Abstract
Glycosylation is an important and common form of posttranscriptional modification of proteins in cells. A vast array of biological functions has been ascribed to glycans during the last decade thanks to a rapid evolution in glycomic technologies. Glycogenes highly expressed at the human ocular surface include families of glycosyltransferases, proteoglycans, glycan degradation proteins, as well as mucins and carbohydrate-binding proteins such as the galectins. On the apical glycocalyx, mucin O-glycans promote boundary lubrication, prevent bacterial adhesion and endocytic activity, and maintain epithelial barrier function through interactions with galectins. The emerging roles attributed to glycans are contributing to the appreciation of their biological capabilities at the ocular surface.
Keywords: Glycobiology, ocular surface, mucin O-glycans, galectin-3, epithelial barrier function
1. Introduction to glycobiology
All cells in nature carry an array of covalently attached carbohydrates or carbohydrate chains generically referred to as glycans. Glycans on the cell surface glycocalyx and on secreted proteins modulate a wide variety of cell–cell, cell-pathogen, and cell–matrix events critical to the function of a multicellular organism and its interaction with the extracellular environment. Glycobiology, a term first coined in the late 1980s, is the scientific discipline that studies the structure, biosynthesis, biology, and evolution of carbohydrates as well as the proteins that recognize them [1]. Glycomics is the technology aimed at understanding how a collection of glycans relates to a particular biological event [2]. Important facts about glycans include the following:
Glycosylation is the most common form of posttranscriptional modification of proteins on cells.
Over half of all proteins are estimated to contain one or more glycan chains.
All cells in nature are covered with a dense and complex array of glycans called glycocalyx.
The glycome or glycan repertoire of a cell is much more diverse than either the genome or proteome.
Glycosyltransferases, enzymes responsible for the biosynthesis of glycans, are expressed in a tissue-specific, temporally regulated manner.
The glycome of a given cell type or organism is dynamic, changing in response to intrinsic and extrinsic signals.
2. Glycome of the human ocular surface epithelia
The genes involved in the addition or modification of carbohydrates in glycoconjugates are generically called glycogenes, and include glycosyltransferases, glycolytic enzymes, sugar nucleotide synthetases, sugar nucleotide transporters, and, in a broader sense, carbohydrate-binding proteins such as lectins [3]. A major development towards obtaining global information relating to glycan biosynthesis, structure, and function has been carried out using focused gene microarrays, termed glycogene-chips, using Affymetrix technology [4]. These chips contain families of glycogenes highly annotated by experts in the field.
The glycogene-chip array has been used to analyze hundreds of glycogenes in human conjunctival epithelial cells obtained by impression cytology [5]. In these experiments, 424 glycogenes were identified in healthy conjunctival epithelium. The largest glycogene groups included several families of glycosyltransferases, proteoglycans, growth factors, glycan degradation proteins, and Notch signaling molecules (Table 1). Mucins and carbohydrate-binding proteins were found to be among the most highly expressed glyogenes in human conjunctiva. In this review, we will focus on the role of mucin O-glycans and carbohydrate-binding proteins in the protection of the ocular surface.
Table 1.
Glycogenes highly expressed in healthy human conjunctival epithelia
|
|
3. Roles for mucin O-glycans in tears
Mucins are a heterogeneous group of extraordinarily large glycoproteins found as major components in all mucous secretions present on wet-surfaced epithelium. It was W. Pigman and co-workers who, in a series of papers between 1960 and 1975, elegantly demonstrated that mucins are structurally characterized by the presence of central tandem repeats of amino acids rich in serine and threonine, to which O-linked glycans attach [6]. At the ocular surface, MUC5AC is the major secreted mucin found in secretory granules within conjunctival goblet cells that, upon fusion with the plasma membrane, are secreted into the tear film.
As determined by fluorometric, high-performance liquid chromatography, the O-glycan profile of human tear mucins consists primarily of core 1 (Galβ1-3GalNAcα1-Ser/Thr)-based structures [7]. More specifically, two thirds of the O-glycan pool in tears consists of monosialylated core 1 structures, being α2-6-sialyl core 1 the predominant O-glycan structure—representing half the O-glycan pool—in human tears. The roles of mucin-type O-glycans in tears are believed to be varied and include hydration, lubrication, and clearance of pathogens and debris.
3.1. Hydration and lubrication
The glycosylated regions of mucins are hydrophilic and contribute to the prevention of ocular surface desiccation by binding water. Mucin hydration is necessary for gelification, a physical property impaired in deglycosylated mucin (or apomucin), which is insoluble in water [8]. The capacity of mucins to form gels is facilitated by their ability to polymerize through cysteine-rich D domains that show sequence homology to the von Willebrand factor. To date, however, there is no evidence that MUC5AC is present in tears as a disulphide-linked multimer. MUC5AC in tears migrates further in SDS-agarose gels than do human stomach, cervix, or gall bladder mucins, and the molecular weight of MUC5AC in tears is smaller than that of conjunctival tissue protein, which suggests processing of MUC5AC upon secretion into tears [9,10].
3.2. Clearance of pathogens
Mucins on mucosal surfaces provide an important innate immune function by detoxifying noxious molecules and by trapping and removing pathogens and particulates from epithelial surfaces [11]. In the tear fluid, mucins bind P. aeruginosa and modulate accessibility of the pathogen to the epithelial glycocalyx [12,13]. The binding site of P. aeruginosa corresponds to a high molecular weight glycoprotein that contains α2-6 sialic acid, as shown by staining with Sambucus nigra agglutinin [14]. As mentioned above, α2-6-sialyl core 1 is the predominant O-glycan structure in human tears, suggesting that mucin O-glycans could potentially act as receptors for P. aeruginosa in tears and mediate bacterial clearance at the ocular surface.
4. Roles for mucin O-glycans in the glycocalyx
In addition to secreted MUC5AC in conjunctival goblet cells, ocular surface epithelia produce the transmembrane mucins MUC1, -4, and -16 on their apical glycocalyces. The predominant O-glycans in these mucins include core 1-based glycan structures [15]. Interestingly, the epithelia contain α2-3 sialyl core 1 as the main O-glycan, in contrast with α2-6 sialyl core 1 in tears.
Transmembrane mucins extend at least 200-500 nm above the plasma membrane, far above other glycoconjugates in the glycocalyx [16,17,18]. Clustered O-glycans are important to maintaining the highly extended and rigid structure of the mucin protein backbone, as they induce the mucin peptide core to adopt a stiff and extended conformation that prevents folding into a globular structure [19]. Densely packed O-glycan chains on transmembrane mucins have been postulated to modulate a variety of biological functions at the ocular surface apical glycocalyx (discussed below).
4.1. Boundary lubrication
Under normal conditions, O-glycans on transmembrane mucins play a role in preventing apical surface adhesion and contribute to the boundary lubrication of opposing membranes in mucosal tissues. The anti-adhesive character of mucins at the ocular surface was initially observed by atomic force microscopy [20]. Using human ocular mucins extracted and purified from human conjunctival tissue using classical mucin purification techniques, Berry et al. demonstrated that the number of mucin-mucin interactions is minimal as compared to mucin-mica interactions. More recently, the role of cell surface O-glycans in providing boundary lubrication has been studied using static and dynamic flow adhesion assays in human corneal epithelial cells treated with benzyl-α-GalNAc, a chemical primer commonly used to suppress the elongation of O-glycan chains [21]. These experiments demonstrated that the cell surface character of corneal epithelial cells producing mucin O-glycans is more anti-adhesive than that of cells with altered O-glycans. Interestingly, negative charges on the cell surface were not found to be involved in preventing adhesion, as would be expected due to negative charge repulsion.
The observation of increased cell surface adhesion after truncation of O-glycan chain elongation is consistent with the concept that O-glycans contribute to the maintenance of an extended structure in transmembrane mucins that prevents cell-cell adhesion. After the synthesis of the hydrophilic carbohydrate side chains is blocked, the extended structure of the membrane-associated mucins may collapse [22] and/or expose a more adhesive, hydrophobic protein backbone that facilitates cellular attachment [23].
4.2. Protection against pathogen colonization
The apical cell layers of the corneal epithelium produce a mucin-rich environment thought to contribute to the prevention of infection. Staphylococcus aureus is one of the most frequent causes of bacterial keratitis. Infection can be severe, leading to corneal ulceration and perforation if not treated effectively. The ability of S. aureus to adhere to the epithelial cell glycocalyx is thought to be one of the first steps in the colonization and infection of wet mucosal surfaces [24,25,26].
Using static and liquid phase adhesion assays, Ricciuto et al. demonstrated that O-glycans in the cell surface glycocalyx limit adherence of S. aureus to corneal epithelial cells [27]. S. aureus bound more predominantly to corneal epithelial cells containing truncated O-glycans, independently of bacterial invasive mechanisms, such as biosynthesis of cell surface adhesins and secretion of virulence factors. A component of the defense mechanism of the ocular surface against pathogen colonization is thought to include the presence of high levels of O-acetyl sialic acid on transmembrane mucins [28,29]. Treatment of corneal epithelial cells with sialidase from Arthrobacter ureafaciens—which hydrolyzes mucin-associated O-acetyl sialic acid—but not from Clostridium perfringens, resulted in an increase in S. aureus adhesion, indicating that O-acetyl groups on transmembrane mucins at the ocular surface constitute an alternative mechanism for preventing mucin degradation and access to specific receptors at the ocular surface. In human conjunctival epithelial cells, induction of mucin expression also results in reduced adherence of nontypeable S. pneumoniae to the cell surface, whereas sialidase treatment enhances adherence [30].
4.3. Barrier function through lectin interactions
As mentioned in Section 2, the glycogene chip array has allowed researchers to determine what glycogenes are among the most highly expressed in human tissues. A critical finding in our laboratory was the identification of galectin-3 as the most highly expressed carbohydrate-binding protein in human conjunctival epithelium, corroborating previous work showing the presence of galectin-3 in corneal and conjunctival epithelia [31,32,33]. Data from the glycogene chip array also indicated that galectin-8 and -9 were present in human conjunctival epithelium, although in much lower concentrations than galectin-3 [5].
Galectins are a family of animal β-galactoside-binding lectins, characterized by their evolutionary conserved carbohydrate-recognition domain (CRD) [34,35]. The biological activities of galectins are commonly associated to their ligand cross-linking properties. Galectin-3, unlike other members of the galectin family, exists as a monomer. However, the N-terminal domain of galectin-3 oligomerizes after ligand binding at the C-terminal domain. Quantitative precipitation studies have shown that galectin-3 promptly precipitates as a pentamer in the presence of multivalent N-acetyllactosamine ligands in a process mediated through the proline- and glycine-rich N-terminal domain [36]. Recent data also indicate that galectin-3 can oligomerize through the C-terminal domain [37]. This association, termed type-C self-association, involves binding of the carbohydrate recognition site of one galectin-3 to another site on the CRD of another galectin-3 molecule to form dimers or oligomers. Oligomerization of galectin-3 occurs on cell surfaces at physiological concentrations of the lectin, resulting in galectin-3 lattices that are robust and resistant to lateral movement of membrane components on the glycocalyx [38].
Based on these findings, we hypothesized that interaction of mucin O-glycans with galectin-3 would result in a highly organized and protective cell surface lattice barrier on the apical glycocalyx of ocular surface epithelial cells. Galactose, in the form of core 1 structures, is a major component of ocular surface mucins and, therefore, could potentially act as a ligand for the carbohydrate-binding domain of galectin-3. This hypothesis was supported by pull-down assays showing MUC1 and MUC16 binding to galectin-3 affinity columns in a galactose-dependent manner, and by immunofluorescence analyses showing apical co-localization of galectin-3 and transmembrane mucins on the ocular surface epithelial glycocalyx [39]. Furthermore, downregulation of mucin O-glycosylation using a stable tetracycline-inducible RNA interfering system to knockdown core 1 β1,3-galactosyltransferase (C1galt1; T-synthase), a critical galactosyltransferase required for the synthesis of core 1 O-glycans, resulted in decreased cell surface O-glycosylation, reduced cell surface galectin-3, and increased corneal epithelial cell permeability as indicated by the rose bengal diagnostic dye [39].
Overall, these results indicate that two barriers contribute to the protection of the ocular surface epithelia against noxious molecules and pathogens—first, the traditional paracellular barrier containing the tight junctions that seal the space between adjacent cells, and, second, the transcellular barrier formed by the association of transmembrane mucins and galectin-3 on the extensive apical glycocalyx of the ocular surface epithelia.
4.4. Prevention of endocytosis and nanoparticle uptake
A major requirement for mucosal surfaces directly challenged by a microbe-rich environment is to be resistant to engulfment of noxious substances and infective particles [40,41]. Endocytic activity and recycling of plasma membrane can be detrimental to the exposed ocular surface, as it could lead to internalization of viruses and bacteria by the most apical cells [42,43].
The thick coat of glycans on the apical glycocalyx is critical to preventing access and uptake of macromolecules and pathogens by mucosal surfaces. Recent work by our group indicates that O-glycans on the apical surface of human corneal epithelial cells contribute to the maintenance of barrier function by preventing endocytosis [44]. Targeted disruption of O-glycosylation by interference with C1galt1 results in increased accumulation of plasma membrane protein in endosomes and increased translocation of negatively charged nanoparticles in a clathrin-dependent manner. These data support a model by which O-glycans on the epithelial glycocalyx bind galectin-3 and limit constitutive endocytosis to promote barrier function (Figure 1). Based on this model, it is possible to speculate that transient manipulation of O-glycans in the glycocalyx could constitute an alternative approach to delivering therapeutic nanoparticles into mucosal surfaces.
Figure 1.
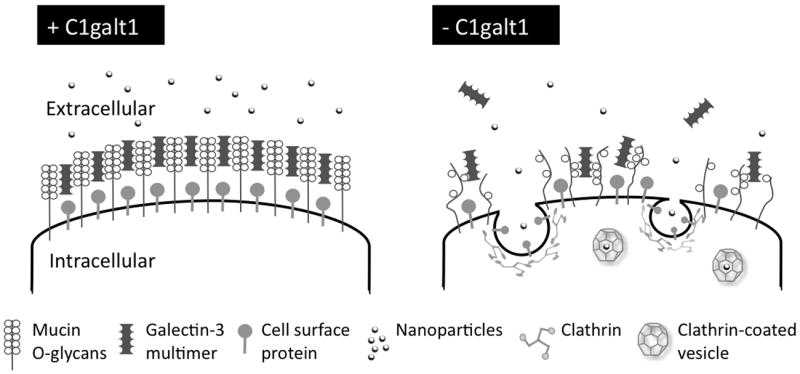
Proposed model for cell surface O-glycans preventing endocytosis in human corneal epithelial cells. Exposed mucosal surfaces limit constitutive endocytosis under physiological conditions to prevent uptake of macromolecules and pathogens. Expression of C1galt1 results in mucin O-glycan biosynthesis and formation of cell surface lattices through interaction with galectin-3 multimers. Abrogation of C1galt1 decreases the availability of mucin carbohydrate ligands in the glycocalyx, causing disruption of galectin-glycan complexes, and triggering clathrin-dependent endocytosis of plasma membrane proteins and extracellular components.
5. Conclusions and future directions
Glycans play a critical role in regulating homeostasis and allowing mucosal surfaces to function effectively in a broad range of environmental conditions. Several families of glycosyltransferases, proteoglycans, glycan degradation proteins, as well as mucins and carbohydrate-binding proteins, are highly expressed at the human ocular surface. Recent work has identified roles for mucin O-glycans in promoting boundary lubrication, protecting the ocular surface against bacterial adhesion, and maintaining epithelial barrier function through interaction with galectin-3. Ongoing research highlights the alteration of glycans in ocular surface disease. In dry eye, several glycogenes are significantly altered and include mucin-type glycosyltransferases, members of the Notch signaling pathway, Wnt signaling molecules, and heparan sulfate sulfotransferases. Unfortunately, the role of the vast majority of glycogenes in maintaining ocular surface health remains poorly characterized. Evaluating their contribution to ocular surface pathology will likely prove to be rewarding.
Acknowledgments
Financial support provided by NIH grant R01EY014847.
Footnotes
There are no conflicts of interest to report.
References
- 1.Varki A, Sharon N. Historical background and overview. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al., editors. Essentials of Glycobiology. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. pp. 1–22. [PubMed] [Google Scholar]
- 2.Bertozzi CR, Sasisekharan R. Glycomics. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al., editors. Essentials of Glycobiology. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. pp. 633–648. [PubMed] [Google Scholar]
- 3.Narimatsu H. Construction of a human glycogene library and comprehensive functional analysis. Glycoconj J. 2004;21:17–24. doi: 10.1023/B:GLYC.0000043742.99482.01. [DOI] [PubMed] [Google Scholar]
- 4.Comelli EM, Head SR, Gilmartin T, Whisenant T, Haslam SM, North SJ, et al. A focused microarray approach to functional glycomics: transcriptional regulation of the glycome. Glycobiology. 2006;16:117–131. doi: 10.1093/glycob/cwj048. [DOI] [PubMed] [Google Scholar]
- 5.Mantelli F, Schaffer L, Dana R, Head SR, Argueso P. Glycogene expression in conjunctiva of patients with dry eye: downregulation of Notch signaling. Invest Ophthalmol Vis Sci. 2009;50:2666–2672. doi: 10.1167/iovs.08-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka K, Bertolini M, Pigman W. Serine and threonine glycosidic linkages in bovine submaxillary mucin. Biochem Biophys Res Commun. 1964;16:404–409. doi: 10.1016/0006-291x(64)90366-3. [DOI] [PubMed] [Google Scholar]
- 7.Guzman-Aranguez A, Mantelli F, Argueso P. Mucin-type O-glycans in tears of normal subjects and patients with non-Sjogren’s dry eye. Invest Ophthalmol Vis Sci. 2009;50:4581–4587. doi: 10.1167/iovs.09-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansil R, Stanley E, LaMont JT. Mucin biophysics. Annu Rev Physiol. 1995;57:635–657. doi: 10.1146/annurev.ph.57.030195.003223. [DOI] [PubMed] [Google Scholar]
- 9.Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84:939–950. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry M, Ellingham RB, Corfield AP. Human preocular mucins reflect changes in surface physiology. Br J Ophthalmol. 2004;88:377–383. doi: 10.1136/bjo.2003.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 12.Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun. 2003;71:3866–3874. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiszig SM, Zaidi TS, Ramphal R, Pier GB. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect Immun. 1994;62:1799–1804. doi: 10.1128/iai.62.5.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aristoteli LP, Willcox MD. The adhesion of Pseudomonas aeruginosa to high molecular weight human tear film species corresponds to glycoproteins reactive with Sambucus nigra lectin. Exp Eye Res. 2006;83:1146–1153. doi: 10.1016/j.exer.2006.06.002. Epub 2006 Jul 1114. [DOI] [PubMed] [Google Scholar]
- 15.Guzman-Aranguez A, Argueso P. Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul Surf. 2010;8:8–17. doi: 10.1016/s1542-0124(12)70213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komatsu M, Carraway CA, Fregien NL, Carraway KL. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245–33254. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- 17.Bramwell ME, Wiseman G, Shotton DM. Electron-microscopic studies of the CA antigen, epitectin. J Cell Sci. 1986;86:249–261. doi: 10.1242/jcs.86.1.249. [DOI] [PubMed] [Google Scholar]
- 18.Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992;17:359–363. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- 19.Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990;15:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 20.Berry M, McMaster TJ, Corfield AP, Miles MJ. Exploring the molecular adhesion of ocular mucins. Biomacromolecules. 2001;2:498–503. doi: 10.1021/bm000145y. [DOI] [PubMed] [Google Scholar]
- 21.Sumiyoshi M, Ricciuto J, Tisdale A, Gipson IK, Mantelli F, Argueso P. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:197–203. doi: 10.1167/iovs.07-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shogren R, Gerken TA, Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry. 1989;28:5525–5536. doi: 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- 23.Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clin Exp Metastasis. 2002;19:339–345. doi: 10.1023/a:1015590515957. [DOI] [PubMed] [Google Scholar]
- 24.Agerer F, Lux S, Michel A, Rohde M, Ohlsen K, Hauck CR. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J Cell Sci. 2005;118:2189–2200. doi: 10.1242/jcs.02328. [DOI] [PubMed] [Google Scholar]
- 25.Hauck CR, Agerer F, Muenzner P, Schmitter T. Cellular adhesion molecules as targets for bacterial infection. Eur J Cell Biol. 2006;85:235–242. doi: 10.1016/j.ejcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Jett BD, Gilmore MS. Host-parasite interactions in Staphylococcus aureus keratitis. DNA Cell Biol. 2002;21:397–404. doi: 10.1089/10445490260099683. [DOI] [PubMed] [Google Scholar]
- 27.Ricciuto J, Heimer SR, Gilmore MS, Argueso P. Cell surface O-glycans limit Staphylococcus aureus adherence to corneal epithelial cells. Infect Immun. 2008;76:5215–5220. doi: 10.1128/IAI.00708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argueso P, Sumiyoshi M. Characterization of a carbohydrate epitope defined by the monoclonal antibody H185: sialic acid O-acetylation on epithelial cell-surface mucins. Glycobiology. 2006;16:1219–1228. doi: 10.1093/glycob/cwl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 30.Williamson YM, Gowrisankar R, Longo DL, Facklam R, Gipson IK, Ades EP, et al. Adherence of nontypeable Streptococcus pneumoniae to human conjunctival epithelial cells. Microb Pathog. 2008;44:175–185. doi: 10.1016/j.micpath.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, et al. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277:42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- 32.Gupta SK, Masinick S, Garrett M, Hazlett LD. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect Immun. 1997;65:2747–2753. doi: 10.1128/iai.65.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hrdlickova-Cela E, Plzak J, Smetana K, Jr, Melkova Z, Kaltner H, Filipec M, et al. Detection of galectin-3 in tear fluid at disease states and immunohistochemical and lectin histochemical analysis in human corneal and conjunctival epithelium. Br J Ophthalmol. 2001;85:1336–1340. doi: 10.1136/bjo.85.11.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 37.Lepur A, Salomonsson E, Nilsson UJ, Leffler H. Ligand induced galectin-3 protein self-association. J Biol Chem. 2012;287:21751–21756. doi: 10.1074/jbc.C112.358002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieminen J, Kuno A, Hirabayashi J, Sato S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem. 2007;282:1374–1383. doi: 10.1074/jbc.M604506200. [DOI] [PubMed] [Google Scholar]
- 39.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284:23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Bijl P, van Eyk AD. Human vaginal mucosa as a model of buccal mucosa for in vitro permeability studies: an overview. Curr Drug Deliv. 2004;1:129–135. doi: 10.2174/1567201043479975. [DOI] [PubMed] [Google Scholar]
- 41.Thoft RA, Friend J. Permeability of regenerated corneal epithelium. Exp Eye Res. 1975;21:409–416. doi: 10.1016/0014-4835(75)90123-2. [DOI] [PubMed] [Google Scholar]
- 42.Shah A, Farooq AV, Tiwari V, Kim MJ, Shukla D. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Mol Vis. 2010;16:2476–2486. [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto N, Petroll MW, Cavanagh HD, Jester JV. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:1348–1355. doi: 10.1167/iovs.04-0542. [DOI] [PubMed] [Google Scholar]
- 44.Guzman-Aranguez A, Woodward AM, Pintor J, Argueso P. Targeted disruption of core 1 betal,3-galactosyltransferase (C1galt1) induces apical endocytic trafficking in human corneal keratinocytes. PloS one. 2012;7:e36628. doi: 10.1371/journal.pone.0036628. [DOI] [PMC free article] [PubMed] [Google Scholar]


