMed] [Google Scholar]
- 14.Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- 15.Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular-system. Circulation. 1991;84(1):1–14. doi: 10.1161/01.CIR.84.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Erlinge D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen Pharmacol. 1998;31(1):1–8. doi: 10.1016/S0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- 17.Hou MY, Moller S, Edvinsson L, Erlinge D. Cytokines induce upregulation of vascular P2Y2 receptors and increased mitogenic responses to UTP and ATP. Arterioscler Thromb Vasc Biol. 2000;20(9):2064–2069. doi: 10.1161/01.ATV.20.9.2064. [DOI] [PubMed] [Google Scholar]
- 18.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 19.Erlinge D. P2Y receptors in health and disease. Adv Pharmacol. 2011;61:417–439. doi: 10.1016/B978-0-12-385526-8.00013-8. [DOI] [PubMed] [Google Scholar]
- 20.Ko H, Carter RL, Cosyn L, Petrelli R, de Castro S, Besada P, Zhou Y, Cappellacci L, Franchetti P, Grifantini M, Van Calenbergh S, Harden TK, Jacobson KA. Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists. Bioorg Med Chem. 2008;16(12):6319–6332. doi: 10.1016/j.bmc.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shneyvays V, Nawrath H, Jacobson KA, Shainberg A. Induction of apoptosis in cardiac myocytes by an A3 adenosine receptor agonist. Exp Cell Res. 1998;243(2):383–397. doi: 10.1006/excr.1998.4134. [DOI] [PubMed] [Google Scholar]
- 22.Safran N, Shneyvays V, Balas N, Jacobson KA, Shainberg A. Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol Cell Biochem. 2001;217:143–152. doi: 10.1023/A:1007209321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Ani D, Jacobson KA, Shainberg A. Characterization of adenosine receptors in intact cultured heart cells. Biochem Pharmacol. 1994;48(4):727–735. doi: 10.1016/0006-2952(94)90050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochhauser E, Cheporko Y, Yasovich N, Pinchas L, Offen D, Barhum Y, Pannet H, Tobar A, Vidne BA, Birk E. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47(1):11–19. doi: 10.1385/cbb:47:1:11. [DOI] [PubMed] [Google Scholar]
- 25.Gao XM, Dart AM, Dewar E, Jennings G, Du XJ. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc Res. 2000;45(2):330–338. doi: 10.1016/S0008-6363(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 26.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284(6):H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 27.Yitzhaki S, Shneyvays V, Jacobson KA, Shainberg A. Involvement of uracil nucleotides in protection of cardiomyocytes from hypoxic stress. Biochem Pharmacol. 2005;69(8):1215–1223. doi: 10.1016/j.bcp.2005.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruoka H, Jayasekara MP, Barrett MO, Franklin DA, de Castro S, Kim N, Costanzi S, Harden TK, Jacobson KA. Pyrimidine nucleotides with 4-alkyloxyimino and terminal tetraphosphate delta-ester modifications as selective agonists of the P2Y4 receptor. J Med Chem. 2011;54(12):4018–4033. doi: 10.1021/jm101591j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunschweiger A, Christa E, Müller CE. P2 Receptors activated by uracil nucleotides—an update. Curr Med Chem. 2006;13:289–312. doi: 10.2174/092986706775476052. [DOI] [PubMed] [Google Scholar]
- 30.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.CIR.93.4.704. [DOI] [PubMed] [Google Scholar]
- 31.Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, McTiernan C. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35(3):537–544. doi: 10.1016/S0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99(10):1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52(3):349–374. [PubMed] [Google Scholar]
- 34.Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42(11):2563–2566. [PubMed] [Google Scholar]
- 35.Kukulski F, Ben Yebdri F, Bahrami F, Fausther M, Tremblay A, Sevigny J. Endothelial P2Y2 receptor regulates LPS-induced neutrophil transendothelial migration in vitro. Mol Immunol. 2010;47(5):991–999. doi: 10.1016/j.molimm.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3(125):ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 38.Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Levesque SA, Martin-Satue M, Sevigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46(2):166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30(2):173–177. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8(8):504–512. [PubMed] [Google Scholar]
- 41.Lefer DJ, Shandelya SM, Serrano CV, Jr, Becker LC, Kuppusamy P, Zweier JL. Cardioprotective actions of a monoclonal antibody against CD-18 in myocardial ischemia-reperfusion injury. Circulation. 1993;88(4 Pt 1):1779–1787. doi: 10.1161/01.CIR.88.4.1779. [DOI] [PubMed] [Google Scholar]
- 42.Ma XL, Tsao PS, Lefer AM. Antibody to CD-18 exerts endothelial and cardiac protective effects in myocardial ischemia and reperfusion. J Clin Invest. 1991;88(4):1237–1243. doi: 10.1172/JCI115427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arai M, Lefer DJ, So T, DiPaula A, Aversano T, Becker LC. An anti-CD18 antibody limits infarct size and preserves left ventricular function in dogs with ischemia and 48-hour reperfusion. J Am Coll Cardiol. 1996;27(5):1278–1285. doi: 10.1016/0735-1097(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 44.Aversano T, Zhou W, Nedelman M, Nakada M, Weisman H. A chimeric IgG4 monoclonal antibody directed against CD18 reduces infarct size in a primate model of myocardial ischemia and reperfusion. J Am Coll Cardiol. 1995;25(3):781–788. doi: 10.1016/0735-1097(94)00443-T. [DOI] [PubMed] [Google Scholar]
- 45.Dove A. CD18 trials disappoint again. Nat Biotechnol. 2000;18(8):817–818. doi: 10.1038/78412. [DOI] [PubMed] [Google Scholar]
- 46.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58(2):88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts R, DeMello V, Sobel BE. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation. 1976;53(3 Suppl):I204–I206. [PubMed] [Google Scholar]
- 48.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13(16):1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 49.Fryer RM, Patel HH, Hsu AK, Gross GJ. Stress-activated protein kinase phosphorylation during cardioprotection in the ischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281(3):H1184–H1192. doi: 10.1152/ajpheart.2001.281.3.H1184. [DOI] [PubMed] [Google Scholar]
- 50.Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, Feuerstein GZ, Thomas H, Maleeff B, Ohlstein EH. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86:692–699. doi: 10.1161/01.RES.86.6.692. [DOI] [PubMed] [Google Scholar]
- 51.Andreka P, Zang J, Dougherty C, Slepak TI, Webster KA, Bishopric NH. Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circ Res. 2001;88(3):305–312. doi: 10.1161/01.RES.88.3.305. [DOI] [PubMed] [Google Scholar]
- 52.Cohen MV, Downey LM. Preconditioning during ischemia: basic mechanisms and potential clinical applications. Cardiol Rev. 1995;3:137–149. doi: 10.1097/00045415-199505000-00003. [DOI] [Google Scholar]
- 53.Dougherty CJ, Kubasiak LA, Prentice H, Andreka P, Bishopric NH, Webster KA. Activation of c-Jun N-terminal kinase promotes survival of cardiac myocytes after oxidative stress. Biochem J. 2002;362(Pt 3):561–571. doi: 10.1042/0264-6021:3620561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ben-Ari Z, Zilbermints V, Pappo O, Avlas O, Sharon E, Greif F, Cheporko Y, Ravid A, Shapiro R, Hochhauser E. Erythropoietin increases survival and attenuates fulminant hepatic failure injury induced by D-galactosamine/lipopolysaccharide in mice. Transplantation. 2011;92(1):18–24. doi: 10.1097/TP.0b013e31821cdea5. [DOI] [PubMed] [Google Scholar]