Abstract
Background
Autologous chondrocyte implantation (ACI) has demonstrated good and excellent results in over 75% of patients up to 10 years after surgery. Reports of longer-term outcomes, however, remain limited.
Questions/purposes
The purposes of this study were to describe the (1) survivorship of ACI grafts; (2) the long-term functional outcomes using validated scoring tools after ACI; and (3) to provide an analysis of potential predictors for failure.
Methods
Two hundred ten patients treated with ACI were followed for more than 10 years. Indications for the procedure included symptomatic cartilage defects in all compartments of the knee unresponsive to nonoperative measures. Mean age at surgery was 36 ± 9 years; mean defect size measured 8.4 ± 5.5 cm2. Outcome scores were prospectively collected pre- and postoperatively at the last followup.
Results
At a mean of 12 ± 2 years followup, 53 of 210 patients (25%) had at least one failed ACI graft. Nineteen of these patients went on to arthroplasty, 27 patients were salvaged with revision cartilage repair, and seven patients declined further treatment; three patients were lost to followup. The modified Cincinnati increased from 3.9 ± 1.5 to 6.4 ± 1.5, WOMAC improved from 39 ± 21 to 23 ± 16, Knee Society Score (KSS) knee score rose from 54 ± 18 to 79 ± 19, and KSS function from 65 ± 23 to 78 ± 17 (all p < 0.0001). The Physical Component of the SF-36 score increased from 33 ± 14 to 49 ± 18, whereas the Mental Component improved from 46 ± 14 to 52 ± 15 (both p < 0.001). Survivorship was higher in patients with complex versus salvage-type lesions (p = 0.03) with primary ACI versus ACI after prior marrow stimulation (p = 0.004) and with concomitant high tibial osteotomy (HTO) versus no HTO (p = 0.01).
Conclusions
ACI provided durable outcomes with a survivorship of 71% at 10 years and improved function in 75% of patients with symptomatic cartilage defects of the knee at a minimum of 10 years after surgery. A history of prior marrow stimulation as well as the treatment of very large defects was associated with an increased risk of failure.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Cartilage is known to have limited intrinsic repair capabilities and cartilage defects can progress to osteoarthritis (OA) [7, 9, 25]. OA is a major source of economic burden in the 21st century, being among the leading causes of disability [4]. The risk of disability from knee OA is as great as that derived from cardiovascular disease [20], a fact that becomes even more concerning when considering that even isolated cartilage defects can cause pain and disability comparable to that of severe OA [24].
Several cartilage repair procedures are in current clinical application, including microfracture [50], osteochondral autograft transfer [22], osteochondral allograft transplantation [17], and autologous chondrocyte implantation (ACI) [5]. Given the economic challenges facing our healthcare system, it appears prudent to choose procedures that provide the most durable long-term outcome. Comparatively few studies have examined long-term outcomes, an important factor when considering the substantial differences in cost and morbidity among the various treatment options.
The purposes of this study were to describe the (1) survivorship of ACI grafts; (2) the long-term functional outcomes using validated scoring tools after ACI; and (3) to provide an analysis of potential predictors for failure. We performed our analyses at a minimum of 10 years after treatment of symptomatic chondral defects of the knee.
Patients and Methods
Patient Demographics
Between March 1995 and March 2010 more than 500 patients were treated at our institution with ACI (Carticel; Genzyme BioSurgery, Cambridge, MA, USA). To be considered for inclusion in the cohort presented here, we included all patients who had completed at least 10 years of followup.
Between March 1995 and March 2001, a total of 210 patients (95 left and 115 right knees) were treated with ACI for symptomatic full-thickness chondral defects of the knee. There were 113 male and 97 female patients with an average age of 36 ± 10 years (range, 8–57 years). An average of 1.7 lesions per knee was treated with a total surface area of 8.4 ± 5.5 cm2 per knee (Table 1).
Table 1.
Patient demographics
| Variable | Number (%) |
|---|---|
| Age (years), mean (SD) | 35.8 (9.6) |
| Men | 113 (53.8) |
| Women | 97 (46.2) |
| Body mass index (kg/m2), mean (SD) | 26.7 (4.6) |
| Number of lesions (%) | |
| 1 | 106 (50.5) |
| 2 | 65 (31.0) |
| 3 | 30 (14.3) |
| 4 | 9 (4.3) |
| Workers compensation | 46 (21.9) |
| Type of cartilage defect, number, size, mean (SD) | |
| Simple (single unipolar grade 3/4 lesion on femur or grade 2 or less on the tibia or patella) | 16 (2.2 ± 2.6 cm2) |
| Complex (multifocal unipolar grade 3/4 chondral lesions on femur, concurrent HTO/TTO, OCD, unipolar lesions on tibia or patella) | 106 (6.2 ± 3.6 cm2) |
| Salvage (bipolar focal chondral lesions, generalized chondromalacia grade 2 or greater) | 88 (11.7 ± 5.9 cm2) |
| Total defect surface area index surgery | 8.4 ± 5.5 cm2 |
HTO/TTO = high tibial osteotomy/tibial tubercle osteotomy; OCD = osteochondritis dissecans.
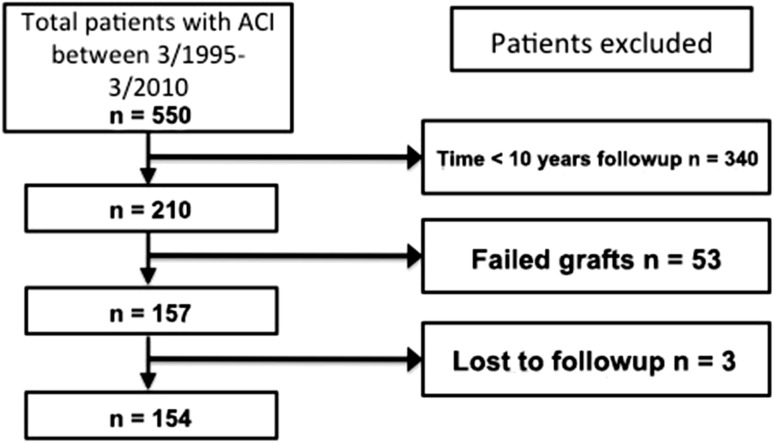
None were lost to followup for the survivorship analysis. Of 157 patients who had successful grafts, we were able to analyze functional scores of 154 patients; three patients were lost to followup (Fig. 1). These patients completed postoperative questionnaires including modified Cincinnati and WOMAC (both, n = 145 [94.2% completion]), Knee Society score [KSS] (n = 122 [79.2%]), SF-36 scores (n = 136 [88.3%], and patient satisfaction (n = 137 [88.7%]).
Fig. 1.
Patient demographics demonstrating the exclusion criteria.
This clinical database was approved by the institutional review board at the initial start date, and all patients provided informed consent at the time they were entered into the database, usually at the time of their index operation.
Thorough clinical examination, MRI, and arthroscopy were completed before consideration of treatment with ACI. Indications included one or more full-thickness chondral defects of the knee in a compliant patient with symptoms matching the defect location and who had failed nonoperative measures. Contraindications to treatment included patients with inflammatory joint disease, unresolved or recent septic arthritis, metabolic or crystal disorders, deficient soft tissue coverage, or OA as evidenced by complete loss of joint space on weightbearing radiographs. Malalignment and meniscal or ligamentous insufficiency were not considered a contraindication to treatment and were addressed in a concurrent or staged fashion.
Patients were divided into three cartilage defect types, namely simple (single unipolar grade 3/4 lesion on femur or grade 2 or less on the tibia or patella), complex (multifocal unipolar grade 3/4 chondral lesions on femur, concurrent high tibial osteotomy [HTO]/tibial tubercle osteotomy [TTO], osteochondritis dissecans, unipolar lesions on tibia or patella), and salvage (bipolar focal chondral lesions, generalized chondromalacia grade 2 or greater).
Surgical Technique
ACI was performed by a single experienced cartilage surgeon as described elsewhere in greater detail [34]. Briefly, autologous chondrocytes were expanded in cell culture from a cartilage biopsy harvested arthroscopically. After a variable time interval in cryopreservation, the cells were implanted into cartilage defects that had been prepared by removing all degenerated tissue as well as the layer of calcified cartilage. A periosteal patch was harvested from the proximal tibia and microsutured to the surrounding cartilage to create a watertight space that was then injected with the chondrocyte suspension at a density of greater than one million cells per square centimeter.
Articular comorbidities such as malalignment, patellar maltracking, and meniscal or ligamentous deficiency were corrected in a staged or concurrent procedure. Patients with more than 3° of malalignment were corrected through a neutralizing osteotomy of the tibia or femur. Concomitant procedures included HTO in 33 (15.7%), TTO in 49 (38 Fulkerson, 11 Maquet) (23.3%), combined HTO/TTO in 15 (7.1%), and distal femoral osteotomy in three patients (1.4%). Anterior cruciate ligament (ACL) repair was performed in 10, lateral collateral ligament (LCL) in one, and combined ACL/LCL in another patient (Table 2).
Table 2.
Concomitant procedures
| Procedure | Number (%) |
|---|---|
| High tibial osteotomy (HTO) | 33 (15.7) |
| Distal femoral varus osteotomy | 3 (1.4) |
| Tibial tubercle osteotomy (TTO) | 49 (23.3) |
| Combined HTO/TTO | 15 (7.1) |
| Ligament reconstruction | 12 (5.7) |
| Meniscal procedures | 18 (8.6) |
We deliberately did not overcorrect by 3° to 5° as recommended by Coventry et al. [8] because our goal was to normalize excessive stresses rather than further unload the respective compartment, except in “kissing” (salvage category) weightbearing lesions, in which we overcorrected by 2° to unload the defects.
Patellofemoral maltracking was addressed with anteromedialization tibial tubercle osteotomy [13, 35]. If patellar subluxation and tilt were diagnosed on physical examination and confirmed on radiographs, and/or CT/MRI, lateral release (n = 130 [62.0%]) and vastus medialis obliquus advancement (n = 39 [18.6%]) were added.
Eighteen (8.6%) patients underwent meniscal procedures, meniscal transplantation in four (2.0%) and partial meniscectomy in 14 (6.7%). Size- and compartment-matched meniscal transplantation (bone block technique) was performed for meniscal deficiency as defined by less than 5 mm of uninterrupted circumferential hoop fibers determined by preoperative MRI and diagnostic arthroscopy.
Eighty-nine of 210 (42.2%) patients had previously undergone attempts at cartilage repair, including microfracture in 13 of 210 (6.2%), abrasion arthroplasty in 30 (14.2%), and drilling in 46 (21.9%) patients (Table 3).
Table 3.
Previous cartilage surgeries
| Procedure | Number (%) |
|---|---|
| Microfracture | 13 (6.2) |
| Abrasion arthroplasty | 30 (14.3) |
| Drilling | 46 (21.9) |
Rehabilitation
Postoperatively, patients were kept on restricted weightbearing that was gradually increased over the course of 8 to 12 weeks. A continuous passive motion machine was used 6 to 8 hours per day for 6 weeks. Physical therapy interventions were slowly advanced as the grafts mature over time with impact activities delayed for 12 to 18 months. The protocol was individually adjusted according to the defect location, concurrent procedures, degree of graft maturation, and previous activity level [36].
Definition of Failure
Failure rates were reported in three categories: graft failure with revision using partial knee arthroplasty or TKA; graft failure with revision cartilage repair; and graft survival but development of new defects elsewhere in the same knee necessitating additional surgery (progression of disease).
Outcome Evaluation
Functional outcomes were evaluated with five validated, generic- and disease-specific instruments measuring changes in symptoms, function, and sports activity level to avoid ceiling and floor effects. Data were routinely collected preoperatively, at 1 and 2 years postoperatively, and then biennially; only the preoperative and final followup data for each patient were analyzed for this study. Patients completed questionnaires including knee-specific (KSS) [26], sports activity-based instruments (modified Cincinnati Rating scale), a generalized nonspecific arthritis score (WOMAC) [3], and the SF-36 health status survey. Patients were also asked to answer questions pertaining to their satisfaction with the procedure. Questionnaires were answered independently by the patients without physician interaction, mostly at home, and mailed in, or patients were contacted by phone by research personnel. Patients presented for clinical, postoperative followup as dictated by standard clinical routine.
The WOMAC osteoarthritis index is a disease-specific, self-administered instrument that was used to measure patient pain, stiffness, and physical function. This health status instrument has been statically tested for reliability, validity, and responsiveness in a number of OA populations [10]. Minimal clinically important difference (MCID) was defined as the smallest difference in score that patients perceive as beneficial [1]. According to previously published studies, the MCID of improvement in patients with OA of the knee has been defined as a 17% to 22% change from baseline WOMAC subscale scores [15].
The patient perception scale of the Cincinnati knee rating system for overall condition was modified as previously described [33] and used to measure self-reported function at baseline and followup on a 10-point scale [2, 41, 42].
Commonly used by orthopaedic surgeons, the KSS health survey captures a diagnosis-based estimate of functional impairment [26]. Two scores (0–100 points) are derived from this survey. The knee score rates only the knee itself and the functional score rates the patient’s ability to walk and climb stairs [26].
The SF-36 health status survey is a widely used generic health instrument that has been validated in the general population and disease-specific populations. The items of the SF-36 instrument include eight health domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. Domain and summary scores range from 0 to 100 points with higher scores pointing toward a better health state [56, 57].
Lastly, the Patient Satisfaction Questionnaire asked patients (1) to rate the operated joint compared with before surgery; (2) to assess their overall satisfaction with their surgery; (3) to assess if they would have the surgery again; and (4) to rate the results of their surgery (all on scales of 1 [best] to 5 [worst]).
Statistics
We determined differences in functional scores (WOMAC, KSS, modified Cincinnati, and SF-36) between two time points (preoperatively and at latest followup) using the Wilcoxon signed rank test. Two-sample Student’s t-test or the Mann-Whitney test was used to assess differences in functional scores between pre- and postoperative questionnaires and one-way analysis of variance for the analysis of the subgroups. We determined survival using the Kaplan-Meier method and calculated 95% confidence intervals for the different groups.
For subgroup analysis, patients were grouped according to the type of osteotomy, type of cartilage defect (simple, complex, salvage), size of the lesion (≤ 5 cm2, 5–10 cm2, 10–15 cm2, > 15 cm2), age, sex, workers compensation status, and whether the defects had been pretreated with marrow stimulation such as microfracture. A chi square test was used to determine differences in survivorship and accepted a p value of 0.05 as significant.
Results
Graft Failures
In total, 53 (25%) patients had at least one failed autologous chondrocyte graft (62 of 362 individual grafts failed [17%]). On further analysis of patients with failed grafts, grafts delaminated in 12 (23%) patients or failed to form adequate tissue (biologic failure) in 20 (38%). Two (4%) patients failed after a traumatic event and five (9%) patients were considered failures as a result of persistent pain although their workup had demonstrated an intact graft. An additional 14 (26%) patients failed not as a result of the transplanted areas, but because of development of new defects in other parts of the knee (progression of disease).
Overall, 19 (9%) of the 210 patients went on to knee arthroplasty within the followup period. Revision cartilage repair procedures were performed in 27 (13%) patients and seven (3%) patients declined further treatment although their grafts had failed.
Reoperation Rate
Of the 210 patients, 142 (67.6%) underwent an additional procedure on the index knee during the followup period (Table 4). In almost half (70 of 142), the indication for reoperation was unrelated to the graft itself (for example, hardware removal); in the other half (72 of 142), treatment was indicated for graft-related issues (for example, graft hypertrophy).
Table 4.
Reoperation rate related and unrelated to ACI graft, number (percent of reoperations)
| Related to ACI graft | Unrelated to ACI | ||||
|---|---|---|---|---|---|
| Microfracture < 25% of graft | Chondroplasty | Lysis with minimal Chondroplasty | Lysis | Others (infection, ACL revision, notchplasty, stich abscess, hardware removal) | |
| No. | 11 (5.2%) | 61 (29.0%) | 46 (25.2%) | 15 (7.1%) | 9 (4.3%) |
| Total number | 142 of 210 patients (67.8%) | ||||
ACI = autologous chondrocyte implantation; ACL = anterior cruciate ligament.
Survivorship Analysis
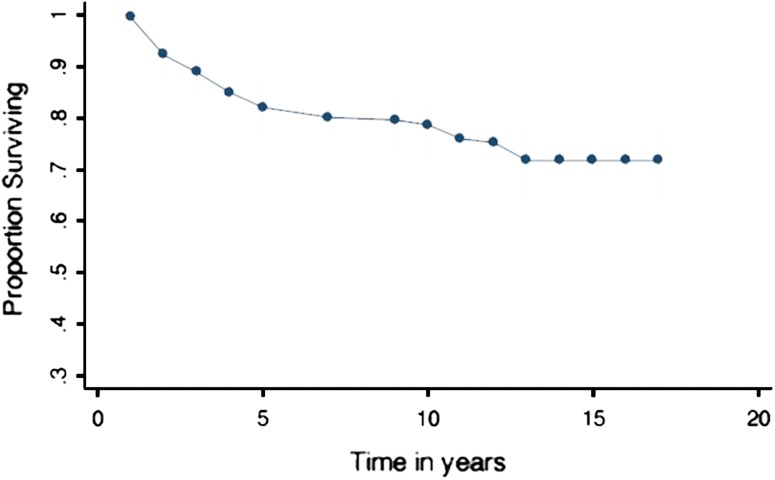
The overall survivorship analysis demonstrated graft survival at 5 years of 79% (95% confidence interval [CI], 76%– 87%), at 10 years of 71% (95% CI, 63%–77%), and at 15 years of 71% (95% CI, 63%–77%) (Fig. 2). For those patients with failed grafts, average time of survival free from revision with knee arthroplasty was 5.4 years (SD 3.4).
Fig. 2.
Overall survivorship of ACI demonstrating graft survival at 5 years of 79% (95% CI, 76%–87%), at 10 years of 71% (95% CI, 63%–77%), and at 15 years of 71% (95% CI, 63%–77%).
Risk Factors for Failure
We completed multiple analysis (Table 5) and point out the most important findings subsequently.
Table 5.
Survivorship analysis comparing various input parameters at 15 years
| Time | Chi square | p value > chi square | |||
|---|---|---|---|---|---|
| Type of cartilage defect | |||||
| Simple | Complex | Salvage | |||
| 15 years (%) (95% CI) | 63 (49–74) | 81 (72–87)* | 61 (27–84)* | 4.06 | 0.004 |
| Age (years) | |||||
| < 30 | 30–45 | > 45 | |||
| 15 years (%) (95% CI) | 84 (66– 93)*,† | 68 (58–76)* | 66 (46–80)† | 3.56,* | 0.02,* |
| 4.36† | 0.05† | ||||
| Sex | |||||
| Female | Male | ||||
| 15 years (%) (95% CI) | 64 (50–76) | 76 (67–83) | 2.66 | 0.10 | |
| Defect Location | |||||
| Patellofemoral | Tibiofemoral | ||||
| 15 years (%) (95% CI) | 70 (49–84) | 73 (60–81) | 0.46 | 0.79 | |
| Defect size | |||||
| 1–15 cm | > 15 cm | ||||
| 15 years (%) (95% CI) | 75 (68–82) | 38 (17–60) | 11.62 | 0.0088 | |
| Prior MFX | |||||
| Yes | No | ||||
| 15 years (%) (95% CI) | 62 (50–72) | 79 (69–87) | 8.45 | 0.0037 | |
| Osteotomy | |||||
| Yes | No | ||||
| 15 years (%) (95% CI) | 88 (72–95) | 66 (57–74) | 6.62 | 0.01 | |
| Workers Compensation | |||||
| Yes | No | ||||
| 15 years (%) (95% CI) | 62 (45–75) | 75 (66–81) | 3.00 | 0.083 | |
* Simple compared with complex cartilage defects; †simple compared with salvage cartilage defects; CI = confidence interval; MFX = microfractrue.
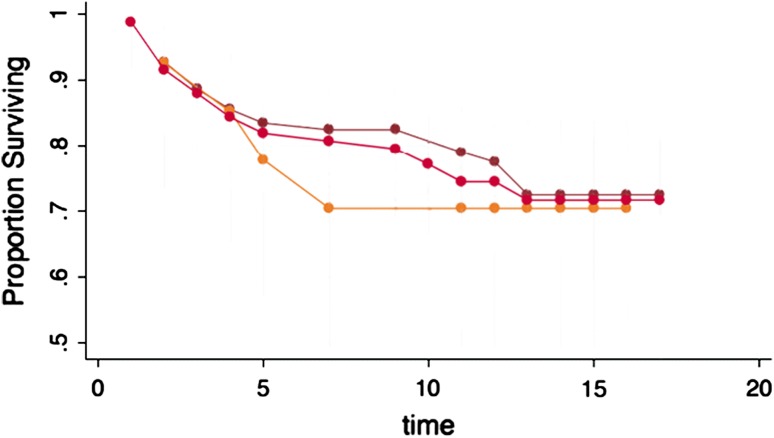
Defect Location
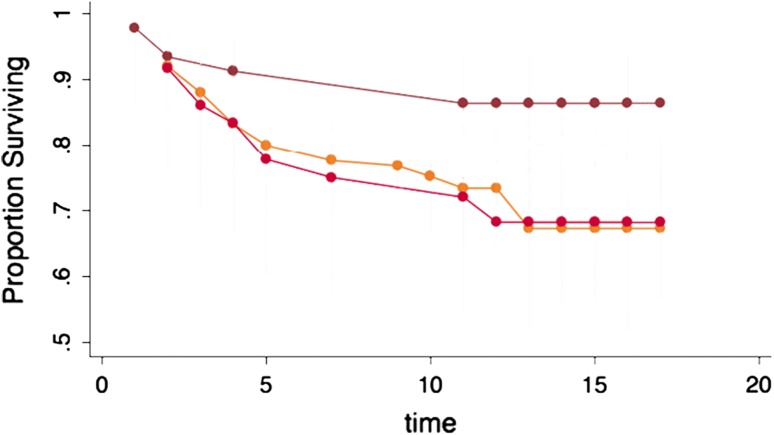
Patellofemoral grafts appeared to fail earlier than grafts in the tibiofemoral compartment (70% versus 79% at 10 years); however, long-term survivorship was not significantly different (70% versus 73% at 15 years) (Fig. 3).
Fig. 3.
The effect of location of the ACI showing no significant differences on the survivorship.
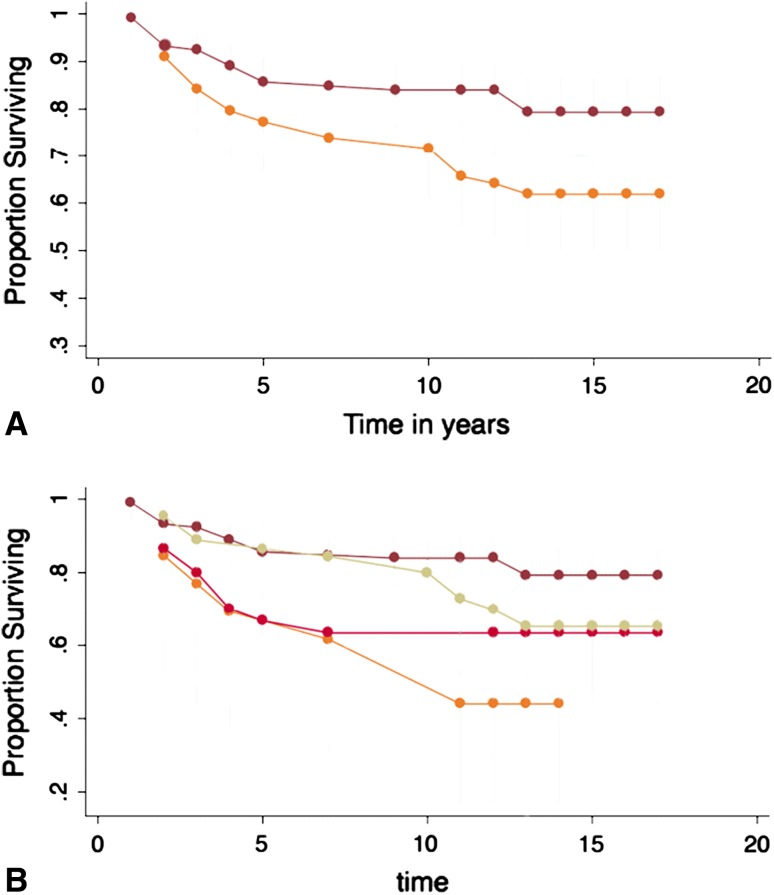
Prior Marrow Stimulation Treatment
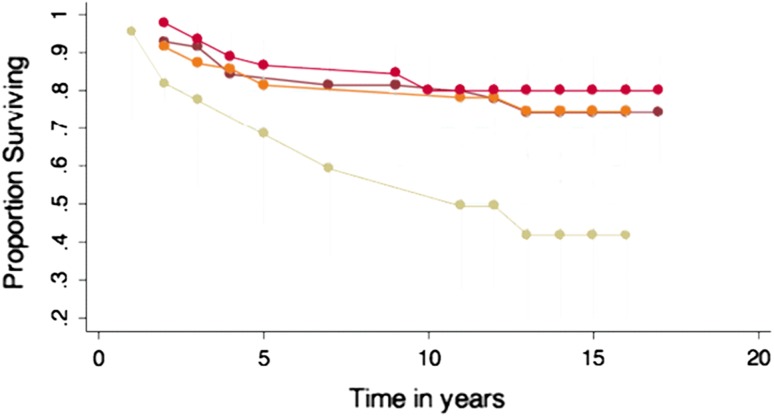
Eighty-nine of 210 patients (42%) had failed prior attempts at cartilage repair with marrow stimulation techniques (MST: microfracture, abrasion arthroplasty, or drilling). Survivorship of ACI was significantly lowered (p = 0.004) when one of these procedures had been performed. At 10 years, 84% (95% CI, 76%–90%) of patients without prior MST had successful grafts, whereas only 66% (95% CI, 55%–75%) of patients with prior MST did. At 15 years, 79% (95% CI, 69%–87%) and 62% (95% CI, 50%–72%) of patients had successful grafts, respectively (Fig. 4A).
Fig. 4A–B.
(A) The effect of prior marrow stimulation treatment to ACI impacted the survivorship of ACI negatively. (B) Survivorship of ACI with prior treatment of microfracture, abrasion arthroplasty, and subchondral drilling showing worst outcome for patients with prior microfracture.
When considering only microfracture specifically (not including drilling or abrasion arthroplasty) in a subgroup analysis, there was a significant (p = 0.006) difference between patients with microfracture versus no microfracture surgery before ACI. At 15 years, ACI graft survival for untreated knees was 79% (95% CI, 69%–87%) compared with 44% (95% CI, 17%–68%) in knees that underwent prior microfracture to the cartilage defect (Fig. 4B).
In patients with successful ACI, there was no difference in clinical outcome scores between knees that had previously been treated with marrow stimulation and those in which ACI was the primary cartilage repair procedure.
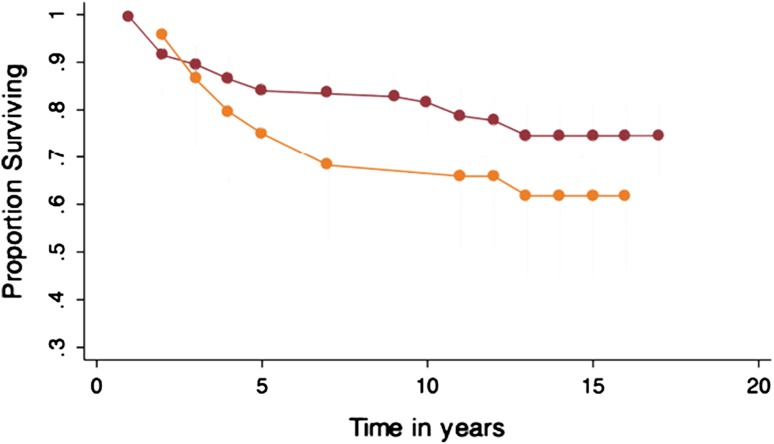
Osteotomy
Concurrent osteotomy significantly (p = 0.01) increased graft survivorship: 15-year survival rate with HTO was 88% (95% CI, 72%–95%) versus 66.0% (95% CI, 57%–74%) without. There were no significant differences in outcome scores between the two groups (Fig. 5).
Fig. 5.
The effect of osteotomy demonstrated a significant improved survivorship on the ACI.
Of the 48 patients who were treated with concurrent HTO, 17 had prior marrow stimulation surgery versus 31 who had no prior marrow stimulation. In the marrow stimulation group, five patients (29%) failed compared with no failures in the other group (p < 0.001).
Defect Size
We first compared outcomes according to total surface area treated per knee (Group 1: 1–5 cm2, Group 2: 5–10 cm2, Group 3: 10–15 cm2, Group 4: > 15 cm2). There were no statistically significant differences in either clinical outcomes or survivorship among Groups 1, 2, and 3. We therefore combined these three groups into one (1–15 cm2) group that was then compared with Group 4, which comprised the largest defects (> 15 cm2). Survivorship was significantly (p < 0.001) lower in patients with a > 15-cm2 surface area transplanted (38%; 95% CI, 17%–60%) in comparison to patients with < 15 cm2 (75%; 95% CI, 68%–82%). However, interestingly some of the postoperative clinical scores (WOMAC, modified Cincinnati) were significantly higher in the patients with lesion > 15 cm2 compared with those who had < 15 cm2 area treated (WOMAC, 10 ± 8 versus 23 ± 16, p = 0.005 and modified Cincinnati, 8 ± 1.3 versus 6.3 ± 1.5, p = 0.02), although preoperative scores were not significantly different (Fig. 6).
Fig. 6.
Survivorship of ACI total surface area (TSA) demonstrated a significant difference between lesions > 15 cm2 in comparison to lesions < 15 cm2.
Age
Patient age negatively impacted implant survival only when a relatively low threshold of 30 years was chosen. Patients younger than 30 years had a significantly higher 15-year survival of 84% (95% CI, 66%–93%) than patients aged 30 to 45 years (68%; 95% CI, 58%–76%) (p = 0.02) and patients older than 45 years (66%; 95% CI, 46%–80%) (p = 0.05). There was no difference between the patients aged 30 to 45 years and 45 years or older (p = 0.86) (Fig. 7).
Fig. 7.
Survivorship of ACI. There was an improved survivorship in patients younger than 30 years.
There was no statistical difference in clinical outcome scores between the age groups, except for WOMAC. Here, significantly (p = 0.002) better scores were reached in the younger patient groups: patients younger than 30 years scored better than patients older than 45 years (18 ± 16 versus 30 ± 15); however, preoperative scores were also significantly (p = 0.003) higher in patients < 30 years (30 ± 18 versus 48 ± 21). There was also a significantly (p = 0.02) better outcome in the WOMAC postoperative score in the group that were aged 30 to 45 years in comparison to > 45 years (22 ± 16 versus 30 ± 15), whereas there was no preoperative difference (p = 0.1).
Workers Compensation
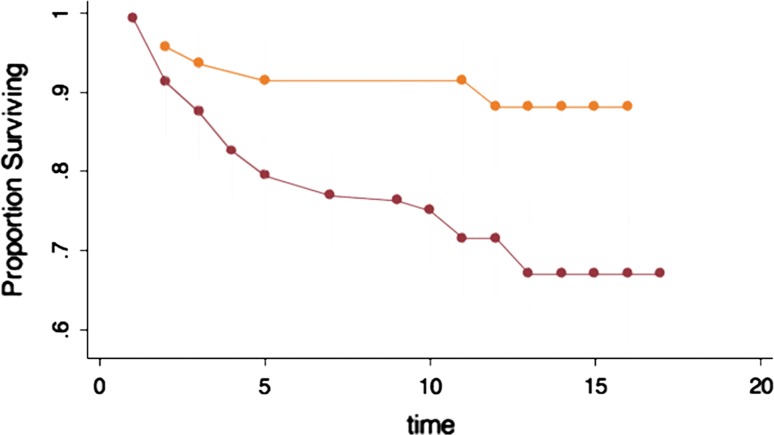
The difference in survivorship according to workers compensation status failed to reach statistical significance (p = 0.08). Survival was 79% versus 66% at 10 years, and 75% versus 62% at 15 years, for patients without and with workers compensation, respectively. Of the 46 patients with workers compensation, more than 50% (24 patients) had previously undergone attempts at cartilage repair with marrow stimulation techniques. In the marrow stimulation group, 10 patients (42%) failed compared with six patients (27%) who had ACI as the primary cartilage repair procedure (Fig. 8).
Fig. 8.
Survivorship of ACI. Workers compensation showed no significant difference.
Patient Satisfaction and Functional Outcomes
At the last followup, 75% of patients were satisfied with the procedure, 12% were neutral, and 11% were dissatisfied. A total of 86% reported that their knee was better than before the surgery, approximately the same in 12%, and worse in 11%. A total of 88% would choose to have ACI again if they could go back in time, 6% were uncertain, and 6% would not undergo the procedure again. A total of 78% rated the result of the surgery as good to excellent, 16% as fair, and 6% as poor (Table 6). The Physical Component of the SF-36 score increased from 33 ± 14 to 49 ± 18, whereas the Mental Component improved from 46 ± 14 to 52 ± 15 (both p < 0.001) (Table 7).
Table 6.
Satisfaction with the procedure at final followup for patients with successful ACI surgery (n = 137)
| Question | Percent |
|---|---|
| Compared with before each surgery, how would you rate your operated joint now? | |
| Better | 85.6 |
| About the same | 11.6 |
| Worse | 10.3 |
| What is your overall satisfaction level with the joint surgery? | |
| Satisfied | 75.3 |
| Neutral | 12.3 |
| Dissatisfied | 11.6 |
| If you could go back in time and make the decision again, would you choose to have your joint surgery? | |
| Yes | 87.7 |
| Uncertain | 6.2 |
| No | 6.2 |
| How would you rate the results of your joint surgery | |
| Good/excellent | 78.1 |
| Fair | 16.4 |
| Poor | 5.5 |
ACI = autologous chondrocyte implantation.
Table 7.
Outcome scores of the SF-36 (n = 136 [88.3%])
| Time | Statistic | Simple | Complex | Salvage | Overall |
|---|---|---|---|---|---|
| Physical Component | |||||
| Pretreatment | Mean | 27.1 | 32.0 | 34.5 | 32.7 |
| SD | 10.5 | 10.9 | 16.3 | 13.6 | |
| At final followup | Mean | 48.8 | 45.6 | 52.9 | 49.0 |
| SD | 21.1 | 18.3 | 17.0 | 18.1 | |
| p value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Mental Component | |||||
| Pretreatment | Mean | 44.5 | 46.6 | 44.9 | 45.7 |
| SD | 11.7 | 12.1 | 15.7 | 13.7 | |
| At final followup | Mean | 52.5 | 49.6 | 54.4 | 51.9 |
| SD | 19.3 | 16.0 | 12.6 | 15.0 | |
| p value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
Patients demonstrated significant improvements in all functional scores. The modified Cincinnati score improved from 3.9 ± 1.5 to 6.4 ± 1.5 (p < 0.0001). WOMAC improved from 39 ± 21 to 23 ± 16 (p < 0.0001) (Table 8). We also observed improvements in the KSS function (65 ± 23 to 78 ± 17, p < 0.0001) and knee pain scale (54 ± 18 to 79 ± 19, p < 0.0001) (Table 8).
Table 8.
Overall outcome scores at the latest followup for patients who had a successful autologous chondrocyte graft
| Outcome score | Preoperative | Postoperative | p value |
|---|---|---|---|
| Modified Cincinnati (n = 145) | 3.9 ± 1.5 | 6.4 ± 1.5 | < 0.0001 |
| KSS pain (n = 122) | 54 ± 18 | 79 ± 19 | < 0.0001 |
| KSS function (n = 122) | 65 ± 23 | 78 ± 17 | < 0.0001 |
| WOMAC (n = 145) | 39 ± 21 | 23 ± 16 | < 0.0001 |
Preoperative and postoperative values are mean ± SD; KSS = Knee Society score.
Discussion
Autologous chondrocyte implantation was described in 1994 as the first application of tissue engineering in orthopaedic surgery with the aim to treat symptomatic cartilage defects by regrowing hyaline-like cartilage. We present the first large cohort of patients with long-term prospective followup after treatment with ACI in the United States. At an average of 12 years, 157 of 210 patients (75%) had successful grafts. Nineteen patients had required revision surgery with knee arthroplasty. An additional 27 patients were salvaged using revision cartilage repair and seven patients with failed grafts elected to forego additional surgery. At the latest followup, 75% of patients reported being satisfied with the procedure and 78% rated their knee as good or excellent; 88% would choose to undergo the surgery again. Our study demonstrated durable outcomes of ACI as detected by patient-reported functional outcome measures and patient satisfaction questionnaires.
Our study had the limitations of a nonrandomized, single-arm patient cohort. Large, long-term randomized trials of cartilage repair techniques are desirable but are complicated by the comparative scarcity of these treatments (less than 2000 ACIs per year in the United States compared with > 500,000 knee arthroplasties); wide variation in defect size, number, and location; and associated comorbidities such as meniscal deficiency and malalignment. Its strengths lie in the single-surgeon design with a consistent approach to indications and treatment, use of prospectively collected data with multiple self-reported instruments independently filled by the patient, and a very high followup rate. The inclusion of every patient treated with ACI at our institution who had completed more than 10 years followup provides a true reflection of clinical reality, whereas many trials enroll less than 5% of all patients presenting with cartilage defects [11].
Steadman et al. [49] published their midterm results with microfracture, reporting that 7 years after surgery, 80% of patients rated their knee as improved. Only patients younger than 45 years with traumatic defects and without ligamentous or meniscal injury were analyzed. These strict inclusion criteria for the study resulted in a comparatively limited study size with only 72 patients treated between 1981 and 1991, an average of less than seven patients per year, which limits generalization of these results to the much more varied pathology presenting in everyday clinical practice. Other mid- to long-term outcome studies using inclusion criteria that are more applicable to clinical practice suggest a deterioration of functional outcome scores after 2 years [28–31, 39, 48]. It is of importance to note that prior microfracture therapy significantly decreased the ACI survivorship in our study to 44% in comparison to 79% graft survival for primary ACI. Only few articles have focused on this important matter but show striking evidence that suggests that prior subchondral surgery, especially microfracture, increases the failure rate of subsequent ACI [37, 44].
Osteochondral allograft transplantation [6, 16] has been used for the treatment of large osteochondral lesions: Gortz et al. [18] reported a 89% survival rate at 5½ years for the management of steroid-induced osteonecrosis of femoral condyles in patients who on average were 24.3 years of age. Comparing the same age group from our study demonstrates a virtually identical survival rate of 91% for ACI.
Outcomes for osteochondral autograft transfer [21, 22, 27] have been promising with good to excellent results in 92% of patients with femoral condylar defects, 87% in tibial resurfacing, and 74% in patellar and/or trochlear procedures [23]. Marcacci et al. [32] reported their midterm followup with comparable results but demonstrated inferior results in larger lesions such as the ones treated in our study with ACI.
Peterson et al. evaluated the long-term outcome of ACI with more than 10 years followup [45]. Patients in this cohort concluded that they were better than before the surgery in 74%, whereas 92% were satisfied with the procedure and would repeat it, findings that are comparable with ours. Especially young patients such as the ones treated in our study seek alternatives to arthroplasty surgery. Of 210 patients who presented with symptomatic cartilage lesions, 191 (91%) were able to preserve their native knee for a minimum of 10 years. Although patients with very large (> 15 cm2) and salvage-type lesions experienced worse graft survival than those with less cartilage damage, the clinical outcomes did not differ significantly in those patients with successful grafts. It is noteworthy that both Peterson’s and our study populations underwent surgery with a first-generation ACI technique that used a periosteal flap. A substantial number of these patients required additional procedures as a result of patch hypertrophy. Patient satisfaction with current second- and third-generation techniques that have a lower rate of subsequent surgery can potentially be expected to be higher.
Although many studies have demonstrated short-term success after various cartilage repair procedures, relatively few have provided information on long-term durability of 10 years and beyond. Such information, however, is essential for the appropriate selection of repair technique. Most patients presenting with cartilage defects are quite young; transient improvement in pain and function in the short term is therefore not adequate in a patient population that measures its remaining period of life and gainful employment in decades. Especially ACI has to demonstrate superior outcomes in the long term to justify its high upfront investment of cost and morbidity compared with other treatment options.
It is well recognized that the treatment of associated articular comorbidities, especially osteotomy for malalignment, is essential for the success of cartilage procedures [43, 46, 51–54]. The respective contribution of the cartilage versus osteotomy procedure to the overall outcome remains controversial. Survival of knees treated with ACI and concomitant osteotomy was 88% at 15 years. This compares favorably with survival rates published in the literature for isolated HTO of 46% to 65% at this time interval [19, 47]. With the numbers available, both female sex and workers compensation status demonstrated worse survival but failed to reach statistical significance. Several recent studies have demonstrated higher failure rates of cartilage repair in women [12, 14, 55], a phenomenon that at this time has not been investigated thoroughly. Workers compensation status generally predicts worse outcomes after many surgical procedures, including rotator cuff repair and knee arthroplasty [38, 40]. In comparison, the differences between patients with and without workers compensation benefits seen in this study were fairly small, a noteworthy finding given the importance of strict compliance with the complex rehabilitation program. Similar results were reported by Yates who also were unable to detect a significant impact of workers compensation status on the outcome of ACI [58]. Furthermore, a much larger percentage of workers compensation patients in our study had undergone previous marrow stimulation than non-workers compensation patients, a confounding factor that most likely is of more importance to the overall results than the actual workers compensation status.
In conclusion, our study demonstrated durable outcomes of ACI that allowed most patients with an age younger than 45 years, defect sizes less than 15 cm2, no prior microfracture, and concomitant HTO for malalignment to preserve their knee with avoidance of knee arthroplasty. Prior microfracture and treatment for very large defects were associated with inferior survivorship and clinical scores.
Acknowledgments
Conflict of interest
One or more of the authors (TM, AHG) certifies that he, or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of less than USD 10,000, from Genzyme BioSurgery/Sanofi (Cambridge, MA, USA). The institution of one or more of the authors (TM, AHG), Brigham and Women's Hospital, Boston, MA, USA, has received during the study period funding from Genzyme BioSurgery/Sanofi.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–138. [PubMed] [Google Scholar]
- 2.Barber-Westin SD, Noyes FR. Objective criteria for return to athletics after anterior cruciate ligament reconstruction and subsequent reinjury rates: a systematic review. Phys Sportsmed. 2011;39:100–110. doi: 10.3810/psm.2011.09.1926. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 4.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15:S230–S235. [PubMed] [Google Scholar]
- 5.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 6.Bugbee WD, Convery FR. Osteochondral allograft transplantation. Clin Sports Med. 1999;18:67–75. doi: 10.1016/S0278-5919(05)70130-7. [DOI] [PubMed] [Google Scholar]
- 7.Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52:2033–2039. doi: 10.1002/art.21148. [DOI] [PubMed] [Google Scholar]
- 8.Coventry MB, Ilstrup DM, Wallrichs SL. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J Bone Joint Surg Am. 1993;75:196–201. doi: 10.2106/00004623-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, Cicuttini FM. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:337–342. doi: 10.1016/j.joca.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635–2641. [PubMed] [Google Scholar]
- 11.Engen CN, Engebretsen L, Aroen A. Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopedic practice. Cartilage. 2010;1:312–319. doi: 10.1177/1947603510373917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filardo G, Kon E, Di Martino A, Patella S, Altadonna G, Balboni F, Bragonzoni L, Visani A, Marcacci M. Second-generation arthroscopic autologous chondrocyte implantation for the treatment of degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2012;20:1704–1713. doi: 10.1007/s00167-011-1732-5. [DOI] [PubMed] [Google Scholar]
- 13.Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176–181. [PubMed] [Google Scholar]
- 14.Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1456–1464. doi: 10.1007/s00167-010-1042-3. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith CH, Boers M, Bombardier C, Tugwell P. Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee. J Rheumatol. 1993;20:561–565. [PubMed] [Google Scholar]
- 16.Gortz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374–1384. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Gortz S, Bugbee WD. Allografts in articular cartilage repair. Instr Course Lect. 2007;56:469–480. [PubMed] [Google Scholar]
- 18.Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468:1269–1278. doi: 10.1007/s11999-010-1250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gstottner M, Pedross F, Liebensteiner M, Bach C. Long-term outcome after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128:111–115. doi: 10.1007/s00402-007-0438-0. [DOI] [PubMed] [Google Scholar]
- 20.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE, Kannel WB. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–358. doi: 10.2105/AJPH.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hangody L, Dobos J, Balo E, Panics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38:1125–1133. doi: 10.1177/0363546509360405. [DOI] [PubMed] [Google Scholar]
- 22.Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(Suppl 2):25–32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hangody L, Vasarhelyi G, Hangody LR, Sukosd Z, Tibay G, Bartha L, Bodo G. Autologous osteochondral grafting—technique and long-term results. Injury. 2008;39(Suppl 1):S32–S39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Heir S, Nerhus TK, Rotterud JH, Loken S, Ekeland A, Engebretsen L, Aroen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231–237. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 25.Hunter W. On the structure and diseases of articulating cartilage. Philos Trans R Soc Lond B Biol Sci. 1743;9:267.
- 26.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 27.Kish G, Hangody L. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2004;86:619; author reply 619–620. [PubMed]
- 28.Kon E, Filardo G, Berruto M, Benazzo F, Zanon G, Della Villa S, Marcacci M. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med. 2011;39:2549–2557. doi: 10.1177/0363546511420688. [DOI] [PubMed] [Google Scholar]
- 29.Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33–41. doi: 10.1177/0363546508323256. [DOI] [PubMed] [Google Scholar]
- 30.Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, Ghanem N, Uhl M, Sudkamp N. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180–1186. doi: 10.1016/j.arthro.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Sudkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Marcacci M, Kon E, Delcogliano M, Filardo G, Busacca M, Zaffagnini S. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med. 2007;35:2014–2021. doi: 10.1177/0363546507305455. [DOI] [PubMed] [Google Scholar]
- 33.Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82:1252–1259. doi: 10.2106/00004623-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Minas T. The role of cartilage repair techniques, including chondrocyte transplantation, in focal chondral knee damage. Instr Course Lect. 1999;48:629–643. [PubMed] [Google Scholar]
- 35.Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005;436:30–39. doi: 10.1097/01.blo.0000171916.40245.5d. [DOI] [PubMed] [Google Scholar]
- 36.Minas T, Chiu R. Autologous chondrocyte implantation. Am J Knee Surg. 2000;13:41–50. [PubMed] [Google Scholar]
- 37.Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902–908. doi: 10.1177/0363546508330137. [DOI] [PubMed] [Google Scholar]
- 38.Misamore GW, Ziegler DW, Rushton JL., 2nd Repair of the rotator cuff. A comparison of results in two populations of patients. J Bone Joint Surg Am. 1995;77:1335–1339. doi: 10.2106/00004623-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 40.Mont MA, Mayerson JA, Krackow KA, Hungerford DS. Total knee arthroplasty in patients receiving Workers’ Compensation. J Bone Joint Surg Am. 1998;80:1285–1290. doi: 10.2106/00004623-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Noyes FR, Barber-Westin SD. Arthroscopic-assisted allograft anterior cruciate ligament reconstruction in patients with symptomatic arthrosis. Arthroscopy. 1997;13:24–32. doi: 10.1016/S0749-8063(97)90206-1. [DOI] [PubMed] [Google Scholar]
- 42.Noyes FR, Barber-Westin SD, Hewett TE. High tibial osteotomy and ligament reconstruction for varus angulated anterior cruciate ligament-deficient knees. Am J Sports Med. 2000;28:282–296. doi: 10.1177/03635465000280030201. [DOI] [PubMed] [Google Scholar]
- 43.Parker DA, Beatty KT, Giuffre B, Scholes CJ, Coolican MR. Articular cartilage changes in patients with osteoarthritis after osteotomy. Am J Sports Med. 2011;39:1039–1045. doi: 10.1177/0363546510392702. [DOI] [PubMed] [Google Scholar]
- 44.Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40:325–331. doi: 10.1177/0363546511425651. [DOI] [PubMed] [Google Scholar]
- 45.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 46.Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25:533–537. doi: 10.1177/036354659702500417. [DOI] [PubMed] [Google Scholar]
- 47.Rinonapoli E, Mancini GB, Corvaglia A, Musiello S. Tibial osteotomy for varus gonarthrosis. A 10- to 21-year followup study. Clin Orthop Relat Res. 1998;353:185–193. doi: 10.1097/00003086-199808000-00021. [DOI] [PubMed] [Google Scholar]
- 48.Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S–19S. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 49.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 50.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(Suppl):S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 51.Sterett WI, Steadman JR. Chondral resurfacing and high tibial osteotomy in the varus knee. Am J Sports Med. 2004;32:1243–1249. doi: 10.1177/0363546503259301. [DOI] [PubMed] [Google Scholar]
- 52.Sterett WI, Steadman JR, Huang MJ, Matheny LM, Briggs KK. Chondral resurfacing and high tibial osteotomy in the varus knee: survivorship analysis. Am J Sports Med. 2010;38:1420–1424. doi: 10.1177/0363546509360403. [DOI] [PubMed] [Google Scholar]
- 53.Tang WC, Henderson IJ. High tibial osteotomy: long term survival analysis and patients’ perspective. Knee. 2005;12:410–413. doi: 10.1016/j.knee.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Tjornstrand B, Egund N, Hagstedt B, Lindstrand A. Tibial osteotomy in medial gonarthrosis. The importance of over-correction of varus deformity. Arch Orthop Trauma Surg. 1981;99:83–89. doi: 10.1007/BF00389742. [DOI] [PubMed] [Google Scholar]
- 55.Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39:2566–2574. doi: 10.1177/0363546511422220. [DOI] [PubMed] [Google Scholar]
- 56.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405–413; discussion 415–420. [DOI] [PubMed]
- 57.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Yates JW Jr. The effectiveness of autologous chondrocyte implantation for treatment of full-thickness articular cartilage lesions in Workers’ Compensation patients. Orthopedics. 2003;26:295–300; discussion 300–391. [DOI] [PubMed]