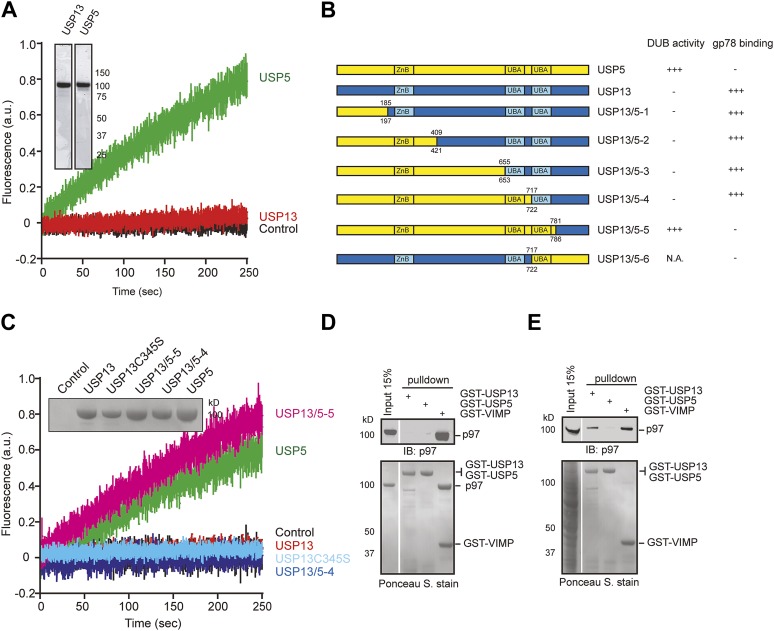
Figure 1. USP13 and USP5 have distinct activities despite sequence homology.
(A) Purified USP13 is inactive whereas USP5 is active. The activities of the purified USP5 and USP13 were measured by the Ub-AFC assay. The Coomassie blue-stained gels show the purified proteins. (B) A schematic illustration of the USP13-USP5 chimeras and a summary of their deubiquitinating activities. (C) The deubiquitinating activity of the indicated USP13-USP5 chimeras as measured by the Ub-AFC assay. The USP13 catalytic inactive mutant C345S was used as a negative control. The inset shows the purified proteins. (D) USP13 does not bind p97 directly. The indicated GST-tagged proteins were immobilized and incubated with recombinant p97. The precipitated proteins were analyzed by immunoblotting (IB). (E) USP13, but not USP5, binds p97 through an adaptor. As in D, except that a whole cell extract was used in replace of p97.