The intestinal stem cell (ISC) niche is a highly complex microenvironment that supports stem cell maintenance and precisely orchestrates the balance between proliferation and differentiation. Here, multiple signaling molecules emanating from both the underlying mesenchyme and crypt-based epithelial cells cooperate to govern stem cell homeostasis (Figure 1). Understanding the cellular and molecular constituents of the niche is of critical importance to harness stem cells for tissue regeneration and to elucidate disease mechanisms. The conserved Wnt, Notch and epidermal growth factor (EGF) signaling pathways are identified regulators of stem cell behavior, however resolution of the cellular players and the impact of signaling molecules on different progenitor populations residing within the niche remain poorly understood. In this issue of Gastroenterology, a group led by Hans Clevers (Farin et al.)[1] specifically address the niche requirement of Paneth cell (PC)-derived Wnt3 in mediating intestinal homeostasis. Analysis of Wnt3-null epithelia in both in vivo, and in vitro cultures resulted in no apparent effect within the intact small intestine, but lack of growth in the culture system. While PCs have been defined to express Wnt6 and 9b in vivo, PCs did not express adequate levels to rescue the phenotype in vitro; instead, addition of the mesenchymally-expressed Wnt2b ligand supported enteroid growth. These findings highlight the robust compensatory signaling support provided by the underlying mesenchymal niche cells despite loss of an epithelial Wnt source.
Figure 1.
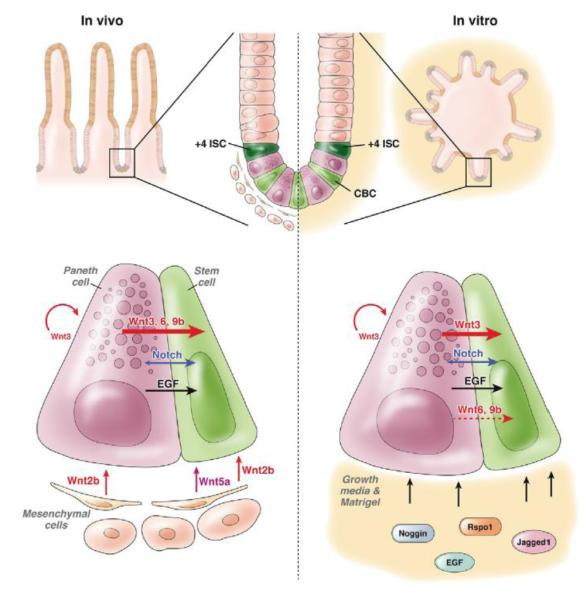
Comparison of in vivo (left) and in vitro (right) signaling support to the intestinal stem cell. Signaling networks between the Paneth cell and stem cell appear to be broadly conserved. However, disruption on one element of a single pathway highlights the important compensation wired into the system where in vivo environments can rely upon mesenchymal cells to maintain signaling homeostasis. Wnt, Notch and signaling pathways are key players in the balance between stem cell maintenance and differentiation.
The stem cell niche regulates intestinal stem cell behavior
The physical invagination of the intestinal crypt provides a functional ISC niche that consists of mesenchymal and epithelial cells within intimate proximity to facilitate tight regulation of the epithelial stem and progenitor populations[2]. Cells within the mesenchyme cluster around the base of crypts and include mesenchymal stem cells, sub-epithelial myofibroblasts, smooth muscle cells of the muscularis mucosae, lymph and vascular endothelial cells, and a variety of bone-marrow derived stromal cells[3]. This microenvironment contains additional factors including nerve cells and extracellular matrix components[4]. The cell census and actual contribution of each cell type in regulating ISCs are not clear. Epithelial niche constituents include both differentiated and progenitor cells, anchored at or near the crypt-base via cellular adhesions. Finely tuned positive and negative feed-back signaling between niche cells regulates stem cell behavior during tissue homeostasis and regeneration[2].
It is believed that the intestinal epithelium is maintained by two distinct pools of ISCs. The first are the quiescent ISCs located at the +4 position[5], more recently defined by their expression of Bmi1, Hopx, mTert and Lrig1[6-9], and the second are the rapidly cycling crypt-based columnar cells (CBCs) expressing the Wnt target gene Lgr5[10]. While the hierarchal relationships between these two ISC pools are only beginning to be uncovered[9, 11, 12], it is evident that each population has different signaling requirements to govern their behavior during tissue homeostasis and regeneration[5]. Lineage tracing experiments verify that cells expressing each of these markers are multipotent stem cells capable of giving rise to the major differentiated epithelial lineages: absorptive enterocytes and secretory enteroendocrine, tuft, goblet and PCs. Unlike other differentiated lineages, PCs are the only one to reside in the crypt-base, directly below quiescent +4 ISCs and interspersed among cycling Lgr5+ CBCs. PCs have a well-established immune role; producing antimicrobial agents that protect the epithelium from bacterial pathogens[13]. Recent evidence supports additional niche functions for PCs including production of important signaling factors--canonical Wnt ligands Wnt3, Wnt6 and Wnt9b, Notch ligands Dll-1 and Dll-4, and EGF[14, 15]-- but their essential role within the ISC niche is unclear.
Paneth cells are a source of signaling ligands
The Wnt signaling pathway is the best characterized regulator of intestinal homeostasis[16]. In the context of canonical Wnt signaling, Wnt receptors internalize secreted Wnt ligands, triggering β-catenin stabilization, nuclear translocation and subsequent transcriptional activation of target genes involved in cell survival and proliferation. While over-activation of Wnt in the intestine is tumorigenic[17], inhibiting Wnt causes loss of proliferative crypts and tissue attrition[18], emphasizing a requirement for its precise modulation. Canonical Wnt signaling is most active in the crypt-base where CBCs and PCs reside. In CBCs, Wnt signaling regulates proliferative capacity, while PC Wnt-target expression influences homing and maturation[19, 20]. Farin et al. further describe a role of Wnt-mediated PC maturation by elegantly demonstrating that forced Wnt3 expression drives progenitors toward a PC fate, thus defining a signaling loop where PCs produce the Wnt ligands that are required for their maturation[1].
PC production of signaling ligands substantiates their regulatory role within the stem cell niche specifically by communicating with the adjacent Lgr5+ CBCs. Despite this, requirement of PC-derived factors within the niche is unclear. Diphtheria toxin-mediated PC ablation does not overtly affect epithelial proliferation nor lineage allocation[21]. However, re-analysis of this model demonstrates incomplete PC loss and yet a concomitant decrease in CBC numbers[15]. While these studies did not definitively specify a niche role for the PC, they provided further support that an intricate relationship exists between Paneth and CBCs populations. The fact that PCs are physically juxtaposed to +4 ISCs presents an intriguing possibility that they may also participate in regulating this population.
Additionally, the importance of CBC and PC communication is evident from in vitro intestinal enteroid[22] cultures. In the absence of mesenchymal cells, single Lgr5-expressing CBCs can form intestinal enteroids at low efficiency, generating structures harboring proliferative crypt-like and differentiated domains similar to native tissue architecture[23]. Enteroids contain all differentiated epithelial lineages present in the intestine, including conserved PC-Lgr5+ CBC spatial relationships. Not surprisingly, recent studies show that ISCs co-cultured with PCs or the addition of exogenous Wnt3a to culture medium vastly improves growth efficiency of Lgr5+ cells[15]. While these observations reinforce that PCs are integral niche components, recent data[24, 25] has called into question the niche function of PCs.
Two recent studies showed that deletion of Math1—a Notch signaling pathway transcription factor required for secretory cell fate specification[26]—resulted in ablation of all intestinal secretory cells, including PCs, leaving the crypt-base populated exclusively by Lgr5+ CBCs[24, 25]. In this context, stem cell function and gene expression are maintained, suggesting PCs are not required to sustain Lgr5+ CBCs in vivo[24, 25]. Furthermore, interpretation of PC niche function in the context of Notch ablation is challenging, as potential Notch signaling contributions by PCs are masked. Indeed, Notch signaling between the CBC and PC is likely important; Notch-1 receptor is highly expressed by the Lgr5+ CBCs[27], whereas PCs express the Notch ligands Dll-1 and Dll-4[15]. These ligands are known to be critical for Notch-mediated homeostasis in the intestinal epithelium, as their genetic removal phenocopies the secretory cell hyperplasia observed upon Notch inhibition[28]. Despite the evidence of intimate PC-CBC cross-regulation, the EGF expression profile of PCs may also indicate a role for regulating +4 ISCs. The recent identification of a quiescent ISC population marked by the Erb inhibitor Lrig1 points to a potential EGF signaling network within the niche[7]. Thus, in order to clearly address the controversial PC niche role, future studies must examine the homeostatic contribution of each signaling factor individually. In light of these challenges, Farin and colleagues used a simplified approach to examine the requirement of a single PC-derived ligand, Wnt3, within the ISC niche. Tissue specific deletion of Wnt3 produced no effect in vivo, but was required for growth and sustainability in vitro[1]. These disparate requirements of Wnt3 pointed to a role for functionally redundant Wnt ligands derived from mesenchymal niche components.
Signaling from unidentified mesenchymal cells contributes to ISC homeostasis
The endogenous intestinal niche contains both epithelial and mesenchymal components, which together produce regulatory growth factors, whereas enteroids are derived solely from epithelial cells that are immersed in Matrigel and a growth factor-rich milieu (Figure 1). The strict dependence on PC-derived Wnt in vitro strongly suggests the underlying mesenchyme provides compensation for loss of Wnt3 in vivo. Indeed, the growth of Wnt3-deficient enteroids was rescued with Wnt2b supplementation, a ligand expressed exclusively in the mesenchyme, or by co-culture with adherent mesenchymal cells[1], directly demonstrating mesenchymal contribution to ISC homeostasis. Currently, a description of the cell census of the mesenchymal niche is incomplete. Multiple mesenchymal cell-types present near the crypt-base are viable niche-cell candidates, most prominently the sub-epithelial myofibroblasts[3], recently shown to sustain human colonoid cultures[29]. Wnt expression in specific intestinal mesenchymal cell-types has not been extensively studied and no mesenchymal cell-type is exclusively localized to the crypt-base in the Wnt2b expression zone[14]. The isolation and characterization of mesenchymal niche cells to determine which cells are important in regulating ISCs is outstanding in the field.
Furthermore, the mechanisms that regulate interactions between mesenchymal and crypt-base epithelial cells remain unclear. Signaling between the mesenchyme and epithelial cells is likely bi-directional[3]. It is possible that crypt-adjacent mesenchymal cells are induced to associate with the niche by signals from the epithelium. Consistently, bone-marrow derived cells home to the base of intestinal crypts after epithelial damage[30]. Further, a recent study shows that unspecified mesenchymal cells are recruited to sites of intestinal tissue regeneration to attenuate crypt-cell proliferation and restore homeostasis through secretion of the non-canonical ligand, Wnt5a[31]. It is apparent that a better understanding of mesenchymal contributions to the niche is essential and will provide additional insight into mechanisms of homeostasis and disease.
Future prospectives
The work by Farin and colleagues highlights the important compensatory mechanisms hardwired into the niche to safeguard against stem cell dysfunction and disease. It is clear that additional redundancy likely exists in vivo, but is not easily uncovered by classical genetics alone. The complexity of the intact niche complicates dissection of redundant signaling components that function in compensatory upregulation of niche-mediated signaling in response to changes in homeostasis. Although the enteroid culture system is more analogous to the exponential growth observed during tissue regeneration than the in vivo homeostatic environment, Farin et al. elegantly demonstrate its utility for elucidating epithelial and mesenchymal signaling contributions to the ISC niche. Understanding the signaling interactions needed for the successful maintenance of the intestine is essential, and the authors fill in another piece of this intricate puzzle.
The identification of novel stem cell markers and the establishment of the in vitro intestinal enteroid culture system have revitalized the ISC field. Combined with exciting advances in generating intestinal tissue from human induced pluripotent stem cells[32] and improved human crypt culturing methods[29, 33], the potential to expand intestinal tissue ex vivo for therapeutic purposes is apparent[34]. The enteroid system also provides a highly tractable platform for applying molecular techniques[35] and live-cell imaging to address numerous outstanding questions regarding ISC biology. Future studies will harness this system to uncover additional mesenchymal-epithelial signaling mechanisms in homeostasis and disease, delineate an ISC hierarchy, specify the niche requirements of the +4 ISC population, explore the influence of the microbiome on ISC behavior[36], identify disease mechanisms and potential therapies, and to develop novel tissue regeneration models. By combining both in vivo and in vitro approaches, the answers to long-standing questions that have hindered the ISC field are now within reach.
Footnotes
The authors state that they have no conflict of interest to disclose.
References
- 1.Farin HF, van Es JH, Clevers H. Redundant Sources of Wnt Regulate Intestinal Stem Cells and Promote Formation of Paneth Cells. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474(7351):318–26. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 3.Powell DW, et al. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–37. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2009;217(2):169–80. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–5. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery RK, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108(1):179–84. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell AE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149(1):146–58. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40(7):915–20. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda N, et al. Interconversion Between Intestinal Stem Cell Populations in Distinct Niches. Science. 2011;334(6061):1420–4. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 11.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–9. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan KS, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109(2):466–71. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356–68. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 14.Gregorieff A, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129(2):626–38. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–8. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306(2):357–63. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247(4940):322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 18.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19(4):379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 19.Batlle E, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111(2):251–63. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 20.van Es JH, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7(4):381–6. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 21.Garabedian EM, et al. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272(38):23729–40. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 22.Stelzner M, et al. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012;302(12):G1359–63. doi: 10.1152/ajpgi.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 24.Durand A, et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci U S A. 2012;109(23):8965–70. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TH, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A. 2012;109(10):3932–7. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shroyer NF, et al. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132(7):2478–88. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 27.Munoz J, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. Embo J. 2012;31(14):3079–91. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrinet L, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140(4):1230–1240. doi: 10.1053/j.gastro.2011.01.005. e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahar N, et al. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One. 2011;6(11):e26898. doi: 10.1371/journal.pone.0026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell AE, et al. Fusion between Intestinal Epithelial Cells and Macrophages in a Cancer Context Results in Nuclear Reprogramming. Cancer Res. 2011;71(4):1497–505. doi: 10.1158/0008-5472.CAN-10-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi H, et al. Wnt5a Potentiates TGF-beta Signaling to Promote Colonic Crypt Regeneration After Tissue Injury. Science. 2012 doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spence JR, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141(5):1762–72. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Howell JC, Wells JM. Generating intestinal tissue from stem cells: potential for research and therapy. Regen Med. 2011;6(6):743–55. doi: 10.2217/rme.11.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo BK, et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat Methods. 2011;9(1):81–3. doi: 10.1038/nmeth.1802. [DOI] [PubMed] [Google Scholar]
- 36.Hooper LV, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291(5505):881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]


