Abstract
Background
Historically, a functional ACL has been a prerequisite for patients undergoing unicondylar knee arthroplasty (UKA). However, this premise has not been rigorously tested.
Questions/purposes
We compared (1) the survivorship free from revision and (2) the failure mechanisms of UKAs in ACL-deficient knees and UKAs in ACL-intact knees performed over the same time interval.
Methods
Between November 2000 and July 2008, a fixed bearing UKA was performed in 72 patients (81 knees) with intraoperatively confirmed ACL deficiency. Five patients (five knees) with preoperative instability underwent ACL reconstruction and were excluded from analysis. Of the remaining 67 patients (76 knees) without preoperative instability, implant status was known for 68 UKAs in 60 patients. Survivorship and failure mechanisms for these knees were compared to those of 706 UKAs in ACL-intact knees performed during the same time interval by the same surgeon using the same implant system. Minimum followup for the ACL-deficient group was 2.9 years (mean, 6 years; range, 2.9–10 years).
Results
Revision rates between UKAs with and without intact ACLs were similar in the absence of clinical instability (p = 0.58). Six-year UKA survivorship was 94% (95% CI: 88%–100%) in ACL-deficient knees and 93% (95% CI: 91%–96%) in ACL-intact knees (p = 0.89). Five knees (7%) in the ACL-deficient group were revised: disease progression (two), loose tibia (one), persistent pain (one), and revised elsewhere/reason unknown (one). Thirty-six knees in the ACL-intact group underwent revision (5%): aseptic loosening (13), revised elsewhere/reason unknown (11), disease progression (three), tibial subsidence/fracture (four), infection (three), pain (one), and lateral compartment overload (one).
Conclusions
At 6 years, deficiency of the ACL in patients without clinical knee instability did not impact the survivorship of UKAs compared to UKAs performed in knees with intact ACLs.
Level of Evidence
Level III, prognostic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Unicondylar knee arthroplasty (UKA) has been a surgical treatment option for managing unicompartmental arthritis of the knee for more than three decades. The procedure has relatively strict contraindications because of higher failure rates in the presence of several clinical variables. Goodfellow and O’Connor [7] reported higher failure rates with mobile bearing implants in knees with ACL deficiency. In their study of 301 mobile bearing procedures, the survivorship was 81% at 6 years in knees without an ACL. The dominant mode of failure was aseptic loosening of the tibial component. Kozinn and Scott [9] believed ACL deficiency should be a relative contraindication to fixed bearing UKA. However, clinical reports in that review did not include data on ACL-deficient knees after UKA.
In this retrospective study, we therefore compared (1) the survivorship using revision of any component as the end point and (2) the failure mechanisms of UKAs in knees with deficient ACLs and UKAs in knees with intact ACLs.
Patients and Methods
Approval from our institutional review board was obtained before initiation of this study and the implant used was FDA approved. The status of the ACL was determined intraoperatively at the time of UKA and previously recorded in our institutional database as intact or deficient. We identified 72 patients (81 knees) as having a UKA in a knee with ACL deficiency. The UKAs were performed by the senior author (GAE) between November 2000 and June 2008 using the Preservation™ unicondylar implant (DePuy, A Johnson & Johnson Company, Warsaw, IN, USA), a fixed bearing UKA implant. All unicondylar implants were sterilized by gas plasma. Before the arthroplasty, patients reported activity limiting pain. Radiographic evidence of ACL deficiency such as offset of the posterior femoral condyle relative to the tibial plateau was not used as exclusion criteria when selecting patients for UKA. Five patients (five knees) reported problems with instability in the knee before their UKA and had clinical instability in examination. These patients underwent ACL reconstruction in conjunction with UKA and were excluded from the analysis. The anterior drawer test performed during the preoperative physical examination was negative for the other 76 knees in the patient cohort. These patients routinely had 5° to 9° varus/valgus laxity but no AP laxity with anterior drawer test.
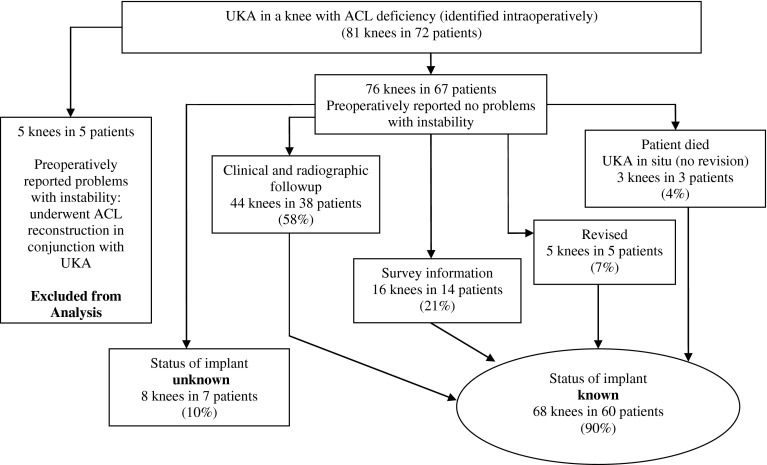
We contacted the remaining 67 patients (76 knees) in an attempt to obtain followup for any patient who had not been seen within 2 years of study initiation. Despite attempts at contact via US mail and telephone, we were unable to ascertain the status of eight UKA implants in seven patients. There was no followup information available for these knees in the clinical database. Two of these patients (two knees) were successfully contacted but did not wish to participate in the study. Thirty-eight patients (44 knees) had complete clinical and radiographic followup, 14 patients (16 knees) had survey information, three patients (three knees) expired but had not undergone revision of their UKA, and five patients (five knees) required conversion to a TKA. Thus, the status of the implant was known for 68 knees in 60 patients (Fig. 1).
Fig. 1.
A flowchart shows the study population of patients with UKA in knees with ACL deficiency.
The 68 UKAs were performed in 60 patients (eight bilateral procedures). There were 29 women and 31 men, with a mean ± SD age of 65 ± 12 years (range, 39–91 years) and a mean BMI of 28.4 ± 6 (range, 18–58). The procedure was performed for arthritis in the medial compartment of 62 knees and in the lateral compartment in six knees. Preoperative radiographs were used to grade the degenerative disease of the knees according to the Ahlbäck classification [1]. A Grade 1 classification is not routinely justification for UKA. In knees with Grade 1 changes, additional evidence of full-thickness cartilage loss from MRI or arthroscopic examination, along with protracted failure of nonoperative treatment, is essential before considering UKA. UKA is reasonable management of a knee with a Grade 2 classification. The knees with ACL deficiency were graded as follows: Grade 1 (two knees), Grade 2 (19 knees), Grade 3 (31 knees), and Grade 4 (13 knees). No knees were classified as having severe bone attrition (Grade 5). Three knees were not assigned an Ahlbäck classification as no preoperative radiograph was available. The mean preoperative arc of motion for these patients was 122° (range, 96°–150°). The preoperative angular deformity (tibiofemoral angle) in the knees was measured on AP weightbearing radiographs (14- × 17-inch cassettes) and ranged from 12° varus to 6° valgus (mean, 2.7° varus ± 4°) for knees undergoing a medial UKA. For the six knees that underwent a lateral UKA, the valgus deformity measured on AP weightbearing radiographs ranged from 11° to 15° (mean, 13° ± 1.7°). The minimum followup was 2.9 years (mean, 6 ± 1.6 years; range, 2.9–10 years). Our surgical technique for UKA in knees with ACL deficiency was slightly modified. The posterior slope of the sagittal tibial resection was reduced from the patient’s native tibial slope to improve stability of the knee in flexion.
During the same time interval, the senior author performed 706 UKAs in 561 patients using the same implant system in knees with intact ACLs. Bilateral procedures were performed in 145 patients. There were 223 men and 338 women, with a mean age of 66 ± 10 years (range, 40–91 years) and a mean BMI of 29.5 ± 6 (range, 16–56). Forty patients in this group did not have a height and/or weight recorded for calculation of their BMI data. Arthritis affected the medial compartment of the knee in 624 knees and the lateral compartment in 82 knees. For knees with medial UKAs, the mean tibiofemoral angle measured preoperatively on AP weightbearing radiographs ranged from 15° varus to 9° valgus (mean, 2.9° varus ± 3.3°). For the knees with lateral UKAs, the preoperative valgus deformity ranged from 2° valgus to 21° valgus (mean, 11° valgus ± 3.5°).
The Kaplan-Meier technique was used to estimate implant survivorship at the 95% CI using revision of the component as the criterion for failure.
Results
There was no difference in revision rate between UKAs with and without intact ACLs, in the absence of clinical instability (p = 0.58). There were five failures of UKAs in ACL-deficient knees (7%); all were revised to TKAs. Two patients (two knees) with medial UKAs were revised for progression of arthritis in the lateral compartment, 31 and 43 months after the UKA. The mechanical axis of these two knees measured after UKA was 13 mm and 10 mm lateral of the knee center. One knee was revised 20 months after the UKA in a patient who reported continued pain, although there was no radiographic evidence of either implant loosening or progression of disease. One knee was revised for a loose cemented tibia 48 months after UKA. The fifth knee revision was performed 104 months after UKA, but the reason for implant failure was unknown as the revision was performed elsewhere.
Thirty-six revisions occurred in the ACL-intact group (5%). The reasons for revisions were as follows: aseptic loosening (13 knees), revised elsewhere and reason unknown (11 knees), progression of disease (three knees), tibial subsidence/fracture (four knees), infection (three knees), pain (one knee), and overload of the lateral compartment (one knee).
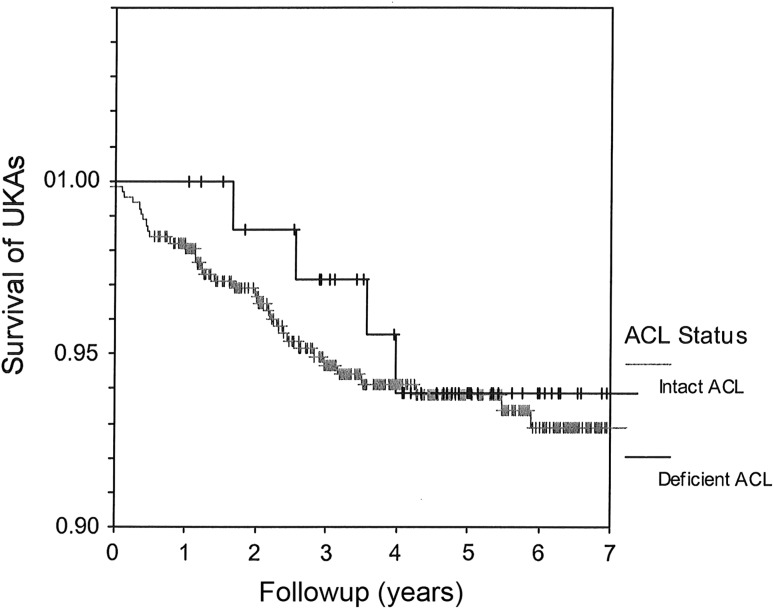
The survivorship at 6 years was 94% (95% CI: 88%–100%; 57 knees remaining at risk) for UKAs in the ACL-deficient knees and 93% (95% CI: 91%–96%; 373 knees remaining at risk) for UKAs in ACL-intact knees (p = 0.89) (Fig. 2).
Fig. 2.
At 6 years, the survivorship is 94% (95% CI, 88%–100%) for UKAs in knees with deficient ACLs and 93% (95% CI, 91%–96%) for UKAs in knees with intact ACLs. + = censored data.
Discussion
Indications and contraindications for UKA have never been clearly defined. In a review article, Kozinn and Scott [9] reported both cruciate ligaments should be intact to ensure the best results with UKA. This recommendation is bolstered in the orthopaedic literature by the results with mobile bearing UKA, but the results with fixed bearing UKA are not consistent.
This study had a number of limitations. First, only 44 knees had complete clinical followup with radiographs. Early evidence of a radiolucency might have been missed in the 16 knees that had only survey information. Late aseptic loosening in knees with ACL deficiency remains a concern as a late mode of failure, and this issue warrants attention in future studies. In addition, the 16 knees with survey information only did not have clinical followup scores.
The reported mechanism of failure after UKA in ACL-deficient knees has been either aseptic tibial loosening or premature polyethylene wear. Goodfellow and O’Connor [7] reported failure of mobile bearing UKA implants in knees with deficient ACL was primarily due to aseptic loosening of the tibial component. Survivorship of 301 Oxford™ UKAs with a deficient ACL was 81% at 6 years. The authors believed eccentric loading of the tibial component caused overload of tibial fixation and early loosening and an intact ACL was a prerequisite for mobile bearing UKA. In a review of 79 Lotus™ implants with a minimum 5-year followup, fixed bearing UKA had an unacceptable early failure rate [6]. On preoperative lateral radiographs reviewed retrospectively, 15 knees had laxity of 10 mm or greater. At mean 7-year followup, 13 of 15 knees with preoperative AP laxity were considered failures and 10 underwent reoperation after a mean 3.5 years. The revisions were for wear and subluxation. No information was available on the method of polyethylene sterilization or the shelf-life of these implants. Others have reported satisfactory results with fixed bearing UKA in ACL-deficient knees. Christensen [5] reported the results of 575 consecutive UKAs using the St Georg® unicondylar implant. A translatory deformity secondary to absence of an ACL was present in many knees, but the overall revision rate was only 1.2%. Cartier et al. [4] reported the results of 132 Marmor implants with minimum 10-year followup. In 10 knees, the ACL was absent at the time of surgery. Seven ACL-deficient knees were asymptomatic, two patients reported slight clinical instability in their knee, and one patient underwent a secondary ACL reconstruction.
One concern with performing UKA in a knee with instability is the potential for accelerated polyethylene wear. Fixed bearing unicondylar implants have a tibial polyethylene with a flat articular surface that makes the knee more prone to a sliding motion as compared to the more contoured surface in the bearing surfaces of a total knee implant. In laboratory studies, Blunn et al. [3] demonstrated a dramatic increase in polyethylene wear resulting from a sliding motion as compared to a rolling motion. In the study by Deschamps and Lapeyre [6], the bearing surface of the implants was relatively flat, similar to the implant in our study. Although they reported wear as a problem with UKA, there were no revisions for accelerated wear in the current study. The implants in our study were sterilized by gas plasma and therefore not prone to problems of oxidation that might accelerate polyethylene wear.
Whenever knee arthroplasty is performed, numerous kinematic variances may occur. Video fluoroscopy has been used to determine femorotibial contact positions after UKA. Argenson et al. [2] analyzed the in vivo kinematics of 20 patients with an intact ACL that underwent UKA with a fixed bearing implant. All knees were clinically successful with no substantial ligamentous laxity. In nine of 17 knees with a medial unicondylar implant, posterior contact in full extension and paradoxical anterior femoral translation were present. The authors postulated progressive laxity of the ACL may occur over time. The altered kinematics also may have been secondary to alterations in soft tissue balance with placement of the components during UKA. The abnormal knee kinematics present in a knee with ACL deficiency may lead to premature failure of the unresurfaced compartment. Two of the knees in our study failed by progression of arthritis in the lateral compartment. Longer followup of unrevised knees in this study will allow us to see if this mode of failure increases.
In the knee with an intact ACL, cartilage wear on the anterior tibial plateau is easily identified when performing UKA. In the ACL-deficient arthritic knee, the wear usually is located more posteriorly along the medial tibial plateau (Fig. 3). Hernigou and Deschamps reported a significant correlation between posterior slope of the tibial implant and outcome of UKA [8]. For our study population, tibial slope reduction became part of our surgical technique. Using our surgical technique for UKA in knees with ACL deficiency, the posterior slope of the sagittal tibial resection was reduced from the patient’s native tibial slope to improve stability of the knee in flexion. This technique modification may have influenced our results, though it is not possible to be certain of this.
Fig. 3.
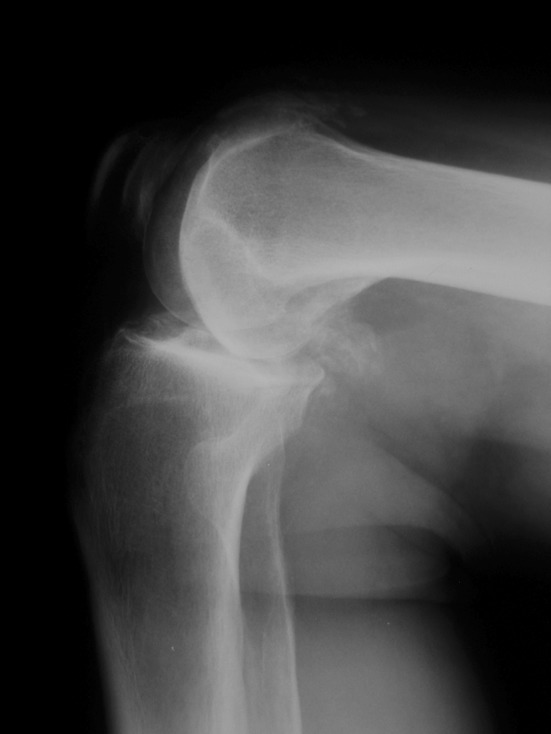
The location of wear in this ACL-deficient knee is on the posterior portion of the tibial plateau.
In our study, the demographics for patients with and without ACL deficiency were similar, all patients underwent fixed bearing UKA, all UKAs were performed by one surgeon over the same time interval using the same implant system, and all polyethylene was sterilized by gas plasma. With consistency between the two study cohorts, we found the absence of a functional ACL at the time of UKA did not increase the revision rate of UKA with a fixed bearing implant as compared to knees with an intact ACL. The failure of five ACL-deficient knees in our study was not related to accelerated polyethylene wear or knee instability as previously reported in the literature with ACL-deficient knees [6, 7]. The failure modes were similar to those with ACL-intact knees. In the ACL-deficient group, two medial UKAs failed due to progression of disease that may have occurred secondary to overcorrection of alignment into valgus and overloading the unresurfaced lateral compartment. The number of ACL-deficient knees that underwent lateral UKA was too small to draw meaningful conclusions. Continued followup is needed to determine whether accelerated wear may impact the survivorship of knees with ACL deficiency.
Footnotes
The institution of the authors has received institutional support from the Inova Health System (Falls Church, VA, USA) and DePuy, A Johnson & Johnson Company (Warsaw, IN, USA). One of the authors certifies that he (GAE), or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount of USD 100,001 to USD 1,000,000 from DePuy and an amount of USD 10,000–USD 100,000 from Smith & Nephew Inc (Memphis, TN, USA). One of the authors certifies that he (GAE) has board membership, consultancy, patents pending, and stocks from TGS Knee Innovations (Plymouth, MN, USA), but he received no monetary payment or benefit during the study period.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ahlbäck S. Osteoarthrosis of the knee: a radiographic investigation. Acta Radiol Diagn (Stockh). 1968;suppl 277:7–72. [PubMed]
- 2.Argenson JN, Komistek RD, Aubaniac JM, Dennis DA, Northcut EJ, Anderson DT, Agostini S. In vivo determination of knee kinematics for subjects implanted with a unicompartmental arthroplasty. J Arthroplasty. 2002;17:1049–1054. doi: 10.1054/arth.2002.34527. [DOI] [PubMed] [Google Scholar]
- 3.Blunn GW, Walker PS, Joshi A, Hardinge K. The dominance of cyclic sliding in producing wear in total knee replacements. Clin Orthop Relat Res. 1991;273:253–260. [PubMed] [Google Scholar]
- 4.Cartier P, Sanouiller JL, Grelsamer RP. Unicompartmental knee arthroplasty surgery: 10-year minimum follow-up period. J Arthroplasty. 1996;11:782–788. doi: 10.1016/S0883-5403(96)80177-X. [DOI] [PubMed] [Google Scholar]
- 5.Christensen N. Unicompartmental prosthesis for gonarthrosis: a nine-year series of 575 knees from a Swedish hospital. Clin Orthop Relat Res. 1991;273:165–169. [PubMed] [Google Scholar]
- 6.Deschamps G, Lapeyre B. [Rupture of the anterior cruciate ligament: a frequently unrecognized cause of failure of unicompartmental knee prostheses. Apropos of a series of Lotus prostheses with follow-up of more than 5 years] [in French] Rev Chir Orthop Reparatrice Appar Mot. 1987;73:544–551. [PubMed] [Google Scholar]
- 7.Goodfellow J, O’Connor J. The anterior cruciate ligament in knee arthroplasty: a risk factor with constrained meniscal prostheses. Clin Orthop Relat Res. 1992;276:245–252. [PubMed] [Google Scholar]
- 8.Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86:506–511. doi: 10.2106/00004623-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kozinn SC, Scott R. Current concepts review: unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71:145–150. [PubMed] [Google Scholar]