Abstract
Background
In response to increasing antibiotic resistance, vancomycin has been proposed as an alternative prophylactic agent in TKA. However, vancomycin requires a prolonged administration time, risks promoting further antibiotic resistance, and can cause systemic toxicity. Intraosseous regional administration (IORA) is known to achieve markedly higher antibiotic concentrations than systemic administration and may allow the use of a lower vancomycin dose.
Questions/purposes
We assessed whether low-dose IORA vancomycin can achieve tissue concentrations equal or superior to those of systemic administration in TKA and compared complications between patients treated with IORA and intravenous vancomycin.
Methods
We randomized 30 patients undergoing primary TKA to receive 250 or 500 mg vancomycin via IORA or 1 g via systemic administration. IORA was performed as a bolus injection into a tibial intraosseous cannula below an inflated thigh tourniquet immediately before skin incision. Subcutaneous fat and bone samples were taken during the procedure and antibiotic concentrations measured.
Results
The overall mean tissue concentration of vancomycin in subcutaneous fat was 14 μg/g in the 250-mg IORA group, 44 μg/g in the 500-mg IORA group, and 3.2 μg/g in the systemic group. Mean concentrations in bone were 16 μg/g in the 250-mg IORA group, 38 μg/g in the 500-mg IORA group, and 4.0 μg/g in the systemic group. One patient in the systemic group developed red man syndrome during infusion.
Conclusions
Low-dose IORA vancomycin results in tissue concentrations equal or superior to those of systemic administration. IORA optimizes timing of vancomycin administration, and the lower dose may reduce the risk of systemic side effects while providing equal or enhanced prophylaxis in TKA.
Level of Evidence
Level I, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-013-3038-z) contains supplementary material, which is available to authorized users.
Introduction
Prophylactic antibiotics dramatically reduce infection rates after arthroplasty. Randomized trials during the 1970s reported deep infection rates of 1% to 2% when prophylactic cephalosporins were given, compared to 7% to 15% with a placebo [5, 11, 18].
However, due to increasing antibiotic resistance in recent decades, cephalosporins may no longer provide adequate prophylaxis [40]. To be effective, prophylactic antibiotics require a spectrum of activity that covers the organisms likely to cause contamination during the procedure [3]. In TKA, the two most common organisms causing infection are Staphylococcus aureus and coagulase-negative staphylococci (CoNS) [12, 27]. Currently, 60% to 90% of CoNS isolates are resistant to cephalosporins [27, 40], and 33% to 56% of S aureus isolates from infected joint arthroplasties are methicillin resistant (MRSA) [23, 26]. Data from the National Nosocomial Infection Surveillance System reported, between 1992 and 2003, the rate of methicillin resistance in S aureus infections rose from 35.9% to 64.4%, an increase of 3.1% per year [21].
Despite increasing methicillin resistance, the vast majority of MRSA and CoNS remain sensitive to vancomycin [27], leading many to propose it as an alternative prophylactic agent in TKA [1, 8, 30, 39]. However, vancomycin has a number of disadvantages. Firstly, it requires a prolonged intravenous administration time, as rapid infusion can cause red man syndrome, consisting of a pruritic, erythematous rash related to histamine release [25]. A typical prophylactic dose of 1 g requires the infusion to be started a minimum of 1 hour before surgery, and failure to achieve this may lead to underdosing [1]. In a review of 18,342 arthroplasty procedures, vancomycin was given with appropriate timing in only 22% of cases, compared to 77% of cases given a cephalosporin [1]. Secondly, widespread use of vancomycin risks promoting further antibiotic resistance [17]. Finally, vancomycin can also cause renal and other systemic toxicity [25].
We previously validated intraosseous regional administration (IORA) of prophylactic cefazolin in TKA [41] and recorded markedly higher tissue concentrations of antibiotic than were achievable with systemic administration. IORA may allow lower vancomycin doses, thereby reducing systemic toxicity and avoiding the difficulties associated with prolonged preoperative infusion times. We hypothesized lower doses of vancomycin via IORA could still achieve tissue concentrations equal or superior to those of systemic administration before TKA. We also compared complications between patients treated with IORA and intravenous vancomycin.
Patients and Methods
Patients undergoing primary TKA at a single institution were eligible for enrollment into this prospective, randomized controlled trial. Inclusion criteria were age of less than 90 years and a primary diagnosis of osteoarthritis. Exclusion criteria were previous compartment syndrome, allergy to an antibiotic used in the study, abnormal cardiac or renal function, or concurrent nephrotoxic medications. From November 2011 to February 2012, 35 patients undergoing primary TKA for osteoarthritis were assessed for enrollment. Three patients were excluded (two patients with significant cardiac dysfunction [aortic stenosis, congestive heart failure], one patient who refused consent), leaving 32 patients who were randomized into three groups using computer-generated random allocations placed in numbered, opaque, sealed envelopes (Table 1). Patients were randomized in the preoperative area to allow appropriate setup in the operative room. Two patients were excluded postrandomization due to technical errors, one patient was given an incorrect dose of systemic vancomycin, and for one patient the intraosseous injection equipment was unavailable, leaving 30 patients available for analysis.
Table 1.
Patient demographics
| Variable | 250-mg IORA group (n = 10) | 500-mg IORA group (n = 10) | 1-g systemic group (n = 10) |
|---|---|---|---|
| Number of males | 5 | 4 | 1 |
| Number of females | 5 | 6 | 9 |
| Age (years)* | 70.8 (49–89) | 71.7 (55–85) | 71.4 (53–83) |
| BMI* | 32.2 (26–36.7) | 30.0 (22–38) | 34.8 (27–51) |
| Tourniquet time (minutes)* | 105 (88–135) | 102 (82–130) | 99 (74–116) |
| Procedure length (minutes skin to skin)* | 101 (85–130) | 97 (78–128) | 97 (74–114) |
| ASA score (points) | 2.7 | 2.2 | 2.7 |
* Values are expressed as mean, with range in parentheses; IORA = intraosseous regional administration; ASA = American Society of Anesthesiologists.
Data from a previous randomized trial comparing IORA versus systemic administration of cefazolin [41] showed mean (± SD) tissue concentrations of cefazolin in subcutaneous fat at different collection intervals ranged from 175.3 (± 110) to 206.3 (± 127) μg/g in the IORA group and from 7.2 (± 4.3) to 12.8 (± 6.6) μg/g in the systemic group. The mean tissue concentration in bone ranged from 75.4 (± 74.2) to 165.6 (± 216.1) μg/g in the IORA group and from 9.2 (± 2.6) to 14.1 (± 8.2) μg/g in the systemic group. Thus, the concentration of cefazolin was approximately 10 times higher using IORA than systemic administration. Using these data, a priori power analysis calculated 10 patients in each arm would provide greater than 80% statistical power to detect the expected fold difference in subcutaneous fat and bone concentrations among the three groups at the 5% significance level when IORA doses that were 25% (250 mg) and 50% (500 mg) of the systemic dose (1 g) were used. While data on pharmacodynamics of vancomycin for prophylaxis are lacking, in treatment models of infection, the area under the concentration-time curve divided by the minimum inhibitory concentration (MIC) is the pharmacokinetic-pharmacodynamic parameter most predictive of efficacy. Therefore, further increases in tissue vancomycin concentrations are likely to enhance the effectiveness of prophylaxis, particularly in organisms with MICs of 1 μg/L or more such as MRSA and CoNS [8]; this suggests the differences used in our power analysis are clinically relevant.
As the study was investigating a new technique, all patients received standard prophylaxis with 1 g systemic cefazolin between 10 and 30 minutes before tourniquet inflation regardless of randomization. All patients underwent limb exsanguination and tourniquet inflation to 250 mm Hg before routine preparation and draping. The tourniquet remained inflated for the entire procedure. TKA was performed using an imageless computer navigation system (Stryker Orthopaedics, Mahwah, NJ, USA).
The first group (250-mg IORA) received 250 mg vancomycin in 200 mL normal saline via IORA using an EZ-IO (Vidacare Corp, San Antonio, TX, USA; FDA-approved) intraosseous cannula placed into the medial aspect of the proximal tibia approximately at the level of the tibial tubercle (Fig. 1), after draping and before skin incision (Video 1; supplemental materials are available with the online version of CORR®). The injection was administered as a bolus immediately after tourniquet inflation, and surgical incision occurred immediately (< 1 minute) after this. The second group (500-mg IORA) received 500 mg vancomycin according to the same protocol, which has been previously described [41]. The third group (1-g systemic) was given 1 g vancomycin systemically through a forearm vein over a 1-hour infusion, beginning 60 to 120 minutes before surgery.
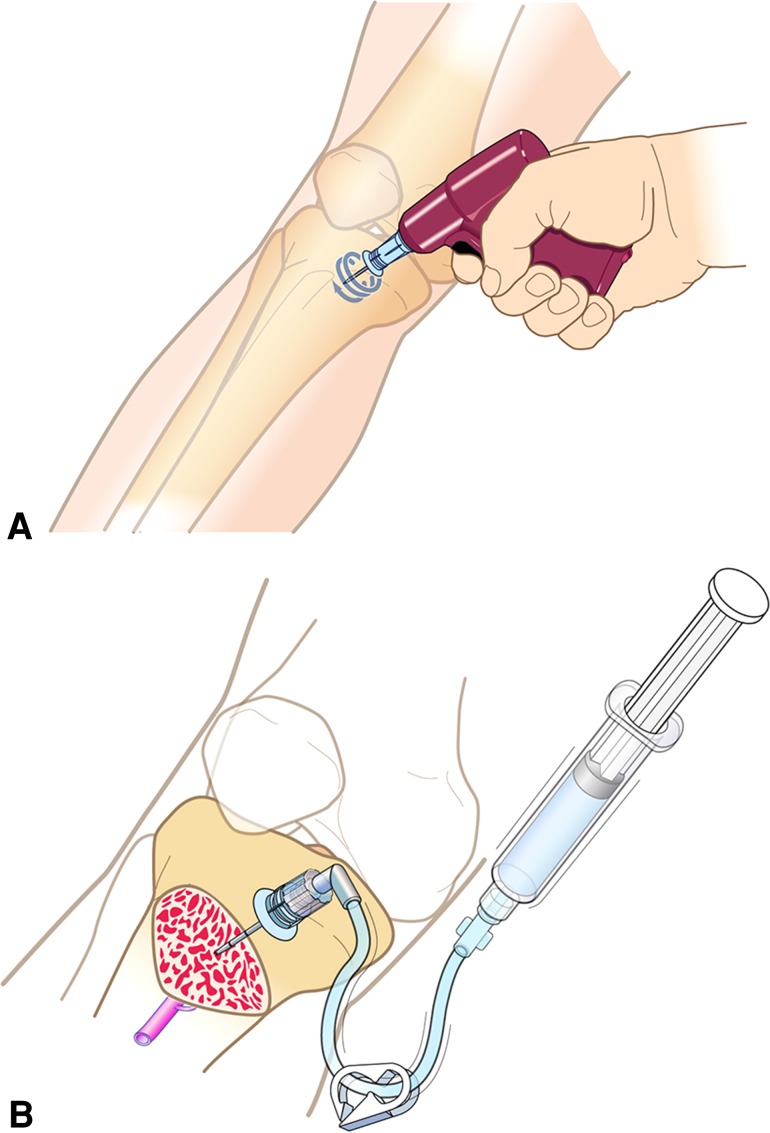
Fig. 1A–B.
Images show (A) insertion of the intraosseous needle using a sterilized driver and (B) the needle in situ allowing injection of the antibiotic, occurring after tourniquet inflation and before skin incision. Reprinted with permission of Vidacare Corp (www.vidacare.com).
Surgery was performed under sedation with combined spinal and epidural anesthesia in 27 patients, spinal anesthesia with a femoral nerve block in two patients, and femoral nerve block alone in one patient. Patients were monitored for clinical signs of red man syndrome throughout the procedure and in particular after tourniquet deflation, and an antihistamine was available for use if required.
Samples of subcutaneous fat and femoral cancellous bone (approximately 0.5 cm3) were taken at four points during the procedure. The first subcutaneous fat sample was taken immediately after skin incision, and subsequently both bone and fat samples were taken at the time of the distal femoral cut, at the time of trialing components, and immediately before closure. Bone samples were taken from the distal femur using a curette. Collection times were recorded for each sample (Table 2). In addition, systemic blood samples were taken at the time of the final tissue sample (while the tourniquet was inflated), then at 1, 4, and 8 hours postdeflation and the morning after the procedure. In previous animal studies of IORA vancomycin, peak systemic concentration occurred 60 to 70 minutes after tourniquet deflation [31].
Table 2.
Tissue concentrations of vancomycin at each sample point
| Sample | 250-mg IORA group | 500-mg IORA group | 1-g systemic group | |||
|---|---|---|---|---|---|---|
| Time (minutes)* | Concentration (μg/g)† | Time (minutes)* | Concentration (μg/g)† | Time (minutes)* | Concentration (μg/g)† | |
| Subcutaneous fat sample | ||||||
| 1 | 0.3 (0.6) | 19.4 (11.7) | 0.1 (0.3) | 50.4 (36) | 0.1 (0.3) | 2.7 (1.0) |
| 2 | 27 (9.0) | 17.0 (12.0) | 24 (6.3) | 52.3 (67) | 24 (7.0) | 4.4 (2.0) |
| 3 | 52 (16.8) | 11.4 (9.1) | 51 (6.9) | 32.0 (18.1) | 54 (10.3) | 3.2 (1.4) |
| 4 | 80 (19.7) | 8.1 (5.6) | 83 (16.7) | 41.1 (36.5) | 81 (11.1) | 2.4 (1.5) |
| Bone sample | ||||||
| 1 | 27 (9.0) | 11.6 (7.9) | 24 (6.3) | 20.7 (23.9) | 24 (7.0) | 3.3 (2.4) |
| 2 | 52 (16.8) | 19.2 (10.2) | 51 (6.9) | 44.0 (66) | 54 (10.3) | 5.3 (2.7) |
| 3 | 80 (19.7) | 18.1 (11.0) | 83 (16.7) | 50.0 (54.1) | 81 (11.1) | 3.5 (2.1) |
Values are expressed as mean, with SD in parentheses; * times are given as minutes after surgical incision; †differences in mean tissue concentrations among the three groups were statistically significant (p < 0.001) for all comparison points after adjustment by sex, age, and time from incision; IORA = intraosseous regional administration.
Tissue samples were rinsed in saline to remove excess blood and stored at −90° C until analyzed. Vancomycin concentrations were analyzed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Each bone sample was crushed with pliers, finely cut further with a scalpel into small particles, weighed, and immersed in phosphate-buffered saline pH 7.3 (ratio of bone to phosphate-buffered saline pH 7.3 was 1:5, w/v) at 4° C overnight to extract vancomycin from the bone. Each fat sample was finely minced with a scalpel, weighed, and treated in the same way as the bone samples. The immersed tissue suspensions were vortexed and centrifuged to precipitate tissue particles. Fifty microliters supernatant was transferred to a 1.5-mL plastic centrifuge tube and 25 μL internal standard (0.25 μg/mL aminopterin) was added. The mixture was then vortexed and 200 μL methanol was added to precipitate the proteins. After centrifugation at 15,000g for 5 minutes, a 50-μL aliquot of clear supernatant was mixed with 500 mL water and transferred to the autosampler 96-well plate. A volume of 10 μL was injected into the LC-MS/MS system. Vancomycin and the internal standard, aminopterin, were resolved on a Luna® C18(2) 5-μm, 50- × 2.0-mm internal diameter column (Phenomenex, Inc, Torrance, CA, USA) using a gradient elution of 0.05% formic acid and methanol. The two compounds were detected using electrospray ionization in the positive mode. The optimized precursor-to-product ion transitions monitored for vancomycin [M + 2H]2+ and aminopterin [M + H]+ were m/z 725.6 → 144.2 and m/z 441.2 → 294.2, respectively. The vancomycin and the internal standard peaks were free of interference from endogenous substances present in blank bone and fat. The standard curve was linear over the concentration range 0.05 to 50 mg/L (r > 0.999), which encompasses clinical concentrations, bias was less than ± 10%, intra- and interday coefficients of variation were less than 10%, and the limit of quantification was 0.05 mg/L. Systemic blood samples were analyzed using homogeneous particle-enhanced turbidimetric inhibition immunoassay on a Dimension Vista® analyzer (Siemens, Erlangen, Germany). All patient samples were analyzed in duplicate, and laboratory analysis was carried out blinded as to the randomization group.
Means, SDs, and 95% CIs were calculated for the concentrations in the different samples. Different tissue samples were pooled according to the surgical steps at which they were taken. Repeat-measure ANOVA was used to compare the average level of concentration across time among groups adjusted by sex, age, and time from incision. The interaction between time from incision and group was also assessed. For those with serum blood sample concentrations of less than 0.8 μg/mL, a random imputation was applied assuming the mean log(concentration) was equal to 0.4, and the SD was derived by the other available records at the same time point.
Results
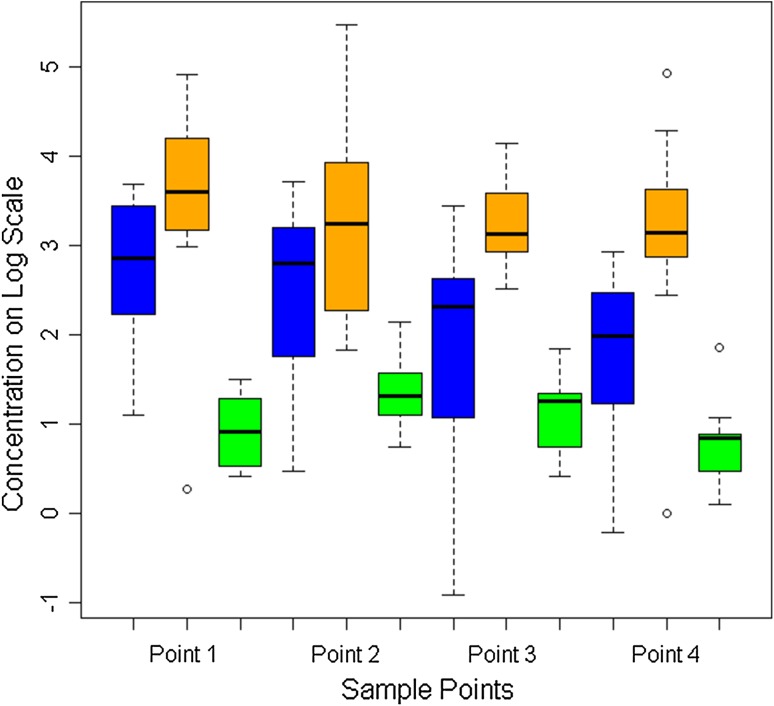
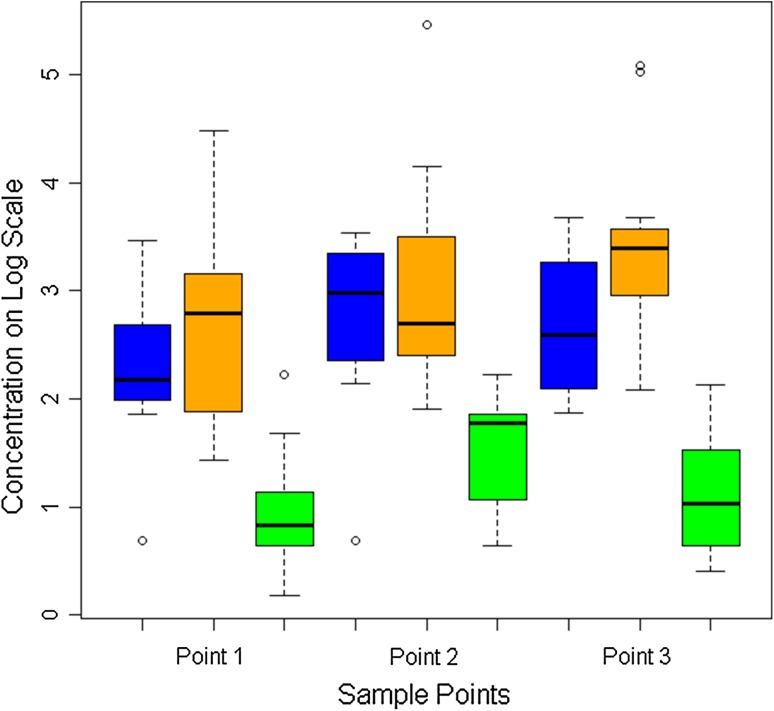
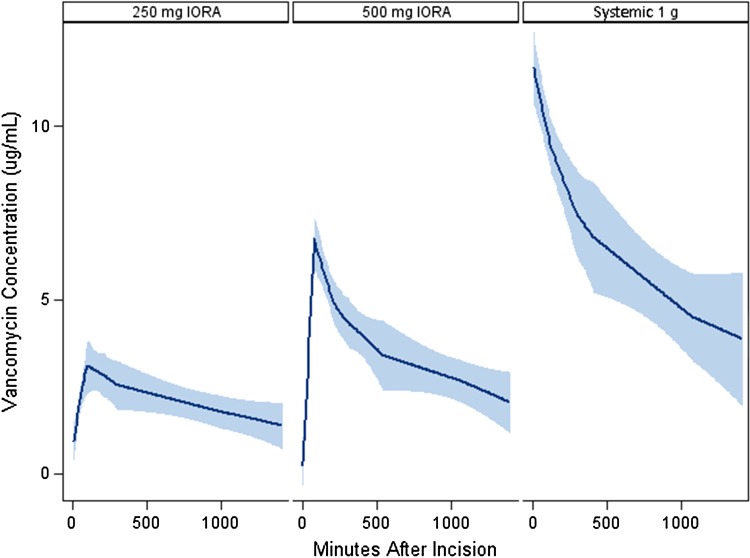
The overall mean tissue concentration of vancomycin in subcutaneous fat was higher in the 250-mg IORA group than in the 1-g systemic group (14 μg/g versus 3.2 μg/g) (p < 0.001, Table 2) and higher in the 500-mg IORA group (44 μg/g) than in both other groups (p < 0.001) (Fig. 2). Similarly, the overall mean tissue concentration of vancomycin in bone was higher in the 250-mg IORA group than in the 1-g systemic group (16 μg/g versus 4.0 μg/g, p < 0.001) and higher in the 500-mg IORA group (38 μg/g) than in both other groups (p < 0.001 (Fig. 3). Of tissue samples in the 1-g systemic group, 25% (16 of 63) were less than 2.0 μg/g, the typical MIC of CoNS against vancomycin. In comparison, 4% of samples (three of 70) in the 250-mg IORA group and 1% (one of 70) in the 500-mg IORA group were below this level. For patients in the IORA groups, vancomycin levels were either not detectable or only slightly raised in intraoperative systemic blood samples taken a mean 86 minutes after injection, indicating generally successful function of the tourniquet (Table 3). After tourniquet deflation, peak vancomycin concentrations in systemic blood were lower for both IORA groups than for the 1-g systemic group (Fig. 4).
Fig. 2.
A graph show the tissue concentrations of vancomycin in subcutaneous fat at each sample point. The 250-mg IORA group is in blue, 500-mg IORA group in orange, and 1-g systemic group in green. Concentrations on log scale: 2 = 7 μg/g, 4 = 55 μg/g, and 6 = 403 μg/g. Box represents the median; horizontal line in box represents the 25% and 75% quartiles; whiskers represent 1.5 times the interquartile range from the box.
Fig. 3.
A graph shows the tissue concentrations of vancomycin in bone at each sample point. The 250-mg IORA group is in blue, 500-mg IORA group in orange, and 1-g systemic group in green. Concentrations on log scale: 1 = 3 μg/g, 2 = 7 μg/g, 3 = 20 μg/g, 4 = 55 μg/g, and 5 = 148 μg/g. Box represents the median; horizontal line in box represents the 25% and 75% quartiles; whiskers represent 1.5 times the interquartile range from the box.
Table 3.
Systemic blood concentrations of vancomycin at each sample point
| Time | Concentration (μg/g) | |||
|---|---|---|---|---|
| 250-mg IORA group | 500-mg IORA group | 1-g systemic group | p value* | |
| Intraoperative† | 0.7 (0.6, 1.06) | 0.6 (0.6, 0.7) | 11.4 (10.2, 13.4) | < 0.001 |
| 1 hour postoperatively | 2.7 (2.1, 3.5) | 6.0 (5.3, 6.9) | 9.4 (8.9, 11.1) | < 0.001 |
| 4 hours postoperatively | 2.6 (1.0, 4.0) | 4.6 (4.2, 4.9) | 7.0 (6.6, 7.8) | < 0.001 |
| 8 hours postoperatively | 1.4 (1.4, 1.4) | 4.0 (2.9, 4.1) | 5.3 (5.1, 5.8) | 0.001 |
| 20 hours postoperatively | 1.4 (1.0, 2.2) | 2.4 (0.6, 2.7) | 3.7 (2.9, 5.3) | < 0.001 |
* ANOVA was used in the analyses; †intraoperative samples were taken a mean of 86 minutes after tourniquet inflation; values are expressed as median, with interquartile range in parentheses; IORA = intraosseous regional administration.
Fig. 4.
A Loess graph shows the systemic blood concentrations of vancomycin with predicted CIs in the three intervention groups.
One patient in the 1-g systemic group developed red man syndrome during vancomycin infusion consisting of erythema, pruritis, and hot flushing. The vancomycin infusion was stopped after 700 mg had been given and the symptoms resolved. The patient remained hemodynamically stable and the procedure was carried out as normal. Tissue and blood samples for this patient were not included in the analysis. No clinical signs of red man syndrome were seen in any patient undergoing IORA; in particular, no signs were seen after tourniquet deflation. Minor transient drops in systolic blood pressure (5–30 mm Hg) were seen after tourniquet deflation in six patients in the 250-mg IORA group, five patients in the 500-mg IORA group, and seven patients in the 1-g systemic group. One patient in the 500-mg IORA group developed a deep vein thrombosis in a peroneal calf vein seen on ultrasound scan at Day 3. He was treated with warfarin, and repeat scan at 6 weeks showed resolution of the clot and the warfarin was discontinued. No deep or superficial infections occurred in either group. One patient in the 250-mg IORA group went on to have a TKA performed on his contralateral knee 2 months after his participation in this study. He was given systemic prophylaxis with 1 g cefazolin. He developed a deep infection 4 weeks postoperatively, which eventually required two-stage revision surgery. The infecting organism was CoNS, resistant to cefazolin.
Discussion
Antibiotic resistance is an increasing problem, and rates of orthopaedic infection due to MRSA or resistant CoNS are rising [39]. This, together with the severe consequences of a deep infection, has led some authors to propose vancomycin as an alternative prophylactic agent in TKA [8], particularly in centers where MRSA rates are high [30, 39]. However, vancomycin has a number of disadvantages, including systemic side effects such as nephrotoxicity and ototoxicity, concerns about promoting further bacterial resistance, and a prolonged administration time. We previously investigated IORA as a method of maximizing tissue concentrations of cefazolin in TKA [41]. This study explored the use of IORA to give a lower dose of a more toxic drug, potentially minimizing its adverse effects. We hypothesized lower doses of vancomycin via IORA could still achieve tissue concentrations equal or superior to those of systemic administration before TKA.
A limitation of our study is, while we saw no evidence of red man syndrome with IORA vancomycin, the number of patients was small and those with significant cardiac disease were excluded. Red man syndrome is an anaphylactoid reaction caused by the degranulation of mast cells resulting in histamine release. It is not an allergic reaction and is independent of preformed immunoglobulin E. It occurs in 30% to 90% of healthy volunteers given vancomycin [36], and symptoms are usually mild and alleviated by use of an antihistamine. Incidence is related to both dosage and rate of infusion; Polk et al. [29] observed the reaction during systemic infusion of 1 g vancomycin in 82% of volunteers, but no reaction occurred when a 500-mg dose was used. Healy et al. [15] noted symptoms in eight of 10 volunteers (80%) given 1 g vancomycin over 1 hour but in only three of 10 volunteers (30%) given the same dose over 2 hours. The absence of red man syndrome seen with IORA vancomycin in our study is likely due to both the lower doses used and the depot effect of the high tissue concentrations that causes antibiotic to be released gradually into the systemic circulation after tourniquet deflation [32]. However, until data on a larger number of patients are available, we recommend patients receiving IORA vancomycin be monitored closely after tourniquet deflation and an antihistamine be available if required.
A second potential limitation of the IORA technique is the lower systemic concentration once the tourniquet is released. Many surgeons routinely continue antibiotics for 24 hours postoperatively, and further systemic vancomycin doses after IORA would still be required to maintain levels. However, due to the high initial concentrations achieved, vancomycin levels in perioperative tissues are likely to remain elevated for some time. Hoddinott et al. demonstrated persistently elevated antibiotic levels in drain fluid the morning following surgery in TKA patients given prophylactic cefazolin via a regional route [19]. Additionally, a distinction should be drawn between antibiotic use for prophylaxis and that for treatment of an established infection. The goal of prophylaxis is to prevent initial bacterial adherence and colonization during the period the wound is open, when contamination is occurring [3]. The critical period therefore when adequate antibiotic concentrations must be present in the tissues is from the time of incision to the time of closure, an outcome achieved in our study in both IORA groups. Moreover, a number of randomized controlled trials have shown no difference in infection rates between a single preoperative dose and continuing antibiotics for 24 hours [13, 16], implying further doses after IORA may be unnecessary.
We found tissue concentrations were three to seven times greater in the 250-mg IORA group than in the systemic group, and given the lower risk of toxicity with lower doses, we would recommend 250 mg as the IORA dose for vancomycin. Vancomycin exhibits concentration-independent killing, and once concentrations are four to five times the MIC, further increases do not alter the killing rate [17, 22]. Surveillance studies from US laboratories report the modal vancomycin MIC is 1.0 μg/mL for MRSA isolates and 2.0 μg/mL for CoNS [37]. Tissue concentrations seen in the systemic group were higher than these MICs but were relatively borderline if one considers up to 50% of vancomycin may be protein bound [33]. Inadequate tissue concentrations have been postulated as the reason why vancomycin appears be less effective against methicillin-sensitive strains of S Aureus than cephalosporins [8, 39], a problem likely to be exacerbated if timing of vancomycin administration is suboptimal [1].
Timing of prophylactic antibiotics is critical to their effectiveness; maximum benefit occurs when given in the 60 minutes before skin incision [6]. As protocols for systemic vancomycin require infusion rates of no greater than 1 g/60 minutes, a prophylactic dose of 1 g needs to be started 1 to 2 hours before surgery [8]. This is difficult to incorporate into operating room protocols [7], and clinical studies show optimal timing of vancomycin is rarely achieved in practice [1, 39]. An advantage of IORA over systemic is that it ensures appropriate timing of administration, and in our study and a previous IORA study [41], very high tissue levels of antibiotic were present immediately after skin incision. A disadvantage is that IORA injection occurs after tourniquet inflation, adding 2 to 4 minutes to overall tourniquet time. The intraosseous needles are also an additional cost.
Regional administration of prophylactic antibiotics in TKA has been investigated previously using teicoplanin, a glycopeptide antibiotic currently unavailable in North America with a similar spectrum of activity to vancomycin. de Lalla et al. [9] compared intravenous regional administration (IVRA) of 400 mg teicoplanin via a foot vein to 800 mg teicoplanin given systemically. IVRA provided tissue concentrations two to 10 times higher than the systemic route. The same authors prospectively evaluated this IVRA protocol in 250 patients undergoing TKA and reported a 0% deep infection rate [10].
While this is the first study of IORA vancomycin in humans, it is well described in the veterinary literature where regional antibiotic administration is commonly used to treat limb infections. Rubio-Martínez et al. [31] compared IORA versus IVRA of vancomycin in 12 horses. No complications were reported, and tissue concentrations achieved with the two routes were similar. That study and a number of other animal studies [4, 24, 34] have demonstrated tissue antibiotic concentrations using the IVRA and IORA routes for administration are equivalent. IORA injections also travel directly into the intravascular space, and in TKA, the main advantages over IVRA are reliability and speed of access. Proximal tibial cannulation using modern intraosseous kits is rapid and reproducible [35] and, in contrast to foot vein cannulation, does not require any changes to standard sterile draping.
Potential complications of intraosseous infusion include fluid extravasation with compartment syndrome related to incorrect needle placement in emergency situations [38]. Needle site infection is rare and correlates with the length of time the needle is left in situ [38]. Fat embolus is a theoretical concern, and subclinical lung microemboli have been seen histologically after intraosseous infusion in some animal studies [14, 28]. However, no measurable effects on ventilation-perfusion performance have been found [20, 28], and other studies report no difference in histologic fat embolus rates between intraosseous and intravenous infusions [20]. To date, no cases of clinical fat emboli associated with intraosseous infusion have been reported in humans [38].
Accepted indications for vancomycin prophylaxis in TKA include β-lactam allergy and known colonization with MRSA [8]. High institutional MRSA prevalence has also been suggested as an indication [30], but the prevalence at which routine prophylaxis with vancomycin becomes beneficial is controversial [39]. Promotion of further antibiotic resistance with routine vancomycin prophylaxis remains a significant concern, as prolonged exposure to sublethal concentrations may promote the emergence of resistant organisms [2]. In theory, low-dose IORA may apply less selection pressure than systemic administration by maximizing tissue levels at the site of action and reducing the overall exposure; however, any advantage is difficult to quantify.
While concerns about the routine use of vancomycin for prophylaxis remain, the use of low-dose vancomycin IORA can achieve higher tissue concentrations than systemic administration without prolonged preoperative infusion times. This may optimize the timing of vancomycin administration and reduce the risk of systemic side effects, while providing equal or enhanced prophylaxis in TKA.
Electronic supplementary material
Acknowledgments
We thank Irene Zeng MSc (Hons) for her assistance with statistical analysis and the Awhina Trust for their funding support, and we thank Dr Kelly Vince for his advice and guidance on the project. We also thank Vidacare Corp for supplying the intraosseous needles without charge.
Footnotes
The institution of one or more of the authors (MZ, GAM) received funding from the Awhina Trust (Auckland, New Zealand), a charitable trust with no relationship to the subject of this article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Procedures and sample collection were performed at North Shore Hospital, Auckland, New Zealand. Sample analysis was performed at Canterbury Health Laboratories, Christchurch, New Zealand.
References
- 1.Bull AL, Worth LJ, Richards MJ. Impact of vancomycin surgical antibiotic prophylaxis on the development of methicillin-sensitive Staphylococcus aureus surgical site infections: report from Australian Surveillance Data (VICNISS) Ann Surg. 2012;256:1089–1092. doi: 10.1097/SLA.0b013e31825fa398. [DOI] [PubMed] [Google Scholar]
- 2.Burgess DS. Pharmacodynamic principles of antimicrobial therapy in the prevention of resistance. Chest. 1999;115(3 suppl):19S–23S. doi: 10.1378/chest.115.suppl_1.19S. [DOI] [PubMed] [Google Scholar]
- 3.Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168. [PubMed] [Google Scholar]
- 4.Butt TD, Bailey JV, Dowling PM, Fretz PB. Comparison of 2 techniques for regional antibiotic delivery to the equine forelimb: intraosseous perfusion vs. intravenous perfusion. Can Vet J. 2001;42:617–622. [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson AK, Lidgren L, Lindberg L. Prophylactic antibiotics against early and late deep infections after total hip replacements. Acta Orthop Scand. 1977;48:405–410. doi: 10.3109/17453677708992017. [DOI] [PubMed] [Google Scholar]
- 6.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 7.Collier PE, Rudolph M, Ruckert D, Osella T, Collier NA, Ferrero M. Are preoperative antibiotics administered preoperatively? Am J Med Qual. 1998;13:94–97. doi: 10.1177/106286069801300208. [DOI] [PubMed] [Google Scholar]
- 8.Crawford T, Rodvold KA, Solomkin JS. Vancomycin for surgical prophylaxis? Clin Infect Dis. 2012;54:1474–1479. doi: 10.1093/cid/cis027. [DOI] [PubMed] [Google Scholar]
- 9.de Lalla F, Novelli A, Pellizzer G, Milocchi F, Viola R, Rigon A, Stecca C, Dal Pizzol V, Fallani S, Periti P. Regional and systemic prophylaxis with teicoplanin in monolateral and bilateral total knee replacement procedures: study of pharmacokinetics and tissue penetration. Antimicrob Agents Chemother. 1993;37:2693–2698. doi: 10.1128/AAC.37.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lalla F, Viola R, Pellizzer G, Lazzarini L, Tramarin A, Fabris P. Regional prophylaxis with teicoplanin in monolateral or bilateral total knee replacement: an open study. Antimicrob Agents Chemother. 2000;44:316–319. doi: 10.1128/AAC.44.2.316-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyon F, Evrard J, Mazas F, Hill C. Long-term results of prophylactic cefazolin versus placebo in total hip replacement. Lancet. 1987;1:860. doi: 10.1016/S0140-6736(87)91635-7. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher N, Sofianos D, Berkes MB, Obremskey WT. Prevention of perioperative infection. J Bone Joint Surg Am. 2007;89:1605–1618. doi: 10.2106/JBJS.F.00901. [DOI] [PubMed] [Google Scholar]
- 13.Garcia S, Lozano ML, Gatell JM, Soriano E, Ramon R, Sanmiguel JG. Prophylaxis against infection. Single-dose cefonicid compared with multiple-dose cefamandole. J Bone Joint Surg Am. 1991;73:1044–1048. [PubMed] [Google Scholar]
- 14.Hasan MY, Kissoon N, Khan TM, Saldajeno V, Goldstein J, Murphy SP. Intraosseous infusion and pulmonary fat embolism. Pediatr Crit Care Med. 2001;2:133–138. doi: 10.1097/00130478-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Healy DP, Sahai JV, Fuller SH, Polk RE. Vancomycin-induced histamine release and “red man syndrome”: comparison of 1- and 2-hour infusions. Antimicrob Agents Chemother. 1990;34:550–554. doi: 10.1128/AAC.34.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heydemann JS, Nelson CL. Short-term preventive antibiotics. Clin Orthop Relat Res. 1986;205:184–187. [PubMed] [Google Scholar]
- 17.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 18.Hill C, Flamant R, Mazas F, Evrard J. Prophylactic cefazolin versus placebo in total hip replacement: report of a multicentre double-blind randomised trial. Lancet. 1981;1:795–796. doi: 10.1016/S0140-6736(81)92678-7. [DOI] [PubMed] [Google Scholar]
- 19.Hoddinott C, Lovering AM, Fernando HC, Dixon JH, Reeves DS. Determination of bone and fat concentrations following systemic cefamandole and regional cefuroxime administration in patients undergoing knee arthroplasty. J Antimicrob Chemother. 1990;26:823–829. doi: 10.1093/jac/26.6.823. [DOI] [PubMed] [Google Scholar]
- 20.Kentner R, Haas T, Gervais H, Hiller B, Dick W. Pharmacokinetics and pharmacodynamics of hydroxyethyl starch in hypovolemic pigs; a comparison of peripheral and intraosseous infusion. Resuscitation. 1999;40:37–44. doi: 10.1016/S0300-9572(98)00121-X. [DOI] [PubMed] [Google Scholar]
- 21.Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis. 2006;42:389–391. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 22.Larsson AJ, Walker KJ, Raddatz JK, Rotschafer JC. The concentration-independent effect of monoexponential and biexponential decay in vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J Antimicrob Chemother. 1996;38:589–597. doi: 10.1093/jac/38.4.589. [DOI] [PubMed] [Google Scholar]
- 23.Lazzarini L, Novelli A, Marzano N, Timillero L, Fallani S, Viola R, de Lalla F. Regional and systemic prophylaxis with teicoplanin in total knee arthroplasty. J Arthroplasty. 2003;18:342–346. doi: 10.1054/arth.2003.50053. [DOI] [PubMed] [Google Scholar]
- 24.Mattson S, Boure L, Pearce S, Hurtig M, Burger J, Black W. Intraosseous gentamicin perfusion of the distal metacarpus in standing horses. Vet Surg. 2004;33:180–186. doi: 10.1111/j.1532-950x.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 25.McNamara DR, Steckelberg JM. Vancomycin. J Am Acad Orthop Surg. 2005;13:89–92. doi: 10.5435/00124635-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Murphy E, Spencer SJ, Young D, Jones B, Blyth MJ. MRSA colonisation and subsequent risk of infection despite effective eradication in orthopaedic elective surgery. J Bone Joint Surg Br. 2011;93:548–551. doi: 10.1302/0301-620X.93B4.24969. [DOI] [PubMed] [Google Scholar]
- 27.Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR. The microbiology of the infected knee arthroplasty. Int Orthop. 2010;34:505–510. doi: 10.1007/s00264-009-0797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlowski JP, Julius CJ, Petras RE, Porembka DT, Gallagher JM. The safety of intraosseous infusions: risks of fat and bone marrow emboli to the lungs. Ann Emerg Med. 1989;18:1062–1067. doi: 10.1016/S0196-0644(89)80932-1. [DOI] [PubMed] [Google Scholar]
- 29.Polk RE, Healy DP, Schwartz LB, Rock DT, Garson ML, Roller K. Vancomycin and the red-man syndrome: pharmacodynamics of histamine release. J Infect Dis. 1988;157:502–507. doi: 10.1093/infdis/157.3.502. [DOI] [PubMed] [Google Scholar]
- 30.Ritter MA, Barzilauskas CD, Faris PM, Keating EM. Vancomycin prophylaxis and elective total joint arthroplasty. Orthopedics. 1989;12:1333–1336. doi: 10.3928/0147-7447-19891001-09. [DOI] [PubMed] [Google Scholar]
- 31.Rubio-Martínez L, López-Sanromán J, Cruz AM, Santos M. San Román F. Medullary plasma pharmacokinetics of vancomycin after intravenous and intraosseous perfusion of the proximal phalanx in horses. Vet Surg. 2005;34:618–624. doi: 10.1111/j.1532-950X.2005.00096.x. [DOI] [PubMed] [Google Scholar]
- 32.Rubio-Martínez LM, López-Sanromán J, Cruz AM, Santos M, Andrés MS, Román FS. Evaluation of safety and pharmacokinetics of vancomycin after intravenous regional limb perfusion in horses. Am J Vet Res. 2005;66:2107–2113. doi: 10.2460/ajvr.2005.66.2107. [DOI] [PubMed] [Google Scholar]
- 33.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(suppl 1):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 34.Scheuch BC, Van Hoogmoed LM, Wilson WD, Snyder JR, MacDonald MH, Watson ZE, Steffey EP. Comparison of intraosseous or intravenous infusion for delivery of amikacin sulfate to the tibiotarsal joint of horses. Am J Vet Res. 2002;63:374–380. doi: 10.2460/ajvr.2002.63.374. [DOI] [PubMed] [Google Scholar]
- 35.Shavit I, Hoffmann Y, Galbraith R, Waisman Y. Comparison of two mechanical intraosseous infusion devices: a pilot, randomized crossover trial. Resuscitation. 2009;80:1029–1033. doi: 10.1016/j.resuscitation.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Sivagnanam S, Deleu D. Red man syndrome. Crit Care. 2003;7:119–120. doi: 10.1186/cc1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenover FC, Moellering RC., Jr The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–1215. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 38.Tobias JD, Ross AK. Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use. Anesth Analg. 2010;110:391–401. doi: 10.1213/ANE.0b013e3181c03c7f. [DOI] [PubMed] [Google Scholar]
- 39.Tyllianakis ME, Karageorgos AC, Marangos MN, Saridis AG, Lambiris EE. Antibiotic prophylaxis in primary hip and knee arthroplasty: comparison between cefuroxime and two specific antistaphylococcal agents. J Arthroplasty. 2010;25:1078–1082. doi: 10.1016/j.arth.2010.01.105. [DOI] [PubMed] [Google Scholar]
- 40.Yamada K, Matsumoto K, Tokimura F, Okazaki H, Tanaka S. Are bone and serum cefazolin concentrations adequate for antimicrobial prophylaxis? Clin Orthop Relat Res. 2011;469:3486–3494. doi: 10.1007/s11999-011-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young SW, Zhang M, Freeman JT, Vince KG, Coleman B. Higher cefazolin concentrations with intraosseous regional prophylaxis in TKA. Clin Orthop Relat Res. 2013;471:244–249. doi: 10.1007/s11999-012-2469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.