Abstract
Corticotropin releasing factor (CRF) and serotonin (5-HT) are strongly linked to stress and anxiety in vertebrates. As a neuromodulator in the brain, CRF has anxiogenic properties often characterized by increased locomotion and stereotyped behavior in familiar environments. We hypothesized that expression of anxiogenic behavior in response to CRF will also be exhibited in a teleost fish. Rainbow trout were treated with intracerebroventricular (icv) injections of artificial cerebrospinal fluid (aCSF), 500 or 2000 ng ovine CRF, or not injected. Treatment with either dose of CRF elicited greater locomotion and pronounced head shaking behavior, but did not influence water column position. Locomotor and head-shaking behaviors may be analogous to the increased stereotypy evoked by icv CRF in rats, and may reflect the expression of stress/anxiety behavior. Injection with either aCSF or CRF produced significant increases in plasma cortisol. The absence of behavioral changes in aCSF injected fish suggests that the behavioral responses following CRF were not due to cortisol. Treatment with 2000 ng CRF significantly increased serotonin, 5-HIAA and dopamine concentrations in the subpallium and raphé, and increased 5-HIAA in the preoptic hypothalamus (POA). Concurrent effects of CRF on central monoamines, locomotion, and headshaking in trout suggest that anxiogenic properties of CRF are evolutionarily conserved. In addition, positive linear correlations between locomotion and serotonergic and dopaminergic function in the subpallium, POA, and raphé nuclei suggest a locomotory function for these monoamines.
Keywords: anxiety, CRF, dopamine, locomotion, Oncorhynchus mykiss, raphé, serotonin, subpallium
Corticotropin-releasing factor (CRF) is a 41 amino acid peptide (Vale et al., 1981) that is released from hypothalamic neurons in response to stress. This endocrine CRF stimulates corticotropin (ACTH) from the pituitary, followed by ACTH stimulation of glucocorticoid secretion from the adrenocortical or interrenal tissue. In addition to this neuroendocrine function, CRF acts centrally to modify neural activity (Lowry et al., 2000), modulate transmitter function (Price and Lucki, 2001; Summers et al., 2003) and alter behavior (Koob and Heinrichs, 1999). These functions of CRF are mediated by CRF1 and CRF2 receptors, which are uniquely distributed in the brain, and are believed to play important roles in stress-induced behavior linked to emotions such as fear and anxiety (Takahashi, 2001).
In addition to anxiety modulating effects of neuropeptides such as CRF (Campbell et al., 2004), other neurotransmitters such as γ-aminobutyric acid (GABA), plus monoamines like serotonin (5-hydroxytryptamine; 5-HT; (Chaouloff, 1994; Gordon and Hen, 2004)) and norepinephrine (NE (Schweimer et al., 2005)) are also a part of the neural mechanisms and circuitry that influence anxiety and behavior. Together, these transmitters shape behavioral responses driven by anxiety primarily through action in limbic regions of the mammalian brain (Bannerman et al., 2003; Kalin et al., 2004; McHugh et al., 2004; Schweimer et al., 2005). The anxiogenic effects of CRF on behavior appear to be evolutionarily conserved, as suggested by increased locomotion in a familiar environment and/or anorexia induced by CRF in a variety of animals including fish (Clements et al., 2002; Clements and Schreck, 2004a; Bernier and Craig, 2005; Bernier, 2006), amphibians (Lowry et al., 1990; Lowry and Moore, 1991; Lowry et al., 1996; Crespi and Denver, 2004), birds (Zhang et al., 2001a; Zhang et al., 2001b) and rodents (Kalivas et al., 1987). The effects of CRF on locomotion as well as on other types of anxious behavior are often found to be mediated by 5-HT (Zhang et al., 2004; Lowry and Moore, 2006). Changes in swimming, stepping or walking, exploring, and/or general locomotor activity in response to CRF may also be accompanied by other behaviors that are indicative of increased anxiety, such as decreased feeding (Crespi et al., 2004), hiding in a dark compartment (Butler et al., 1990), and burying noxious objects (Korte et al., 1993).
Like other vertebrates, fish synthesize two stress-related neuropeptides, CRF and urotensin, which are part of a family of CRF-like peptides that also include the urocortins and sauvagine (Lovejoy and Balment, 1999; Craig et al., 2005). Fish initiate stress responses from the hypothalamic-pituitary-interrenal (HPI; homologue of the mammalian adrenal or HPA) axis, when CRF stimulates secretion of ACTH from the pituitary (Rotllant et al., 2000; Pepels et al., 2004; Pepels and Balm, 2004). Furthermore, CRF and resultant HPI secretory products also have central effects on behavior in salmonids. In Chinook salmon (Oncorhynchus tshawytscha), intracerebroventricular (icv) CRF stimulates increased locomotion and altered downstream movement (Clements et al., 2002; Clements et al., 2004a). The CRF effects on anxiogenic locomotor activity in salmon have been hypothesized to be mediated by GABA, dopamine (DA) and serotonin (5-HT) systems (Clements et al., 2003; Clements and Schreck, 2004b). Other studies have also linked these transmitter systems to stress responsiveness in salmonid fish (Winberg and Nilsson, 1993; Winberg et al., 1997; Winberg and Lepage, 1998).
The purpose of these experiments was to corroborate the effect of CRF on locomotion in another salmonid, the rainbow trout (Oncorhynchus mykiss), and to determine if CRF-induced hyperlocomotion coincided with altered changes in monoaminergic activity in specific brain regions. Locomotion in rainbow trout appears to be sensitive to HPI axis activity, as this species exhibits elevated locomotory response to cortisol implants (Gregory and Wood, 1999). We hypothesized that trout subjected to an icv injection of CRF would show increased anxiety in a familiar environment (as measured by increased locomotion and water column preference). Furthermore, we hypothesized that fish receiving icv CRF injections would have higher levels of serotonergic activity in brain regions associated with anxiety and locomotion, including the subpallium and raphé. As the subpallium appears to be an evolutionary precursor of striatum and amygdala (Northcutt and Davis, 1983), we also predicted that dopaminergic activity in this region would increase to reflect a striatal dopaminergic influence on locomotor activity.
Material and methods
Subjects and Housing
Juvenile rainbow trout (Oncorhynchus mykiss; raised from eggs) weighing 130 ± 30 g were housed in a large indoor circular tank under natural light conditions prior to experimentation (Gavins Point National Fish Hatchery, Yankton, SD). All experiments were conducted in a manner that minimized suffering and the number of animals used, in accordance with the Declaration of Helsinki and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23), under approved protocol by University of South Dakota IACUC. Fish were fed daily with Nelson’s Silver Cup sinking trout feed at a rate of 1% body weight per day.
Experimental fish were allowed to acclimate for 10 days in individual compartments of 75 gallon glass aquaria with a flow through water system and aeration. Each tank was individually lit and separated into 4 equal sized compartments by the insertion of 3 opaque barriers. This was done to stimulate territorial association with each individual’s compartment, and to inhibit social interaction between fish.
Experimental design
Locomotion (time spent moving forward) and water column position for each fish was observed and recorded for five minutes each, prior to and following injection. After the initial observation, 24 fish (N = 8 each group) were anesthetized, and injected with of one of the following: 0 (injection control; aCSF only), 500, or 2,000 ng CRF (ovine corticotrophin releasing factor; Sigma, St. Louis) in 2μl of artificial cerebrospinal fluid (aCSF). The dose of icv CRF was chosen to be comparable to that of earlier work on Chinook salmon, based on the relevant literature, taking into account body size (Clements et al., 2002). Eight other fish served as untreated controls that were assessed for behavior but did not receive anesthesia or icv injection.
Anesthesia
Fish were anesthetized in a volume of 10 liters of water and 5g methane tricane sulfonate (MS222) to make a concentration of 500 mg/l. The time to loss of equilibrium was consistent (~13 sec) for each fish. To facilitate recovery after injection, fresh water was artificially passed across the gills. To accomplish this, the entire fish was manually moved back and forth through the water column until equilibrium was recovered. Full recovery from anesthesia was accomplished in two to four minutes. To ensure adequate delivery of icv injected CRF, post-injection behavioral observations were carried out 25 minutes after equilibrium was recovered, similar to previous experiments (Clements et al., 2002).
Injections
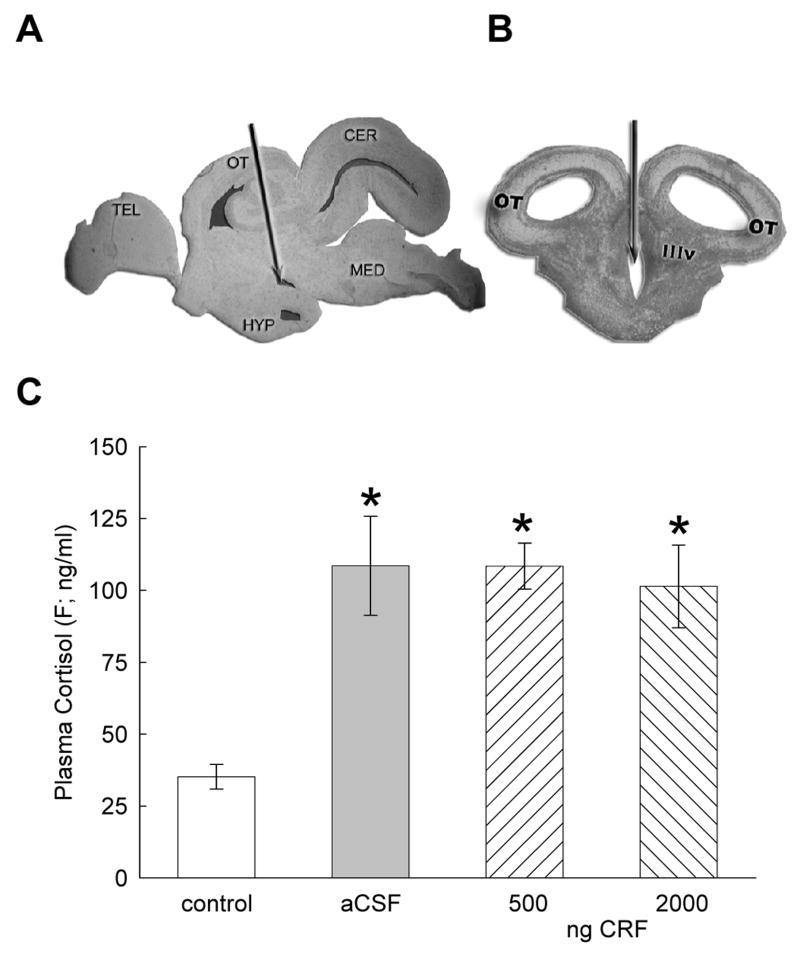
Anesthetized fish were injected post-orbitally (26 gauge), along the midline to a depth of 7 mm into the 3rd ventricle of the brain (icv; Fig. 1A, B). Prior to experimentation, methylene blue was injected to determine the accuracy of the icv procedure. To minimize leakage of CRF or aCSF from the injection site, 2 μl of fluid was injected slowly, and 5 seconds was allowed to elapse after the injection before the needle was withdrawn.
Figure 1.
Photomicrographs of A sagittal view of the trout brain indicating the third ventricle injection site (arrows) for icv administration of CRF or aCSF; B coronal view (TEL = telencephalon, OT = optic tectum, HYP = hypothalamus, IIIv = third ventricle, CER = cerebellum, MED = medulla). C Mean plasma cortisol (F ± SEM) concentrations are influenced by icv injections of CRF or aCSF. Trout injected icv with 500 or 2000 ng CRF (hatched bars) had significantly elevated plasma F (* indicate significance; P < 0.05) concentrations, as did trout injected with artificial cerebrospinal fluid (aCSF, gray bars), as compared with uninjected controls (clear bars).
Cortisol Analysis
Immediately following the second behavioral observation, fish were anesthetized as previously described, had blood samples taken from the caudal vein (21 gauge needle), and then were killed by rapid decapitation. Plasma samples were frozen (−80°C) until analysis. Sample preparation for ELISA analysis of cortisol begins with 1 μl of steroid displacement reagent added to 99 μl of plasma, to extract cortisol from any binding proteins, and then vortexed for 10 s. Then 5 μl of this solution is added to 900 μl of assay buffer and vortexed for 10 s. Next, 100 μl of this solution is added to a 96 well plate, followed by addition of 50 μl of both cortisol conjugate and cortisol antibody solution (R&D Systems cortisol kit). The plate is then covered and incubated on a horizontal shaker for two hours at room temperature. Following the incubation, the plate is aspirated and rinsed 3 times with washing buffer. After washing, 200 μl of pNPP substrate is then added to all wells before covering the plate and incubating at room temperature for one hour. Following this incubation, 50 μl of stop solution is added to all wells and the plate is placed immediately into a plate reader (Biotech Instruments, EL800) and absorption is measured. Data are transformed and checked against control values to determine actual cortisol concentration and are represented as nanograms cortisol per ml plasma (ng/ml).
Analysis of Monoamines
Brains were removed from the fish intact in the cranium, stored at −80°C, and sliced coronally (300 μm) in a temperature controlled (−12°C) cryostat (Leica-Jung 1800, Wetzlar, Germany). Slices were thaw-mounted on glass microscope slides. The individual brain areas sampled were microdissected from the appropriate sections using a 500 μm diameter punch. To examine the role of CRF on anxiety and locomotion, monoamines were measured in limbic forebrain regions including the dorsolateral pallium (DL), subpallium (Vc), preoptic area of the hypothalamus (POA) and mid to hindbrain region of 5-HT producing cells in the raphé using HPLC with electrochemical detection. The DL is a putative homologue of the mammalian hippocampus (Carruth et al., 2000; Northcutt, 2006). The amygdala is a complex structure that includes nuclei derived from both limbic and striatal elements (Laberge et al., 2006). As such the dorsomedial pallium (DM), and the commissural (Vc) and supracommissural (Vs) nuclei of the ventral area of the teleost telencephalon are likely to be subregions homologous to mammalian amygdalar nuclei (Northcutt, 2006). Based on recent neuroanatomical work in fish and amphibians, the Vc was chosen for analysis as it represents a likely combination of striatal and amygdalar functions (Northcutt et al., 1983; Laberge et al., 2006). The striatum is important for motivated locomotion, and the amygdala is an important site of extrahypothalamic CRF production in mammals. Locations of brain regions were based on a stereotaxic atlas for trout and salmonid maps of brain neurochemistry (Billard and Peter, 1982; Carruth et al., 2000).
Dopamine (DA), norepinephrine (NE), epinephrine (Epi), and the DA catabolite: 3,4-dihydroxyphenylacetic acid (DOPAC); serotonin (5-HT); and its catabolite: 5-hydroxyindoleacetic acid (5-HIAA) were measured using high performance liquid chromatography (HPLC) with electrochemical detection (Renner and Luine, 1984; Renner and Luine, 1986; Emerson et al., 2000). Punched samples were expelled into 60 μl of sodium acetate buffer (pH 5.0) containing 5 × 10−8 M DHBA (9.4 pg/μl dihydroxybenzylamine; internal standard). Each sample was frozen, then thawed and centrifuged at 15 000 × g for 3 min. The supernatant was removed and 45 μl was injected into a chromatographic system (Waters Associates, Milford, MA) and analyzed electrochemically with an LC-4B potentiostat (Bioanalytical Systems, West Lafayette, IN). The electrode potential was set at +0.6 V with respect to an Ag/AgCl reference electrode. The tissue pellet remaining from each sample was dissolved in 110 ml 0.4 N NaOH and protein content was assayed (Bradford, 1976).
Calculations and Statistical Analysis
The concentrations of the amines and amine metabolites were calculated with respect to the mean peak height values corrected for volume and internal standard using CSW32 software (DataApex Ltd., Czech Republic). All amine standards were obtained from Sigma Chemical Co. (St Louis, MO). The resulting pg values for the amines were divided by μg protein to yield pg amine/μg protein.
Behavioral results were analyzed using a two-way Analysis of Variance (ANOVA) in a repeated measures design to compare locomotion and water column position before and after treatment. The effect of icv CRF on plasma cortisol and central monoamines were compared separately using one-way ANOVA followed by Duncan’s multiple range tests. The relationships between monoamine concentrations and locomotion, in addition to CRF dose and monoamine concentration, were analyzed further using linear regression. For dose by monoamine comparisons, the data for 0 ng CRF dose was constructed in two ways (1: aCSF injection alone; 2: aCSF injection plus uninjected controls), and then evaluated by separate regression analyses. As uninjected and aCSF controls produced very similar results following ANOVA, both types of regression also produced similar results. The analyses using 1) aCSF injection alone invariably produced stronger regression correlation, based on r2 values, but we have reported the more conservative results that include 2) aCSF injection plus uninjected controls as the combined data for 0 ng CRF dose.
Results
While icv injections of saline or CRF stimulated elevated plasma cortisol concentrations, only icv CRF injections, at either dose, influenced behavior. Similarly, icv CRF also stimulated elevated monoamine concentrations in subpallium, preoptic area, and raphé. There was no effect at any dose on NE or Epi in any brain region tested. However, the effect of CRF on DA, 5-HT or 5-HIAA in subpallium, preoptic area, and raphé was only measured after icv injection of the highest dose. Despite this, there were positive correlations between DA, 5-HT or 5-HIAA concentrations in subpallium, POA and raphé and locomotion, and positive correlations between CRF dose and DA, 5-HT and/or 5-HIAA in subpallium, POA and raphé.
Cortisol
As expected, the stress hormone cortisol was significantly elevated (F3,25 = 10.024, P < 0.0001) following icv injection with CRF (Fig. 1C). However, all three groups of fish that received an icv injection (aCSF, 500 ng, 2000 ng CRF) showed similar increases in plasma cortisol when compared to control fish.
Behavior
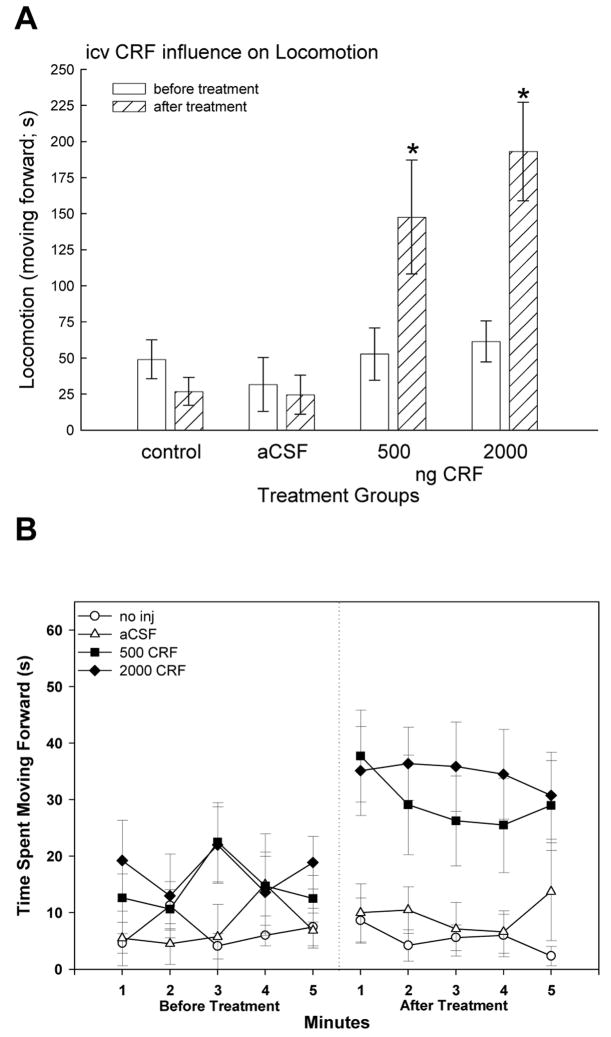
Locomotion time increased significantly (two-way repeated measures ANOVA; F3,28 = 5.83, P < 0.003) in both CRF groups compared before and after CRF treatment (Fig. 2A). While none of the groups were significantly different prior to treatment (one-way ANOVA; F3,28 = 1.02, P > 0.4), after CRF injection trout exhibited increased total locomotion time when compared withcontrols animals treated icv with aCSF (one-way ANOVA; F3,28 = 5.91, P < 0.003;), and only CRF treated fish showed an increase in locomotion (Fig. 2B).
Figure 2.
Time spent moving forward (in seconds ± SEM) before (clear bars) and after treatment (hatched bars) during the five minute observation periods. There were no differences in locomotion between groups prior to injection, and those groups that were not injected or injected icv with artificial cerebrospinal fluid (aCSF) did not change their forward movement significantly. However, trout inject icv with 500 or 2000 ng CRF had significantly elevated forward locomotion (* indicate significance; P < 0.05) compared with pretreatment measurements and with injected and uninjected controls.
While locomotion was significantly influenced by CRF treatment, position in the water column was not (two-way repeated measures ANOVA; F3,23 = 1.84, P > 0.17). Fish swam throughout the entire tank showing no preference for open water versus edges, or the middle of the water column versus the upper or lower portions.
However, 75% of fish that received injections of CRF at both doses, but not aCSF, showed a previously undocumented “head shaking” behavior, which may be analogous to increased stereotypy. Head shaking frequency was 1.675 shakes/minute for trout treated with 500 ng CRF and 0.9/minute for 2000 ng CRF. For the entire five minute bout, there was 8.375 (mean ± 2.58) head shakes for trout injected icv with 500 ng CRF and 4.50 (mean ± 1.86) for fish injected with the higher 2000 ng dose. The mean frequency for the lower CRF dose was not statistically (t14 = 1.22, P > 0.24) greater than head shaking frequency in trout treated with the higher icv dose of CRF. This CRF-induced behavior is characterized by flaring the gill covers near the top of the water column, opening the mouth wide, and shaking the head quickly and violently, typically lasting for three to five seconds.
Dorsolateral Pallium
In the dorsolateral region of the pallium, putatively homologous to the mammalian hippocampus (Carruth et al., 2000), concentrations of the DA catabolite DOPAC were elevated (but only at the P < 0.07 level of significance, F3,17 = 2.8; data not shown) following 500 ng CRF when compared to the injection controls (aCSF). However, there were no significant effects of CRF on DA, 5-HT, or 5-HIAA in the DL or hippocampal pallium.
Dorsomedial Pallium
The DM is a region that contains elements homologous to part of the mammalian amygdalar complex (Northcutt, 2006). Monoamine concentrations or activity were not significantly affected in this part of the limbic pallium by icv CRF or aCSF.
Subpallium
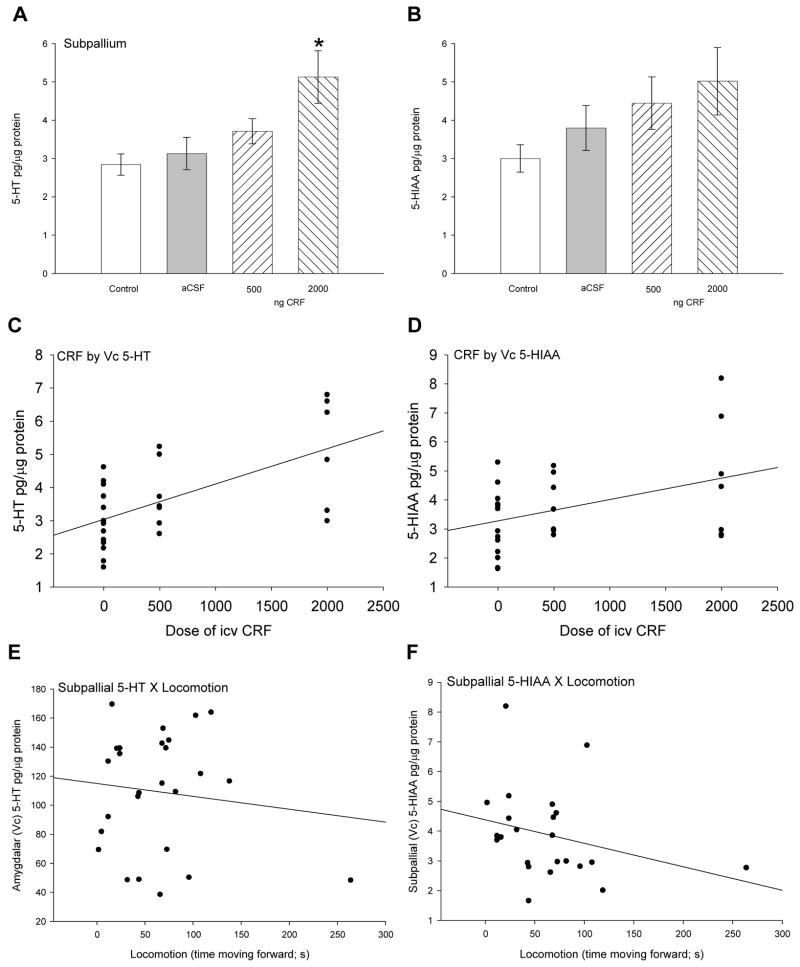
In the commissural (Vc) nuclei of the ventral area of the telencephalon or subpallium, perhaps containing putative homologues of subregions of mammalian amygdala and striatum, significantly increased 5-HT (F3,27 = 5.07, P < 0.007) concentrations were elicited by the highest dose (2000 ng) of CRF only (Fig. 3A). The serotonergic catabolite 5-HIAA was not significantly (F3,27 = 1.79, P > 0.175) influenced by injection of aCSF or CRF, but the trend toward an increase with CRF treatment suggested a pattern of increased serotonergic activity in the subpallium (Fig. 3B). The patterns for 5-HT and 5-HIAA suggest dose dependent effects of CRF on serotonergic activity, corroborated by significant positive correlations with CRF dose for both 5-HT (linear regression: r2 = 0.38, F1,26 = 15.81, P < 0.001; Fig. 3C) and 5-HIAA (linear regression: r2 = 0.17, F1,26 = 5.32, P < 0.029; Fig. 3D). However, no correlation between 5-HT (linear regression: r2 = 0.00, F1,25 = 0.003, P > 0.95; Fig. 3E) or 5-HIAA (linear regression: r2 = 0.08, F1,25 = 1.9, P > 0.18; Fig. 3F) in Vc and locomotory behavior was evident.
Figure 3.
Mean (± SEM) A serotonin (5-HT) and B serotonin catabolite 5-HIAA concentrations in the commissural (Vc) nuclei of the ventral area of the subpallial region of the telencephalon. The Vc nuclei constitute putative amygdalar/striatal regions in the teleost. Trout injected icv with 2000 ng CRF (hatched bars) had significantly more 5-HT (* indicates significance; P < 0.05) than those given 500 ng CRF, artificial cerebrospinal fluid (aCSF, gray bars) or uninjected controls (clear bars). Dose of CRF injected icv (0 = uninjected + aCSF controls, 500 ng, or 2000 ng) is positively correlated with C 5-HT (linear regression: r2 = 0.38, P < 0.001) and D 5-HIAA (linear regression: r2 = 0.17, P < 0.029) in the subpallium. No correlations between E 5-HT (linear regression: r2 = 0.00, P > 0.95) or F 5-HIAA (linear regression: r2 = 0.08, P > 0.18) concentrations in subpallium and locomotion exist in rainbow trout.
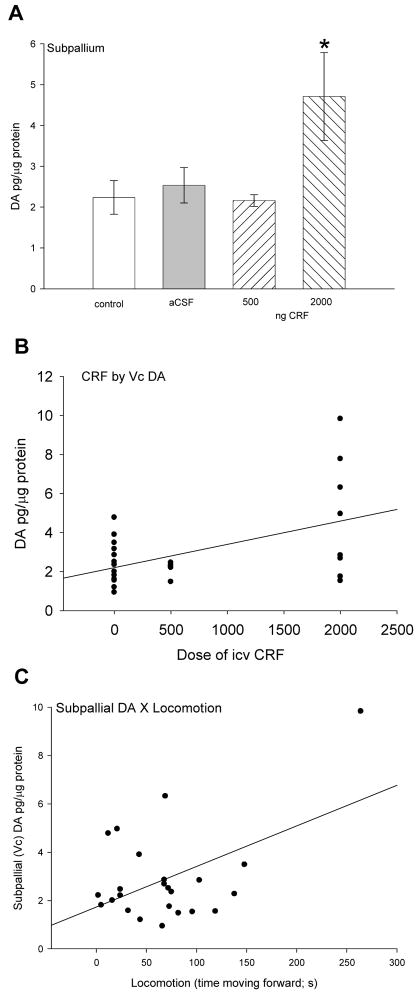
Similarly, DA in subpallium was significantly (F3,27 = 3.24, P < 0.04) elevated by the 2000 ng dose of CRF only (Fig. 4A). Similar to 5-HT and 5-HIAA there was a significant positive correlation (linear regression: r2 = 0.26, F1,26 = 9.02, P < 0.006; Fig. 4B) between CRF dose and Vc dopamine. In contrast to subpallial serotonin parameters, there was a significant linear regression between subpallial DA and locomotion (linear regression: r2 = 0.26, F1,25 = 7.23, P < 0.013; Fig. 4C). No significant changes in DOPAC or other monoamines in Vc were measured.
Figure 4.
Mean A dopamine (DA ± SEM) concentrations in subpallium. Trout injected icv with 2000 ng CRF (hatched bars) had significantly more DA (* indicates significance; P < 0.05) than those given 500 ng CRF, artificial cerebrospinal fluid (aCSF, gray bars) or uninjected controls (clear bars). Dose of CRF injected icv (0 = uninjected + aCSF controls, 500 ng, or 2000 ng) is positively correlated with B DA (linear regression: r2 = 0.26, P < 0.006) in the subpallium. In contrast to serotonergic parameters, a positive correlation between C DA (linear regression: r2 = 0.26, P < 0.013) concentrations in Vc and locomotion in rainbow trout suggests that DA in the subpallium is involved in locomotion.
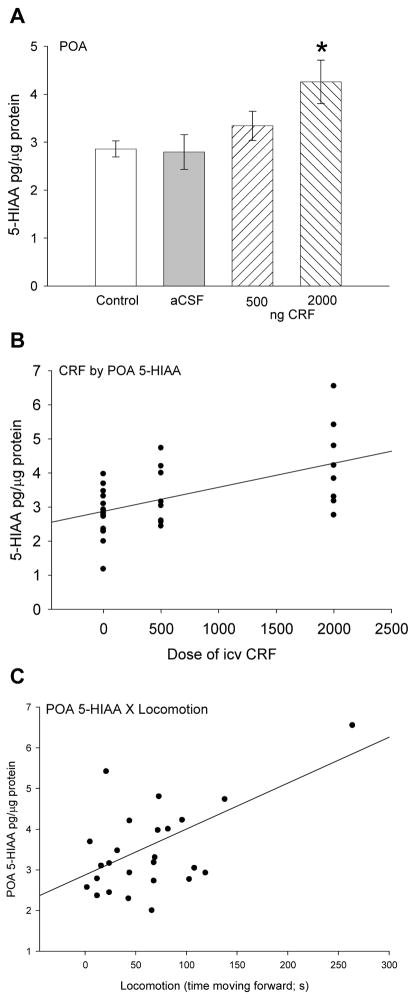
POA
In the preoptic region of the hypothalamus there was no change in the concentration of 5-HT elicited by injection of either aCSF or CRF. However, concentrations of 5-HIAA were significantly elevated (F3,28 = 5.69, P < 0.004) by the 2000 ng dose of CRF (Fig. 5A). Similar to 5-HT and 5-HIAA in the subpallium there was a significant positive correlation (linear regression: r2 = 0.31, F1,28 = 12.3, P < 0.002; Fig. 5B) between CRF dose and 5-HIAA in the POA. In addition, there was also a positive linear correlation between 5-HIAA in POA and locomotory behavior (linear regression: r2 = 0.24, F1,25 = 7.6, P < 0.011; Fig. 5C).
Figure 5.
Mean A serotonin catabolite 5-hydroxyindoleacetic acid (5-HIAA ± SEM) concentrations in the hypothalamic preoptic area (POA). Trout injected icv with 2000 ng CRF (hatched bars) had significantly more 5-HIAA (* indicates significance; P < 0.05) than those given 500 ng CRF, artificial cerebrospinal fluid (aCSF, gray bars) or uninjected controls (clear bars). Dose of CRF injected icv (0 = uninjected + aCSF controls, 500 ng, or 2000 ng) is positively correlated with B 5-HIAA (linear regression: r2 = 0.31, P < 0.002) concentration in the POA. A positive correlation also exists between C 5-HIAA (linear regression: r2 = 0.24, P < 0.011) concentrations in POA and locomotion in rainbow trout.
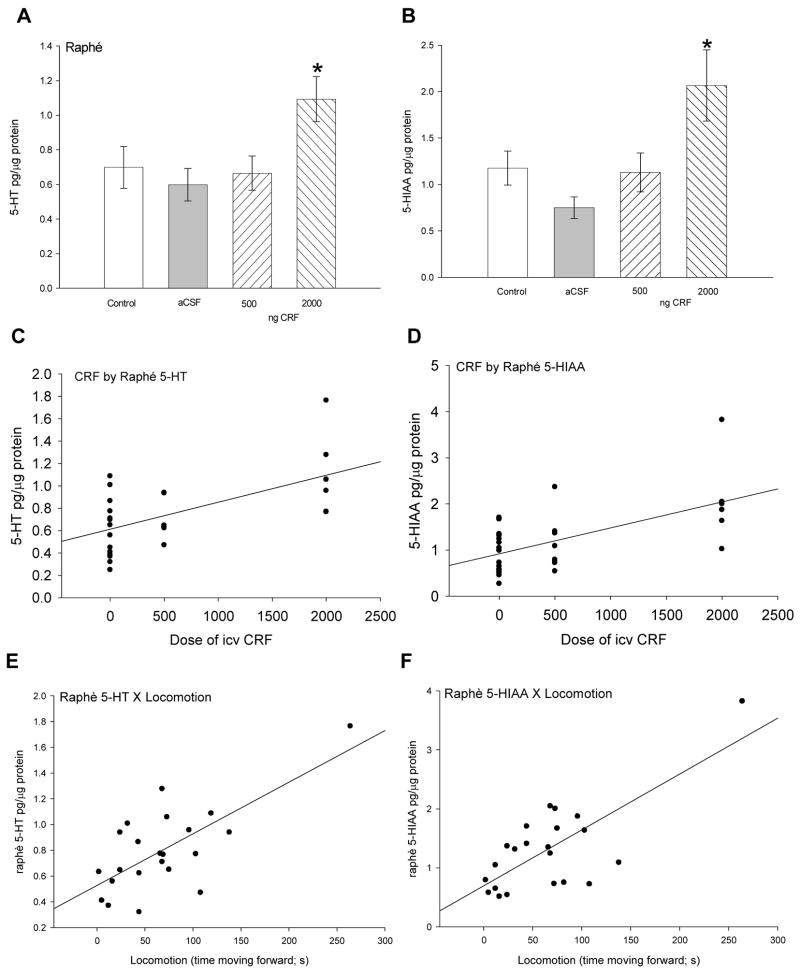
Raphé
Treatment with the highest dose of CRF (2000 ng), but not the lower dose (500 ng), or aCSF, stimulated a significant (F3,29 = 3.94, P < 0.019) increase in 5-HT in the raphé nuclei compared to uninjected controls (Fig. 6A). A dose-dependent effect of CRF on raphé 5-HT concentration is suggested by a significant positive correlation of CRF dose with 5-HT (linear regression: r2 = 0.50, F1,26 = 19.3, P < 0.0001; Fig. 6C). In addition, 5-HT in the raphé was positively correlated (linear regression: r2 = 0.50, F1,25 = 20.1, P < 0.0002) with locomotion (Fig. 6E). Similarly, 5-HIAA was significantly (F3,28 = 5.69, P < 0.004) elevated only by the highest dose of CRF, and not by the lower dose or aCSF injection alone (Fig. 6B). A similar dose-dependent relationship between CRF and raphé 5-HIAA concentration is also suggested by a significant positive correlation between these two variables (linear regression: r2 = 0.45, F1,26 = 16.06, P < 0.0007; Fig. 6D). In raphé, 5-HIAA also showed a significant positive linear correlation with trout swimming (linear regression: r2 = 0.55, F1,25 = 24.0, P < 0.0001; Fig. 6F).
Figure 6.
Mean (± SEM) A serotonin (5-HT) and B 5-HIAA concentrations in the raphé nucleus of the rainbow trout brain. Trout injected icv with 2000 ng CRF (hatched bars) had significantly more 5-HT (* indicate significance; P < 0.05) than those given 500 ng CRF, artificial cerebrospinal fluid (aCSF, gray bars) or uninjected controls (clear bars). Dose of CRF injected icv (0 = uninjected + aCSF controls, 500 ng, or 2000 ng) is positively correlated with C 5-HT (linear regression: r2 = 0.50, P < 0.0001) and D 5-HIAA (linear regression: r2 = 0.45, P < 0.0007) in the trout subpallium. In addition, E 5-HT (linear regression: r2 = 0.50, P < 0.0002) and F 5-HIAA (linear regression: r2 = 0.55, P < 0.0001) concentrations in subpallium were positively correlated with locomotion in rainbow trout.
Similar to serotonin parameters in the raphé, DA showed a significant (F3,28 = 5.58, P < 0.005) increase at the highest dose of CRF compared to control fish and those treated icv with aCSF and the lowest dose of CRF (Fig. 7A). There is a positive correlation (linear regression: r2 = 0.35, F1,27 = 14.59, P < 0.001) between CRF dose and DA concentrations in the raphé (Fig. 7B). In addition, DA concentrations in the raphé were also positively correlated (linear regression: r2 = 0.26, F1,25 = 8.4, P < 0.008) with time spent moving forward (Fig. 7C). No significant changes in any other monoamine concentration in the raphé were measured.
Figure 7.
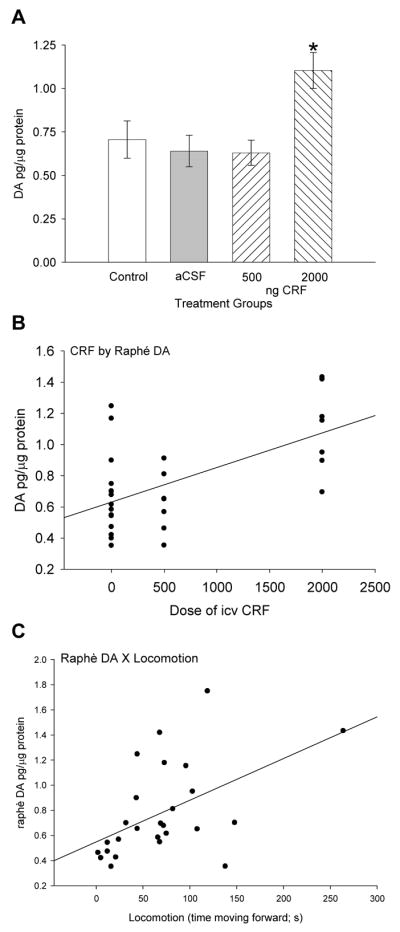
Mean A dopamine (DA ± SEM) concentrations in raphé. Trout injected icv with 2000 ng CRF (hatched bars) had significantly more DA (* indicates significance; P < 0.05) than those given 500 ng CRF, artificial cerebrospinal fluid (aCSF, gray bars) or uninjected controls (clear bars). Dose of CRF injected icv (0 = uninjected + aCSF controls, 500 ng, or 2000 ng) is positively correlated with B DA (linear regression: r2 = 0.35, P < 0.001) concentration in the raphé. A positive correlation also exists between C DA (linear regression: r2 = 0.26, P < 0.008) concentrations in raphé and locomotion in rainbow trout.
Discussion
The neuropeptide CRF, at a dose of 2000 ng delivered icv, stimulated increased serotonergic and dopaminergic activity in amygdalo-striatal pallium, POA and raphé of rainbow trout (Oncorhynchus mykiss; Figs. 3A, 4A, 5A, 6A, B, 7A). Plasma cortisol concentrations were elevated by CRF injection, but also in aCSF injected trout (Fig. 1C), suggesting that cortisol was not the primary cause of behavioral changes observed. Concurrent to the effects on monoaminergic activity, 500 or 2000 ng CRF elicited increased locomotion (Figs. 2A, B). The locomotor activity results for trout, as measured by time spent moving forward, are similar to those in which icv CRF injection in chinook salmon increased locomotion under both simulated stream conditions or in a tank (Clements et al., 2002; Clements et al., 2004a).
There are two possible, but incompatible, ways of interpreting our data. First, based on analysis from ANOVA alone, the results suggest that monoamines played no role in mediating CRF-induced locomotion. This is because while both doses of CRF stimulated locomotion, only the 2000 ng dose of CRF stimulated increased DA, 5-HT, and 5-HIAA (and DOPAC, NE and Epi did not change with icv CRF injection). Considered in this way, our data do not support the recent evidence in Chinook salmon that CRF-induced locomotion is mediated by 5-HT (Clements et al., 2003; Clements et al., 2004b), at least not 5-HT in dorsolateral pallium (DL), subpallium (Vc), POA, and raphé nuclei.
The second possible interpretation of the data considers regressions showing positive correlations between dose of CRF and DA, 5-HT and/or 5-HIAA; and positive regression correlations between these monoamines and locomotion. Positive regression correlations in subpallium (DA only), POA (5-HIAA) and raphé (DA, 5-HT and 5-HIAA) suggest that monoamines are involved in mediating CRF-induced locomotion. With this more inclusive analysis, our results tend to corroborate those of experiments employing pharmacological manipulation in salmon (Clements et al., 2003; Clements et al., 2004b), and suggest that 5-HT and DA appear to be involved in mediating trout locomotion (Figs. 4B,C, 5B,C, 6C–F, 7B,C), while cortisol does not appear to be involved (Fig. 1C). These regression correlations are not conclusive, and suggest that monoamines in specific regions only explain between 17 and 55% of the factors contributing to locomotion. However, since more than one region and more than one transmitter are involved, a regression explaining substantially more than half the variability in locomotive behavior would be very unlikely. It is also important to note that we have not tested the specificity of our icv CRF results with a CRF receptor antagonist, which leaves open the possibility that CRF is acting non-specifically or through other receptors. However, the CRF1&2 receptor antagonist α-helical-CRF used in a related species (Chinook salmon) blocked icv CRF stimulation of locomotion (Clements et al., 2002). The results in salmon suggest that a non-specific effect in rainbow trout is unlikely. If the second broader interpretation of CRF stimulating monoamines and locomotion is accurate, we demonstrate that exogenous CRF in the trout brain stimulated increases in serotonergic activity in conjunction with increased locomotion (Figs. 5A–C, 6A–F). Furthermore, we demonstrate that icv CRF also stimulated increased DA concentrations in subpallium and raphé, which are correlated with locomotion as well (Figs. 4A–C, 7A–C). Previously DA has been suggested to only be involved in mediating GABA but not CRF-induced locomotion in salmon (Clements et al., 2004b).
Working with another salmonid, Chinook salmon, Clements and Schreck demonstrated in CRF treated animals that concurrent administration of fluoxetine (a selective 5-HT reuptake inhibitor) potentiated locomotory behavior, while concurrent administration of a 5-HT1A receptor antagonist (NAN190) reduced CRF-induced locomotion (Clements et al., 2003). These results suggest that 5-HT is important in mediating the locomotory response to CRF. Our results demonstrate that such an increase in 5-HT does exist in rainbow trout treated with icv CRF, at least at the 2000 ng dose. Increased serotonergic activity induced by CRF treatments were measured in subpallium (Fig. 3A–D), POA (Fig. 5A–B: 5-HIAA only), and the raphé (Fig. 6 AD), with positive CRF dose by monoamine concentration regressions suggesting dose responsiveness. For serotonergic activity, only POA and raphé showed a positive relationship with locomotion, suggesting that the role of 5-HT in locomotion is site specific. A broadly generalized role of 5-HT in arousal and locomotion has been hypothesized for mammals (Jacobs and Fornal, 1999), which does not appear to be supported in our model. While the CRF-induced effect was regionally specific, an increase in both 5-HT and 5-HIAA, especially in raphé, suggests up-regulation of overall serotonergic activity in specified regions. The increased serotonergic activity in raphé following CRF treatment was accompanied by increased DA, suggesting the possibility of cross-regulation between these two monoamine systems (Korzan et al., 2001).
Previous studies in salmon suggested no evidence for CRF-induced hyperactivity mediated by dopaminergic systems (Clements et al., 2004b). However, elevated DA in the striatal pallium is generally associated with increased locomotion (Jones et al., 1981; Wickens, 1990). Similarly in fish, dopaminergic receptor agonists (apomorphine) added to the tank water also stimulate increased locomotion (Mok and Munro, 1998). The striatum (and presumably the striatal pallium) includes dense dopaminergic innervation as well as GABAergic inter- and projection neurons. In salmon, icv treatment with a GABAA receptor agonist (muscimol) potentiated CRF-induced locomotory responses(Clements et al., 2004b). However, while a DA2 receptor antagonist (haloperidol) inhibited GABA-induced locomotion, it had no effect on CRF-induced locomotion. This suggested that DA did not play a role in locomotion induced by CRF in salmon. In other vertebrates, locomotory activity induced by CRF also does not appear to be dependent upon activation of mesolimbic dopaminergic systems (Koob et al., 1984; Swerdlow and Koob, 1985; Swerdlow et al., 1986). Our results, however, demonstrate that the highest dose of icv CRF increased DA in both striatal subpallium (Fig. 4A) and raphé (Fig. 7A), and there was a positive linear correlation between CRF dose and DA concentration in both regions (Figs. 4B, 7B). However, at variance with the previous studies listed above, DA in the limbic Vc is positively, but only weakly correlated with locomotion (Fig. 4B), suggesting a minor role (r2 = 0.26, therefore the relationship between DA and locomotion only explains 26% of the variance) for mesolimbic DA in CRF-induced locomotion in trout. On the other hand CRF directly injected into the ventral tegmental area does stimulate locomotor behavior in rats (Kalivas et al., 1987). While teleosts do not possess discrete dopaminergic tegmental nuclei, DA in the raphé was elevated by icv CRF and also correlated significantly with forward locomotion in trout, suggesting a direct role for DA in CRF-induced hyperlocomotion (Fig. 7B). We suggest that the change in serotonergic and dopaminergic activity in conjunction with locomotion is a product of the anxiogenic properties of CRF in salmonids, as has been demonstrated in other vertebrate models (Bagdy, 1998; To et al., 1999; Carrasco and Van de Kar, 2003).
Anxious behavior in vertebrates typically occurs in anticipation of a fearful stimulus and may include conditioning to fear (Buwalda et al., 2005). However anxiety may also represent innate unlearned fear (Davis and Shi, 1999). Anxious behavior appears to be mediated by the bed nucleus of the stria terminalis (BNST) in mammals, and is influenced by CRF (Campbell et al., 2004) which is produced locally in the BNST and the central nucleus of the amygdala (CeA). In rats icv CRF yields a dose dependant decrease in novel open field exploration, suggesting increased anxiety. However, in a familiar environment, or during social interaction, CRF treated rats exhibit increased investigation and exploration, demonstrating that anxiogenic effects produced by CRF are dependent on context (Dunn and Berridge, 1990; Campbell et al., 2004). In rainbow trout (Fig. 3) and chinook salmon, CRF stimulates dramatically increased exploratory locomotion, which also appears to be context dependent (Clements et al., 2002; Clements et al., 2004a). In icv CRF treated chinook salmon placed in an artificial stream, downstream locomotion was limited to a subgroup of fish, but for those traveling downstream, locomotion was more rapid compared to controls (Clements et al., 2004a). The context dependent effects of CRF also appear to be species specific. For example, CRF injected salmon preferred the center of the tank, but CRF did not influence relative position of trout in the water column. The indiscriminate swimming pattern exhibited by CRF-treated trout might at first suggest that the additional locomotion was not anxiogenic, as swimming was not confined to the edges. However, the magnitude of increase in locomotion and relative size of the tank was such that CRF-treated trout were compelled to utilize the entire tank. Further experimentation in a larger test arena may elucidate spatial preferences induced by CRF treatment.
In addition, while social interaction influenced CRF dependent locomotion in rats, it did not in chinook salmon (Campbell et al., 2004; Clements et al., 2004a). Like salmon and rats, icv CRF in rainbow trout stimulates locomotion in a familiar environment, and this effect is independent of glucocorticoid actions (Figs. 1C, 2A, B). However, like rats, but unlike salmon, social interaction influences CRF dependent activity in rainbow trout (Carpenter et al., 2006). During socially aggressive interaction CRF stimulates increased locomotion generally. In addition, only trout that become dominant following the social interaction show a greater locomotory response during both attacks and retreats (Carpenter et al., 2006).
The confluence of anxiety and locomotory behavior suggests an interaction between DA mediated activity in the striatum and CRF plus 5-HT effects in the amygdala. In fish, the striatal and amygdalar portions of the pallium may be anatomically or evolutionarily undifferentiated and located together in or near the ventral commissural nuclei of the telencephalon (Wullimann and Rink, 2002; Northcutt, 2006). Significantly, rainbow trout exhibit elevated DA, 5-HT and 5-HIAA in Vc following icv CRF injection, suggesting that both locomotor and anxiogenic systems have been stimulated. We presume that CRF stimulation of increased subpallial DA (Fig. 4A), a positive regression correlation between CRF dose and DA concentration (Fig. 4B), and a positive correlation between DA concentration and locomotion (Fig. 4C) reflects changes in the striatal portion of the Vc (Rink and Wullimann, 2001). Conversely, increased serotonergic activity in the Vc may reflect amygdalar influence on anxiety.
Trout injected icv with CRF had elevated serotonergic and dopaminergic activity, which may have induced the previously unreported head shaking behavior that we measured. This behavior, characterized by flaring the gill covers near the top of the water column, opening the mouth wide, and shaking the head quickly and violently, was evident within the first minute of behavioral observation. This first recorded incidence of head shaking was therefore 26 minutes after icv CRF treatment, which may not have been the first time it occurred. The frequency of headshaking was variable, less than two times per minute on average, and the behavior typically lasted only three to five seconds. Therefore, the CRF treated trout did not spend the majority of their time occupied with this behavior. In trout treated with CRF, head shaking was also observed during socially aggressive interaction (Carpenter et al., 2006), however head shaking has never been observed in aCSF-treated or untreated trout. While we cannot rule out head shaking as reaction to a noxious stimulus, during social interactions head shaking was never performed at the expense of another behavior (Carpenter et al., 2006). The cause this behavior is unknown, but could be related directly to CRF, DA, 5-HT or some other related factor.
As previously noted, although we have demonstrated the increase in serotonergic activity formerly hypothesized (Clements et al., 2003; Clements et al., 2004b), increased 5-HT, 5-HIAA and DA were only measured at the highest dose (2000 ng) of CRF. While these results may be mitigated somewhat by significant regression correlations between CRF dose and monoaminergic activity in subpallium, POA and raphé, they also likely suggest that other factors or brain regions may be involved in eliciting behavioral responses. For example, previous studies have suggested glutamate is important for maintaining rhythmic motor output in lamprey (Takahashi and Alford, 2002), and glucocorticoids have been suggested to elicit hyperactive locomotion in trout (Øverli et al., 2002a).
Experiments on genetically derived proactive and reactive trout suggest that corticosteroid reactivity is primarily responsible for locomotor response to territorial intrusion, which is higher in fish that respond to stress with a greater cortisol response (Pottinger and Carrick, 2001; Øverli et al., 2002b). In addition, studies with exogenous corticosteroids in a variety of vertebrates imply that adrenal/interrenal steroids stimulate hyperlocomotion (Challet et al., 1995; Breuner et al., 1998; Cash and Holberton, 1999; Øverli et al., 2002a). However, our results, like those for salmon, suggest that the HPI axis and cortisol were not involved in stimulating increased locomotion (Clements et al., 2002). In addition, cortisol implants had no affect on aerobic swimming performance in rainbow trout (Gregory et al., 1999). While Clements et al. showed no difference in plasma cortisol between saline and CRF injection (Clements et al., 2002), our results suggest that the icv injection protocol was stressful (Höglund et al., 2001), stimulating elevated cortisol in all injected groups compared to uninjected fish (Fig. 2). Trout injected with aCSF did have increased cortisol levels, but also an absence of increased locomotory activity, which eliminates cortisol as the causative factor.
Conclusions
In conclusion, icv CRF in rainbow trout stimulates locomotion possibly through complex interactions of serotonergic and dopaminergic activity in discrete brain regions. In addition, although icv injection protocol was stressful and stimulated elevated glucocorticoids, icv CRF did not contribute to further increases in plasma cortisol concentrations. Furthermore, cortisol stimulated by icv injection alone (with the vehicle aCSF) did not induce elevated locomotion. This suggests that stress-induced cortisol alone does not stimulate hyperlocomotion in trout. While CRF-induced hyperactivity in rainbow trout may be only directly related to locomotion per se, we suggest that for trout, like other vertebrates, CRF is an anxiogenic neuropeptide, and that its effects on 5-HT and DA in amygdalo-striatal complex and raphé, and on locomotion, may reflect its anxiogenic properties.
Acknowledgments
We would like to thank the Gavins Point National Fish Hatchery in Yankton, South Dakota for their gracious donation of fish, time and effort, without which this experiment would not have been possible. We would like to thank Nick Bernier for his help with a photomicrograph of a trout brain. This work was supported by NIH Grant P20 RR15567, and a South Dakota Board of Regents Fellowship (to REC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagdy G. Serotonin, anxiety, and stress hormones. Focus on 5-HT receptor subtypes, species and gender differences. Ann N Y Acad Sci. 1998;851:357–363. doi: 10.1111/j.1749-6632.1998.tb09009.x. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bernier NJ, Craig PM. CRF-related peptides contribute to stress response and regulation of appetite in hypoxic rainbow trout. Am J Physiol Regul Integr Comp Physiol. 2005;289:R982–R990. doi: 10.1152/ajpregu.00668.2004. [DOI] [PubMed] [Google Scholar]
- Bernier NJ. The corticotropin-releasing factor system as a mediator of the appetite-suppressing effects of stress in fish. Gen Comp Endocrinol. 2006;146:45–55. doi: 10.1016/j.ygcen.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Billard R, Peter RE. A stereotaxic atlas and technique for nuclei of the diencephalon of rainbow trout (Salmo gairdneri) Reprod Nutr Dev. 1982;22:1–25. doi: 10.1051/rnd:19820101. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Greenberg AL, Wingfield JC. Noninvasive corticosterone treatment rapidly increases activity in Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) Gen Comp Endocrinol. 1998;111:386–394. doi: 10.1006/gcen.1998.7128. [DOI] [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav. 2004;77:447–455. doi: 10.1016/j.pbb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Carpenter RE, Watt MJ, Forster GL, Ling TJ, Summers CH. Corticotropin-releasing factor modulates monoaminergic activity, aggression and cortisol in rainbow trout. Soc Neurosci Abs. 2006;32:61.4. [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Jones RE, Norris DO. Cell density and intracellular translocation of glucocorticoid receptor- immunoreactive neurons in the kokanee salmon (Oncorhynchus nerka kennerlyi) brain, with an emphasis on the olfactory system. Gen Comp Endocrinol. 2000;117:66–76. doi: 10.1006/gcen.1999.7391. [DOI] [PubMed] [Google Scholar]
- Cash WB, Holberton RL. Effects of exogenous corticosterone on locomotor activity in the red-eared slider turtle, Trachemys scripta elegans. J Exp Zool. 1999;284:637–644. doi: 10.1002/(sici)1097-010x(19991101)284:6<637::aid-jez5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Challet E, Le Maho Y, Robin JP, Malan A, Cherel Y. Involvement of corticosterone in the fasting-induced rise in protein utilization and locomotor activity. Pharmacol Biochem Behav. 1995;50:405–412. doi: 10.1016/0091-3057(94)00287-s. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Influence of physical exercise on 5-HT1A receptor- and anxiety-related behaviours. Neurosci Lett. 1994;176:226–230. doi: 10.1016/0304-3940(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Clements S, Moore FL, Schreck CB. Evidence that acute serotonergic activation potentiates the locomotor-stimulating effects of corticotropin-releasing hormone in juvenile chinook salmon (Oncorhynchus tshawytscha) Horm Behav. 2003;43:214–221. doi: 10.1016/s0018-506x(02)00027-2. [DOI] [PubMed] [Google Scholar]
- Clements S, Schreck CB. Central administration of corticotropin-releasing hormone alters downstream movement in an artificial stream in juvenile chinook salmon (Oncorhynchus tshawytscha) Gen Comp Endocrinol. 2004a;137:1–8. doi: 10.1016/j.ygcen.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Clements S, Schreck CB. Evidence that GABA mediates dopaminergic and serotonergic pathways associated with locomotor activity in juvenile chinook salmon (Oncorhynchus tshawytscha) Behav Neurosci. 2004b;118:191–198. doi: 10.1037/0735-7044.118.1.191. [DOI] [PubMed] [Google Scholar]
- Clements S, Schreck CB, Larsen DA, Dickhoff WW. Central administration of corticotropin-releasing hormone stimulates locomotor activity in juvenile chinook salmon (Oncorhynchus tshawytscha) Gen Comp Endocrinol. 2002;125:319–327. doi: 10.1006/gcen.2001.7707. [DOI] [PubMed] [Google Scholar]
- Craig PM, Al Timimi H, Bernier NJ. Differential increase in forebrain and caudal neurosecretory system corticotropin-releasing factor and urotensin I gene expression associated with seawater transfer in rainbow trout. Endocrinology. 2005;146:3851–3860. doi: 10.1210/en.2005-0004. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ. Ontogeny of corticotropin-releasing factor effects on locomotion and foraging in the Western spadefoot toad (Spea hammondii) Horm Behav. 2004;46:399–410. doi: 10.1016/j.yhbeh.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Emerson AJ, Kappenman DP, Ronan PJ, Renner KJ, Summers CH. Stress induces rapid changes in serotonergic activity: Restraint and exertion. Behav Brain Res. 2000;111:83–92. doi: 10.1016/s0166-4328(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Hen R. The serotonergic system and anxiety. Neuromolecular Med. 2004;5:27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- Gregory TR, Wood CM. The effects of chronic plasma cortisol elevation on the feeding behavior, growth, competitive ability and swimming performance of juvenile rainbow trout. Physiol Biochem Zool. 1999;72:286–295. doi: 10.1086/316673. [DOI] [PubMed] [Google Scholar]
- Höglund E, Kolm N, Winberg S. Stress-induced changes in brain serotonergic activity, plasma cortisol and aggressive behavior in Arctic charr (Salvelinus alpinus) is counteracted by L-DOPA. Physiol Behav. 2001;74:381–389. doi: 10.1016/s0031-9384(01)00571-6. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ, Wu M. Injections of dopaminergic, cholinergic, serotoninergic and GABAergic drugs into the nucleus accumbens: effects on locomotor activity in the rat. Neuropharmacology. 1981;20:29–37. doi: 10.1016/0028-3908(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp The. 1987;242:757–763. [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Swerdlow N, Seeligson M, Eaves M, Sutton R, Rivier J, Vale W. Effects of alpha-flupenthixol and naloxone on CRF-induced locomotor activation. Neuroendocrinology. 1984;39:459–464. doi: 10.1159/000124021. [DOI] [PubMed] [Google Scholar]
- Korte SM, Bouws GA, Bohus B. Central actions of corticotropin-releasing hormone (CRH) on behavioral, neuroendocrine, and cardiovascular regulation: brain corticoid receptor involvement. Horm Behav. 1993;27:167–183. doi: 10.1006/hbeh.1993.1013. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Summers TR, Ronan PJ, Renner KJ, Summers CH. The role of monoaminergic nuclei during aggression and sympathetic social signaling. Brain Behav Evol. 2001;57:317–327. doi: 10.1159/000047250. [DOI] [PubMed] [Google Scholar]
- Laberge F, Mnhlenbrock-Lenter S, Grunwald W, Roth G. Evolution of the amygdala: New insights from studies in amphibians. Brain, Behavior and Evolution. 2006;67:177–187. doi: 10.1159/000091119. [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Deviche P, Moore FL. Effects of corticotropin-releasing factor (CRF) and opiates on amphibian locomotion. Brain Res. 1990;513:94–100. doi: 10.1016/0006-8993(90)91093-v. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Moore FL. Corticotropin-releasing factor (CRF) antagonist suppresses stress- induced locomotor activity in an amphibian. Horm Behav. 1991;25:84–96. doi: 10.1016/0018-506x(91)90041-f. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Moore FL. Regulation of behavioral responses by corticotropin-releasing factor. Gen Comp Endocrinol. 2006;146:19–27. doi: 10.1016/j.ygcen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Rose JD, Moore FL. Corticotropin-releasing factor enhances locomotion and medullary neuronal firing in an amphibian. Horm Behav. 1996;30:50–59. doi: 10.1006/hbeh.1996.0008. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Mok EY, Munro AD. Effects of dopaminergic drugs on locomotor activity in teleost fish of the genus Oreochromis (Cichlidae): involvement of the telencephalon. Physiol Behav. 1998;64:227–234. doi: 10.1016/s0031-9384(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J Comp Neurol. 2006;494:903–943. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Davis RE. Telencephalic organization in ray-finned fishes. In: Davis RE, Northcutt RG, editors. Fish Neurobiology Volume 2, Higher Brain Areas and Functions. University of Michigan Press; Ann Arbor: 1983. pp. 203–236. [Google Scholar]
- Øverli Ø, Kotzian S, Winberg S. Effects of cortisol on aggression and locomotor activity in rainbow trout. Horm Behav. 2002a;42:53–61. doi: 10.1006/hbeh.2002.1796. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Pottinger TG, Carrick TR, Overli E, Winberg S. Differences in behaviour between rainbow trout selected for high- and low-stress responsiveness. J Exp Biol. 2002b;205:391–395. doi: 10.1242/jeb.205.3.391. [DOI] [PubMed] [Google Scholar]
- Pepels PP, Balm PH. Ontogeny of corticotropin-releasing factor and of hypothalamic-pituitary-interrenal axis responsiveness to stress in tilapia (Oreochromis mossambicus; Teleostei) Gen Comp Endocrinol. 2004;139:251–265. doi: 10.1016/j.ygcen.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Pepels PP, Van Helvoort H, Wendelaar Bonga SE, Balm PH. Corticotropin-releasing hormone in the teleost stress response: rapid appearance of the peptide in plasma of tilapia (Oreochromis mossambicus) J Endocrinol. 2004;180:425–438. doi: 10.1677/joe.0.1800425. [DOI] [PubMed] [Google Scholar]
- Pottinger TG, Carrick TR. Stress responsiveness affects dominant-subordinate relationships in rainbow trout. Horm Behav. 2001;40:419–427. doi: 10.1006/hbeh.2001.1707. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner KJ, Luine V. Analysis of temporal and dose-dependent effects of estrogen on monoamines in brain nuclei. Brain Res. 1986;366:64–71. doi: 10.1016/0006-8993(86)91281-3. [DOI] [PubMed] [Google Scholar]
- Renner KJ, Luine VN. Determination of monoamines in brain nuclei by high performance liquid chromatography with electrochemical detection: young vs. middle aged rats Life Sci. 1984;34:2193–2199. doi: 10.1016/0024-3205(84)90320-5. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rotllant J, Balm PH, Ruane NM, Perez-Sanchez J, Wendelaar-Bonga SE, Tort L. Pituitary proopiomelanocortin-derived peptides and hypothalamus-pituitary-interrenal axis activity in gilthead sea bream (Sparus aurata) during prolonged crowding stress: differential regulation of adrenocorticotropin hormone and alpha-melanocyte-stimulating hormone release by corticotropin-releasing hormone and thyrotropin-releasing hormone. Gen Comp Endocrinol. 2000;119:152–163. doi: 10.1006/gcen.2000.7508. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur J Pharmacol. 2005;507:117–124. doi: 10.1016/j.ejphar.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Summers CH, Kampshoff JL, Ronan PJ, Lowry CA, Prestbo AA, Korzan WJ, Renner KJ. Monoaminergic activity in subregions of raphe nuclei elicited by prior stress and the neuropeptide corticotropin-releasing factor. J Neuroendocrinol. 2003;15:1122–1133. doi: 10.1111/j.1365-2826.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Separate neural substrates of the locomotor-activating properties of amphetamine, heroin, caffeine and corticotropin releasing factor (CRF) in the rat. Pharmacol Biochem Behav. 1985;23:303–307. doi: 10.1016/0091-3057(85)90574-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF. The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacol Biochem Behav. 1986;25:233–248. doi: 10.1016/0091-3057(86)90261-3. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF1 and CRF2 receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Alford S. The requirement of presynaptic metabotropic glutamate receptors for the maintenance of locomotion. J Neurosci. 2002;22:3692–3699. doi: 10.1523/JNEUROSCI.22-09-03692.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To CT, Anheuer ZE, Bagdy G. Effects of acute and chronic fluoxetine treatment of CRH-induced anxiety. Neuroreport. 1999;10:553–555. doi: 10.1097/00001756-199902250-00020. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wickens J. Striatal dopamine in motor activation and reward-mediated learning: steps towards a unifying model. J Neural Transm Gen Sect. 1990;80:9–31. doi: 10.1007/BF01245020. [DOI] [PubMed] [Google Scholar]
- Winberg S, Lepage O. Elevation of brain 5-HT activity, POMC expression, and plasma cortisol in socially subordinate rainbow trout. Am J Physiol. 1998;274:R645–R654. doi: 10.1152/ajpregu.1998.274.3.R645. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson A, Hylland P, Soderstöm V, Nilsson GE. Serotonin as a regulator of hypothalamic-pituitary-interrenal activity in teleost fish. Neurosci Lett. 1997;230:113–116. doi: 10.1016/s0304-3940(97)00488-6. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson GE. Roles of brain monoamine transmitters in agonistic behaviour and stress reactions, with particular reference to fish. Comp Biochem Physiol. 1993;106C:597–614. [Google Scholar]
- Wullimann MF, Rink E. The teleostean forebrain: a comparative and developmental view based on early proliferation, Pax6 activity and catecholaminergic organization. Brain Res Bull. 2002;57:363–370. doi: 10.1016/s0361-9230(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Zhang R, Nakanishi T, Ohgushi A, Ando R, Yoshimatsu T, Denbow DM, Furuse M. Interaction of corticotropin-releasing factor and glucagon-like peptide-1 on behaviors in chicks. Eur J Pharmacol. 2001a;430:73–78. doi: 10.1016/s0014-2999(01)01363-2. [DOI] [PubMed] [Google Scholar]
- Zhang R, Nakanishi T, Ohgushi A, Ando R, Yoshimatsu T, Denbow DM, Furuse M. Suppression of food intake induced by corticotropin-releasing factor family in neonatal chicks. Eur J Pharmacol. 2001b;427:37–41. doi: 10.1016/s0014-2999(01)01109-8. [DOI] [PubMed] [Google Scholar]
- Zhang R, Tachibana T, Takagi T, Koutoku T, Denbow DM, Furuse M. Serotonin modifies corticotropin-releasing factor-induced behaviors of chicks. Behav Brain Res. 2004;151:47–52. doi: 10.1016/j.bbr.2003.08.005. [DOI] [PubMed] [Google Scholar]