Abstract
Cells of a cloned line of murine virus-induced erythroleukemia were stimulated to differentiate along the erythroid pathway by dimethyl sulfoxide at concentrations that did not inhibit growth. A rise in the number of benzidine-positive normoblasts was accompanied by increased synthesis of heme and hemoglobin and a decrease in the malignancy of the cells. This action of dimethyl sulfoxide, which was reversible, may represent the derepression of leukemic cells to permit their maturation.
Full text
PDF
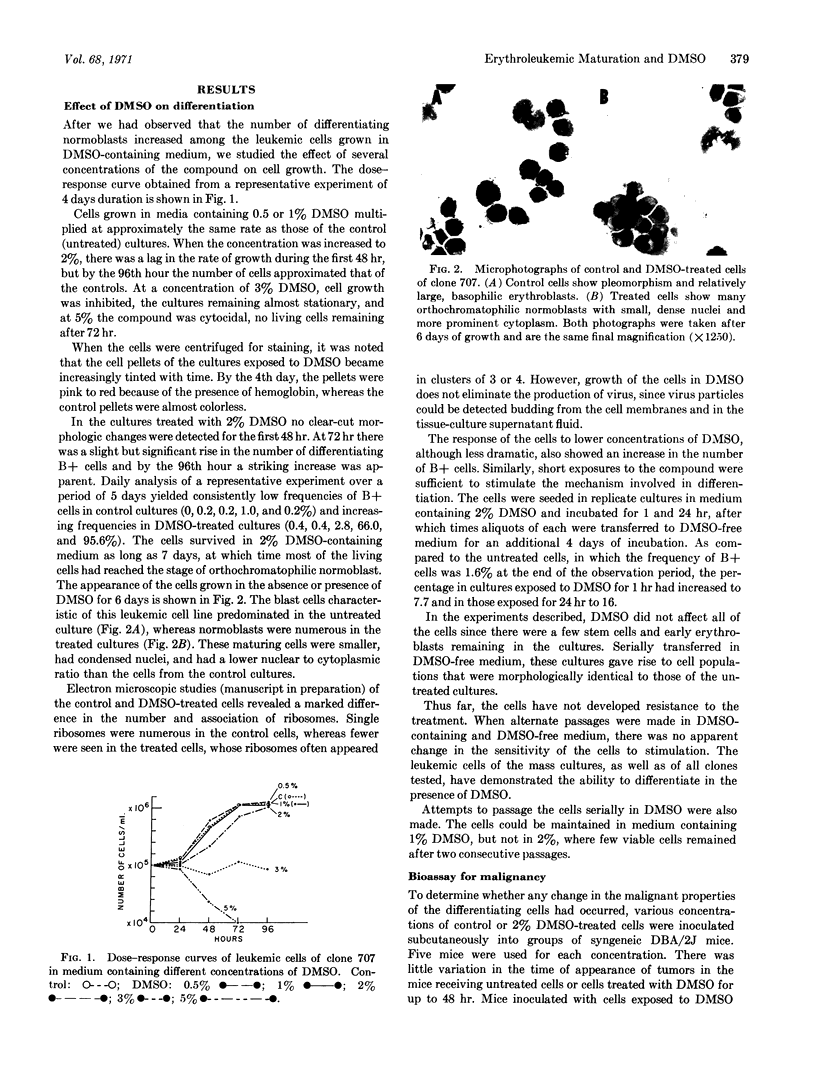
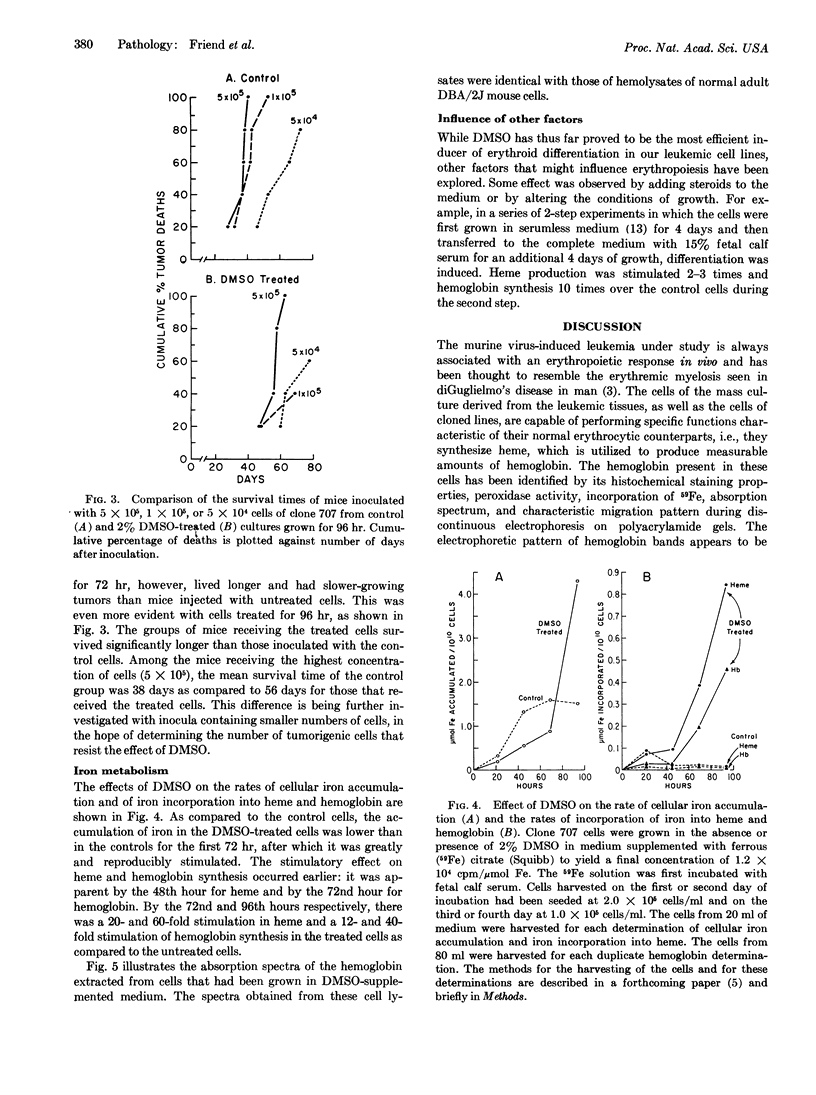
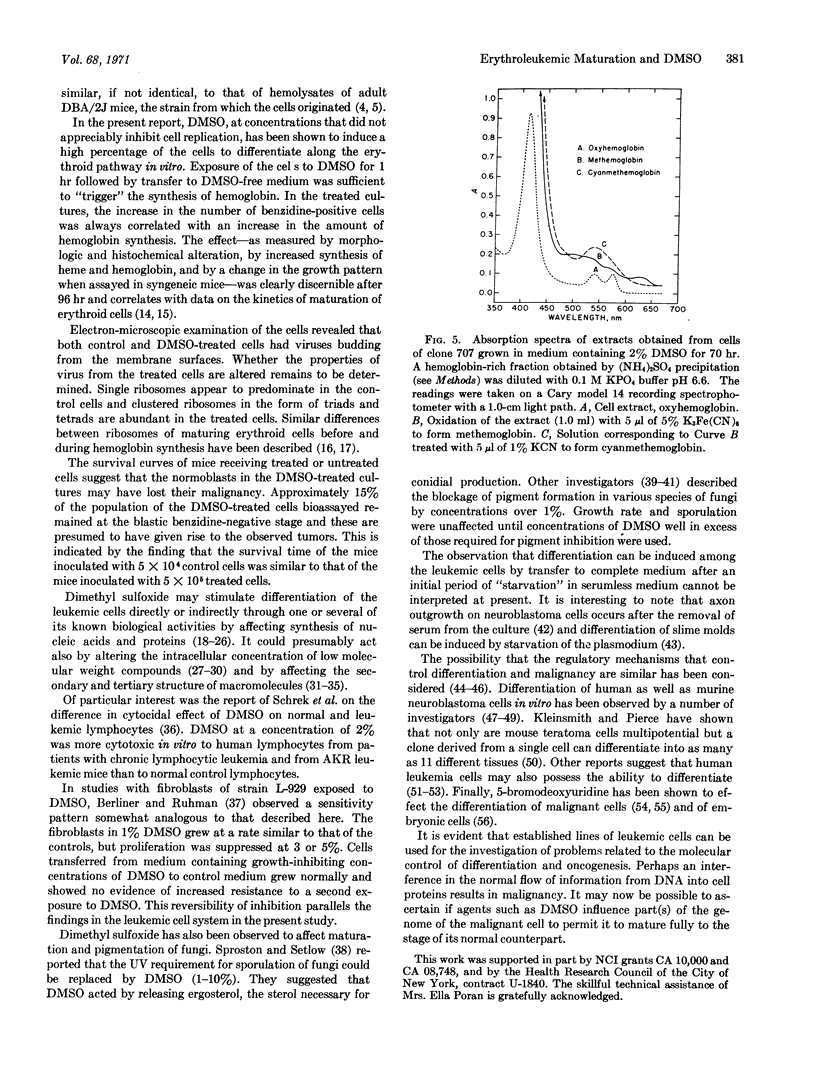

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstey M. S., Parkman P. D. Enhancement of polio-RNA infectivity by dimethylsulfoxide. Proc Soc Exp Biol Med. 1966 Nov;123(2):438–442. doi: 10.3181/00379727-123-31508. [DOI] [PubMed] [Google Scholar]
- Ashwood-Smith M. J. Radioprotective and cryoprotective properties of dimethyl sulfoxide in cellular systems. Ann N Y Acad Sci. 1967 Mar 15;141(1):45–62. doi: 10.1111/j.1749-6632.1967.tb34865.x. [DOI] [PubMed] [Google Scholar]
- Bean G. A., Rambo G. W., Klarman W. L. Influence of dimethyl sulfoxide (DMSO) on conidial pigmentation and consequent UV light sensitivity of aflatoxin producing strains of Aspergillus flavus. Life Sci. 1969 Nov 15;8(22):1185–1191. doi: 10.1016/0024-3205(69)90173-8. [DOI] [PubMed] [Google Scholar]
- Berliner D. L., Ruhmann A. G. The influence of dimethyl sulfoxide on fibroblastic proliferation. Ann N Y Acad Sci. 1967 Mar 15;141(1):159–164. doi: 10.1111/j.1749-6632.1967.tb34877.x. [DOI] [PubMed] [Google Scholar]
- Clarkson B., Strife A., De Harven E. Continuous culture of seven new cell lines (SK-L1 to 7) from patients with acute leukemia. Cancer. 1967 Jun;20(6):926–947. doi: 10.1002/1097-0142(196706)20:6<926::aid-cncr2820200603>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Coleman A. W., Coleman J. R., Kankel D., Werner I. The reversible control of animal cell differentiation by the thymidine analog, 5-bromodeoxyuridine. Exp Cell Res. 1970 Feb;59(2):319–328. doi: 10.1016/0014-4827(70)90606-3. [DOI] [PubMed] [Google Scholar]
- FARNES P., TROBAUGH F. E., Jr Observations on leukemic marrow explants in well cultures. J Lab Clin Med. 1961 Apr;57:568–573. [PubMed] [Google Scholar]
- Franz T. J., Van Bruggen J. T. A possible mechanism of action of DMSO. Ann N Y Acad Sci. 1967 Mar 15;141(1):302–309. doi: 10.1111/j.1749-6632.1967.tb34895.x. [DOI] [PubMed] [Google Scholar]
- Friend C., Patuleia M. C., De Harven E. Erythrocytic maturation in vitro of murine (Friend) virus-induced leukemic cells. Natl Cancer Inst Monogr. 1966 Sep;22:505–522. [PubMed] [Google Scholar]
- Friend C., Rossi G. B. The phenomenon of differentiation in murine irus-induced leukemic cells. Proc Can Cancer Conf. 1969;8:171–182. [PubMed] [Google Scholar]
- Friend C., Rossi G. B. Transplantation immunity and the suppression of spleen colony formation by immunization with murine leukemia virus preparations (Friend). Int J Cancer. 1968 Jul 15;3(4):523–529. doi: 10.1002/ijc.2910030413. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN M. N., BURDMAN J. A., JOURNEY L. J. LONG-TERM TISSUE CULTURE OF NEUROBLASTOMAS. II. MORPHOLOGIC EVIDENCE FOR DIFFERENTIATION AND MATURATION. J Natl Cancer Inst. 1964 Jan;32:165–199. [PubMed] [Google Scholar]
- Gerhards E., Gibian H. The metabolism of dimethyl sulfoxide and its metabolic effects in man and animals. Ann N Y Acad Sci. 1967 Mar 15;141(1):65–76. doi: 10.1111/j.1749-6632.1967.tb34867.x. [DOI] [PubMed] [Google Scholar]
- Hagemann R. F. Effect of dimethyl sulfoxide on RNA synthesis in S-180 tumor cells. Experientia. 1969 Dec 15;25(12):1298–1300. doi: 10.1007/BF01897511. [DOI] [PubMed] [Google Scholar]
- Hagemann R. F., Evans T. C. Inhibition of DNA synthesis in sarcoma-180 tumor cells in vitro by dimethyl sulfoxide. Proc Soc Exp Biol Med. 1968 Jul;128(3):648–650. doi: 10.3181/00379727-128-33088. [DOI] [PubMed] [Google Scholar]
- KLEINSMITH L. J., PIERCE G. B., Jr MULTIPOTENTIALITY OF SINGLE EMBRYONAL CARCINOMA CELLS. Cancer Res. 1964 Oct;24:1544–1551. [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- Kisch A. L. Dimethyl sulfoxide enhancement of transformation by polyoma virus. Virology. 1969 Jan;37(1):32–41. doi: 10.1016/0042-6822(69)90303-1. [DOI] [PubMed] [Google Scholar]
- LOBUE J., DORNFEST B. S., GORDON A. S., HURST J., QUASTLER H. Marrow distribution in rat femurs determined by cell enumeration and Fe59 labeling. Proc Soc Exp Biol Med. 1963 Apr;112:1058–1062. doi: 10.3181/00379727-112-28250. [DOI] [PubMed] [Google Scholar]
- Markert C. L. Neoplasia: a disease of cell differentiation. Cancer Res. 1968 Sep;28(9):1908–1914. [PubMed] [Google Scholar]
- Montagnier L., Goldé A., Vigier P. A possible subunit structure of Rous sarcoma virus RNA. J Gen Virol. 1969 Apr;4(3):449–452. doi: 10.1099/0022-1317-4-3-449. [DOI] [PubMed] [Google Scholar]
- NEUMAN R. E., TYTELL A. A. Serumless medium for cultivation of cells of normal and malignant origin. Proc Soc Exp Biol Med. 1960 Jun;104:252–256. doi: 10.3181/00379727-104-25797. [DOI] [PubMed] [Google Scholar]
- Pierce G. B. Differentiation of normal and malignant cells. Fed Proc. 1970 May-Jun;29(3):1248–1254. [PubMed] [Google Scholar]
- RIFKIND R. A., DANON D., MARKS P. A. ALTERATIONS IN POLYRIBOSOMES DURING ERYTHROID CELL MATURATION. J Cell Biol. 1964 Sep;22:599–611. doi: 10.1083/jcb.22.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammler D. H., Zaffaroni A. Biological implications of DMSO based on a review of its chemical properties. Ann N Y Acad Sci. 1967 Mar 15;141(1):13–23. doi: 10.1111/j.1749-6632.1967.tb34861.x. [DOI] [PubMed] [Google Scholar]
- Ritter P. O., Kull F. J., Jacobson K. B. Aminoacylation of Escherichia coli valine transfer ribonucleic acid by Neurospora crassa phenylalanyl transfer ribonucleic acid synthetase in tris(hydroxymethyl)aminomethane hydrochloric acid and potassium cacodylate buffers. Effect of salts, dimethyl sulfoxide, ethanol, and 2-mercaptoethanol. J Biol Chem. 1970 Apr 25;245(8):2114–2120. [PubMed] [Google Scholar]
- Schrek R., Elrod L. M., Batra K. V. Cytocidal effect of dimethyl sulfoxide on normal and leukemic lymphocytes. Ann N Y Acad Sci. 1967 Mar 15;141(1):202–213. doi: 10.1111/j.1749-6632.1967.tb34880.x. [DOI] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Jacob F. 5-bromodeoxyuridine-induced differentiation of a neuroblastoma. Proc Natl Acad Sci U S A. 1970 Sep;67(1):247–254. doi: 10.1073/pnas.67.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silagi S., Bruce S. A. Suppression of malignancy and differentiation in melanotic melanoma cells. Proc Natl Acad Sci U S A. 1970 May;66(1):72–78. doi: 10.1073/pnas.66.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproston T. Setlow RB: Ergosterol and substitutes for the ultraviolet radiation requirement for conidia formation in Stemphylium solani. Mycologia. 1968 Jan-Feb;60(1):104–114. [PubMed] [Google Scholar]
- Steinberg A. The employment of dimethyl sulfoxide as an antiinflammatory agent and steroid-transporter in diversified clinical diseases. Ann N Y Acad Sci. 1967 Mar 15;141(1):532–550. doi: 10.1111/j.1749-6632.1967.tb34922.x. [DOI] [PubMed] [Google Scholar]
- Stock B. H., Hansen A. R., Fouts J. R. Stimulation of hepatic microsomal aniline p-hydroxylase activity by dimethyl sulfoxide administration to rats. Toxicol Appl Pharmacol. 1970 May;16(3):728–739. doi: 10.1016/0041-008x(70)90078-5. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- Tillman R. W., Bean G. A. Tentative identification and influence of sterols and dimethyl sulf-oxide (DMSO) on the growth and survival of Fusarium roseum. Mycologia. 1970 May-Jun;62(3):428–436. [PubMed] [Google Scholar]
- Tovell D. R., Colter J. S. The interaction of tritium-labelled mengo virus RNA and L cells: the effects of DMSO and DEAE-dextran. Virology. 1969 Apr;37(4):624–631. doi: 10.1016/0042-6822(69)90280-3. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Stabilization of enveloped viruses by dimethyl sulfoxide. J Virol. 1968 Sep;2(9):953–954. doi: 10.1128/jvi.2.9.953-954.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Rich A., Hall C. E. Electron Microscope Studies of Ribosomal Clusters Synthesizing Hemoglobin. Science. 1962 Dec 28;138(3548):1399–1403. doi: 10.1126/science.138.3548.1399. [DOI] [PubMed] [Google Scholar]