Abstract
Longitudinal studies of objectively measured physical activity are lacking in older adults. We tested whether objective measures of total daily activity decline more rapidly in older adults. This prospective, observational cohort study included 519 community-dwelling older persons from across metropolitan Chicago participating in the Rush Memory and Aging Project. Repeated total daily activity measures (leisure and non-leisure physical activity) were derived from actigraphic recordings for up to 10 days. Generalized estimating equation models which controlled for demographics measures were employed. At baseline, age was inversely related with the level of total daily activity (Estimate, −0.014, S.E., 0.002, p<0.001). During up to 6 years of follow-up, total daily activity declined by about 0.070 × 105 activity counts/day/yr (Estimate −0.065, S.E. 0.005, p<0.001). Total daily activity declined 3% more rapidly for each additional year of age at baseline (Estimate −0.002, S.E. 0.001, p=0.027). Thus, total daily activity declined almost twice as fast in an individual 91 years old at baseline versus an individual 71 years old. A higher level of education was associated with a slower rate of decline (Estimate 0.004, S.E. 0.002, p<0.018). The associations of age and education with the rate of declining total daily activity were unchanged when controlling for baseline level of motor and cognitive function, other late-life activities and chronic health conditions. These data suggest that total daily activity in very old adults declines more rapidly with increasing age. Thus, physical inactivity is likely to become a larger problem in our aging population.
Keywords: Aging, Actigraphy, Physical Activity, Total Daily Activity
1. INTRODUCTION
To meet the growing personal and social burden of declining health in our aging population, public health efforts have focused on increasing physical activity, a modifiable behavior with a wide range of potential health benefits.(O'Donovan et al., 2010; D Paterson, Jones, & Rice, 2007) There is sparse data about the trajectory of declining physical activity in very old adults.(Timmons et al., 2010) Available studies have typically relied on self-reported physical activity questionnaires which are imprecise, lack sufficient variability, and are subject to recall bias, particularly in very old adults who may develop cognitive impairment.(J. William Langston, 2006; Siderowf & Lang, 2012; Stessman, Hammerman-Rozenberg, Cohen, Ein-Mor, & Jacobs, 2009) Furthermore, few studies have accounted for both leisure and non-leisure physical activity which is essential since the latter may constitute the majority of daily physical activity for many very old adults.(Dong, Block, & Mandel, 2004; Lin et al., 2011; Robertson, 2013) Thus, in the absence of studies employing validated objective measures of total daily physical activity, the natural history of physical activity in older adults and its role as a modifiable risk factor will remain unclear.(Baker, Francis, Soares, Weightman, & Foster, 2011; Daviglus Ml & et al., 2011)
To test the hypothesis that total daily activity declines more rapidly with increasing age, we used data from more than 500 older adults from the Memory and Aging Project. Actigraphsa were worn on the non-dominant wrist and recorded all movements 24 hours/day for up to 10 days for 2 or more annual assessments. First, we documented longitudinal changes in total daily activity and then examined whether the rate of change varied with age, sex or education.
2. METHODS
2.1 Participants
Participants were from the Rush Memory and Aging Project. The study recruited residents of continuous care retirement communities, subsidized housing, and through local Churches and social service agencies serving minorities and low-income elderly in metropolitan Chicago. The aim was to recruit a cohort that was comparable to the 80-year old population in Chicago at the time of the 1990 census in terms of education (33% with 12 years or less), gender (75% women) and racial and ethnic minorities (10%). Written informed consent was obtained, and the study was conducted in accordance with the latest version of the Declaration of Helsinki and was approved by Rush University Medical Center Institutional Review Board.
Rolling admission for the Memory and Aging Project began in 1997, actigraphy data collection was added in August, 2005. Of 1336 living participants since actigraphy was added, 41 refused testing and 400 are awaiting testing. Participants undergoing actigraphy were older, had more education and better cognition, less disability, but had lower motor function and more chronic health conditions compared to participants without actigraphy. The groups did not differ by gender or levels of self-report physical activity. Of 895 participants assessed with actigraphy, there were 590 with 1 or more follow-up actigraphy assessments and eligible for these analyses. Of these 33 had missing clinical data and 38 had dementia at the time of the first Actical cycle leaving 519 for these analyses. These participants were younger, had higher levels of cognition, motor function and self-report physical activity with fewer chronic health conditions and less disability than cases with only 1 assessment with actigraphy. The two groups did not differ with respect to gender or education (results not shown).
2.2 Assessment of Total Daily Activity
Total daily activity was measured with actigraphs. Actical®; (Philips Healthcare,,Andover, MA) is a portable, battery-operated activity monitor similar in size to a wristwatch which was worn on the non-dominant wrist for 24 hours/day. Actical accelerometers generate a signal proportional to the magnitude and duration of the detected motion. After its signal is digitized, it is rectified, integrated across 15 seconds and rounded to the nearest integer, to create an “activity count” for each 15-second period with motion.
Total daily activity was the average sum of all activity counts recorded during all days for which activity was recorded. We evaluated the day-to-day reliability of actigraphy by performing a variance components analysis for actigraphy obtained at baseline in the 895 participants with up to 10 days of data. The between-subject variance estimate was 2.40 and within-subject variance estimate was 0.45, such that less than one fifth (16%) of the total variance in daily actigraphy data was due to daily variation, suggesting the measure was stable across multiple days (intra-class correlation coefficient=0.824).
For illustrative purposes, using standardized instructions and structured tasks, investigators have reported activity counts for several tasks including sitting and writing for 3 minutes (43 activity counts), floor sweeping for 3 minutes (1, 721activity counts) and walking for 5 minutes at 2.5 m.p.h. (2, 355 activity counts).(Heil, 2006) Due to the large number of counts accumulated over 24 hours, raw counts in the current study were divided by 1×105 (about 1 Standard deviation [SD]) to facilitate presentation and interpretation of the results.
2.3 Other Covariates
Age in years at the time of actigraphy was computed from self-reported date of birth and date of actigraphy collection. Sex and years of education were recorded at the study entry. Clinical diagnoses were made using a multi-step process, as previously described.(Bennett et al., 2012) Cognitive function testing included 21 performance tests, 19 of which were summarized into a composite measure of global cognition using z scores as described previously.(Bennett et al., 2012) Participants were then evaluated in person by an experienced physician who diagnosed dementia,(McKhann et al., 1984) stroke,(Adams et al., 1993) Parkinson’s disease(J. W. Langston et al., 1992) based on published criteria. Self-report assessment of late-life physical activity was based on questions adapted from the 1985 National Health Interview Survey and expressed as hours of activity/week. (A. S. Buchman et al., 2009) Frequency of participation in social activity was based on 6 items involving social interaction over the past year.(A. S. Buchman et al., 2009) Frequency of participation over the past year in cognitively stimulating activities was based on 7 cognitive activities. (A. S. Buchman et al., 2009) Motor function was based on eleven motor performance tests which were scaled and averaged to obtain a summary measure.(A.S. Buchman et al., 2011) Activities of daily living were assessed using a modified version of the Katz scale.(Katz & Akpom, 1976) Self-reported vascular risk factors and vascular diseases were used in these analyses.(Boyle et al., 2005)
2.4 Statistical Analysis
Pearson correlations were used to examine the associations between total daily activity, age and education. T-tests were used to compare men and women. Generalized linear models fit using the method of generalized estimating equations (GEE) were used to summarize cross-sectional and longitudinal information about level of, and the annual rate of change in total daily activity.(Liang & Zeger, 1986; Zeger, Liang, & Albert, 1988) The models included a term for study time, measured as time in years from the study baseline. There were also terms for age (centered at the approximate mean of 81), sex (1 for male, 0 for female) and education (centered at the mean of 14) at baseline as well as terms for the interaction of each of these measures with study time. We added terms for other covariates as well as their interactions with time to see if these covariates affected the associations of age and education with the rate of declining in total daily activity. Model validation was performed graphically and analytically and there was no evidence of nonlinearity or non-proportionality. Programming was done in SAS.(SAS Institute Inc, 2002–2003)
3. RESULTS
3.1 Descriptive Properties of Total Daily Activity at Baseline
There were 519 participants (N=394, 76% female) with an average age of 81.6 (SD=6.84, range 56–99) and 14.8 years of education (SD=2.94 years, range 7–28; 29.5% 12 years or less); 29% reported income lower than $25K, 39% had income between $25K and $50K, and 32% had income over $50K and median income between $35K and $50K. Men had more vascular diseases than women but did not differ with respect to vascular risk factors or level of disability (results not shown). Additional baseline clinical characteristics are included in Table 1. On average, total daily activity was measured for 9 days (9.3 days; SD=1.1 days. Range 2–11). Total daily activity ranged from 0.16 × 105 counts/day to 11.06 × 105 counts/day (mean: 3.15 × 105 counts/ day; SD = 1.47 × 105 counts/ day). At baseline, total daily activity was associated with age (r=−0.23, p<0.001) but not with education (r=−0.02, p=0.710) and did not differ between men and women (t[183]=1.50, p=0.134).
Table 1.
Characteristics of the Cohort at Baseline (N=519)
| Variable a | Mean (SD) or N (%) |
|---|---|
| Cognitive Function (composite) | 0.23 (0.49) |
| Motor Function (composite) | 1.0 (0.29) |
| Disability (max 6) | 0.19 (0.69) |
| Physical Activities (hours/week) | 3.4 (3.80) |
| Social Activities (max 6) | 2.6 (0.59) |
| Cognitive Activities (max 7) | 3.2 (0.64) |
| Vascular risk factors | 1.2 (0.82) |
| Hypertension | 300 (58%) |
| Diabetes mellitus | 82 (16%) |
| Smoking | 198 (38%) |
| Vascular diseases | 0.4 (0.74) |
| Myocardial infarction | 62 (12%) |
| Congestive heart failure | 28 (6%) |
| Claudication | 77 (15%) |
| Stroke | 52 (11%) |
Abbreviations: SD, standard deviation; Cognitive Function: A composite measure of cognition was obtained by averaging z scores of 19 cognitive tests (a higher score indicates a higher level of cognition). BMI: calculated as weight in kilograms divided by height in meters squared. Motor Function: A composite motor measure was obtained by averaging scaled scores for 11 motor performance measures. These included grip and pinch strength as well as seven performance-based tests of lower extremity function and two aspects of motor performance were tested in the upper extremities (a higher score indicates higher or better motor function. Disability: 6 basic activities of daily living (ADLs) were assessed using a modified version of the Katz scale. A higher score indicates greater disability Physical Activity: Self-reported frequency (hours/week) of participation in 5 physical activities [walking for exercise, gardening or yardwork, calisthenics or general exercise, bicycle riding, and swimming or water exercise]. Social Activity: Self-reported frequency of participation in 6 items about activities involving social interaction[1) go to restaurants, sporting events or teletract, or play bingo, 2) go on day or overnight trips, 3) do unpaid volunteer work, 4) visit relatives or friends houses, 5) participate in senior groups, 6) attend religious services]; a higher score indicates more frequent participation. Cognitive Activity: Self reported frequency of participation in 7 cognitive activities, a higher score indicates more frequent participation. Vascular Risk Factors: sum of smoking, diabetes, and hypertension self-reported. Vascular Diseases: sum of myocardial infarction, congestive heart failure, claudication and stroke self-reported.
3.2 Demographic Measures and the Level and Rate of Change of Total Daily Activity
A generalized linear model fit using generalized estimating equations (GEE) was used to summarize both the cross-sectional and longitudinal associations of total daily activity with demographic measures. This model included 3 terms to assess the effect of age on total daily activity: age (at study entry), time (annual rate of change in total daily activity) and age × time (interaction).
At baseline, age was inversely related to the level of total daily activity (Table 2, Age). Thus, for each additional year above the mean of 81 years, the level of total daily activity was 0.014 × 105 activity counts/day lower (about 0.4%/yr).
Table 2.
Level and Rate of Change in Total Daily activity and Demographic Measures *
| Term | Estimate (S.E., p-value) |
|---|---|
| Time | −0.065 (0.006, <0.001) |
| Age | −0.014 (0.002, <0.001) |
| Sex | −0.047 (0.041, 0.2497) |
| Education | −0.004 (0.006, 0.5590) |
| Time × Age | −0.002 (0.001, 0.0264) |
| Time × Sex | 0.000 (0.012, 0.9903) |
| Time × Education | 0.004 (0.002, 0.0182) |
Estimated from a generalized estimating equation (GEE) models.
During up to 6 years of follow-up (mean 3.3 years (SD=1.15 years), total daily activity declined by about 0.07 × 105 activity counts/day/yr or about 2%/yr (Table 2, Time). Thus the predicted annual rate of decline in total daily activity is more than 5× larger than what would be expected based solely on the cross-sectional effect estimated at baseline (Table 2, estimated effect of Age/yr versus Time).
These models also included terms which examined whether declining total daily activity varied with demographic measures. There was a significant interaction between the annual rate of decline of total daily activity and age (Table 2, Time × Age) suggesting that the rate of decline during the study was more rapid in individuals who were older at study entry.
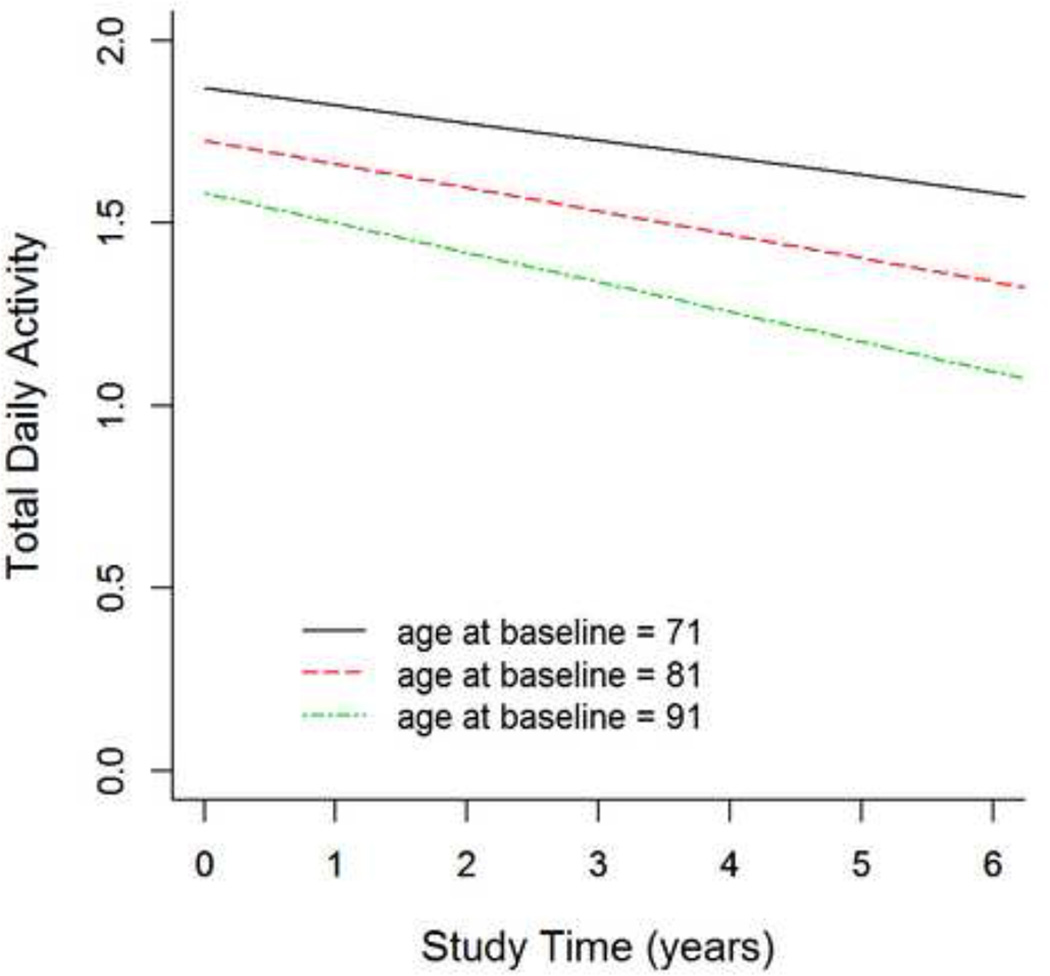
Figure 1 illustrates the effect of age on the trajectory of declining total daily activity for 3 average participants age 71, 81 and 91 years old respectively at study entry. Total daily activity declined by about 3% more rapidly per year (about 0.002 unit/yr) for each year above 81 years old at baseline (Table 2, Time × Age). Thus, total daily activity declined almost 2× faster in an individual 91 years old at study entry versus an individual 71 years old.
Figure 1. Interaction of Age at Study Entry and Annual Rate of Declining Total Daily Activity.
This figure shows the effect of age (x axis) on total daily activity (y axis) for 3 average participants, female with 14 years of education, ages 71 (blue), 81 (red) and 91 (green) years at baseline of the current study.
Although motor function may differ between men and women across the lifespan, there was no significant difference in the rate of change in total daily activity during follow-up in men compared to women (Table 2, Time × Sex). By contrast, higher levels of education were associated with about a 6% slower rate of decline for each year of education (Table 2, Model D: Time × Education). Comparing the estimates for the interactions of age (Time × Age) and education (Time × education) with the rate of change in total daily activity shows that each additional year of education years was similar to a participant being 2 years younger at study entry.
The rate of declining total daily activity might be more rapid in participants with motor disorders. In sensitivity analyses the associations of age and education with declining total daily activity were unchanged when we excluded cases with a history of PD or stroke at study entry (results not shown).
3.3 Age, the Rate of Change of Total Daily Activity and Age-Related Health Conditions
Diverse physiologic systems essential for producing physical activity can be affected by age-related health conditions and could be associated with the rate of declining total daily activity. Therefore we repeated the core model (Table 2, Model D) to examine whether common functional impairments or health conditions, assessed at the time of the intial actigraphy, might account for the more rapid rate of declining total daily activity with increasing age described above.
Recent work has suggested that not only physical activity but a wider range of late-life activities may affect the level of total daily activity.(A. S. Buchman et al., 2009) The association of age and rate of change in total daily activity was unchanged after adjusting for self-report physical activity as well as the frequency of social and cognitive activities (Table 3, Models A–C).
Table 3.
Association of Age and Education with the Rate of Change in Total Daily Activity after Adjusting for Potential Confounders
| Models | Terms Added to Core Model * | Estimate (S.E., p-value) |
Estimate (S.E., p-value) |
|---|---|---|---|
| A | Self-Report Physical Activity | Time × Age −0.002 (0.001,0.021) |
Time × Education 0.004 (0.002,0.014) |
| B | Self-Report Cognitive Activity | Time × Age −0.002 (0.001,0.026) |
Time × Education 0.004 (0.002,0.057) |
| C | Self-Report Social Activity | Time × Age −0.002 (0.001,0.036) |
Time × Education 0.004 (0.002,0.022) |
| D | Cognitive Function | Time × Age −0.002 (0.001,0.022) |
Time × Education 0.005 (0.002,0.018) |
| E | Motor Function | Time × Age −0.002 (0.001,0.033) |
Time × Education 0.004 (0.002,0.013) |
| F | Disability | Time × Age −0.002 (0.001,0.019) |
Time × Education 0.004 (0.002,0.020) |
| G | Chronic Health Conditions | Time × Age −0.002 (0.001,0.021) |
Time × Education 0.005 (0.002,0.009) |
Core Model: Estimated from a separate generalized estimating equation (GEE) model which also included terms for the annual rate of total daily activity decline (Time) as well as for age, sex, education as well as their interaction with the annual rate of change in total daily activity. (Table 2, Model D). Physical Activity: Self-reported frequency of participation in 5 physical activities (hours/week), a higher score indicates more frequent participation. Cognitive Activity: Self reported frequency of participation in 7 cognitive activities, a higher score indicates more frequent participation. Social Activity: Self-reported frequency of participation in 6 items about activities involving social interaction, a higher score indicates more frequent participation. Cognitive Function: Composite measure of cognition based on performances on 19 cognitive tests (a higher score indicates a higher level of cognition). Motor Function: Composite measure summarizing 11 motor performance tests. Disability: Six basic activities of daily living (ADLs) were assessed using a modified version of the Katz scale. A higher score indicates greater disability Chronic Health Conditions: included terms for the sum of 3 vascular risk factors (hypertension, diabetes and smoking) and sum of 4 vascular diseases (myocardial infarction, congestive heart failure, claudication and stroke).
Motor and cognitive abilities can affect physical activity. The association of age and total daily activity was unchanged when we controlled for level of motor and cognitive function as well as disability at study entry (Table 3, Models D–F). Chronic health conditions can affect physical activity. The association of age and rate of change in total daily activity was unchanged when we controlled for vascular risk factors and vascular diseases (Table 3, Model G). Controlling for these covariates did not affect the association of education and the rate of declining total daily activity (Table 3). Next, we repeated these analyses using the average value for each of the covariates during the same period of actical follow-up. The estimates for the associations of age and education with the rate of change in total daily activity were unchanged (results not shown).
4. DISCUSSION
In a group of more than 500 community-dwelling older adults, repeated objective measures of total daily activity, based on actigraphic recordings of all movements (leisure and non-leisure physical activity) 24 hours/day for up to 10 days, showed that the rate of declining total daily activity accelerated with increasing age and was slower for individuals with more education. These data suggest that physical inactivity is likely to become a larger problem in our aging population.
Longitudinal studies which have employed objective physical activity measures in very old adults are lacking. Until recently, objective measures of physical activity could only be obtained in the laboratory setting, leading to gaps in our knowledge since older more debilitated individuals, were not tested in these studies.(Donald Paterson & Warburton, 2010; Warburton, Charlesworth, Ivey, Nettlefold, & Bredin, 2010) Moreover, laboratory testing in older adults may not reflect the level and patterns of physical activity in the community-setting (Kaye et al., 2011) In prior publications in this cohort we have demonstrated the feasibility of long term actigraphic recordings in very old adults living in the community-setting and provided evidence that an objective measure, total daily activity, which captures all daily leisure and non-leisure physical activity, is associated with adverse health outcomes and has independent effects when compared with traditional self-reported physical activity. (A. S. Buchman et al., 2012; Aron S. Buchman, Yu, Boyle, Shah, & Bennett 2012; Shah, Buchman, Leurgans, Boyle, & Bennett, 2012)
The current study extends these previous reports by providing evidence that total daily activity declines in very old adults and that the rate of decline was more rapid in older participants. The magnitude of decline documented in the current longitudinal analysis was larger than expected based on cross sectional analysis. Previous studies have also reported that cross-sectional estimates of age-related decline may underestimate the rate of decline documented in longitudinal studies.(Desrosiers, Hebert, Bravo, & Rochette, 1998) In the current study, the exclusion of individuals with dementia at baseline may have resulted in a restricted range of total daily activity at baseline due to a healthy volunteer effect accounting for the underestimate of age-associated decline derived from baseline assessments. The accelerating decline of total daily activity with increasing age observed in the current study may reflect the accumulation of impairments in multiple physiologic functions which are essential for the production of physical activity. Consistent with this idea, aerobic capacity as well as pulmonary function show accelerated decline with increasing age in older adults.(Dontas, Jacobs, Corcondilas, Keys, & Hannan, 1984; Rabbitt, Lunn, Pendleton, & Yardefagar, 2011) Thus, objective measures of the trajectory of physical activity in older adults might serve as an important index of overall health. Recent work suggests that several years prior to death, older adults undergo a marked acceleration of functional decline.(Robert S. Wilson et al., 2012) Thus, the acceleration of declining physical activity with increasing age may reflect impending death rather than advancing age. In contrast to age, more education was associated with slower rate of declining total daily activity raising the possibility that there may be additional behavioral or biologic factors which may provide “resilience” or motor reserve to counter age-related declining total daily activity.(R.S. Wilson et al., 2013) While these data fill an important gap in our knowledge about declining total daily activity in old age, further work is needed to identify additional risk factors and to explicate their underlying biology.
While there is widespread recognition of the importance of physical activity as an essential health intervention even in old age, accumulating evidence underscores the adverse health consequences of physical inactivity and a sedentary lifestyle. (Narici & Maffulli, 2010; Stamatakis, Hamer, & Dunstan, 2011) Thus, the current finding that declining total daily activity accelerates with aging suggests that the public health challenge of physical inactivity is likely to increase in our aging population. To counter the untoward effects of accelerating physical inactivity, it may be very important to focus on non-leisure physical activity which may constitute the major source of physical activity in many very old adults.(Lawlor, Taylor, Bedford, & Ebrahim, 2002) Thus, older persons, for whom participation in formal exercise may be constrained because of underlying health problems, may nonetheless benefit from increases in their levels of non-exercise physical activities. Thus, public health efforts to facilitate a more active lifestyle in older adults, should be expanded to include a much wider spectrum of non-exercise movement-based activities including habitual daily physical activity, leisure-time physical and social activities, and even fidgeting.(Davis et al., 2011) Additionally, it is important to note that while the analyses in this study focused on total daily physical activity, continuous activity recordings include all periods of both activity and rest during 24 hours. Recent work in this cohort and by others suggests that additional novel metrics can be derived from these recordings including the duration of periods of physical activity and their distribution over the course of the day. Thus, like sleep, not only the amount of physical activity, but its distribution or “fragmentation” may be an important determinant of health and may be amenable to modification.(Chastin et al., 2010; Lim et al., 2011) It is likely that with falling cost, further miniaturization and increasingly sophistication of the devices which are available that their use will become more commonplace providing new physical activity metrics which have the potential for new ways to facilitate more active lifestyles in older adults. Ultimately, the efficacy of suggestions derived from observational studies, like the current study, need to be tested in randomized controlled clinical trials.
The main strength of this study is the use of actigraphy to provide repeated objective measures of total daily activity in very old adults in the community-setting. In addition, this study was able to adjust for traditional self-reported measures of physical activity and other late-life activities in a relatively large number of older persons who may be more representative of the cognitive and physical function spectrum observed in the community-setting. An additional strength is that robust measurements of both cognition and motor function and other possible confounders, evaluated as part of a uniform clinical evaluation were also examined.
There are several limitations to this study. Participants are a volunteer cohort, and given their health status, high educational and socioecomic status of some of the participants, the current findings may underestimate the rate of declining physical activity in the general population of older adults. While the preponderance of females in this study approximates the population in metropolitan Chicago, the lack of sex differences might be due to power. There are limitations to the actigraphs used in this study: certain types of activities may not be measured depending on where the device is located on the body, the device used in the current study did not differentiate the types of activities that were performed, activity while awake or asleep could not be differentiated and removal of the device cannot always be distinguished from periods of no activity. While the devices used in the current study provided objective measures of total daily activity, further studies are needed to delineate the determinants of leisure and non-leisure physical activity in older adults.
5. CONCLUSIONS
In more than 500 community-dwelling older persons followed for up to 6 years, a novel objective measure of total daily activity based on leisure and non-leisure activity declined. The rate of declining total daily activity was more rapid in older participants and slower in those with more education. Thus, the public health challenge of physical inactivity and the number of individuals who transition into a more sedentary lifestyle is likely to grow larger in our aging population. Interventions to modify non-leisure activities in very old adults may provide an important way to facilitate a more active lifestyle.
Acknowledgments
We thank all the participants in the Rush Memory and Aging Project. We also thank Traci Colvin, RN and Tracey Nowakowski, MS for project coordination; John Gibbons, MS for data management; Wenqing Fan, MS for statistical programming and the staff of the Rush Alzheimer’s Disease Center.
Source of Funding: This work was supported by National Institute on Aging grants R01AG17917 (DAB), and R01AG24480 (ASB), Illinois Department of Public Health, and Robert C. Borwell Endowment Fund. The study sponsors had no role in the study design, data collection, analyses or preparation of manuscripts.
Conflict of Interest: We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated. We certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified above. (ASB, RSW, LY, BDJ, PAB, DAB)
ASB receives support from the NIH [R01AG17917 (Co-Investigator), P30AG10161 (Co-Investigator), R01AG24480 (PI), R01AG043379 (PI), R01NS078009 (PI)].
RSW serves as a Consulting Editor for Aging, Neuropsychology, and Cognition andPsychology and Aging; has served as a consultant for Pain Therapeutics, Inc.; and receives research support from NIH (R01AG024871 [principal investigator], P30AG10161 [coinvestigator], R01AG11101 [co-investigator], R01AG15819 [co-investigator], U24AG026395 [co-investigator], R01AG017917 [co-investigator], R01AG009966 [co-investigator], R01AG034374 [co-investigator], and RC2AG036547 [co-investigator]).
LY receives research support from NIH [R01AG033678 (co-investigator), R01AG024871 (co-investigator), R01AG024871 (co-investigator), P30AG010161 (co-investigator), R01HL096944 (co-investigator), R01AG036042 (co-investigator), R01AG038651 (co-investigator).
BDJ receives research support from NIH R01AG17917 (co-Investigator), P30AG10161 (co-Investigator) and R01AG033678 (co-Investigator). He served as a consultant to the Alzheimer’s Association and Partners.
PAB receives research support from the NIH (R01AG034374 [principal investigator], R01AF034119 [co-investigator], and R01AG033678 [principal investigator]).
DAB serves on the editorial board of Neurology; has received honoraria for non-industry sponsored lectures; has served as a consultant to Danone, Inc., Wilmar Schwabe GmbH & Co., Eli Lilly, Inc., Schlesinger Associates, Geson Lehrman Group; and receives research the NIH [P30AG10161 (PI), R01AG17917 (principal investigator), R01AG15819 (principal investigator)], Illinois Department of Public Health (principal investigator), Robert C. Borwell Endowment Fund (principal investigator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Baker PR, Francis DP, Soares J, Weightman AL, Foster C. Community wide interventions for increasing physical activity. Cochrane Database Syst Rev. 2011;(4):CD008366. doi: 10.1002/14651858.CD008366.pub2. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and Findings From the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Arvanitakis Z, Kelly J, Bienias JL, Bennett DA. Parkinsonian signs in subjects with mild cognitive impairment. Neurology. 2005;65(12):1901–1906. doi: 10.1212/01.wnl.0000188878.81385.73. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Wilson RS, Fleischman DA, Leurgans S, Bennett DA. Association between late-life social activity and motor decline in older adults. Arch Intern Med. 2009;169(12):1139–1146. doi: 10.1001/archinternmed.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of Motor Measures More Strongly Predict Adverse Health Outcomes in Old Age: The Rush Memory and Aging Project, a Community-Based Cohort Study. BMC Medicine. 2011;9:42. doi: 10.1186/1741-7015-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman Aron S, Yu Lei, Boyle Patricia A, Shah Raj C, Bennett David A. Total Daily Physical Activity and Longevity in Old Age. Arch Intern Med. 2012;172:444–446. doi: 10.1001/archinternmed.2011.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastin Sebastein FM, Baker Katherine, Jones Diana, Burn David, Granat Malcolm H, Rochester Lynn. The pattern of habitual sedentary behavior is different in advanced Parkinson's disease. Movement Disorders. 2010;25(13):2114–2120. doi: 10.1002/mds.23146. [DOI] [PubMed] [Google Scholar]
- Daviglus Ml, Plassman BL, Pirzada A, et al. Risk factors and preventive interventions for alzheimer disease: State of the science. Archives of Neurology. 2011;68(9):1185–1190. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- Davis Mark, Fox Kenneth, Hillsdon Melvyn, Coulson Jo, Sharp Debbie, Stathi Afroditi, Thompson Janice. Getting out and about in older adults: the nature of daily trips and their association with objectively assessed physical activity. International Journal of Behavioral Nutrition and Physical Activity. 2011;8(1):116. doi: 10.1186/1479-5868-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers J, Hebert R, Bravo G, Rochette A. Comparison of cross-sectional and longitudinal designs in the study of aging of upper extremity performance. J Gerontol A Biol Sci Med Sci. 1998;53(5):B362–B368. doi: 10.1093/gerona/53a.5.b362. [DOI] [PubMed] [Google Scholar]
- Dong Linda, Block Gladys, Mandel Shelly. Activities Contributing to Total Energy Expenditure in the United States: Results from the NHAPS Study. International Journal of Behavioral Nutrition and Physical Activity. 2004;1(1):4. doi: 10.1186/1479-5868-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontas AS, Jacobs DR, Jr, Corcondilas A, Keys A, Hannan P. Longitudinal versus cross-sectional vital capacity changes and affecting factors. J Gerontol. 1984;39(4):430–438. doi: 10.1093/geronj/39.4.430. [DOI] [PubMed] [Google Scholar]
- Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport. 2006;77(1):64–80. doi: 10.1080/02701367.2006.10599333. [DOI] [PubMed] [Google Scholar]
- Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- Kaye JA, Maxwell SA, Mattek N, Hayes TL, Dodge H, Pavel M, Jimison HB, Wild K, Boise L, Zitzelberger TA. Intelligent systems for assessing aging changes: home-based, unobtrusive, and continuous assessment of aging. J Gerontol B Psychol Sci Soc Sci. 2011;(66 Suppl 1) doi: 10.1093/geronb/gbq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- Langston JWilliam. The parkinson's complex: Parkinsonism is just the tip of the iceberg. Annals of Neurology. 2006;59(4):591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Taylor M, Bedford C, Ebrahim S. Is housework good for health? Levels of physical activity and factors associated with activity in elderly women. Results from the British Women's Heart and Health Study. Journal of Epidemiology and Community Health. 2002;56(6):473–478. doi: 10.1136/jech.56.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lim AGP, Yu Lei, Costa Madalena D, Buchman Aron S, Bennett David A, Leurgans Sue E, Saper Clifford B. Quantification of the Fragmentation of Rest-Activity Patterns in Elderly Individuals using a State Transition Analysis. Sleep. 2011;34:1569–1581. doi: 10.5665/sleep.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Yu-Pei, Huang Ying-Hsiang, Lu Feng-Hwa, Wu Jin-Shang, Chang Chih-Jen, Yang Yi-Ching. Non-leisure time physical activity is an independent predictor of longevity for a Taiwanese elderly population: an eight-year follow-up study. BMC Public Health. 2011;11(1):428. doi: 10.1186/1471-2458-11-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Narici Marco V, Maffulli Nicola. Sarcopenia: characteristics, mechanisms and functional significance. British Medical Bulletin. 2010;95(1):139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- O'Donovan Gary, Blazevich Anthony J, Boreham Colin, Cooper Ashley R, Crank Helen, Ekelund Ulf, Fox Kenneth R, Gately Paul, Giles-Corti Billie, Gill Jason MR, Hamer Mark, McDermott Ian, Murphy Marie, Mutrie Nanette, Reilly John J, Saxton John M, Stamatakis Emmanuel. The ABC of Physical Activity for Health: A consensus statement from the British Association of Sport and Exercise Sciences. Journal of Sports Sciences. 2010;28(6):573–591. doi: 10.1080/02640411003671212. [DOI] [PubMed] [Google Scholar]
- Paterson D, Jones G, Rice C. Ageing and physical activity: evidence to develop exercise recommendations for older adults. Can J Public Health. 2007;98:S69–S108. [PubMed] [Google Scholar]
- Paterson Donald, Warburton Darren. Physical activity and functional limitations in older adults: a systematic review related to Canada's Physical Activity Guidelines. International Journal of Behavioral Nutrition and Physical Activity. 2010;7(1):38. doi: 10.1186/1479-5868-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt Patrick, Lunn Mary, Pendleton Neil, Yardefagar Ghasem. Terminal Pathologies Affect Rates of Decline to Different Extents and Age Accelerates the Effects of Terminal Pathology on Cognitive Decline. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66B(3):325–334. doi: 10.1093/geronb/gbr026. [DOI] [PubMed] [Google Scholar]
- Robertson Ian H. A noradrenergic theory of cognitive reserve: implications for Alzheimer's disease. Neurobiology of Aging. 2013;34(1):298–308. doi: 10.1016/j.neurobiolaging.2012.05.019. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT® Software for Unix, Version (9.18) Cary, NC: SAS Institute Inc; 2002–2003. [Google Scholar]
- Shah Raj, Buchman Aron, Leurgans Sue, Boyle Patricia, Bennett David. Association of total daily physical activity with disability in community-dwelling older persons: a prospective cohort study. BMC Geriatrics. 2012;12(1):63. doi: 10.1186/1471-2318-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderowf Andrew, Lang Anthony E. Premotor Parkinson's disease: Concepts and definitions. Movement Disorders. 2012;27(5):608–616. doi: 10.1002/mds.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol. 2011;57(3):292–299. doi: 10.1016/j.jacc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- Stessman Jochanan, Hammerman-Rozenberg Robert, Cohen Aaron, Ein-Mor Eliana, Jacobs Jeremy M. Physical Activity, Function, and Longevity Among the Very Old. Archives of Internal Medicine. 2009;169(16):1476–1483. doi: 10.1001/archinternmed.2009.248. [DOI] [PubMed] [Google Scholar]
- Timmons James A, Knudsen Steen, Rankinen Tuomo, Koch Lauren G, Sarzynski Mark, Jensen Thomas, Keller Pernille, Scheele Camilla, Vollaard Niels BJ, Nielsen Søren, Åkerström Thorbjörn, MacDougald Ormond A, Jansson Eva, Greenhaff Paul L, Tarnopolsky Mark A, van Loon Luc JC, Pedersen Bente K, Sundberg Carl Johan, Wahlestedt Claes, Britton Steven L, Bouchard Claude. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. Journal of Applied Physiology. 2010;108(6):1487–1496. doi: 10.1152/japplphysiol.01295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DER, Charlesworth S, Ivey A, Nettlefold L, Bredin SSD. A systematic review of the evidence for Canada's Physical Activity Guidelines for Adults. Int J Behav Nutr Phys Act. 2010;7:39. doi: 10.1186/1479-5868-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel RP, Yu L, Buchman AS, Schneider JA, Bennett DA. Neural Reserve, Neuronal Density in the Locus Coeruleus, and Cognitive Decline. Neurology. 2013 doi: 10.1212/WNL.0b013e3182897103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson Robert S, Segawa Eisuke, Buchman Aron S, Boyle Patricia A, Hizel Loren P, Bennett David A. Terminal Decline in Motor Function. Psych & Aging. 2012 doi: 10.1037/a0028182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]