Abstract
Background
Improper acetabular component orientation in THA has been associated with increased dislocation rates, component impingement, bearing surface wear, and a greater likelihood of revision. Therefore, any reasonable steps to improve acetabular component orientation should be considered and explored.
Questions/purposes
We therefore sought to compare THA with a robotic-assisted posterior approach with manual alignment techniques through a posterior approach, using a matched-pair controlled study design, to assess whether the use of the robot made it more likely for the acetabular cup to be positioned in the safe zones described by Lewinnek et al. and Callanan et al.
Methods
Between September 2008 and September 2012, 160 THAs were performed by the senior surgeon. Sixty-two patients (38.8%) underwent THA using a conventional posterior approach, 69 (43.1%) underwent robotic-assisted THA using the posterior approach, and 29 (18.1%) underwent radiographic-guided anterior-approach THAs. From September 2008 to June 2011, all patients were offered anterior or posterior approaches regardless of BMI and anatomy. Since introduction of the robot in June 2011, all THAs were performed using the robotic technique through the posterior approach, unless a patient specifically requested otherwise. The radiographic cup positioning of the robotic-assisted THAs was compared with a matched-pair control group of conventional THAs performed by the same surgeon through the same posterior approach. The safe zone (inclination, 30°–50°; anteversion, 5°–25°) described by Lewinnek et al. and the modified safe zone (inclination, 30°–45°; anteversion, 5°–25°) of Callanan et al. were used for cup placement assessment. Matching criteria were gender, age ± 5 years, and (BMI) ± 7 units. After exclusions, a total of 50 THAs were included in each group. Strong interobserver and intraobserver correlations were found for all radiographic measurements (r > 0.82; p < 0.001).
Results
One hundred percent (50/50) of the robotic-assisted THAs were within the safe zone described by Lewinnek et al. compared with 80% (40/50) of the conventional THAs (p = 0.001). Ninety-two percent (46/50) of robotic-assisted THAs were within the modified safe zone described by Callanan et al. compared with 62% (31/50) of conventional THAs p (p = 0.001). The odds ratios for an implanted cup out of the safe zones of Lewinnek et al. and Callanan et al. were zero and 0.142, respectively (95% CI, 0.044, 0.457).
Conclusions
Use of the robot allowed for improvement in placement of the cup in both safe zones, an important parameter that plays a significant role in long-term success of THA. However, whether the radiographic improvements we observed will translate into clinical benefits for patients—such as reductions in component impingement, acetabular wear, and prosthetic dislocations, or in terms of improved longevity—remains unproven.
Level of Evidence
Level III, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Improper implantation of the cup during THA has been associated with several complications, including dislocation [1, 4, 5, 21, 26, 28], component impingement [1, 33, 38, 40], leg length discrepancy [29], altered hip biomechanics [21], accelerated bearing surface wear [6, 11, 21, 25, 40], and revision surgery. To ascertain what constitutes good alignment, several safe zones for inclination and anteversion have been described, including those of Lewinnek et al. [26] and Callanan et al. [5].
To try to minimize the complications caused by cups placed outside those safe zones, several methods have been developed to improve the accuracy and consistency of placement of acetabular components in the correct three-dimensional (3-D) orientation. The conventional technique of using manually manipulated instrumentation remains the most widely used for implantation of the acetabular component. Multiple intraoperative anatomic landmarks have been described to guide placement of the acetabular cup into the safe zone. These landmarks include the transverse acetabular ligament [3, 20], the acetabular notch [12], the anterosuperior iliac spine with the sciatic notch [27], and alignment guides [14], to name a few.
Computerized technology has been introduced as high-technology instrumentation for THAs including image-assisted navigation, imageless navigation, and robotic-assisted computer navigation [18, 24, 31, 41]. The purported advantage of this technology is that the robot assists the user to follow the navigated plan for cup positioning, and the guided process could result in potentially more accurate reaming, keeping the reamer centered between the anterior and posterior acetabular walls.
We therefore determined whether use of the robot made it more likely for the acetabular component to be placed in the safe zones of Lewinnek et al. and Callanan et al., as compared with the conventional technique in THA.
Patients and Methods
Subjects
This study is a matched-pair controlled study using retrospectively collected data for THAs done between September 2008 and September 2012. Patients who underwent the posterior-approach THA by the senior surgeon (BGD), and who had proper postoperative supine AP radiographs of the pelvis and cross-table lateral radiographs of the hip were included in the study. Patients were excluded if they had missing postoperative radiographs or radiographs showing a rotated or tilted pelvis [34]. The robot was introduced in our practice in June 2011. Between September 2008 and June 2011, all patients were offered conventional posterior-approach THA or radiographic-guided anterior-approach THA, regardless of his or her BMI or anatomy. After introduction of the robot, between June 2011 and September 2012, all THAs were performed using the robotic-assisted technique through the posterior approach, unless a patient specifically requested otherwise.
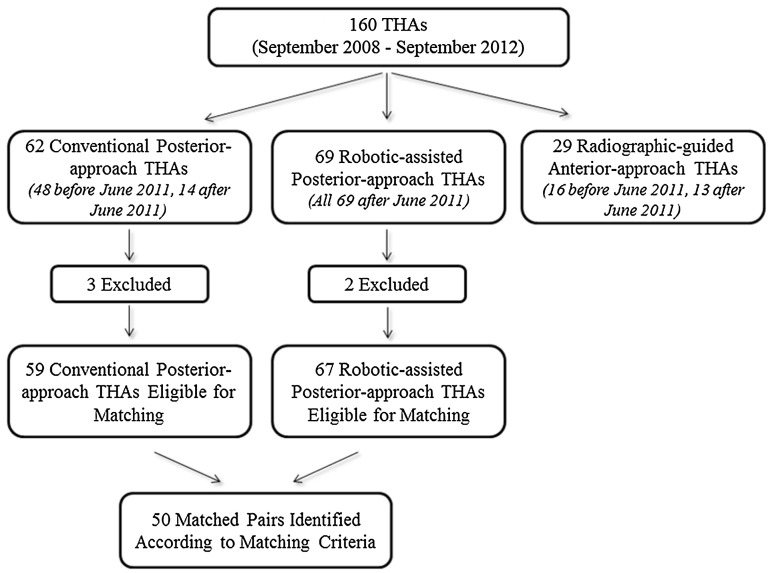
Sixty-seven patients (of 160; 41.9%) underwent robotic-assisted posterior-approach THAs (robotic THA) and 59 (of 160; 36.9%) underwent conventional posterior-approach THAs (conventional THA) who met the inclusion and exclusion criteria (Fig. 1). The matching process was done before collecting the radiographic measurements by one blinded observer (AYS) and was for sex, age ± 5 years, and BMI ± 7 units in that order. When more than one patient who had a conventional THA could be matched to one patient who had a robotic THA, the patient who had the conventional THA who was closest in terms of age (then BMI, in that order of priority) to a patient who had a robotic THA was chosen. Fifty patients who had robotic THAs were manually matched to a control group of 50 patients who had conventional THAs. No more than 50 patients could be matched owing to the small number of patients in each group, and the lack of matches for the remaining nine patients who had conventional THAs and 17 who had robotic THAs were within the matching criteria discussed previously. Investigational review board approval was obtained before initiation of this study.
Fig. 1.
The flow chart shows the total number of THAs performed during the study period and the numbers of THAs performed using each technique.
Surgical Techniques
Preoperative planning using plain radiographs to determine component position and sizes, level of the neck cut, and amount of leg lengthening or shortening needed was done for patients scheduled for THA. For the robotic THAs, we used the MAKOTM robotic hip system (MAKOplasty® total hip application; MAKOTM Surgical Corporation, Ft. Lauderdale, FL, USA), which is robotic-assisted computer navigation that uses the RIO® (Robotic Arm Interactive Orthopedic System) for reaming the acetabulum during bone preparation and cup placement. For the robotic THAs, CT scans of the involved hip and knee were obtained preoperatively for all patients. A 3-D patient-specific model of the pelvis and proximal femur was created by the robotic system that was used to guide performance of the THA. The robotic THA was performed with the patient in the lateral position using the standard mini-posterior approach. The system detected patient-specific landmarks intraoperatively to register the femur and acetabulum and help determine the position of the pelvis and proximal femur. This system used a haptic robotic arm that guided acetabular reaming and cup placement and provided the surgeon with feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the robotic software, and all measurements were done on the coronal (functional) plane of the body as described by Murray [30].
Conventional THAs were performed with the patient in the lateral position using the standard mini-posterior approach. Use of the mini-posterior approach does not limit our ability to place the cup in the desired inclination position. The acetabular cup position was assessed using an alignment guide, which provided an estimate of inclination and anteversion. This guide could be attached to the handle that is connected to the reamer and the handle that is connected to the final cup. The transverse acetabular ligament, anterior and posterior acetabular walls, and the sciatic notch were used in conjunction with this alignment guide to help assess the acetabular cup position.
The target inclination and anteversion angles in both groups were 40° and 20°, respectively.
Cup Implants and Operative Time
For conventional THAs, the acetabular cup implant used was the R3 cup (Smith & Nephew®, London, UK). For the robotic THAs, the acetabular cup implant used was the Restoris Trinity cup (Corin Group PLC®, Cirencester, UK). The senior surgeon (BGD) was familiar and comfortable performing the conventional THA through the posterior approach. After introduction of the robotic system, the senior author (BGD) switched to robotic THAs (unless a patient specifically requested otherwise), which necessitated switching to the Restoris Trinity cup (Corin Group PLC®). The total operating room time and surgical time were recorded for all patients in both groups.
Radiographic Measurements
The radiographic measurements were done using the Trauma-CadTM software (build number 2.2.535.0, 2012, Voyant Health®, Petach-Tikva, Israel). This software allows measurement of cup inclination and version on the AP view of the pelvis, with measurements done on the coronal plane of the pelvis [30, 36] (Fig. 2). The accuracy of this software in measuring parameters on radiographs has been reported [22, 35, 37]. This software allows for measurement of version but does not specify whether the cup is anteverted or retroverted. To overcome this limitation, the cross-table lateral radiographs of all patients were reviewed, and all patients were found to have anteverted cups using the Woo and Morrey technique [39].
Fig. 2.
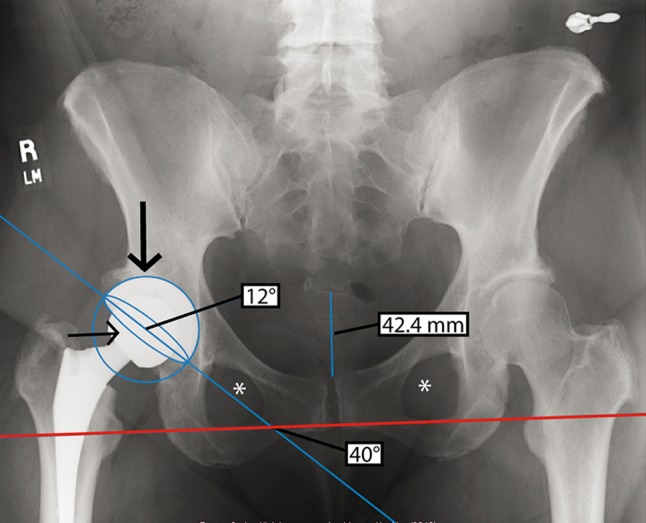
An AP radiograph shows the radiographic measurements of inclination and anteversion angles in a female patient. The coccyx is in line with the symphysis pubis and the obturator foramina are symmetric (asterisks). The distance from the sacrococcygeal junction to the top of the symphysis pubis is 42.4 mm. The angle formed by the intersection of the interobturator reference line (red) and the bisecting line (blue) is the inclination angle (40°). The concentric circle (large arrow) and ellipse (small arrow) measure the anteversion angle (12°).
The radiographic measurements were performed by two different observers (YFE and IBB) who were blinded to which surgery was performed (robotic THA versus conventional THA) and from each other’s results. Measurements of inclination and anteversion angles for both groups were done twice by each observer with both measurements done 2 months apart. There were 16 interobserver and intraobserver correlation measures in both groups, showing strong correlations for all measurements (r > 0.82 and p < 0.001 in all).
We compared age, sex, BMI, side, operating room time, surgical time, and inclination and anteversion angles between patients in both groups (Table 1).
Table 1.
Demographics, cup angles, and operation times
| Demographic | Robotic THA | Conventional THA | p value |
|---|---|---|---|
| Mean ± SD (95% CI) | Mean ± SD (95% CI) | ||
| Age (years) | 56.8 ± 7.9 (54.6–59) | 56.7 ± 8.1 (54.5–59) | 0.942 |
| BMI (kg/m2) | 28.3 ± 3.9 (27.2–29.3) | 28.7 ± 5.0 (27.3–30.1) | 0.632 |
| Cup inclination (degrees) | 40.0 ± 3.2 (39.1–40.8) | 42.6 ± 5.4 (41.1–44.1) | 0.004 |
| Cup anteversion (degrees) | 16.7 ± 3.0 (15.9–17.6) | 13.3 ± 7.0 (11.3–15.3) | 0.002 |
| Operating room time (minutes) | 162.3 ± 28.7 (154.3–170.2) | 158.8 ± 22.5 (152.5–165) | 0.496 |
| Surgical time (minutes) | 109.8 ± 25.5 (102.7–116.8) | 101.5 ± 21.6 (95.5–107.5) | 0.084 |
| Sex | |||
| Male | 19 | 19 | 1.000 |
| Female | 31 | 31 | |
| Side | |||
| Right | 26 | 28 | 0.841 |
| Left | 24 | 22 | |
Statistical Analysis
The average cup inclination and anteversion angles were calculated for the robotic and conventional THA groups from the observers’ measurements along with standard deviation (SD) and 95% CI measurements for each group. Calculation of the number of hips that were in the safe zones of Lewinnek et al. (inclination, 30°–50°; anteversion, 5°–25°) [26] and Callanan et al. (inclination, 30°–45°; anteversion, 5°–25°) [5] regarding inclination, anteversion, and a combination of both were done for both groups.
Statistical analysis was done for interobserver and intraobserver reliabilities of the inclination and anteversion measurements in both groups using the Pearson correlation coefficient test. Independent t-tests were performed to compare both groups for age, BMI, operating room time, surgical time, and inclination and anteversion angles. Fisher’s exact test was used to compare both groups regarding the number of hips in the safe zones of Lewinnek et al. and Callanan et al., and to compare both groups for sex and surgical side. Odds ratios and 95% CI were reported for cups placed outside the safe zones.
Results
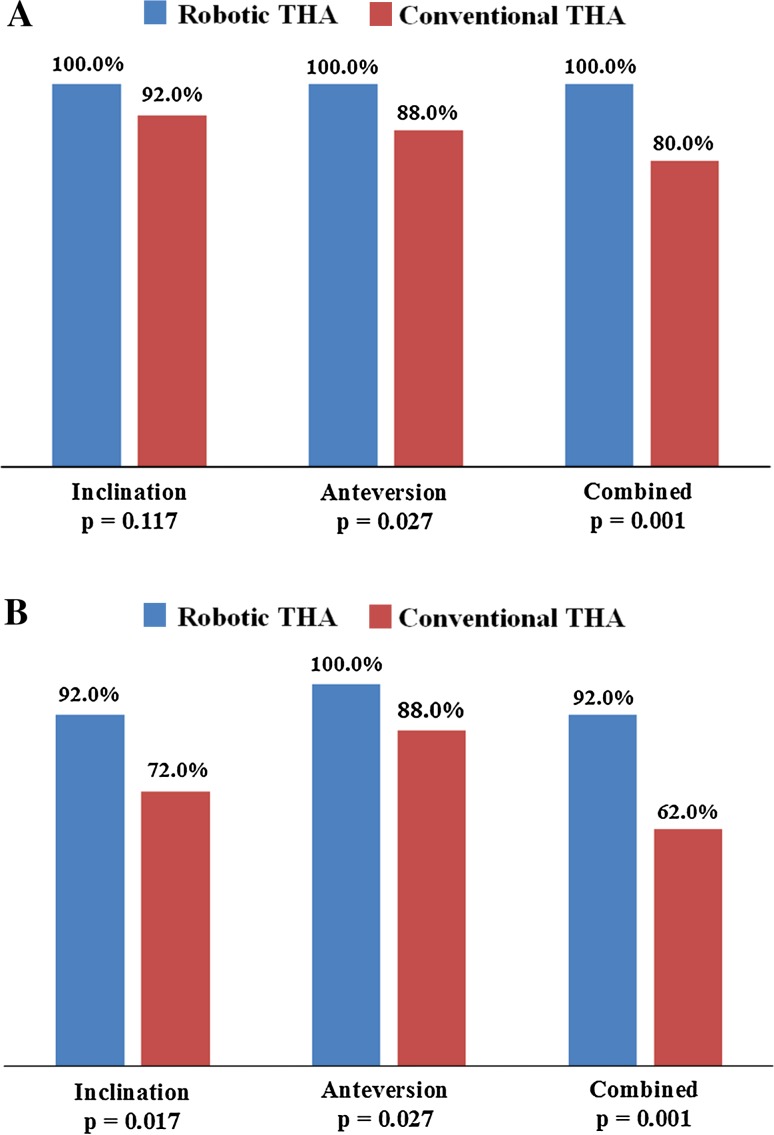
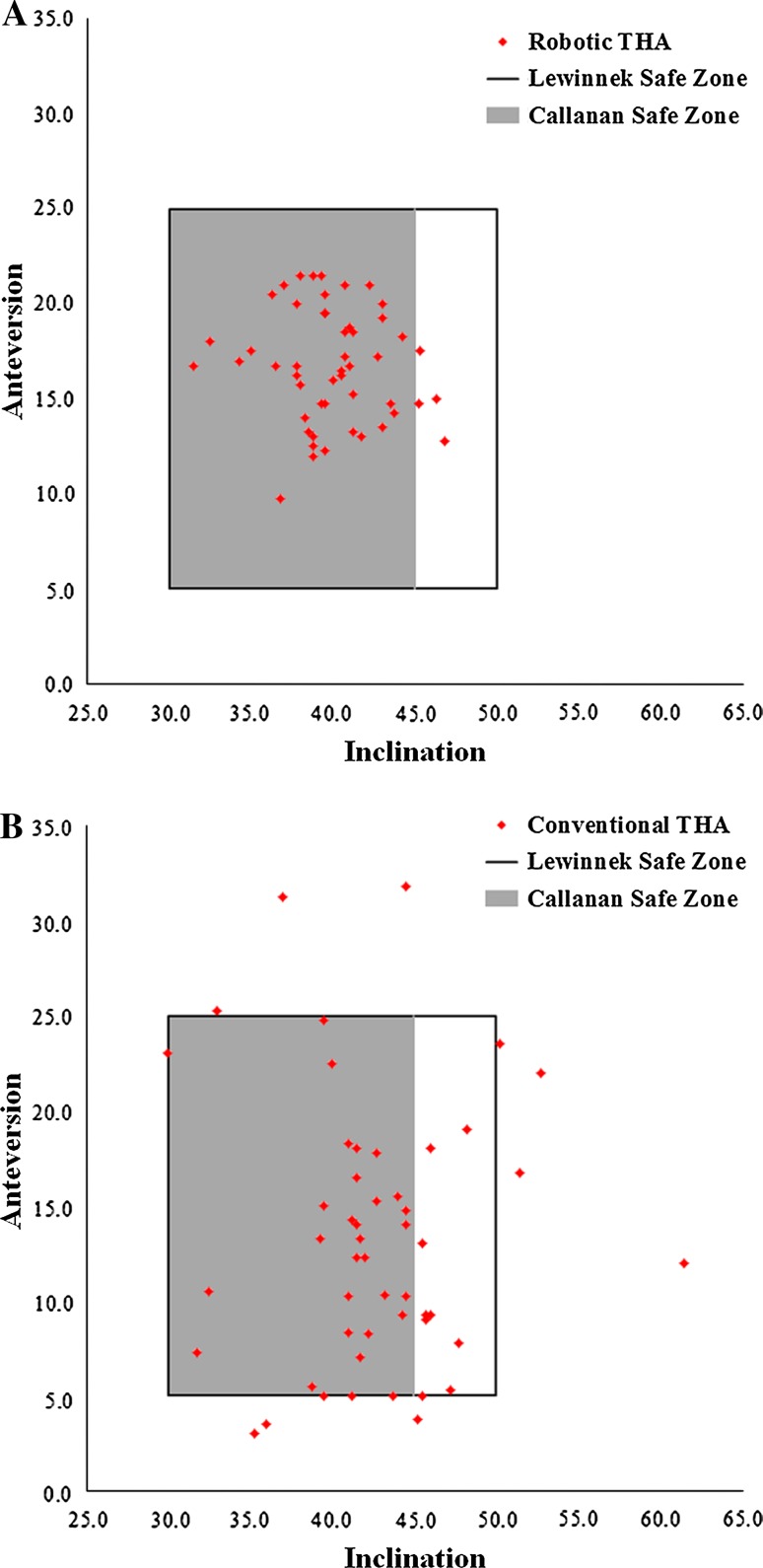
Robotically placed cups were more likely to be in both safe zones compared with conventionally placed cups. One hundred percent (50/50) of cups in the robotic THA group were in the safe zone of Lewinnek et al. [26], compared with 80% (40/50) of cups in the conventional THA group (p = 0.001) (Fig. 3A). Ninety-two percent (46/50) of cups in the robotic THA group were in the safe zone of Callanan et al. [5], compared with 62% (31/50) of cups in the conventional THA group (p = 0.001) (Figs. 3B, 4). The odds ratios for the implanted cup that was outside the safe zones of Lewinnek et al. and Callanan et al. were zero and 0.142, respectively (95% CI, 0.044, 0.457).
Fig. 3A–B.
The clustered column charts show the percentages of robotic-assisted cups and conventional cups in the safe zones of (A) Lewinnek et al. and (B) Callanan et al.
Fig. 4A–B.
Scatterplots of the (A) robotic-assisted and (B) conventional cups in the safe zones of Lewinnek et al. and Callanan et al. are shown.
There was only one intraoperative technical complication related to the robotic system, in which the senior surgeon judged the cup placement guidance by the robotic system to be outside the safe zone based on the intraoperative bony landmarks, the transverse acetabular ligament, and the patient’s lateral position. He repositioned the cup using the conventional technique, and the postoperative measurements of that cup were within the safe zones of Lewinnek et al. and Callanan et al. There were no intraoperative technical complications in the conventional THA group.
Discussion
Acetabular cup positioning in THA is critical to ensure stability of the prosthetic hip and longevity of the implant. Component malposition has been associated in numerous reports of complications, including impingement, dislocation, accelerated wear, and revision surgery [1, 4–6, 11, 21, 25, 26, 28, 29, 33, 38, 40]. Some studies have offered recommended cup orientation ranges, most suggesting 30° to 50° inclination [5, 25, 26, 38] and 0° to 30° anteversion [5, 23, 26, 38]. The safe zone established by Lewinnek et al. [26] is the most widely used range of acceptable angles with inclination of 30° to 50° and anteversion of 5° to 25°, and was used in our study to compare our results with the most common safe zone reported in previous publications. Callanan et al. suggested a modified safe zone with inclination of 30° to 45° and anteversion of 5° to 25° [5]. They suggested a lower upper limit of inclination (45° instead of 50° suggested by Lewinnek et al.) based on the study by Leslie et al. [25] that showed increased wear and edge loading in THAs with a hard-on-hard bearing surface with an abduction angle greater than 45°. We used the safe zone suggested by Callanan et al. because several reports have suggested that steeper cups increase polyethylene and metal wear, and in our opinion, reducing the inclination safe zone to 30° to 45° would accommodate evidence in the literature [8, 25, 38]. We therefore sought to determine the proportion of cups placed in the safe zones using robotic THA and conventional THA, and we compared acetabular component accuracy between the two systems.
The major limitation of our study was the lack of clinical data at short- or long-term. The system adds expense, and we will not know whether that expense is justified until studies show improvements that patients can perceive, such as reduced dislocation rates or a lower likelihood of revision. The robotic system was a capital expenditure by the hospital, and its cost is unknown to us. However, there was no additional cost to the patients or patients’ insurance companies. All our radiographic measurements were done on the coronal plane of the pelvis as described by Murray [30] and were compared with the safe zone described by Lewinnek et al. [26]. However, Lewinnek et al. defined the safe zone for cup placement with measurements done on the anterior pelvic plane of the pelvis and not the coronal plane. Despite this limitation, their safe zone has been widely used even in the radiographic coronal plane [7, 13, 17, 18]. Combined anteversion (cup anteversion + stem anteversion) has been described as being critical for stability in THA with the optimal range of 25° to 45° being the accepted safe zone [9]. We used different types of acetabular components in each group because of introduction of the robotic system to our practice and the compatibility of this system with certain implant types. Our small sample of patients in each group (50 in each) added to the limitations of this study. Another limitation was that we assessed acetabular cup position without taking into account femoral anteversion. Femoral component anteversion measurement is possible on CT scans that involve the hip and the knee simultaneously, which would add radiation and cost to the patients. The dose of radiation from the CT scan per patient in this study was 60 mGy, and was consistent and standardized throughout all the cases. The senior surgeon (BGD) is an experienced high-volume consultant for the robotic company, and results in this study may not apply to lower-volume or less-experienced surgeons. Use of the robotic technology needs to be validated in future multiple-surgeon independent series. Finally, some selection bias might have been part of patient selection, especially after introduction of the robot.
The use of robotic-assisted THA provided good accuracy and reproducibility in placing the cup in the safe zones in our patients. Similar studies comparing computer-assisted THA with conventional THA have shown greater accuracy in cup placement in the safe zone. Hohmann et al. [15] compared imageless navigation with manual implantation of acetabular cups using the direct lateral approach, and measured cup angles postoperatively on CT scans. Of cups in the navigation group, 76.7% (23/30) were placed in the safe zone of Lewinnek et al. compared with 20% (6/30) using the manual technique (p = 0.01) [15]. Parratte and Argenson [31] compared cup positioning using imageless computer-assisted navigation with freehand cup placement, using the supine anterolateral approach. Computer navigation provided greater accuracy in placing the cup in the safe zone of Lewinnek et al. with 20% (6/30) outliers compared with 57% (17/30) outliers in the freehand group (p = 0.002) [31]. Kalteis et al. [19] compared conventional alignment guides with imageless navigation in cup placement using the supine anterolateral approach. Eleven of 22 cups in the conventional group were placed outside the safe zone of Lewinnek et al. compared with three of 23 in the navigation group (p = 0.003) [19].
Callanan et al. reported on acetabular cup positioning performed by several experienced surgeons during a 5-year period [5]. Using the conventional THA technique, they reported 47% of cups were in their modified safe zone [5]. Our results were superior using the conventional (62%) and robotic techniques (92%) in placing the cup in their modified safe zone. Determining the 3-D position of the pelvis intraoperatively is challenging [7, 13, 14, 16, 27]. Pelvic tilt, obesity, and hip flexion contracture play a significant role in judging the position of the pelvis and subsequently placement of the cup [5, 13, 14, 41]. Alignment jigs and bony and soft tissue landmarks have been used for intraoperative orientation with varying degrees of accuracy and reproducibility [2, 3, 10, 14, 20, 32]. The introduction of computer-assisted surgery for THA has provided a useful tool for orthopaedic surgeons to improve accuracy in placing the cup in the safe zone and to prevent long-term adverse outcomes. Despite the improved accuracy using navigation, additional cost, operating room time, and duration of surgery have limited widespread acceptance of computer-assisted systems [7, 18].
Robotic-assisted THA was consistent in placing the acetabular cup in the safe zones of Lewinnek et al. and Callanan et al. with minimal intraoperative technical complications. However, whether the radiographic improvements we observed will translate into clinical benefits for patients, such as reductions in component impingement, acetabular wear, and prosthetic dislocations, or in terms of improved longevity, remains unproven. Further studies are needed to investigate the short- and long-term clinical outcomes, possible long-term complications, and cost-effectiveness of robotic-assisted THA.
Acknowledgments
We thank Zachary Finley BA, Ryan Baise BS, Jennifer C. Stone MA, and Anthony P. Trenga BA for assistance with data collection and analysis and literature review.
Footnotes
The institution of one or more of the authors (BGD) has received, during the study period, funding from Mako Corporation (Fort Lauderdale, FL, USA) in an amount less than USD 10,000. The institution of one author (BGD) has received, during the study period, funding from Arthrex Inc (Naples, FL, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the American Hip Institute, Westmont, IL, USA.
References
- 1.Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63:214–218. doi: 10.1302/0301-620X.63B2.7217144. [DOI] [PubMed] [Google Scholar]
- 2.Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88:883–886. doi: 10.1302/0301-620X.88B7.17577. [DOI] [PubMed] [Google Scholar]
- 3.Beverland D. The transverse acetabular ligament: optimizing version. Orthopedics. 2010;33:631. doi: 10.3928/01477447-20100722-22. [DOI] [PubMed] [Google Scholar]
- 4.Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87:762–769. doi: 10.1302/0301-620X.87B6.14745. [DOI] [PubMed] [Google Scholar]
- 5.Callanan MC, Jarrett B, Bragdon CR, Zurakowski D, Rubash HE, Freiberg AA, Malchau H. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res. 2011;469:319–329. doi: 10.1007/s11999-010-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 7.Digioia AM, 3rd, Jaramaz B, Plakseychuk AY, Moody JE, Jr, Nikou C, Labarca RS, Levison TJ, Picard F. Comparison of a mechanical acetabular alignment guide with computer placement of the socket. J Arthroplasty. 2002;17:359–364. doi: 10.1054/arth.2002.30411. [DOI] [PubMed] [Google Scholar]
- 8.Dorr LD. Acetabular cup position: the imperative of getting it right. Orthopedics. 2008;31:898–899. doi: 10.3928/01477447-20080901-10. [DOI] [PubMed] [Google Scholar]
- 9.Dorr LD, Malik A, Dastane M, Wan Z. Combined anteversion technique for total hip arthroplasty. Clin Orthop Relat Res. 2009;467:119–127. doi: 10.1007/s11999-008-0598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein NJ, Woolson ST, Giori NJ. Acetabular component positioning using the transverse acetabular ligament: can you find it and does it help? Clin Orthop Relat Res. 2011;469:412–416. doi: 10.1007/s11999-010-1523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34:19–26. doi: 10.1007/s00264-009-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha YC, Yoo JJ, Lee YK, Kim JY, Koo KH. Acetabular component positioning using anatomic landmarks of the acetabulum. Clin Orthop Relat Res. 2012;470:3515–3523. doi: 10.1007/s11999-012-2460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22:151–159. doi: 10.1016/j.arth.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Hassan DM, Johnston GH, Dust WN, Watson G, Dolovich AT. Accuracy of intraoperative assessment of acetabular prosthesis placement. J Arthroplasty. 1998;13:80–84. doi: 10.1016/S0883-5403(98)90079-1. [DOI] [PubMed] [Google Scholar]
- 15.Hohmann E, Bryant A, Tetsworth K. A comparison between imageless navigated and manual freehand technique acetabular cup placement in total hip arthroplasty. J Arthroplasty. 2011;26:1078–1082. doi: 10.1016/j.arth.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Jaramaz B, DiGioia AM, 3rd, Blackwell M, Nikou C. Computer assisted measurement of cup placement in total hip replacement. Clin Orthop Relat Res. 1998;354:70–81. doi: 10.1097/00003086-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop Relat Res. 2004;426:174–179. doi: 10.1097/01.blo.0000141903.08075.83. [DOI] [PubMed] [Google Scholar]
- 18.Kalteis T, Handel M, Bathis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT-based navigation? J Bone Joint Surg Br. 2006;88:163–167. doi: 10.1302/0301-620X.88B2.17163. [DOI] [PubMed] [Google Scholar]
- 19.Kalteis T, Handel M, Herold T, Perlick L, Baethis H, Grifka J. Greater accuracy in positioning of the acetabular cup by using an image-free navigation system. Int Orthop. 2005;29:272–276. doi: 10.1007/s00264-005-0671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalteis T, Sendtner E, Beverland D, Archbold PA, Hube R, Schuster T, Renkawitz T, Grifka J. The role of the transverse acetabular ligament for acetabular component orientation in total hip replacement: an analysis of acetabular component position and range of movement using navigation software. J Bone Joint Surg Br. 2011;93:1021–1026. doi: 10.1302/0301-620X.93B8.25720. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy JG, Rogers WB, Soffe KE, Sullivan RJ, Griffen DG, Sheehan LJ. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530–534. doi: 10.1016/S0883-5403(98)90052-3. [DOI] [PubMed] [Google Scholar]
- 22.Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32:815. doi: 10.3928/01477447-20090922-08. [DOI] [PubMed] [Google Scholar]
- 23.Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14:509–513. doi: 10.1016/S0883-5403(99)90110-9. [DOI] [PubMed] [Google Scholar]
- 24.Leenders T, Vandevelde D, Mahieu G, Nuyts R. Reduction in variability of acetabular cup abduction using computer assisted surgery: a prospective and randomized study. Comput Aided Surg. 2002;7:99–106. doi: 10.3109/10929080209146021. [DOI] [PubMed] [Google Scholar]
- 25.Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop Relat Res. 2009;467:2259–2265. doi: 10.1007/s11999-009-0830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 27.McCollum DE, Gray WJ. Dislocation after total hip arthroplasty: causes and prevention. Clin Orthop Relat Res. 1990;261:159–170. [PubMed] [Google Scholar]
- 28.Morrey BF. Difficult complications after hip joint replacement: dislocation. Clin Orthop Relat Res. 1997;344:179–187. [PubMed] [Google Scholar]
- 29.Murphy SB, Ecker TM. Evaluation of a new leg length measurement algorithm in hip arthroplasty. Clin Orthop Relat Res. 2007;463:85–89. doi: 10.1097/BLO.0b013e318126c08f. [DOI] [PubMed] [Google Scholar]
- 30.Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75:228–232. doi: 10.1302/0301-620X.75B2.8444942. [DOI] [PubMed] [Google Scholar]
- 31.Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty: a prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89:494–499. doi: 10.2106/JBJS.F.00529. [DOI] [PubMed] [Google Scholar]
- 32.Saxler G, Marx A, Vandevelde D, Langlotz U, Tannast M, Wiese M, Michaelis U, Kemper G, Grutzner PA, Steffen R, von Knoch M, Holland-Letz T, Bernsmann K. The accuracy of free-hand cup positioning: a CT based measurement of cup placement in 105 total hip arthroplasties. Int Orthop. 2004;28:198–201. doi: 10.1007/s00264-004-0542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shon WY, Baldini T, Peterson MG, Wright TM, Salvati EA. Impingement in total hip arthroplasty a study of retrieved acetabular components. J Arthroplasty. 2005;20:427–435. doi: 10.1016/j.arth.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 34.Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res. 2003;407:241–248. doi: 10.1097/00003086-200302000-00033. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130:1429–1432. doi: 10.1007/s00402-010-1046-y. [DOI] [PubMed] [Google Scholar]
- 36.Wan Z, Malik A, Jaramaz B, Chao L, Dorr LD. Imaging and navigation measurement of acetabular component position in THA. Clin Orthop Relat Res. 2009;467:32–42. doi: 10.1007/s11999-008-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8. doi: 10.1186/1749-799X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22:815–821. doi: 10.1016/j.orthres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64:1295–1306. [PubMed] [Google Scholar]
- 40.Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305–313. doi: 10.1016/S0883-5403(00)90601-6. [DOI] [PubMed] [Google Scholar]
- 41.Ybinger T, Kumpan W, Hoffart HE, Muschalik B, Bullmann W, Zweymuller K. Accuracy of navigation-assisted acetabular component positioning studied by computed tomography measurements: methods and results. J Arthroplasty. 2007;22:812–817. doi: 10.1016/j.arth.2006.10.001. [DOI] [PubMed] [Google Scholar]