Abstract
Background
Surgical treatment of pelvic tumors with or without acetabular involvement is challenging. Primary goals of surgery include local control and maintenance of good quality of life, but the procedures are marked by significant perioperative morbidity and complications.
Questions/purposes
We wished to (1) evaluate the frequency of infection after limb salvage surgical resection for bone tumors in the pelvis; (2) determine whether infection after these resections is associated with particular risk factors, including pelvic reconstruction, radiotherapy or chemotherapy, type of resection, and age; and (3) analyze treatment of these infections, particularly with respect to the need of additional surgery or hemipelvectomy.
Methods
From 1975 to 2010, 270 patients with pelvic bone tumors (149 with chondrosarcoma, 40 with Ewing’s sarcoma, 27 with osteosarcoma, 18 with other primary malignant tumors, 11 with metastatic tumors, and 25 with primary benign tumors) were treated by surgical resection. Minimum followup was 1.1 years (mean, 8 years; range, 1–33 years). The resection involved the periacetabular area in 166 patients. In 137 patients reconstruction was performed; in 133 there was no reconstruction. Chart review ascertained the frequency of deep infections, how they were treated, and the frequency of resection arthroplasty or hemipelvectomies that occurred thereafter.
Results
A total of 55 patients (20%) had a deep infection develop at a mean followup of 8 months. There were 20 infections in 133 patients without reconstruction (15%) and 35 infections in 137 patients with reconstruction (26 %). Survivorship rates of the index procedures using infection as the end point were 87%, 83%, and 80% at 1 month, 1 year, and 5 years, respectively. Infection was more common in patients who underwent pelvic reconstruction after resection (univariate analysis, p = 0.0326; multivariate analysis, p = 0.0418; odds ratio, 1.7718; 95% CI, 1.0243–3.0650); no other risk factors we evaluated were associated with an increased likelihood of infection. Despite surgical débridements and antibiotics, 16 patients (46%) had the implant removed and five (9%) underwent external hemipelvectomy (four owing to infection and one as a result of persistent infection and local recurrence).
Conclusions
Infection is a common complication of pelvic resection for bone tumors. Reconstruction after resection is associated with an increased risk of infection compared with resection alone, without significant difference in percentage between allograft and metallic prosthesis. When infection occurs, it requires removal of the implant in nearly half of the patients who have this complication develop, and external hemipelvectomy sometimes is needed to eradicate the infection.
Level of Evidence
Level IV, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Primary bone tumors of the pelvis are rare lesions that are difficult to treat [9, 16, 26, 33]. At the time of presentation, these tumors often are large and technically challenging because of numerous neurovascular structures and the urinary and intestinal tracts. In the past, most malignant tumors in the pelvis were treated with hindquarter amputation [11, 32]. With improvements in chemotherapy and surgical techniques, limb-preserving procedures have emerged as viable surgical modalities [10, 17, 23, 26, 31, 33] and, in most cases, these have replaced ablative surgery. Because the anatomy of the pelvis is complex, resection of pelvic tumors requires extensive exposure to identify and preserve major neurovascular structures and obtain adequate margins. Extensive resection of pelvic sarcomas often necessitates reconstruction to avoid severe functional disabilities as a result of loss of the acetabulum, an incomplete pelvic ring, and loss of the abductor musculature [16, 40].
Primary goals of surgery include local tumor control and maintenance of good quality of life, but surgery can be complicated neurologic damage, visceral or vascular injury, large soft tissue defects, and complications related to the reconstruction (aseptic loosening, fracture, nonunion), difficulty with ambulation, infection, and delayed wound healing [1–4, 6–10, 12–27, 29–31, 33–36, 38–43, 45, 46]. Infection, often associated with wound complications, is the most frequent postoperative complication after pelvic resection, ranging from 10% to 47%, and sometimes calls for multiple surgical débridement and soft tissue reconstructive procedures [1, 3, 4, 8, 10, 12, 13, 18–20, 23, 25–27, 29, 30, 34, 36, 38, 40, 45, 46].
Few studies with adequate followup have investigated the incidence and sequelae of infection after resection of pelvic bone tumors [26, 27].
We therefore performed an observational study of a large number of patients with primary pelvic bone tumors, surgically treated in a specialized oncologic center and followed for a mean of more than 8 years to analyze the frequency and sequelae of infection. Specifically, we sought to (1) evaluate the frequency of infection after limb salvage surgical resection for bone tumors in the pelvis; (2) determine whether infection after these resections is associated with particular risk factors, including pelvic reconstruction, radiotherapy or chemotherapy, type of resection, and age; and (3) analyze treatment of these infections, particularly with respect to the need of additional surgery or hemipelvectomy.
Patients and Methods
In this retrospective study, we examined the records of 315 patients who underwent limb salvage surgical resection for pelvic bone tumors at our institution from 1975 to 2010. Criteria for inclusion in the study were (1) confirmed histologic diagnosis of a pelvic bone tumor; (2) treatment consisting of limb salvage surgical resection with uniform antibiotic prophylaxis; and (3) minimum followup of 1 year. Eight patients did not fulfill the criteria, whereas information regarding the clinical characteristics, treatment, and outcome of 33 patients was not complete and so these patients were excluded from the study. Moreover, four patients (of the remaining 274) died postoperatively as a result of surgical complications not associated with infection (cardiac arrest in two, pulmonary insufficiency as a result of massive pleural effusion in one, and acute pulmonary insufficiency associated with cardiac arrest and multiorgan failure in one), Therefore, 270 (87% of the original 315) patients treated for their pelvic bone tumor and followed for a mean of 8.3 years (range, 1–33 years) were included in the study. We had complete followup for the last 2 years for all 270 patients. There were 159 males and 111 females with a mean age of 39.1 years (range, 4.7–81.8 years) (Table 1). All patients or guardians gave written informed consent at the time of admission to be included in scientific studies. The ethical committee of our institute gave institutional review board approval to this retrospective study.
Table 1.
Baseline characteristic and operative data
| Variable | All patients (n = 270) |
|---|---|
| Sex | |
| Males (number; %) | 159 (59) |
| Females (number; %) | 111 (41) |
| Age at diagnosis (years; SD) | 39 (± 18.2) |
| Mean followup (years; SD) | 8.3 (± 7.4) |
| Surgical technique | |
| Pelvic resection without reconstruction (number; %) | 133 (49) |
| Pelvic resection followed by reconstruction (number; %) | 137 (51) |
| Bone resection | |
| Type I (number; %) | 63 (23) |
| Type II (number; %) | 23 (9) |
| Types I–II (number; %) | 41 (15) |
| Types I–II + proximal femur (number; %) | 1 (< 1) |
| Type III (number; %) | 25 (9) |
| Types I–II–III (number; %) | 23 (9) |
| Types II–III (number; %) | 78 (29) |
| Types I–IV (number; %) | 16 (6) |
| Margins | |
| Wide (number; %) | 168 (62) |
| Wide but contaminated (number; %) | 42 (16) |
| Marginal (number; %) | 38 (14) |
| Intralesional (number; %) | 22 (8) |
| Treatments other than surgery | |
| Chemotherapy | 67 (25) |
| Radiotherapy plus chemotherapy | 40 (15) |
| Radiotherapy | 9 (3) |
| Preoperative selective arterial embolization | 8 (3) |
Histologic diagnosis was established by trocar biopsy or open surgical biopsy (95 biopsies had been performed in other hospitals before admission in our center) and all histologic sections were reviewed by experienced pathologists at our institution with special interest in musculoskeletal oncology to confirm the diagnosis. Primary malignant bone tumors were found in 234 patients (chondrosarcomas in 149, Ewing’s sarcoma in 40, osteosarcoma in 27, spindle cell sarcoma in six, fibrosarcoma in four, malignant fibrous histiocytoma in three, angiosarcoma in two, lymphoma in two, and leiomyosarcoma in one), 25 had primary benign bone tumors (giant cell tumor in 12; osteoblastoma in four; fibroma in three; exostoses in two; and desmoplastic fibroma, aggressive fibromatosis, benign fibrous histiocytoma, and aneurysmal bone cyst in one each), and 11 patients had metastatic tumors.
Pelvic location of bone tumors was classified according to Enneking and Dunham [17] (Table 1). Thirty-six patients had undergone surgical treatment before being admitted to our institution for relapse or inadequate treatment. Surgical treatment was performed according to the principles of musculoskeletal tumor surgery and the decision regarding type of treatment was obtained with a high degree of consensus among the oncologic council that included orthopaedic oncology surgeons, medical oncologists, pathologists, and radiologists. Decisions were made for pelvic resection without reconstruction for 133 patients (49%) and pelvic resection followed by reconstruction for 137 patients (51%). In general, patients with pelvic stability and maintenance of limb length after a Type I resection (usually with pelvic ring continuity), patients with an ischiopubic tumor or Types II and III resection with minor acetabular involvement, and patients with poor general conditions did not receive reconstruction. In general, the indications to perform reconstruction included resection of weightbearing or moving elements (such as the hip) because it may result in pelvic instability and unsatisfactory functional results, young patients, primary sarcomas or benign aggressive tumors with intention to cure, solitary pelvic bone metastasis in patients with “favorable” cancers such as thyroid, renal, and breast cancer with long life expectancies, and the availability of materials relative to the timing of surgery. In the 35 patients who had no acetabular involvement, reconstruction was performed with allograft only (24 Type I resections, 10 Types I to IV, one Type III). Pelvic allografts were procured in sterile form in an operating room from organ donors by a surgical team and subsequently preserved at −80° C until use in our cell and musculoskeletal tissue bank. No final irradiation for sterilization was performed. At the time of the surgery, the allograft was thawed for approximately 1 hour in a rifampicin solution at 37°C and then implanted. Allografts were fixed with screws and/or plates. In the 10 patients with sacral involvement, spinal instrumentation with pedicle screws was performed. Acetabular resections were reconstructed with prosthetic composite allografts in 59 cases (27 hip prostheses with conventional acetabular cups, 33 hip prostheses with stemmed acetabular cups), with allograft only in 16, a prosthesis only in 10, a saddle prosthesis in 11, and iliofemoral arthrodesis in six. The margins were defined based on the worst margin: wide if a continuous shell of healthy tissue could be seen around the tumor (168 patients [62%]); marginal if the plane of resection was along the pseudocapsule (38 patients [14%]; six had benign tumors); intralesional when pathologic tissue was present in a margin (22 patients [8%]; 10 had benign tumors); and contaminated if either a wide specimen was intralesional in some small area or broken with spilling of tumor content during surgery but additional tissue was removed (42 patients with a finally wide margin [16%]) (Table 1). Treatments other than surgery were used according to histologic diagnosis: chemotherapy in 67 patients (25%), radiotherapy plus chemotherapy in 40 (15%), radiotherapy in nine (3%), and preoperative selective arterial embolization in eight (3%) (Table 1). In all patients, a 5-day regimen of intravenous antibiotic prophylaxis including cefazolin (infusion of 1000 mg during a 12-hour period twice daily) and tobramycin (3 mg/kg/day, in three equally divided doses every 8 hours) starting 1 hour preoperatively was administered. The protocol has been designed to be clinically effective against Gram-positive and Gram-negative bacteria. The records of all patients were reviewed and special attention was given to the presence of wound complications or deep infections requiring revision surgery. The criteria used to establish infection that involved skin and subcutaneous tissue of the incision were: (1) purulent drainage, (2) organisms isolated from a culture of fluid or tissue from the incision, and (3) presence of pain or swelling or redness. Characteristics of infections including cultured organism, number of bacteria, the time to onset after surgery, and treatment and outcome were recorded. Infections were classified as postoperative (if the onset was less than 1 month after surgery), early (from 30 days to 6 months after surgery), or late (after 6 months from surgery).
Patients with malignant tumors were followed up postoperatively at regular intervals of 3 months for the first 2 years, every 6 months for the next 3 years, and once a year after 5 years, whereas patients with benign tumors were followed every 6 months for the first 3 years and then annually. The followup time was calculated from the date of surgical resection to the most recent followup or until the time of death.
Survivorship of the index procedures using infection as the end point was analyzed using the Kaplan-Meier analysis [28]. Categorical variables were expressed as the number of occurrences and percentage of the total patients in a category. The curves were compared with the log-rank test [37], where the starting point was surgery and the end point was the occurrence of complications. Multivariate analysis was performed to investigate independent risk factors for infection using the Cox regression model with stepwise forward procedure [37]. Type of resection, resection with and without reconstruction, and treatment with radiotherapy or chemotherapy in addition to surgery were evaluated. Statistical significance was assigned to p values less than 0.05. Data were recorded in a Microsoft Excel 2003 (Microsoft, Richmond, WA, USA) spreadsheet and analyzed using MedCalc software Version 11.1 (MedCalc Software, Mariakerke, Belgium).
Results
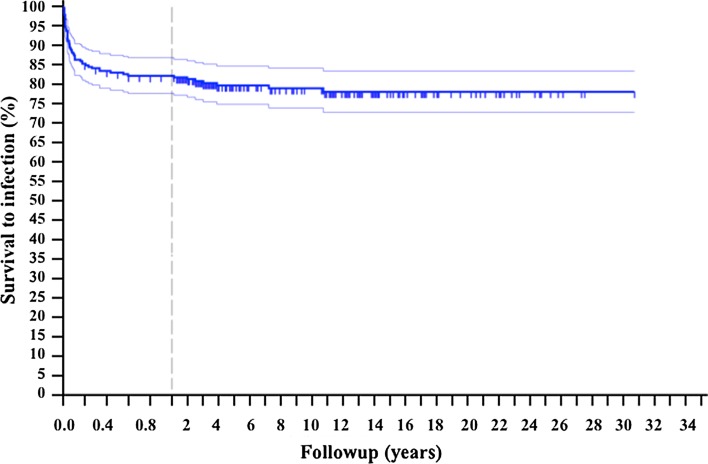
The overall survival status varied according to histologic diagnosis (Table 2). Fifty-five of 270 patients (20.4%) had infections at a mean of 8 months (range, 4 days to 128 months) after surgery. There were 33 postoperative infections (60.0%), 12 early-onset infections (21.8%), and 10 late-onset infections (18.2%). Survivorship rates of the index procedures using infection as the end point were 87% at 1 month, 83% at 6 months, 82% at 1 year, and 80% at 5 years (Fig. 1). Infections were multimicrobial in 21 patients (38.2%) and monomicrobial in 34 patients (61.8%). Gram-negative bacteria grew in culture of 25 patients (37.3%) and Gram-positive bacteria in 42 (62.7%).
Table 2.
Clinical and followup details of the patients with surgical resection for pelvic bone tumors
| Diagnosis | Patients/followup (years) | Age (mean years)/sex (male: female) | Survival status | LR | Metastases | |||
|---|---|---|---|---|---|---|---|---|
| NED | AWD | DWD | DOD | |||||
| Chondrosarcoma | 149/9.8 | 48/85:64 | 97 | 9 | 26 | 17 | 35 | 29 |
| Ewing’s sarcoma | 40/6 | 18/27:13 | 20 | 3 | 16 | 1 | 7 | 17 |
| Osteosarcoma | 27/5.8 | 31/14:13 | 13 | 2 | 11 | 1 | 8 | 12 |
| Spindle cell sarcoma | 6/1.9 | 46/3:3 | 2 | 1 | 3 | – | 2 | 5 |
| Fibrosarcoma | 4/10 | 33/3:1 | 3 | – | – | 1 | – | – |
| Malignant fibrous histiocytoma | 3/6.4 | 48/2:1 | – | – | 2 | 1 | – | 2 |
| Angiosarcoma | 2/1.9 | 65/0:2 | – | 1 | 1 | – | – | 2 |
| Lymphoma | 2/7.2 | 44/0:2 | – | 1 | – | 1 | – | 1 |
| Leiomyosarcoma | 1/12.7 | 21/1:0 | 1 | – | – | – | – | – |
| Metastatic tumors | 11/1 | 32/8:3 | 2 | 1 | 8 | – | 1 | 8 |
| Giant cell tumor | 12/11.2 | 33/8:4 | 10 | – | 2 | – | 3 | – |
| Osteoblastoma | 4/14 | 32/3:1 | 4 | – | – | – | – | – |
| Fibroma | 3/10.7 | 17/3:0 | 3 | – | – | – | – | – |
| Exostoses | 2/13 | 16/0:2 | 2 | – | – | – | – | – |
| Desmoplastic fibroma | 1/3.4 | 27/1:0 | 1 | – | – | – | – | – |
| Aggressive fibromatosis | 1/11.8 | 24/1:0 | 1 | – | – | – | 1 | – |
| Benign fibrous histiocytoma | 1/14.8 | 33/0:1 | 1 | – | – | – | – | – |
| Aneurysmal bone cyst | 1/3.1 | 11/0:1 | 1 | – | – | – | – | – |
NED = no evidence of disease; AWD = alive with disease; DWD = died with disease; DOD = died of other disease; LR = local recurrence.
Fig. 1.
The Kaplan-Meier curve shows the overall survival to infection of the entire group. Patients were censored based on whether a deep infection was reported during followup. Considering that most of the events happened during the first year, the graph shows more accurately this range of followup.
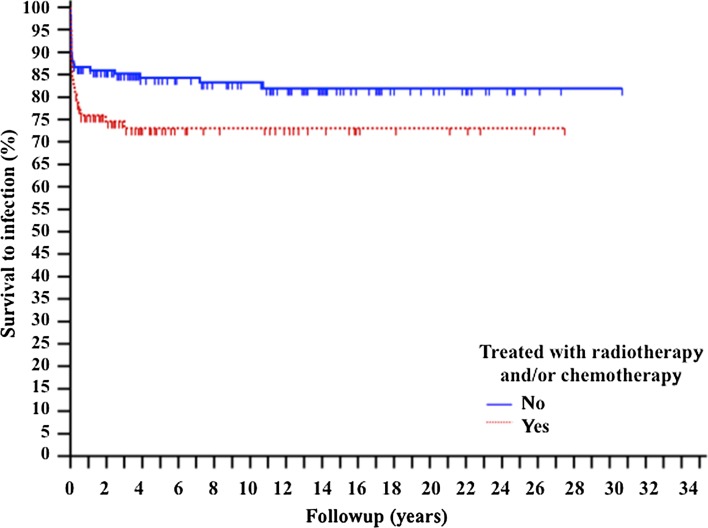
Infection was more common in patients who had reconstruction after limb salvage infection; we identified no other risk factors associated with an increased likelihood of infection. A total of 15% of patients (20 of 133) treated without reconstruction had infections develop, compared with 26% of patients treated with reconstruction (35 of 137, p = 0.0326; odds ratio [OR], 0.5168; 95% CI, 0.3020–0.8841) (Fig. 2). Infection was not more likely in patients who received radiation therapy or chemotherapy (p = 0.0509) (Fig. 3), in different tumor sites (periacetabular or other, p = 0.4558) (Fig. 4), or in patients of different ages (younger or older than 40 years, p = 0.8098) (Fig. 5). In the multivariate Cox regression analysis, only the presence of pelvic reconstruction emerged as an independent significant prognostic factor on infection (p = 0.0418; OR, 1.7718; 95% CI, 1.0243–3.0650). Considering the role of this variable, the group has been subdivided into the different types of reconstruction and rate of infection assessed. Of the 137 patients treated with reconstruction, 35 had infections develop: 14 of 51 treated with a massive allograft (28%), 12 of 59 with an allograft prosthetic composite (20%), three of 10 with a prosthesis only (30%), four of 11 with a saddle prosthesis (36%), and two of six with arthrodesis (33%).
Fig. 2.
The Kaplan-Meier curve shows the survival to infection of the entire group depending on whether an infection developed in patients who underwent pelvic reconstruction. The statistical analysis shows a p of 0.0326.
Fig. 3.
The Kaplan-Meier curve shows the survival to infection of the entire group depending on whether an infection developed in patients who received radiotherapy or chemotherapy. The statistical analysis shows a p value of 0.0509.
Fig. 4.
Kaplan-Meier curve showing the survival to infection of the entire group depending on whether an infection did or did not develop in patients treated with resection that involved the periacetabular area (red line) or other type of resection (blue line). The statistical analysis shows a p of 0.4558.
Fig. 5.
The Kaplan-Meier curve shows the survival to infection of the entire group depending on whether an infection developed in patients aged older or younger than 40 years. The statistical analysis shows a p of 0.8098.
Thirty-five patients (of 55 who had an infection develop) had pelvic reconstruction, and despite surgical treatment and antibiotics, 16 of them required implant removal (46%). External hemipelvectomy as a final treatment was necessary in five of 55 patients (9%) as a result of infection in four and a concomitant presence of infection and local recurrence in one. In 44 patients with infection (80%), surgical débridement was done in addition to culture-guided antibiotics. In 25 patients, one surgical débridement was sufficient, whereas repeated débridements were necessary in 19 patients. No surgical procedure was associated with antibiotic therapy in the other 11 patients; four patients who were inoperable were treated with wound medication with materials capable of protecting, promoting, and accelerating wound healing, three were treated with wound or fistula irrigation, two received topic antimicrobials, and two refused further treatments.
Discussion
Surgical treatment of pelvic bone tumors is challenging. Some studies have shown that there is no difference between limb salvage techniques and external hemipelvectomy in terms of survival rate of patients with malignant pelvic bone tumors [35, 47, 49]; therefore, limb salvage techniques have been used more frequently, even for advanced tumors in which a complete cure and functional recovery after resection are difficult to achieve [2, 9, 33]. Unfortunately, diagnosis of pelvic bone tumors is usually late [9, 33], and the choice of adequate surgical treatment can be particularly difficult because of the size of the tumor and its relationship to important adjacent structures. As in the current series, the most frequent malignant tumor is chondrosarcoma [5, 44], a chemotherapy- and radiotherapy-resistant tumor, in which the role of surgical margins is important [15, 43]. Limb salvage surgery for such patients, especially when pelvic reconstruction is needed, is an extensive procedure associated with a considerable rate of local complications [1–4, 6–10, 12–27, 29–31, 33–36, 38–43, 45, 46]. The current clinical knowledge regarding the role of infection as a complication after limb salvage surgery for pelvic tumors is based on numerous retrospective clinical studies [1, 3, 4, 8, 10, 12, 13, 18–20, 23, 25–27, 29, 30, 34, 36, 38, 40, 45, 46]. The variety of treatments and inability to use prospective clinical trials because of the rarity of these tumors are the main obstacles in treating these patients. We therefore sought to study a relatively large number of patients treated for pelvic tumors and collected during a long time. Specifically, we (1) evaluated the incidence of infection and its characteristics; (2) determined the effect of type of resection, presence of pelvic reconstruction, additional treatment (radiation and chemotherapy), and age at development; and (3) analyzed the sequelae of infection in this complex patient population. Although this study is relatively large, Type II statistical error remains possible for some of the end points we considered. However, we believe that the long-term followup increases the power of our analysis. Moreover, the rarity of these primary tumors of the pelvis may increase the value of our study. Second, because of the relatively small number of patients in some of our subgroups, we could not analyze all confounding variables with a multivariate regression model; in fact, we had the choice to reduce the number of variables to increase the value of our analysis. Third, we included only patients who had surgical resection of the pelvis for pelvic bone tumors; this resulted in the exclusion of numerous patients who underwent pelvic surgery (ie, soft tissue tumors, external hemipelvectomy as primary treatment), but increased the homogeneity of our study population, a tradeoff we considered favorable. Fourth, owing to the retrospective nature of the study, we could not evaluate the correlation between wound complications and deep infection rate. Moreover, we did not want to run a large number of post hoc analyses to assess the influence of numerous variables, many of which (such as surgical time, wide wound exposure, extensive soft tissue stripping, local hematoma formation, and poor skin flap blood supply) have been studied before [24, 42].
The 20% infection rate in our series of patients is lower than reported rates in some series [3, 4, 18, 26, 27, 36, 39, 41, 45, 46] and greater or comparable to the experience reported in other studies [1, 8, 12, 13, 19, 20, 29, 30, 38] (Table 3). The overall infection rate in many series varied from 10% to 47% after internal hemipelvectomy [1, 3, 4, 8, 10, 12, 13, 18–20, 23, 25–27, 29, 30, 34, 36, 38–41, 45, 46], whereas it ranged from 11% to 38% in patients who had resection and endoprosthetic reconstruction [1, 3, 12, 19, 20, 26, 27, 36, 45, 46]. Abudu et al. [3] reported a 26% infection rate in a series of 35 patients undergoing endoprosthetic reconstruction with saddle prostheses. A similar infection rate (30%) was reported in a series of 98 patients treated by resection and reconstruction with custom-made pelvic endoprostheses [27]. To our knowledge, there are no published studies focused on infection as a complication of pelvic resection for bone tumors. Known data for infections usually are derived from small case series (patients randomized for histologic diagnosis or tumor site or type of reconstruction), and this probably is attributable to the rarity of the disease and the absence of a unique modality of treatment. As reported by others [8, 27], most infections occurred within 2 years of surgery with a median time to infection of 6 months.
Table 3.
Major series of pelvic resection and data from the current study
| Study | Year | Number of patients | Type of pelvic reconstruction | Infection (%) |
|---|---|---|---|---|
| Aboulafia et al. [1] | 1995 | 17 | Saddle prosthesis | 18 |
| Windhager et al. [45] | 1996 | 21 | Different types (mainly saddle and custom-made prostheses) | 21 |
| Abudu et al. [3] | 1997 | 35 | Saddle prosthesis | 26 |
| Wirbel et al. [46] | 1999 | 39 | Megaprosthetic replacement | 26 |
| Cottias et al. [12] | 2001 | 17 | Saddle prosthesis | 18 |
| Langlais et al. [30] | 2001 | 13 | Structural pelvic allograft | 18 |
| Ozaki et al. [35] | 2002 | 12 | Custom-made hemipelvic prosthesis | 25 |
| Hillmann et al. [26] | 2003 | 110* | Different types: | |
| No reconstructions (35 patients) | 6 | |||
| Amputation (9 patients) | 22 | |||
| Hip transposition (17 patients) | – | |||
| Pelvic prosthesis (16 patients) | 38 | |||
| Massive allograft (13 patients) | 39 | |||
| Autograft (12 patients) | 8 | |||
| Allograft prosthetic composite (8 patients) | 88 | |||
| Aljassir et al. [4] | 2005 | 27 | Saddle prosthesis | 37 |
| Delloye et al. [13] | 2007 | 24 | Structural pelvic allograft | 13 |
| Guo et al. [20] | 2007 | 28 | Hemipelvic prosthesis | 14 |
| Jaiswal et al. [27] | 2008 | 98 | Hemipelvic prosthesis | 30† |
| Biau et al. [8] | 2009 | 13 | Femoral autograft | 15 |
| Guo et al. [19] | 2010 | 45 | Different types: | |
| Modular hemipelvic prosthesis (27 patients) | 11 | |||
| Saddle prosthesis (4 patients) | 25 | |||
| Devitalized tumor bone (5 patients) | 20 | |||
| Arthrodesis (3 patients) | – | |||
| Amputation (6 patients) | – | |||
| Gebert et al. [18] | 2011 | 62 | Hip transposition | 32 |
| Laffosse et al. [29] | 2012 | 10 | Femoral autograft | 10 |
| Puri et al. [38] | 2012 | 26 | No prosthetic or allograft reconstruction | 19 |
| Current study | – | 270 | Different types: | |
| No reconstruction (133 patients) | 15 | |||
| Saddle prosthesis (11 patients) | 36 | |||
| Prosthesis only (10 patients) | 30 | |||
| Arthrodesis (6 patients) | 33 | |||
| Massive allograft (51 patients) | 28 | |||
| Allograft prosthetic composite (59 patients) | 20 |
* Forty-nine had reconstructions, nine had amputations, 17 had hip transposition, 35 had no reconstruction; †12% superficial infection, 18% deep infection.
The presence and type of pelvic reconstruction may influence incidence of infection. In our series, we found that only the presence of pelvic reconstruction was statistically significant at multivariate analysis as an independent prognostic factor on infection. Puri et al. [38] reported their experience for 26 patients with nonmetastatic Ewing’s sarcoma of the pelvis treated with surgical resection, with an infection rate of 19% (four patients including one patient with sepsis and omental gangrene who died during the immediate perioperative period). They attributed this low incidence of infection to the fact that 50% of their patients did not undergo any form of reconstruction. There are few published retrospective reviews regarding surgical treatment of pelvic bone tumors with more than 50 patients [26, 50], with obvious differences among subgroups, tumor size, resection type, and surgical and adjuvant treatments. Zeifang et al. [50] reported that biologic reconstructions were associated with higher complication rates than endoprosthetic reconstructions, whereas Hillmann et al. [26], analyzing 110 patients with pelvic tumors, reported a similar incidence of infection (38%) between biologic and prosthetic reconstructions. Infection is one of the most problematic complications that can occur in patients with pelvic allografts [13, 27, 48]. Reported infection rates after massive pelvic allograft reconstruction have ranged from 12.5% to 38.5% in series ranging from 13 to 24 patients [7, 13, 26, 30]. In our series we found 28% and 20% infection rates after reconstruction with massive allografts and allograft prosthetic composite, respectively (Table 3), suggesting that these did not change the risk of infection compared with the other reconstructive approaches used, although certainly a comparative trial would be a way to get a more definitive answer to this question. The role of chemotherapy and radiation in the development of infection is unclear. To exclude pelvic reconstruction as a verified risk factor, we analyzed the series of 62 patients with periacetabular tumors treated with pelvic resection and hip transposition, without allograft or prosthetic reconstruction in 45 patients, reported by Gebert et al. [18]. In their statistical analysis, among a list of risk factors that included clinical stage, surgical procedure, chemotherapy, and radiotherapy, none of these was associated with infection and other postoperative complications [18]. Only age older than 50 years was found to be a significant negative predictor of postoperative complications [18]. Moreover, homogeneous series of chemosensitive histologic diagnoses (Ewing’s sarcoma or osteosarcoma) in the pelvis showed an incidence of postoperative infection or wound healing problems ranging from 19% to 31% despite that all patients received chemotherapy and/or radiation [38]. In our series, even if the treatment with chemotherapy and/or radiation showed a statistically significant difference at univariate analysis, it lost its significance at multivariate analysis as an independent prognostic factor of infection. Other series showed no statistically significant difference in the rate of infection between tumors confined to the acetabulum and those involving the acetabulum and ilium or pubis [27, 35]. In our series, an association between the rate of infection and type of resection (periacetabular versus other types) was not found. Despite the similar complication rate, Puri et al. did not reconstruct any of the resections that excluded the acetabulum [38], whereas others advocate restoring the pelvic ring after Type I or Types I to IV pelvic resections [6, 41]. In our series, half of the patients with Type I or Types I to IV pelvic resections underwent reconstruction because of a massive bone defect and pelvic instability to avoid complications, including a shortened limb or limb discrepancy, pain, and diastasis of the symphysis pubis.
In our series, infections resolved after one or more surgical débridements in combination with antibiotic therapy in 80% of the patients. However, in 46% of patients with pelvic reconstruction, the implants were removed. External hemipelvectomy as a final treatment rarely was needed. There is no unanimous opinion regarding the appropriate treatment of deep infection. Puri et al. [38] reported that three (of four) infections resolved after wound lavage. One patient with ipsilateral femur autograft and prosthetic implant achieved healing of an infection with extensive, early débridement and lavage and appropriate antibiotic therapy [29]. In a series of saddle prosthetic reconstructions after pelvic tumor resections, four of six patients responded to débridement and irrigation, whereas one patient with infection had chronic drainage and in another patient, implant removal was necessary [4]. In another series, nine of 17 patients who underwent resection and prosthetic reconstruction [27], had deep infections develop and were treated by wound débridement, washout, and antibiotics and their infections resolved. Of the remaining eight, six had a Girdlestone procedure, one had a hindquarter amputation, and one had a two-stage revision, all of which were successful [27]. In another study, after treatment of deep infection of a hemipelvic prosthesis with implant removal and its substitution with an antibiotic-impregnated bead and spacer, two of three patients achieved healing, however, the third patient underwent an external hemipelvectomy because of persistent infection [36]. In a previous study from our institute concerning pelvic reconstruction with massive allograft, implants were removed in six of eight patients with deep infections [14]. Two of these infections were related to contaminated allografts, which raises the question regarding whether irradiation of the allograft might reduce the risk of infection, even if it might imply an increase of failure risk [34].
The infection rate is still high after pelvic resection and it can have dire consequences for the patient. Pelvic reconstruction was the only independent significant prognostic factor on infection, and often (46% of the time) requires removal of the reconstruction once an infection occurs. However external hemipelvectomy occasionally is needed after limb salvage surgery for pelvic bone tumors. More efforts should be made to decrease postoperative infections to enhance the limb salvage techniques. Starting from the role of pelvic reconstruction on infection rate, additional studies should be done expanding the variable list, assessing the role of operative time, blood loss, tumor size, BMI, wound complications, the extension into the sacrum and lower spine, and the type of material used for reconstruction.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aboulafia AJ, Buch R, Mathews J, Li W, Malawer MM. Reconstruction using the saddle prosthesis following excision of primary and metastatic periacetabular tumors. Clin Orthop Relat Res. 1995;314:203–213. [PubMed] [Google Scholar]
- 2.Aboulafia AJ, Malawer MM. Surgical management of pelvic and extremity osteosarcoma. Cancer. 1993;71(10 suppl):3358–3366. doi: 10.1002/1097-0142(19930515)71:10+<3358::AID-CNCR2820711738>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 3.Abudu A, Grimer RJ, Cannon SR, Carter SR, Sneath RS. Reconstruction of the hemipelvis after the excision of malignant tumours: complications and functional outcome of prostheses. J Bone Joint Surg Br. 1997;79:773–779. doi: 10.1302/0301-620X.79B5.6749. [DOI] [PubMed] [Google Scholar]
- 4.Aljassir F, Beadel GP, Turcotte RE, Griffin AM, Bell RS, Wunder JS, Isler MH. Outcome after pelvic sarcoma resection reconstructed with saddle prosthesis. Clin Orthop Relat Res. 2005;438:36–41. doi: 10.1097/00003086-200509000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Angelini A, Guerra G, Mavrogenis AF, Pala E, Picci P, Ruggieri P. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106:929–937. doi: 10.1002/jso.23173. [DOI] [PubMed] [Google Scholar]
- 6.Beadel GP, McLaughlin CE, Wunder JS, Griffin AM, Ferguson PC, Bell RS. Outcome in two groups of patients with allograft-prosthetic reconstruction of pelvic tumor defects. Clin Orthop Relat Res. 2005;438:30–35. doi: 10.1097/01.blo.0000180048.43208.2f. [DOI] [PubMed] [Google Scholar]
- 7.Bell RS, Davis AM, Wunder JS, Buconjic T, McGoveran B, Gross AE. Allograft reconstruction of the acetabulum after resection of stage-IIB sarcoma: intermediate-term results. J Bone Joint Surg Am. 1997;79:1663–1674. doi: 10.2106/00004623-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Biau D, Thévenin F, Dumaine V, Babinet A, Tomeno B, Anract P. Ipsilateral femoral autograft reconstruction after resection of a pelvic tumor. J Bone Joint Surg Am. 2009;91:142–151. doi: 10.2106/JBJS.G.01061. [DOI] [PubMed] [Google Scholar]
- 9.Campanacci M, Capanna R. Pelvic resections: the Rizzoli Institute experience. Orthop Clin North Am. 1991;22:65–86. [PubMed] [Google Scholar]
- 10.Capanna R, van Horn JR, Guernelli N, Briccoli A, Ruggieri P, Biagini R, Bettelli G, Campanacci M. Complications of pelvic resections. Arch Orthop Trauma Surg. 1987;106:71–77. doi: 10.1007/BF00435417. [DOI] [PubMed] [Google Scholar]
- 11.Carter SR, Eastwood DM, Grimer RJ, Sneath RS. Hindquarter amputation for tumours of the musculoskeletal system. J Bone Joint Surg Br. 1990;72:490–493. doi: 10.1302/0301-620X.72B3.2341454. [DOI] [PubMed] [Google Scholar]
- 12.Cottias P, Jeanrot C, Vinh TS, Tomeno B, Anract P. Complications and functional evaluation of 17 saddle prostheses for resection of periacetabular tumors. J Surg Oncol. 2001;78:90–100. doi: 10.1002/jso.1127. [DOI] [PubMed] [Google Scholar]
- 13.Delloye C, Banse X, Brichard B, Docquier PL, Cornu O. Pelvic reconstruction with a structural pelvic allograft after resection of a malignant bone tumor. J Bone Joint Surg Am. 2007;89:579–587. doi: 10.2106/JBJS.E.00943. [DOI] [PubMed] [Google Scholar]
- 14.Donati D, Di Bella C, Frisoni T, Cevolani L, DeGroot H. Alloprosthetic composite is a suitable reconstruction after periacetabular tumor resection. Clin Orthop Relat Res. 2011;469:1450–1458. doi: 10.1007/s11999-011-1799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donati D, El Ghoneimy A, Bertoni F, Di Bella C, Mercuri M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J Bone Joint Surg Br. 2005;87:1527–1530. doi: 10.1302/0301-620X.87B11.16621. [DOI] [PubMed] [Google Scholar]
- 16.Enneking WF. Pelvis. In: Enneking WF, editor. Musculoskeletal Tumor Surgery. New York, NY: Churchill-Livingstone; 1983. pp. 483–490. [Google Scholar]
- 17.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731–746. [PubMed] [Google Scholar]
- 18.Gebert C, Wessling M, Hoffmann C, Roedl R, Winkelmann W, Gosheger G, Hardes J. Hip transposition as a limb salvage procedure following the resection of periacetabular tumors. J Surg Oncol. 2011;103:269–275. doi: 10.1002/jso.21820. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, Li D, Tang X, Ji T. Surgical treatment of pelvic chondrosarcoma involving periacetabulum. J Surg Oncol. 2010;101:160–165. doi: 10.1002/jso.21442. [DOI] [PubMed] [Google Scholar]
- 20.Guo W, Li D, Tang X, Yang Y, Ji T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin Orthop Relat Res. 2007;461:180–188. doi: 10.1097/BLO.0b013e31806165d5. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, Sun X, Ji T, Tang X. Outcome of surgical treatment of pelvic osteosarcoma. J Surg Oncol. 2012;106:406–410. doi: 10.1002/jso.23076. [DOI] [PubMed] [Google Scholar]
- 22.Guo Z, Li J, Pei GX, Li XD, Wang Z. Pelvic reconstruction with a combined hemipelvic prostheses after resection of primary malignant tumor. Surg Oncol. 2010;19:95–105. doi: 10.1016/j.suronc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Ham SJ, Schraffordt Koops H, Veth RP, van Horn JR, Eisma WH, Hoekstra HJ. External and internal hemipelvectomy for sarcomas of the pelvic girdle: consequences of limb-salvage treatment. Eur J Surg Oncol. 1997;23:540–546. doi: 10.1016/S0748-7983(97)93173-5. [DOI] [PubMed] [Google Scholar]
- 24.Han I, Lee YM, Cho HS, Oh JH, Lee SH, Kim HS. Outcome after surgical treatment of pelvic sarcomas. Clin Orthop Surg. 2010;2:160–166. doi: 10.4055/cios.2010.2.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrington KD. The use of hemipelvic allografts or autoclaved grafts for reconstruction after wide resections of malignant tumors of the pelvis. J Bone Joint Surg Am. 1992;74:331–341. [PubMed] [Google Scholar]
- 26.Hillmann A, Hoffmann C, Gosheger G, Rodl R, Winkelmann W, Ozaki T. Tumors of the pelvis: complications after reconstruction. Arch Orthop Trauma Surg. 2003;123:340–344. doi: 10.1007/s00402-003-0543-7. [DOI] [PubMed] [Google Scholar]
- 27.Jaiswal PK, Aston WJ, Grimer RJ, Abudu A, Carter S, Blunn G, Briggs TW, Cannon S. Peri-acetabular resection and endoprosthetic reconstruction for tumours of the acetabulum. J Bone Joint Surg Br. 2008;90:1222–1227. doi: 10.1302/0301-620X.90B9.20758. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 29.Laffosse JM, Pourcel A, Reina N, Tricoire JL, Bonnevialle P, Chiron P, Puget J. Primary tumor of the periacetabular region: resection and reconstruction using a segmental ipsilateral femur autograft. Orthop Traumatol Surg Res. 2012;98:309–318. doi: 10.1016/j.otsr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Langlais F, Lambotte JC, Thomazeau H. Long-term results of hemipelvis reconstruction with allografts. Clin Orthop Relat Res. 2001;388:178–186. doi: 10.1097/00003086-200107000-00025. [DOI] [PubMed] [Google Scholar]
- 31.Mankin HJ, Doppelt S, Tomford W. Clinical experience with allograft implantation: the first ten years. Clin Orthop Relat Res. 1983;174:69–86. [PubMed] [Google Scholar]
- 32.Masterson EL, Davis AM, Wunder JS, Bell RS. Hindquarter amputation for pelvic tumors: the importance of patient selection. Clin Orthop Relat Res. 1998;350:187–194. [PubMed] [Google Scholar]
- 33.Mavrogenis AF, Soultanis K, Patapis P, Guerra G, Fabbri N, Ruggieri P, Papagelopoulos PJ. Pelvic resections. Orthopedics. 2012;35:e232–e243. doi: 10.3928/01477447-20120222-03. [DOI] [PubMed] [Google Scholar]
- 34.Ozaki T, Hillmann A, Bettin D, Wuisman P, Winkelmann W. High complication rates with pelvic allografts: experience of 22 sarcoma resections. Acta Orthop Scand. 1996;67:333–338. doi: 10.3109/17453679609002326. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki T, Hillmann A, Lindner N, Blasius S, Winkelmann W. Chondrosarcoma of the pelvis. Clin Orthop Relat Res. 1997;337:226–239. doi: 10.1097/00003086-199704000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Ozaki T, Hoffmann C, Hillmann A, Gosheger G, Lindner N, Winkelmann W. Implantation of hemipelvic prosthesis after resection of sarcoma. Clin Orthop Relat Res. 2002;396:197–205. doi: 10.1097/00003086-200203000-00030. [DOI] [PubMed] [Google Scholar]
- 37.Petrie A. Statistics in orthopaedic papers. J Bone Joint Surg Br. 2006;88:1121–1136. doi: 10.1302/0301-620X.88B9.17896. [DOI] [PubMed] [Google Scholar]
- 38.Puri A, Gulia A, Jambhekar NA, Laskar S. Results of surgical resection in pelvic Ewing’s sarcoma. J Surg Oncol. 2012;106:417–422. doi: 10.1002/jso.23107. [DOI] [PubMed] [Google Scholar]
- 39.Rodl RW, Hoffmann C, Gosheger G, Leidinger B, Jurgens H, Winkelmann W. Ewing’s sarcoma of the pelvis: combined surgery and radiotherapy treatment. J Surg Oncol. 2003;83:154–160. doi: 10.1002/jso.10256. [DOI] [PubMed] [Google Scholar]
- 40.Satcher RL, Jr, O’Donnell RJ, Johnston JO. Reconstruction of the pelvis after resection of tumors about the acetabulum. Clin Orthop Relat Res. 2003;409:209–217. doi: 10.1097/01.blo.0000057791.10364.7c. [DOI] [PubMed] [Google Scholar]
- 41.Schwameis E, Dominkus M, Krepler P, Dorotka R, Lang S, Windhager R, Kotz R. Reconstruction of the pelvis after tumor resection in children and adolescents. Clin Orthop Relat Res. 2002;402:220–235. doi: 10.1097/00003086-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Senchenkov A, Moran SL, Petty PM, Knoetgen J, 3rd, Clay RP, Bite U, Barnes SA, Sim FH. Predictors of complications and outcomes of external hemipelvectomy wounds: account of 160 consecutive cases. Ann Surg Oncol. 2008;15:355–363. doi: 10.1245/s10434-007-9672-5. [DOI] [PubMed] [Google Scholar]
- 43.Sheth DS, Yasko AW, Johnson ME, Ayala AG, Murray JA, Romsdahl MM. Chondrosarcoma of the pelvis: prognostic factors for 67 patients treated with definitive surgery. Cancer. 1996;78:745–750. doi: 10.1002/(SICI)1097-0142(19960815)78:4<745::AID-CNCR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Shin K-H, Rougraff BT, Simon MA. Oncologic outcomes of primary bone sarcomas of the pelvis. Clin Orthop Relat Res. 1994;304:207–217. [PubMed] [Google Scholar]
- 45.Windhager R, Karner J, Kutschera HP, Polterauer P, Salzer-Kuntschik M, Kotz R. Limb salvage in periacetabular sarcomas: review of 21 consecutive cases. Clin Orthop Relat Res. 1996;331:265–276. doi: 10.1097/00003086-199610000-00038. [DOI] [PubMed] [Google Scholar]
- 46.Wirbel RJ, Schulte M, Maier B, Mutschler WE. Megaprosthetic replacement of the pelvis: function in 17 cases. Acta Orthop Scand. 1999;70:348–352. doi: 10.3109/17453679908997823. [DOI] [PubMed] [Google Scholar]
- 47.Wirbel RJ, Schulte M, Mutschler WE. Surgical treatment of pelvic sarcomas: oncologic and functional outcome. Clin Orthop Relat Res. 2001;390:190–205. doi: 10.1097/00003086-200109000-00022. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida Y, Osaka S, Mankin HJ. Hemipelvic allograft reconstruction after periacetabular bone tumor resection. J Orthop Sci. 2000;5:198–204. doi: 10.1007/s007760050151. [DOI] [PubMed] [Google Scholar]
- 49.Yuen A, Ek ET, Choong PF. Research: is resection of tumours involving pelvic ring justified? A review of 49 consecutive cases. Int Semin Surg Oncol. 2005;2:9. doi: 10.1186/1477-7800-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeifang F, Buchner M, Zahlten-Hinguranage A, Bernd L, Sabo D. Complications following operative treatment of primary malignant bone tumours in the pelvis. Eur J Surg Oncol. 2004;30:893–899. doi: 10.1016/j.ejso.2004.05.023. [DOI] [PubMed] [Google Scholar]