Abstract
During agonistic interactions between male Anolis carolinensis, perception of a visual sign stimulus (darkened eyespots) not only inhibits aggression and promotes initial attainment of dominant social status, but also evokes distinct neuroendocrine responses in each opponent. This study was designed to examine the effect of eyespot manipulation on behavior and social rank during a second interaction between opponents that had previously established a natural dyadic social hierarchy. Prior to a second interaction, eyespots of familiar size-matched combatants were manipulated to reverse information conveyed by this visual signal. Eyespots on the previously dominant male were masked with green paint to indicate low aggression and social status. Previously subordinate males had their eyespots permanently marked with black paint to convey high aggression and status. Opponents were then repaired for a second 10 min interaction following either one or three days of separation. Aggression was generally decreased and social status between pairs remained reasonably consistent. Unlike rapidly activated monoaminergic activity that occurs following the initial pairing, most brain areas sampled were not affected when animals were re-introduced, regardless of visual signal reversal or length of separation between interactions. However in males with “normal” eyespot color, dominant males had reduced serotonergic activity in CA3 and raphé, while subordinate males exhibited elevated CA3 dopaminergic activity. Reversing eyespot color also reversed serotonergic activity in raphé and dopaminergic activity in CA3 after three days of separation. The results suggest that males remember previous opponents, and respond appropriately to their previous social rank in spite of eyespot color.
1. Introduction
Species that live in social groups or occur in close territorial proximity often establish strong social hierarchies between members or neighbors [7,13,14,55,66,86]. Among territorially competitive male Anolis carolinensis lizards, visual sign stimuli (eyespots) and/or previous experience modify aggressive behavior during interactions that help establish or affirm social rank [69,70]. Memory of previous opponents helps to stabilize rank relationships between individuals, influencing future social interactions between the same or new opponents [70]. Established dominant-subordinate relationships between male A. carolinensis remain intact, even when males from a dyad have been separated for seven days and then reintroduced [41]. Males reintroduced to the same dominant opponent exhibit diminished aggression, similar to the effect caused by rapid eyespot darkening during interactions with a novel opponent [15,34]. However, males made subordinate in an initial interaction exhibit a 50% increase in aggressive behavior (compared to original interaction) when faced with a different opponent known to be socially dominant [15]. Similarly, in social interactions where male A. carolinensis were highly mismatched with regard to size, smaller males invaded territories of larger opponents and engaged them in physical fighting, despite losing 90% of the interactions [31]. This strongly argues against simple behavioral inhibition due to losing (loser effects) being responsible for the stability of social rank. Taken together, these studies suggest that male A. carolinensis remember previous opponents for up to seven days, and that social rank is formed in relation to a specific individual. However, after ten days behavioral output by both A. carolinensis combatants suggests the plasticity of rank formation has returned [15].
Even in hierarchical groups this relative plasticity of social rank typically results from repeated interactions of the same opponents, because the basic social interaction occurs between two individuals. Dominant – subordinate relationships, often established through agonistic interactions, may be modified by social sign stimuli [52,53]. This is also the case in dominant – subordinate ranking between pairs of A. carolinensis [1,4,19,69]. Male A. carolinensis utilize aggressive interactions to establish territories during their reproductive season [4,14,18], and those that exhibit more aggression usually become socially dominant [69]. During agonistic encounters between two males, sympathetically activated darkening of postorbital skin (eyespot formation) in both males acts as a potent social sign stimulus, influencing aggressive behavior and the outcome of the interaction [38,40]. Male A. carolinensis viewing a novel opponent with darkened eyespots exhibit fewer aggressive acts [37–40]. Moreover, individuals with the most rapid eyespot formation become dominant in virtually all paired interactions between novel opponents [40,41,69,71]. In addition, artificially darkening the eyespot region of one male in a combative pair facilitates achievement of dominant status for that individual [40]. Therefore, formation of the eyespot signal plays a role in aggression [38], rank formation [40] and social stability [70]. This contextually plastic social sign stimulus is controlled by epinephrine and norepinephrine (NE) from the adrenal gland and sympathetic nerve terminals, which binds to β2-adrenergic receptors to cause darkening of postorbital skin while the rest of the body remains green [16,22,25,83]. More rapid eyespot darkening corresponds with more rapid production of high intensity aggression, which facilitates dominant social status [34,40,69–71]. Eyespot formation occurs under all stressful conditions [62,71], but in the context of social dominance relationships, eyespots may allow displaying male A. carolinensis to convey neuroendocrine condition and aggressive intent without engaging in physical interaction [18,34].
Previous social experience [26,55–57,59], social memory [15,41], and sign stimuli [34,39,40] may all influence aggressive interaction and social rank relationships [70]. However, the value of the eyespot signal in the formation of social rank has only been tested thus far in pairs of male A. carolinensis that are naïve to one another. The purpose of this study was to determine the relative potency of visual signals (eyespots) and experience/memory to mediate social rank and aggressive relationships during subsequent interactions between familiar pairs of male Anolis carolinensis. Artificial darkening of eyespots inhibits opponent aggression in dyads interacting for the first time [15,40,41]. We therefore investigated the effect of artificially reversing the eyespot signal prior to the second interaction, such that dominant males had eyespots masked and were made to appear less threatening, while their subordinate opponents were given darkened eyespots to convey elevated aggressive intent. We hypothesized that such visual signal manipulation may suppress aggressive output by dominants while encouraging higher aggression from subordinates, possibly allowing reversal of previously established individual social status. As central and plasma monoaminergic activity, influence behavior, eyespot coloration, and social status [16,33,35,37–41,71,73]we also measured changes in neurotransmitter and hormonal activity following a second social interaction. For example, during aggressive encounters, temporally and anatomically specific activation and reuptake of 5-HT is highly correlated with eyespot celerity, aggression and social status [8–11,41,68,69,71,73,75,76]. Results from these and other recent experiments suggest that the mechanisms regulating eyespots, behavior, autonomic function and social status are strongly influenced by central serotonergic and dopaminergic activity [33,37,41,73]. We hypothesized that most of the neurotransmitter and endocrine changes associated with aggression and social rank accrue to initial interactions between naïve opponents, and are already established as part of the neuroendocrine milieu prior to a secondary social interaction [73]. We therefore predicted that relatively few neuroendocrine changes would be evident following a subsequent interaction between familiar opponents with rank appropriate eyespot coloration. In contrast, we hypothesized that reversal of eyespot coloration would promote change in social rank and be reflected in greater changes in central monoaminergic and peripheral catecholaminergic activity, similar to those observed following initial interactions between naïve males bearing artificially altered eyespots.
2.0 Materials and methods
2.1. Animals
Adult male (>60 mm snout-vent length) Anolis carolinensis were obtained from a commercial supplier (Thibodaux Live Supplies; Thibodaux, LA, USA). Each lizard was weighed, measured and placed individually into one half of a terrarium. Each side (25 × 25 × 25 cm) contained a diagonally placed wooden perch and was separated from the other half by an opaque divider [38]. Animals that were placed adjacently, on opposite sides of the divider, were matched for size (mean weight varied by less than 6 mg, and mean snout-vent length varied by 2 mm) to minimize the influence of body size on behavioral interactions [80]. There were no significant differences in mean initial weights or snout-vent lengths between pairs or isolated controls. Lights, temperature (14L32°C:10D20°C) and relative humidity (70 – 80%) were regulated to maintain gonadal activity [42]. Lizards were fed live crickets and watered ad libitum.
2.2. Experimental Design
Following one week of acclimation, males were tested for reproductive condition. Only males responding with courtship behavior when presented with a female [24] were included in the study, as reproductive state and androgen levels are usually associated with aggressive capacity [24,46]. Naïve pairs were introduced by removing the opaque divider. They were allowed to interact for two hours, during which behavior was recorded for first 10 minutes, with social status for each animal was re-examined and confirmed at two hours. Once status was verified (after 2 h) males were captured and the eyespot region of each lizard was covered with non-toxic paint (Accent Acrylic Paints, Bloomsbury, NJ). Dominant and subordinate males received one of two treatments: 1) postorbital skin of dominant males was painted black, to darken the eyespot region, and painted green on subordinate lizards (N = 16 for each group) to hide the formation of natural eyespot [37–39]; 2) Conversely, eyespots of dominant males were painted green, covering the area to hide the formation of natural eyespot, and artificially darkened eyespots were added to the subordinate lizard (N = 16 for each group) [37–39]. When eyespots are hidden by green paint, the animal is visually similar to a subordinate male in the sense that subordinate males have a significantly longer latency to eyespot darkening [70,71], and therefore are more often viewed without darkened eyespots. Conversely, black paint mimics the rapid onset of eyespot formation in dominant males by artificially affixing the eyespot prior to the encounter. One group of males were painted with water, (controls; N = 10) and never exposed to an opponent, and therefore isolated from behavioral interaction. Each male was placed back in a sterilized aquarium on the opposite side to that in which it participated in the first social interaction, and cages were placed in a new location in the room. Either one or three days later the divider was again removed and behavior was manually recorded for ten minutes. Observations were made with room lights off and cage lights on. Darkened room, cage illumination and distance of observers from cages (1.5 m) minimized observer effects on lizard behavior [67]. All behavioral observations were performed between 12:30 pm and 3:00 pm central time. This window of observation was selected because room humidity and temperature were consistent at 32°C. All animal experiments were executed in a manner that minimized suffering and the number of animals used, in accordance with the Declaration of Helsinki and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23), under approved protocol by University of South Dakota IACUC.
2.3. Aggressive Behavior
All paired males interacted aggressively in their first interaction. Aggressive displays of A. carolinensis have been previously described [4,5,12,14,18,19]. All displays performed in assertion or challenge context [12,19] were counted during a ten minute period [38]. Behavioral records included approaches, bites, and extension of dewlap with head nods. Maximally aggressive display behavior (sagittal expansion) includes a combination of lateral compression of the rib cage, nuchal crest expansion, dewlap extension, sagittal spatial positioning, and head nods. Animals were scored with an approach every time they slowly decreased the distance between themselves and their opponent and the opponent did not move away. Approaches often precede aggressive displays, and always precede biting behavior. Winning an antagonistic encounter is accomplished by a greater frequency of and shorter latency to aggressive acts (such as biting) by a given male, and by chasing and displacing the opponent. Fight-winning males were confirmed to be socially dominant by attainment of the most superior perching position (the highest position directly under the overhead light), continued displacement of the subordinate individual from the superior perch position and from other locations within the cage, by lighter body coloration and reduced latency to eyespot formation during interaction one [34].
2.4. Plasma catecholamine assay
Within 5 seconds of completion of the experiment (immediately following the second 10 min interaction) animals were decapitated and blood was collected immediately from the body and head into heparinized microcapillary tubes. The tubes were then centrifuged to separate blood cells and plasma. An internal standard, DHBA (dihydroxybenzylamine; 100 ng/ml), was added along with a relatively small volume (25–100 μl) of plasma into a 1.5 ml syringe-filter cartridge (EG&G WALLAC/AKRON) with 50 mg of acid-washed aluminum oxide (BAS) [43]. Immediately upon the addition of 1 ml Tris buffer (1.86 M, pH 8.65) samples were vortexed and capped, then rotated for 10 min and vortexed again. The supernatant was aspirated through the filter and the alumina was then washed and vortexed 4 times with 1 ml H2O to which a small volume of pH 7 buffer was added and aspirated to near dryness each time. A 200 μl microcentrifuge tube was placed on the cartridge as a receiver tube and centrifuged to remove any residual fluid. A new receiver tube was placed on the cartridge and 100 μl 0.1 N perchloric acid (HClO4) was added to the sample. At this time, the samples were vortexed for 30 seconds, allowed to stand for 3–5 min and vortexed again. The cartridge and receiver tube were centrifuged until no fluid remained in the cartridge. Sixty μl of HClO4 extract was injected directly into an HPLC system (Waters) and analyzed electrochemically with an LC-4B potentiostat (BAS). The electrode potential was set at +0.6 V with respect to an Ag/AgCl reference electrode. Mobile phase consisted of 14 g citric acid, 8.6 g sodium acetate, 110 mg 1-octanesulfonic acid (sodium salt), 150 mg EDTA disodium salt, and 100 ml methanol in 1 L of deionized water. Flow rate was maintained at 1.0 ml/min.
2.5. Analysis of central monoamines
Brains were obtained by rapidly decapitating subjects within five seconds of capture at the end of the 10 minute interaction, and were immediately frozen on dry ice. Frozen heads were sliced coronally in 300 μm sections and brain nuclei were identified utilizing a stereotaxic atlas, brain map of tyrosine hydroxylase activity, and brain punch atlas [20,45,45,76,76], then microdissected from slices [39]. Nuclei known to be associated with stress and aggression were sampled and included hippocampus (CA, contains large pyramidal cells [50], nucleus accumbens, medial amygdala, lateral amygdala (ventrolateral portion of the anterior dorsal ventricular ridge), anterior hypothalamus (preoptic area) and raphé.
Dopamine and its catabolite dihydroxyphenylacetic acid (DOPAC), serotonin (5-HT) and its catabolite 5-hydroxyindoleacetic acid (5-HIAA) were measured using HPLC with electrochemical detection [75]. Briefly, microdissected samples were expelled into 60 μl of a sodium acetate buffer (pH 5.1) containing an internal standard (α-methyl dopamine; Sigma, St. Louis, MO), freeze-thawed and centrifuged at 15,000 × g for 2 min. Following centrifugation, 2 μl of a 1 mg/ml ascorbate oxidase solution (Sigma, St. Louis, MO) was added to each sample. The supernatant was removed and 40 μl was injected into a chromatographic system (Waters Associates, Inc.) and analyzed with an ESA 5200 Coulochem II liquid chromatography system with electrochemical detection (ESA, Bedford, MA) using two electrodes at reducing, then oxidizing potentials of −40 and +320 mV [49]. The sodium phosphate-10% acetonitrile mobile phase was brought to a final pH of 2.9. Separation of the monoamines was achieved with a 10 cm × 4.6 mm reverse phase, 3 μm particle size, Hypersil ODS column (Keystone Scientific, Bellefonte, PA), and mobile phase flow rate maintained at 1 ml/min with a Waters 515 HPLC pump. Sample peak areas were quantified by comparison to standard solutions of known concentrations (dopamine, DOPAC, 5-HT and 5-HIAA Sigma, St. Louis, MO) and corrected for recovery of the internal standard using interpretation software (CSW32, DataApex, Czech Rep.).
Figures presented depict ratios of catabolite to transmitter (i.e., 5-HIAA/5-HT) as an estimate of monoaminergic turnover and activity. Monoaminergic activity is often approximated by the ratio of the catabolite to transmitter, such as 5-HIAA/5-HT. This is especially important for analyzing serotonergic and dopaminergic activity in studies of behavior or stress [35,37,68]. Serotonin will serve as the example to describe the implications of the ratio data for both dopamine and serotonin. As accessible 5-HT is often greater than demand for individual or even multiple behavioral events, 5-HT levels usually remain unchanged. Constant 5-HT levels may also occur when synthesis is rapidly elevated in response to stress. Behaviorally or stress induced changes in the catabolite 5-HIAA are often seen along with changes in 5-HIAA/5-HT [85]. However, the ratio is a more direct index of serotonergic activity than catabolite levels per se, because variance related to tissue sampling, and to total levels of 5-HT and 5-HIAA, are reduced [65]. Although changes in ratio are often a result of changes in 5-HIAA levels, experiments that immediately follow behavioral interaction may discern a reduction in 5-HT concentrations reflecting recent release, and have more effect than 5-HIAA levels on the ratio.
2.6. Statistical analyses
Comparison among samples within the three-way design (eyespot painting × days of separation × social status) was achieved via three-way ANOVA. Within either one or three day separation treatments, plasma catecholamine concentrations and monoaminergic activity of behaviorally interacting groups were compared against controls by separate one-way ANOVA (followed by Dunnett’s posthoc analysis). Within-status comparisons of frequency of aggression after either one or three days were made by separate one-way ANOVA with repeated measures. Posthoc comparisons between interacting pairs were done by paired t-test for all parameters. Comparisons between animals of similar eyespot state were done by independent t-tests, as were comparisons of similar treatment between one and three days of separation between interactions.
3. Results
3.1. Behavior
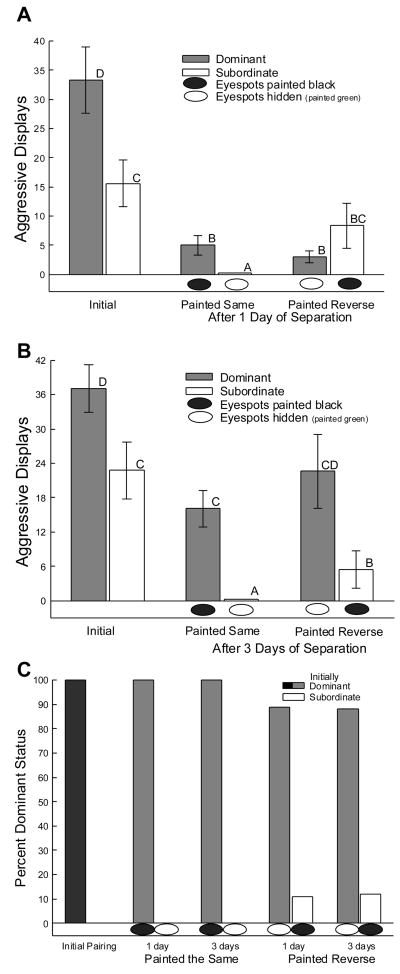
All paired males responded behaviorally to one another during both timed interactions (Figs. 1A, B). During the initial interaction (Figs. 1A, B, C: leftmost pair of bars), more aggressive (tp = 5.0, P < 0.0001, Fig. 1A; tp = 3.85, P < 0.001, Fig. 1B) males became dominant [34,69,70]. The presence or absence of eyespots affected agonistic behavior between the two combatants during the second interaction, which depended on rank and the number of days of separation of the dyad between the initial and second interactions (3 way ANOVA main effect: F7,56 = 4.86, P < 0.0001; 1 day separation: Fig. 1A; 3 days separation: Fig. 1B). In the second interaction, known dominant males bearing darkened eyespots expressed significantly more aggressive displays than facing their previously subordinate (white bars) opponent without eyespots (i.e. hidden by green paint) after both one (tp = 2.99, P < 0.04; Fig. 1A: middle pair of bars) and three days (tp = 5.06, P < 0.001; Fig. 1B: middle bars) of separation. Subordinates in painted same group for both one and three days of separation displayed only submissive behaviors such as flattening or hiding during the 2nd interaction. Initially subordinate males, when confronting their familiar initially dominant opponent with hidden eyespots, increased agonistic displays compared to subordinate males viewing an initially dominant opponent with darkened eyespots; at both one (ti = 2.2, P < 0.05; Fig. 1A: rightmost pair of bars) and three days (ti = 2.51, P < 0.026; Fig. 1B: rightmost pair of bars). However, it is important to point out that these dominant males (with eyespots hidden by green paint) were significantly more aggressive after 3 days of separation that after one. That means that dominant males, even without eyespots, are more aggressive than their subordinate counterparts after three days of separation (3 way ANOVA, time effect: F1,56 = 6.2, P < 0.016), but not after one day of separation. So, initially dominant males challenged by an opponent with darkened eyespots demonstrated fewer agonistic behaviors compared to previously subordinate males after one day, but not three days, apart. Thus, males viewing an opponent with eyespot signals not corresponding to previously established rank exhibited more aggressive displays than males viewing opponents with eyespot signaling corresponding to previously held rank.
Figure 1.
Aggressive displays (mean ± SEM) with full sagittal expansion A) initially and one day of separation (Top); B) initially and three days of separation (Middle) during a 10 minute period. During the initial interaction (leftmost pair of bars; A, B) more aggressive males became dominant (gray bars). Aggressive displays were significantly more frequent among males viewing a previously subordinate (white bars) opponent without eyespots (i.e. hidden by green paint, white oval below bar) for both one (A) and three days (B) of separation (middle pairs of bars). Moreover, a subordinate male viewing a previously dominant opponent with hidden eyespots increased aggressive displays compared to subordinates that viewed their previously dominant male with eyespots darkened with black paint (black oval below bar). C) After both one and three days of separation the second interaction did not reverse social standings when dominant male’s eyespots where darkened and subordinate male’s where hidden. However, when eyespot signal was reversed in respect to the original social status ~10% of both one and three day subordinate males became dominant (Bottom). Means with no common superscript letters (e.g. A vs B or B vs C; not A vs AB or B vs BC) are significantly different.
Social rank was very stable over the time frame of these experiments (three days; Fig. 1C), [70]. Aggressive displays in which full sagittal expansion was achieved [19] were significantly inhibited in all groups of males one day after introduction (One way repeated measures ANOVA, dominant males: F2,11 = 22.68, P < 0.0005; subordinate: F2,12 = 5.06, P < 0.025; Fig. 1 top). Diminished aggressive interaction between familiar combatants has been demonstrated previously to reflect individual recognition of conspecifics in this species of lizard [15]. Those individuals defined as dominant following the first interaction remained dominant in subsequent interactions in which the eyespots were painted commensurate with their initial rank (Fig. 1C). That is, dominant males with eyespots darkened by black paint, which is similar to very quickly darkening eyespots of some dominant males, remained 100% dominant. Subordinate males with hidden eyespots, simulating slowly reacting eyespot darkening, remained 100% subordinate in the second interaction. Even when the eyespot colors were reversed, i.e., black (darkened) eyespots on subordinate males and green (hidden) eyespots on dominant males, the previously established social rank order was generally maintained. However, among these reverse-painted pairs, a little over 10% (11% at one day, and 12% at three days) of subordinate males inverted their social standing to become dominant against a previously superior opponent (Fig. 1C).
3.2. Plasma catecholamines
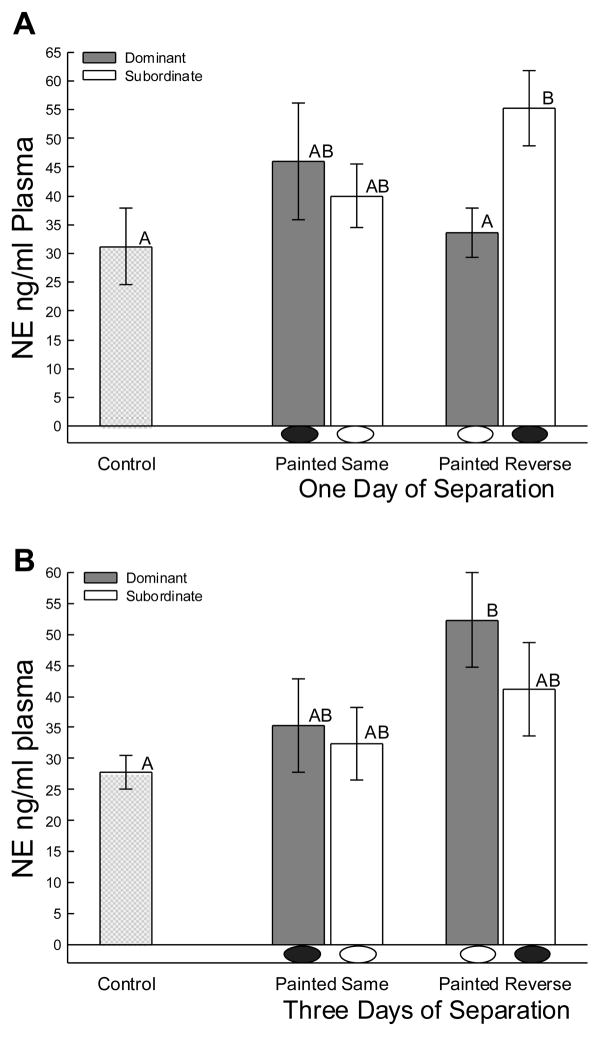
Although plasma catecholamines are typically increased by aggressive social interaction [38,40,48], it was previously unknown whether reintroduction of former combatants would influence this fast acting stress response. Importantly, not all male A. carolinensis responded to a former competitor with elevated plasma NE concentrations (Fig. 2). All animals that had their eyespots painted in concert with their rank following the initial interaction showed no significant elevations in plasma NE following the second interaction (Fig. 2A, B; middle bars). However, when the eyespots were painted converse to their social rank (green eyespots on dominant males, and black on subordinate males), the plasma catecholaminergic stress response was activated (3-way ANOVA main effect: F7,44 = 2.29, P < 0.045), at least in one animal of the pair (Fig. 2A, B; right bars). The pattern of significantly (3-way ANOVA paint x status interaction: F1,44 = 7.74, P < 0.008) elevated plasma in reverse-painted animals was opposite at one and three days. Concentrations of plasma NE were elevated in previously subordinate males that viewed an initially dominant opponent with hidden eyespots one day after rank was established when compared to opponent (tp = 3.7, P < 0.007) and control animals (One-way ANOVA F2,16 = 3.7, P < 0.046; Fig. 2A; right bars). It is noteworthy that these reverse-painted subordinate males were significantly more aggressive than similar males painted to match their rank (Fig 1A). Three days after interaction, only previously dominant males viewing an initially subordinate male with eyespots darkened exhibited an increase in NE compared to controls (One-way ANOVA F2,19 = 3.4, P < 0.05; Fig. 2B; right bars). Similarly, it is the reverse-painted dominant males at three days that are most aggressive (Fig 1B) that show elevated NE, which is also elevated (tp = 2.15, P < 0.047) compared to dominant reverse painted males at one day of separation.
Figure 2.
Mean (± SEM) concentrations of plasma norepinephrine (NE) were influenced only in animals when eyespots were painted opposite of their original status (black on subordinate males, black oval below bar; or hidden by green paint on dominant males, white oval below bar) in secondary social aggressive interactions after both A) one and B) three days of separation. Increased noradrenergic responsiveness appeared to be influenced by relatively increased aggression, but not by the absolute amount of aggression viewed or performed.. Means without common superscript letters (e.g. A vs B, but not A vs AB) are significantly different.
3.3. Central monoamines
The effects of social reintroduction on central indoleamines and catecholamines were limited to the medial hippocampal cortex and raphé. Serotonergic and dopaminergic parameters were not significantly different in nucleus accumbens, medial amygdala, lateral amygdala (the ventrolateral portion of the anterior dorsal ventricular ridge), anterior hypothalamus (preoptic area) when compared by treatment group, previous social status or days of separation.
3.3.1. Hippocampal nuclei
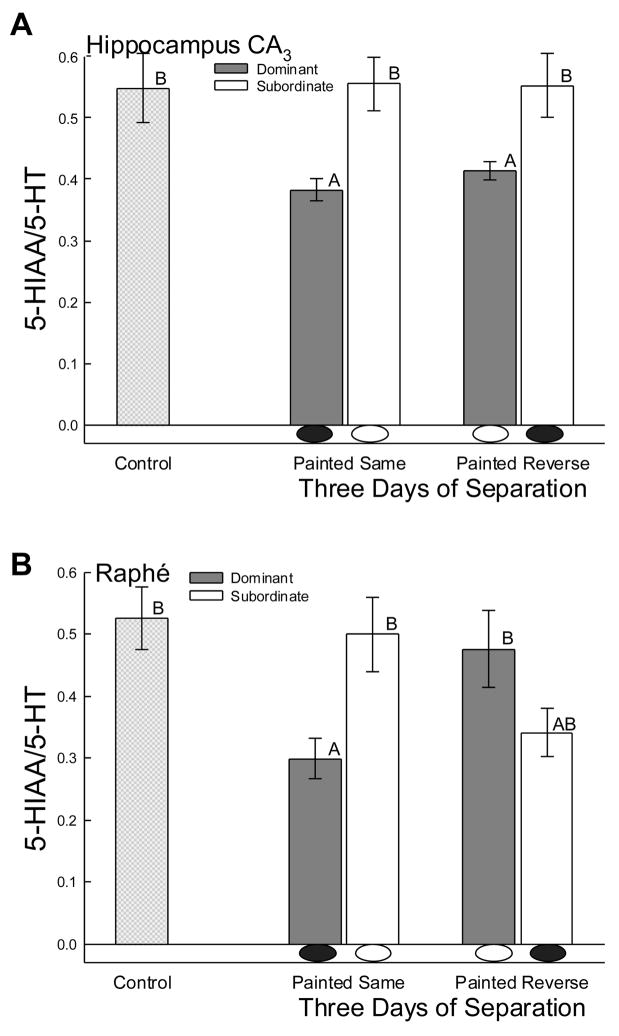
In the hippocampus aggressive social interaction usually elevates serotonergic activity [73,78]. After reintroduction to the same opponent, no hippocampal elevation of serotonergic activity occurred. However, in the CA3 region of the hippocampus (containing large pyramidal cells), the pattern of serotonergic activity (estimated by 5-HIAA/5-HT ratio) after three days of separation was significantly (F2,18 = 4.86, P < 0.003) higher in subordinate than dominant animals (Fig. 3A). This difference was not due to elevated 5-HIAA/5-HT in subordinates, because they showed no difference compared to controls after one or three days of separation. The change in ratio was produced by a significant decrease of 5-HT in subordinate males (F2,22 = 5.34, P < 0.013) coupled with a tendency toward reduced 5-HIAA in dominant males (F2,25 = 2.76, P < 0.083). In contrast to unchanged ratio in subordinates, dominant males, regardless of how their eyespots were painted, had significantly reduced serotonergic activity in the CA3 (Figure 3A).
Figure 3.
Mean levels of serotonergic activity (the ratio of 5-HIAA to 5-HT; ± SEM) are remain mostly unchanged A) hippocampal CA3 and B) raphé nuclei, following a second social interaction between familiar opponents. A) However after three days of separation, in hippocampal CA3 dominant males had significantly lower serotonergic activity when compared to controls and subordinate males, regardless of eyespot signaling. B) In addition after three days separation, and regardless of status, serotonergic activity in raphé was significantly reduced in males that viewed an opponent without eyespots (hidden by green paint; white oval below bar) compared to controls or to males that viewed an opponent with darkened eyespots (black oval below bar). Means with no common superscript letter (A vs B) are significantly different.
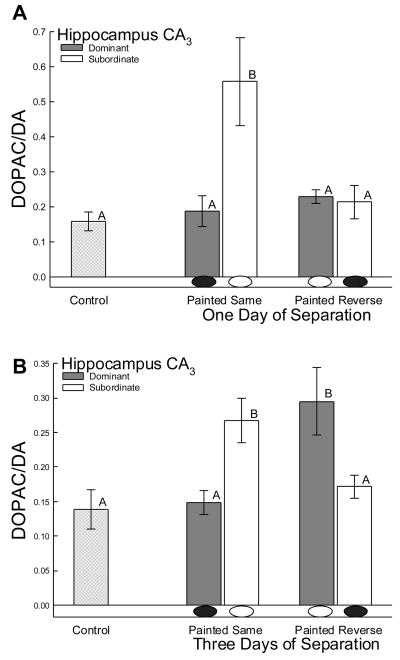
Dopaminergic activity in the CA3 region remained unchanged after one day of separation in all animals except subordinate males viewing their previously dominant opponent with eyespots darkened (3 way ANOVA main effect: F7,54 = 6.4, P < 0.0001; Fig. 4A). After one day of separation, those subordinate males with eyespots painted green to imitate subordinate rank showed significantly elevated DOPAC/DA ratio compared to all other animals (3 way ANOVA paint effect: F1,54 = 14.4, P < 0.0001; rank effect: F1,54 = 19.9, P < 0.0001; One-way ANOVA for comparison to controls: F2,18 = 7.23, P < 0.0001; Fig. 4A). There were no group wise changes in DA or DOPAC to explain the effect on ratio, but there was elevated DA in dominant males painted appropriately for status (black paint) compared to subordinates (t = 2.6, P < 0.035), and increased DOPAC in dominant males painted in reverse (green paint) compared to subordinates (t = 2.3, P < 0.05), and to controls (t = 2.3, P < 0.04). However, after three days of separation both dominant and subordinate males viewing an opponent with eyespots darkened exhibited a significant increase in dopaminergic activity (3 way ANOVA paint by rank interaction: F1,54 = 23.5, P < 0.0001) when compared to isolated controls (One-way ANOVA: F2,18 = 5.64, P < 0.001) and males viewing an opponent without eyespots present (Fig. 4B). In this case the changes in ratio may be due to a significant effect (One-way ANOVA: F2,23 = 10.5, P < 0.001) of DA in dominant males painted to look dominant (black paint) compared to controls and dominants painted in reverse (green), and a tendency toward an increase in DOPAC (One-way ANOVA: F2,20 = 3.1, P < 0.066) for dominant males painted in reverse.
Figure 4.
Mean levels of dopaminergic activity (the ratio of DOPAC to DA; ± SEM) are stimulated in hippocampal CA3 of subordinate males with eyespots painted to match their social rank (i.e. hidden by green paint; white oval below bar), following a second social interaction, after separation from their dominant co-combatant for A)one day and B) three days. B) After 3 days, hippocampal CA3 dopaminergic activity was elevated, regardless of status, by viewing an opponent with darkened eyespots (black oval below bar). Means with no common superscript letter (A vs B) are significantly different
3.3.2. Raphé nuclei
Similar to serotonergic activity in hippocampal CA3, the secondary interaction between familiar combatants did not stimulate serotonergic activity in the raphé (Fig. 3B). A significant decrease was measured (F2,18 = 3.93, P < 0.009), but only among animals with eyespots darkened by black paint facing a known subordinate opponent with hidden eyespots. This complex, eyespot color-driven change in ratio appeared to be caused by significantly decreased 5-HIAA in all subordinates (One-way ANOVA: F2,24 = 3.91, P < 0.034) and dominant males painted appropriately for status (black paint; One-way ANOVA: F2,24 = 4.13, P < 0.029) Serotonergic activity levels in the raphé were almost significantly lower for males viewing an opponent with eyespot signaling regardless of status after three days of separation when compared to controls and males viewing an opponent with eyespots darkened (for males with reversed painting (tp = 1.9, P < 0.07; Fig. 3B).
4. Discussion
When combined with knowledge from previous studies, the results of these experiments clearly indicate that a second interaction with a familiar opponent is substantially different, behaviorally and physiologically, from the initial interaction that establishes social rank. The secondary interaction appears to be much less stressful. Formation of social status relationships follows a four step pattern of aggressive interactions, that progress from rank-distinctive neuroendocrine function in disposition through differential motivation, a very stressful aggression phase, and finally resolution [78]. The qualities (behaviorally and neurochemically) necessary for successful social domination are present prior to the initial social interaction, and can be used to predict the outcome [34,73]. As supported in these experiments (Fig. 1C), social rank is relatively stable [15,70] but not immutable, such that 10–50% of social rank relationships can be reversed, depending on the social and neuroendocrine conditions [41,70]. Some of the stability appears to extend from social recognition of individual territorial competitors [15]. The familiarity, and recognition, between opponents appears to be a fundamental factor influencing the results we observed and measured here. What is more, the memory of opponents and their status is more important for determining subsequent social behavior and rank than the social sign stimuli presented by the eyespots. We also believe that firmly established of rank relationships, not greatly affected by eyespot color change, nor aggressively disputed, constrained stimulation of serotonergic activity that is usually evident following social antagonism in specific regions of the aggression neurocircuitry [35,37,39,49,68,70,72,73,75–79].
4.1. Agonistic behavior and social status response to a second social interaction
Social antagonism between closely adjacent male A. carolinensis is rarely observed in a natural setting [28,30]. However, male A. carolinensis partition the natural habitat by means of intense territorial contests and enduring rivalry [29]. Previous work suggests that the presence of eyespots, whether artificially or naturally obtained, effectively influences agonistic behavior, social status, plasma catecholamine and central monoamine activity in male A. carolinensis (Figs. 1A, B, C; 2A, B; 3B; 4B) [33–35,37–39,41,70,71]. The eyespots as a signal for A. carolinensis may have the most relevant social value during distant or short-term interaction when social rank relationships are first established. Eyespots may also facilitate rank maintenance or reaffirmation. As male A. carolinensis territories generally do not overlap in the wild [30,54,64], eyespot formation could provide a remote indicator of internal neuroendocrine condition representative of putative aggressive intent and social status [27,34,68–71] and thereby reduce the amount of physical aggressive interaction [37–39] needed to determine initial social hierarchy.
Investigation of the functional value of eyespots relative to initial establishment, maintenance, or reaffirmation of social rank by opponent recognition was predicated by earlier work investigating repeated social interaction between the same and new opponents [15,41]. Specifically, social hierarchies are very stable and remained intact between individuals after separation for up to ten days [15,41,70]. Foster et al. directly tested the recognition of individual males by allowing size matched males to establish dominant-subordinate social ranks and then re-pairing these individuals with their familiar or an unfamiliar opponent after three and ten days of separation [15]. Males reintroduced to the same opponent after three days maintained the original social rank. However, after ten days all males interacted as if they were naïve to one another. After three days of separation, subordinate males were introduced to a new dominant opponent (achieved dominant status in the previous pairing with a different male) to test whether the subordinate males were exhibiting behavioral inhibition due to losing the fight (loser effect). Surprisingly, subordinate males introduced to this previously unknown dominant individual increased their aggressive behavior by 50% when compared to their original interaction. This strongly argues against a loser effect being responsible for the stability of social rank once it is determined. Results from this experiment demonstrate that males painted in a way similar to how the eyespots naturally form maintain the original social rank hierarchy through modification of their agonistic behavior (Fig. 1C). Dominant and subordinate males show a significant reduction in aggressive behavior as a consequence of recognition of their social opponent and rank status [15]. Additionally, in our experiments where eyespots were painted to express the opposite social signal of the original dominant-subordinate forming interaction, the majority of males did not change their behavioral expression or rank during the second interaction (Fig. 1C). That is, rank relationships are relatively stable regardless of the social signals conveyed [70]. However, subordinate males viewing their previously dominant opponent without the eyespot signal reversed their social standing in ~10 percent of second interactions (Fig. 1C). This was consistent over one or three days of separation. Together, this suggests that male A. carolinensis use both previous experience and the relative influence of eyespot signaling to modify behavior. The dramatic decrease in eyespot signal potency on established ranks during a second interactions compared to naïve pairings suggest that males may be using the signal during initial interaction possibly to conserve time and energy in their three dimensional habitat. This seems to be supported by the drastic modulation of behavioral response to an opponent because of the apparent recognition of that individual. Moreover this inhibition of behavior towards recognized neighbors could also explain the low levels of aggression reported in field studies.
It is important to note that eyespots are only one type of potently modifiable sign stimulus among many other signals employed by green anoles. Stereotypical behaviors, dewlap and/or nuchal crest extension, lateral compression, body color and eyespots are the most obvious signals, and are used for different functions, and produce different neurochemical output in male and female Anolis carolinensis [1,19,69,79]. Highly ritualized behavioral displays are characterized by three display action patterns of head nods and dewlap extension, with tempo but not cadence distinguishing function [58]; the imparted messages depending on context plus the rank and sex of the lizard [1,19,70,79]. It may be that different components from a multilayered signal redundancy, which includes the eyespots, are important for opponent recognition in A. carolinensis, as has been demonstrated for mate recognition in frogs [63]. A combination of signals, and the stress they elicit, appear to be necessary for activation of rank dependent neural and endocrine responses during initial interactions [70]. The chronology of eyespot responses, both initiation and termination, differs between dominant and subordinate males [34,36]. Rank also appears to influence the responsiveness of eyespot expression to nonsocial stressors [62]. The confluence of timing for eyespot and body coloration presents different messages depending on rank [21–23,40,69,70]. A darkened eyespot on a proactive dominant green male inhibits aggression and stimulates catecholamine release [36,40,69,70]. Conversely, eyespots darken about the same time as overall body color darkens in reactive subordinate males, which limits stress responsiveness in their opponents, at least during the initial interaction. Results of the study reported here, combined with previous work suggest that the male A. carolinensis are capable of remembering previous opponents and their social rank, and modifying their behavior in response. Moreover, the influence of eyespot signal during agonistic social interactions, which has been demonstrated to be powerful in naïve interactions [40], is not as potent at modifying behavior in subsequent interactions as the memory of specific individuals in previously established hierarchies. Therefore, a combination of opponent recognition and eyespots may facilitate maintenance of rank by reducing opponent aggression.
4.2. Catecholamine response to a second social interaction
Establishment of social status and behavioral interactions between male A. carolinensis have been described in detail by previous studies [4,5,12,14,18,19]. More recent work has reported that the latency to eyespot formation is tightly correlated with and a strong indicator with dominant status [27,34,41,70,71]. In addition, recent work manipulating the expression (presence or absence) of the eyespot signal results in a profound effect on aggressive behavior and plasma catecholamines in lizards interacting with a real opponent [33,35,40] or a mirror image [37–39]. While behavioral responses were similar in mirror vs. real opponent experiments, opposing patterns of plasma catecholamine responses were elicited by each paradigm. Males viewing a mirrored (reflected self-image) opponent with hidden eyespots had significantly elevated levels of plasma catecholamines compared both to controls and to males viewing a reflected image with eyespots darkened [38]. In contrast, in experiments using paired males, a male viewing an opponent with darkened eyespots had significantly elevated levels of plasma catecholamines when compared to controls and males viewing an opponent with hidden eyespots [40]. In the study reported here, the primary plasma catecholamine (NE) was influenced by presence or absence of the social signal when the eyespots were painted in reverse to the natural formation. Most animals in the second interaction showed no significant change in plasma NE (Fig. 2A, B). This suggests that a subsequent social interaction is not as stressful as the first. However, for the reverse painted animals, one combatant in the pair showed elevated plasma NE, which was not related to the absolute amount of aggression viewed or performed [70]. It was however, associated with relatively increased aggressive behavior (Fig. 1A, B). Specifically, after one day of separation subordinate males reintroduced to a dominant opponent with its eyespot region masked had significantly increased plasma NE (Fig. 2A) compared to their opponent and control animals. These reverse-painted subordinate males also had significantly greater aggression then subordinate males painted in concert with their social rank after both one and three days of separation before the second interaction. In contrast, after three days of separation it was the initially dominant male that expressed elevated NE and aggression levels compared to controls when viewing a previously subordinate with darkened eyespots (Fig. 2B). It may be that elevated plasma catecholamines are important for stimulating, at least in part, relatively elevated levels of aggression in both dominant and subordinate males. Taken together this suggests that an aggressive male’s sympathetic response may be activated in a context dependent manner. Specifically, viewing or performing aggression combined with eyespot color and memory of previous opponent and status all influence the NE response of males during subsequent agonistic interactions.
4.3. Central monoaminergic response to a second social interaction
The most striking difference between this study, in which monoamines were measured after a second aggressive social interaction, and previous studies that examined monoaminergic activity immediately after an initial social interaction is the degree of serotonergic activity. Recent work has demonstrated that experience modifies behavior in this species, perhaps via parallel modifications of sensory and limbic circuitry [87,88]. Unlike previous studies that have repeatedly demonstrated specifically activated serotonergic systems in centers of aggression neurocircuitry, in the study reported here we measured no change in any serotonergic parameter nucleus accumbens, medial amygdala, lateral amygdala or anterior hypothalamus. These regions particularly, but also other regions, have been demonstrated to rapidly, or for some regions more slowly, increase serotonergic output and/or metabolism [35,37,39,49,68,70,72,73,75–79]. Aggressive social interactions that determine social status produce different hormonal and central neurochemical patterns, particularly of serotonergic and dopaminergic activity, dependent on status of each individual [33,35,40,68–70,73,75–78]. For the most part, we did not measure those changes here.
Furthermore, changes in serotonin (5-HT) concentration can alter social status in male A. carolinensis [41,73]. Moreover, peripheral stress hormones also influence central serotonergic activity [74]. What is more, the behavioral, monoaminergic, hormonal and autonomic responses in male A. carolinensis appear to be modulated by a visual indicator of social status and stress: eyespots [70]. Multiple experiments have demonstrated that during aggressive interactions between paired males, the first individual to achieve eyespots becomes dominant in virtually all encounters [40,69–71], and that pharmacologically elevating central 5-HT levels delays eyespot formation [41]. Previous studies investigating the role of eyespot signals on social status and central serotonergic activity in paired interactions have found that in subordinate animals viewing an opponent with darkened eyespots, serotonergic activity was elevated in hippocampus [35]. In contrast, males that viewed opponents with hidden eyespots (painted green) and became dominant had increased serotonergic activity in raphé [35]. For male Anolis carolinensis in this study, most brain regions (nucleus accumbens, medial amygdala, lateral amygdala and anterior hypothalamus) showed no stress- or aggression-induced elevation of serotonergic or dopaminergic activity at all. In addition, for hippocampus and raphé, where monoaminergic activity was affected, 10 out of 16 treated groups were not significantly different from controls (Figs. 3A, B; 4A, B). Like the data for plasma NE, the monoamine results suggest that a subsequent interaction between familiar opponents is not as stressful as the initial interaction.
However, rank and eyespot presence did influence the central monoamines. For example, dominant males regardless of eyespot color showed diminished serotonergic activity compared to subordinate animals in the large celled pyramidal region (CA3) of the hippocampus after three days of separation (Fig. 3A). Decreased serotonergic activity in the hippocampus may follow recognition of a familiar subordinate opponent, acting as a potent mechanism for stress relief. The hippocampus is invariably involved when aggression stimulates elevated serotonergic activity [75,76]. In addition, the CA3 region of the hippocampus also appears to be involved in social learning during aggression, through preferential modification of NR2B subunits of NMDA receptors there [50,70]. The hippocampus is important for spatial learning in lizards, which can be enhanced by social stress [2,6]. Importantly for our work, the hippocampus is also important for social recognition in rats [47]. This factor may be involved in the monoaminergic changes in CA3 measured during our study.
Decreased CA3 serotonergic activity in dominant males is reminiscent of lower serotonergic activity measured prior to aggression in proactive males, who exhibit faster recovery from stress, more immediate courtship, and quicker aggression, and are presumed to become socially dominant in interactions with novel opponents [73]. In that study however, hippocampal CA3 only trended toward lower serotonergic activity. As the results from our experiment (Figs 3, 4) suggest very little monoaminergic stimulation from secondary interactions between familiar opponents, it is tempting to compare post-secondary monoamine levels with those measured prior to aggression (as above), but it should be noted that proactive trout and rats respond to stress with larger increases in brain serotonergic activity than reactive individuals [60,82]. In A. carolinensis proactive dominant males respond to the stress of aggression with greater increases in serotonergic activity in nucleus accumbens, and faster serotonergic increases to restraint stress in raphé [44,73]. Conversely, reactive subordinate males exhibited a greater decrease in serotonergic activity in raphé in response to aggression [73].
In the study reported here, dominant and probably subordinate males exhibited reduced serotonergic activity in raphé after three days of separation (Fig. 3B). Inhibited serotonergic activity in raphé has been demonstrated to last three days following initial aggressive interaction in dominant and subordinate males [44]. The distinction in raphé at this time after a second interaction depended on eyespot coloration, such that male viewing an opponent without eyespots (masked with paint) exhibited reduced serotonergic activity compared to controls and males viewing an opponent with darkened eyespots (Fig. 3B). The raphé appears to retain the effects of social stress over a long period, perhaps contributing to the effects on social memory. In addition however, the raphé appears to respond rapidly to short-term stimuli as well [44]. Thus, while there are few changes in brain serotonergic activity following a second interaction with a familiar opponent, those that occur do not appear to be increases stimulated by a second stressful social interaction, but rather are represented by reductions in serotonergic activity, modulated by visual sign stimuli (raphé) and previous social status (hippocampal CA3) respectively [39].
Dopaminergic activity is also influenced by social status and social interactions [3,17,32,51,84]. Dopaminergic activity in hippocampal nuclei of various vertebrate species has also been shown to be critical for spatial memory formation and retrieval [61,81]. Hippocampal dopaminergic activity of aggressive male A. carolinensis viewing a reflected opponent (without eyespots) is significantly decreased compared to controls and submissive males [39]. In paired interactions, both dominant and submissive males exhibited decreased dopamine levels in the hippocampus compared to controls [33]. In our study, hippocampal dopaminergic activity following a second social interaction was increased in subordinate males with hidden eyespots viewing their previously dominant opponent (bearing darkened eyespots) both one and three days after the original interaction (painted same; Fig. 4A, B). In contrast, dominant males only exhibited elevated hippocampal dopaminergic activity after three days, and then only when viewing a previously subordinate opponent with darkened eyespots (painted reverse; Fig. 4B). Perhaps, as time elapses between interactions, eyespot reversal has a greater stimulatory effect on dopaminergic activity in dominant males, matching the level of aggressive behavior shown at this time. Combined, these results suggest that changes in hippocampal dopaminergic activity during secondary agonistic encounters are specifically influenced by the creation of social status in the first interaction, and may be linked to opponent recognition and information conveyed by visual sign stimuli. Moreover, changes in hippocampal dopaminergic activity do not appear to be strictly status dependent, but instead may reflect the effects of social interaction and previous status in a context dependent manner.
4.4. Conclusions
Subsequent aggressive social interactions differ from initial encounters that establish social rank. The reintroduction of familiar combatants results in reduced aggressive behavior, and very limited hormonal and neurochemical responses to the reunion, suggesting a less stressful interaction. However, the results of these experiments are also accord with earlier work demonstrating that male A. carolinensis use eyespots signal as a visual indicator of the potential speed of neuroendocrine responses to stress, and by extension convey willingness to fight and likelihood of more intense aggression to yield elevated rank and subsequently improved physical condition and overall fitness. The result is decreased agonistic physical contact and potential injury in determining social hierarchy [34,38,40,41,71]. Moreover, this work supports earlier studies in which males re-modify their behavioral response in a second interaction according to opponent familiarity and their status in relation to that individual [15]. To that end, the current experiments show that the potency of the eyespot signal observed in naïve pairs is not as strong in subsequent interactions, and therefore does not exert as much influence as memory of a familiar opponent. However, this experiment does expose some plasticity in relationships of established social standing via eyespot signaling. Plasma NE levels were influenced by the presence or absence of the social signal, and the relative amount of increased aggression. Serotonergic activity in hippocampal CA3 was lower in dominant males regardless of the presence or absence of the social signal on their opponent. Serotonergic activity decreased in raphé when eyespots were darkened and dopaminergic activity increased in hippocampus CA3 when eyespots were hidden. The limited changes in monoaminergic activity appear to be derived from the stability of the social hierarchy, and the alterations to monoaminergic activity that did occur may reflect the maintenance of rank or perception of the eyespot signal.
Acknowledgments
This work was supported by NIH grants P20 RR15567; R03 MH068303 & R01 DA019921 (GLF), R03 MH068364 (MJW), F31 MH64983 & F32 MH074222 (WJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews TJ, Summers CH. Aggression, and the acquisition and function of social dominance in female Anolis carolinensis. Behaviour. 1996;133:1265–1279. [Google Scholar]
- 2.Bartolomucci A, de Biurrun G, Czeh B, Van Kampen M, Fuchs E. Selective enhancement of spatial learning under chronic psychosocial stress. Eur J Neurosci. 2002;15:1863–1866. doi: 10.1046/j.1460-9568.2002.02043.x. [DOI] [PubMed] [Google Scholar]
- 3.Cabib S, D’Amato FR, Puglisi-Allegra S, Maestripieri D. Behavioral and mesocorticolimbic dopamine responses to non aggressive social interactions depend on previous social experiences and on the opponent’s sex. Behav Brain Res. 2000;112:13–22. doi: 10.1016/s0166-4328(00)00157-1. [DOI] [PubMed] [Google Scholar]
- 4.Crews D. Inter- and intraindividual variation in display patterns in the lizard, Anolis carolinensis. Herpetelogica. 1975;31:37–47. [Google Scholar]
- 5.Crews D. The hormonal control of behavior in a lizard. Sci Am. 1979;241:180–187. doi: 10.1038/scientificamerican0879-180. [DOI] [PubMed] [Google Scholar]
- 6.Day LB, Crews D, Wilczynski W. Effects of medial and dorsal cortex lesions on spatial memory in lizards. Behav Brain Res. 2001;118:27–42. doi: 10.1016/s0166-4328(00)00308-9. [DOI] [PubMed] [Google Scholar]
- 7.de Villiers MS, van Jaarsveld AS, Meltzer DG, Richardson PR. Social dynamics and the cortisol response to immobilization stress of the African wild dog, Lycaon pictus. Horm Behav. 1997;31:3–14. doi: 10.1006/hbeh.1997.1314. [DOI] [PubMed] [Google Scholar]
- 8.Deckel AW. Behavioral changes in Anolis carolinensis following injection with fluoxetine. Behav Brain Res. 1996;78:175–182. doi: 10.1016/0166-4328(95)00246-4. [DOI] [PubMed] [Google Scholar]
- 9.Deckel AW, Fuqua L. Effects of serotonergic drugs on lateralized aggression and aggressive displays in Anolis carolinensis. Behav Brain Res. 1998;95:227–232. doi: 10.1016/s0166-4328(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 10.Deckel AW, Jevitts E. Left vs. right-hemisphere regulation of aggressive behaviors in Anolis carolinensis: Effects of eye-patching and fluoxetine administration. J Exp Zool. 1997;278:9–21. [Google Scholar]
- 11.Deckel AW, Lillaney R, Ronan PJ, Summers CH. Lateralized effects of ethanol on aggression and serotonergic systems in Anolis carolinensis. Brain Res. 1998;807:38–46. doi: 10.1016/s0006-8993(98)00718-5. [DOI] [PubMed] [Google Scholar]
- 12.DeCourcy KR, Jenssen TA. Structure and use of male territorial headbob signals by the lizard Anolis carolinensis. Anim Behav. 1994;47:251–262. [Google Scholar]
- 13.Edwards PJ. Plumage variation, territoriality and breeding displays of the golden plover Pluvialis apricaria in Southwest Scotland. Ibis. 1982;124:88–95. [Google Scholar]
- 14.Evans LT. A study of social hierarchy in the lizard, Anolis carolinensis. J Genet Psychol. 1936;48:88–111. [Google Scholar]
- 15.Forster GL, Watt MJ, Korzan WJ, Renner KJ, Summers CH. Opponent recognition in male green anoles, Anolis carolinensis. Anim Behav. 2005;69:733–740. [Google Scholar]
- 16.Goldman JM, Hadley ME. In vitro demonstration of adrenergic receptors controlling melanophore responses of the lizard, Anolis carolinensis. J Pharmacol Exp Ther. 1969;166:1–7. [PubMed] [Google Scholar]
- 17.Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg B, Noble GK. Social behavior of the American chameleon (Anolis carolinensis) Physiol Zool. 1944;17:392–439. [Google Scholar]
- 19.Greenberg N. A neuroethological study of display behavior in the lizard, Anolis carolinensis (Reptilia, Lacertilia, Iguanidae) Am Zool. 1977;17:191–201. [Google Scholar]
- 20.Greenberg N. A forebrain atlas and stereotaxic technique for the lizard, Anolis carolinensis. J Morphol. 1982;174:217–236. doi: 10.1002/jmor.1051740210. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg N. Central and autonomic aspects of aggression and dominance in reptiles. In: Ewert JP, Capranica RR, Ingle DJ, editors. Advances in Vertebrate Neuroethology. Plenum: New York; 1983. pp. 1135–1143. [Google Scholar]
- 22.Greenberg N. Ethological aspects of stress in a model lizard, Anolis carolinensis. Integ and Comp Biol. 2002;42:526–540. doi: 10.1093/icb/42.3.526. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg N. Sociality, stress, and the corpus striatum of the green anolis lizard. Physiol Behav. 2003;79:429–440. doi: 10.1016/s0031-9384(03)00162-8. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg N, Crews D. Endocrine and behavioral responses to aggression and social dominance in the green anole lizard, Anolis carolinensis. Gen Comp Endocrinol. 1990;77:246–255. doi: 10.1016/0016-6480(90)90309-a. [DOI] [PubMed] [Google Scholar]
- 25.Hadley ME, Goldman JM. Physiological color changes in reptiles. Am Zool. 1969;9:489–504. doi: 10.1093/icb/9.2.489. [DOI] [PubMed] [Google Scholar]
- 26.Höglund E, Kolm N, Winberg S. Stress-induced changes in brain serotonergic activity, plasma cortisol and aggressive behavior in Arctic charr (Salvelinus alpinus) is counteracted by L-DOPA. Physiol Behav. 2001;74:381–389. doi: 10.1016/s0031-9384(01)00571-6. [DOI] [PubMed] [Google Scholar]
- 27.Höglund E, Korzan WJ, Watt MJ, Forster GL, Summers TR, Johannessen HF, Renner KJ, Summers CH. Effects of L-DOPA on aggressive behavior and central monoaminergic activity in the lizard Anolis carolinensis, using a new method for drug delivery. Behav Brain Res. 2005;156:53–64. doi: 10.1016/j.bbr.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Jenssen TA, Greenberg N, Hovde KA. Behavioral profile of free-ranging lizards, Anolis carolinensis, across breeding and post-breeding seasons. Herp Monogr. 1995;9:41–62. [Google Scholar]
- 29.Jenssen TA, Lovern MB, Congdon JD. Field-testing the protandry-based mating system for the lizard, Anolis carolinensis: Does the model organism have the right model? Behav Ecol Sociobiol. 2001;50:162–172. [Google Scholar]
- 30.Jenssen TA, Nuñez SC. Spatial and breeding relationships of the lizard, Anolis carolinensis: Evidence of intrasexual selection. Behaviour. 1998;135:1003. [Google Scholar]
- 31.Jenssen TA, Decourcy KR, Congdon JD. Assessment in contests of male lizards (Anolis carolinensis): how should smaller males respond when size matters? Anim Behav. 2005;69:1325–1336. [Google Scholar]
- 32.Kaplan JR, Manuck SB, Fontenot MB, Mann JJ. Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis) Neuropsychopharmacology. 2002;26:431–443. doi: 10.1016/S0893-133X(01)00344-X. [DOI] [PubMed] [Google Scholar]
- 33.Korzan WJ, Forster GL, Watt MJ, Summers CH. Dopaminergic activity modulation via aggression, status and a visual social signal. Behav Neurosci. 2006;120 doi: 10.1037/0735-7044.120.1.93. in press. [DOI] [PubMed] [Google Scholar]
- 34.Korzan WJ, Øverli Ø, Summers CH. Future social rank: forecasting status in the green anole (Anolis carolinensis) Acta Ethologica. 2006;9:48–57. [Google Scholar]
- 35.Korzan WJ, Summers CH. Serotonergic response to social stress and artificial social sign stimuli during paired interactions between male Anolis carolinensis. Neuroscience. 2004;123:835–845. doi: 10.1016/j.neuroscience.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Korzan WJ, Summers CH. Behavioral diversity and neurochemical plasticity: Selection of stress coping strategies that define social status. Brain Behav Evol. 2007;70 doi: 10.1159/000105489. in press. [DOI] [PubMed] [Google Scholar]
- 37.Korzan WJ, Summers TR, Ronan PJ, Renner KJ, Summers CH. The role of monoaminergic nuclei during aggression and sympathetic social signaling. Brain Behav Evol. 2001;57:317–327. doi: 10.1159/000047250. [DOI] [PubMed] [Google Scholar]
- 38.Korzan WJ, Summers TR, Ronan PJ, Summers CH. Visible sympathetic activity as a social signal in Anolis carolinensis: Changes in aggression and plasma catecholamines. Horm Behav. 2000;38:193–199. doi: 10.1006/hbeh.2000.1619. [DOI] [PubMed] [Google Scholar]
- 39.Korzan WJ, Summers TR, Summers CH. Monoaminergic activities of limbic regions are elevated during aggression: Influence of sympathetic social signaling. Brain Res. 2000;870:170–178. doi: 10.1016/s0006-8993(00)02420-3. [DOI] [PubMed] [Google Scholar]
- 40.Korzan WJ, Summers TR, Summers CH. Manipulation of visual sympathetic sign stimulus modifies social status and plasma catecholamines. Gen Comp Endocrinol. 2002;128:153–161. doi: 10.1016/s0016-6480(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 41.Larson ET, Summers CH. Serotonin reverses dominant social status. Behav Brain Res. 2001;121:95–102. doi: 10.1016/s0166-4328(00)00393-4. [DOI] [PubMed] [Google Scholar]
- 42.Licht P. Regulation of the annual testis cycle by photoperiod and temperature in the lizard, Anolis carolinensis. Ecology. 1971;52:240–252. [Google Scholar]
- 43.Lin PYT, Bulawa MC, Wong P, Lin L, Scott J, Blank CL. The determination of catecholamines, indoleamines and related enzymatic activities using three micron liquid chromatography. J Liq Chromatogr. 1984;7:509. [Google Scholar]
- 44.Ling T, Forster GL, Korzan WJ, Renner KJ, Summers CH, Watt MJ. Rapid neuroendocrine responses to restraint stress differ with social status. Soc Neurosci Abs. 2005;31:57–8. [Google Scholar]
- 45.Lopez KH, Jones RE, Seufert DW, Rand MS, Dores RM. Catecholaminergic cells and fibers in the brain of the lizard Anolis carolinensis identified by traditional as well as whole-mount immunohistochemistry. Cell Tissue Res. 1992;270:319–337. doi: 10.1007/BF00328017. [DOI] [PubMed] [Google Scholar]
- 46.Lovern MB, McNabb FM, Jenssen TA. Developmental effects of testosterone on behavior in male and female green anoles (Anolis carolinensis) Horm Behav. 2001;39:131–143. doi: 10.1006/hbeh.2000.1637. [DOI] [PubMed] [Google Scholar]
- 47.Maaswinkel H, Baars AM, Gispen WH, Spruijt BM. Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol Behav. 1996;60:55–63. doi: 10.1016/0031-9384(95)02233-3. [DOI] [PubMed] [Google Scholar]
- 48.Matt KS, Moore MC, Knapp R, Moore IT. Sympathetic mediation of stress and aggressive competition: Plasma catecholamines in free-living male tree lizards. Physiol Behav. 1997;61:639–647. doi: 10.1016/s0031-9384(96)00500-8. [DOI] [PubMed] [Google Scholar]
- 49.Matter JM, Ronan PJ, Summers CH. Central monoamines in free-ranging lizards: differences associated with social roles and territoriality. Brain Behav Evol. 1998;51:23–32. doi: 10.1159/000006526. [DOI] [PubMed] [Google Scholar]
- 50.Meyer WN, Keifer J, Korzan WJ, Summers CH. Social stress and corticosterone regionally upregulate limbic N-methyl-D-aspartate receptor (NR) subunit type NR2A and NR2B in the lizard Anolis carolinensis. Neuroscience. 2004;128:675–684. doi: 10.1016/j.neuroscience.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 51.Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol. 2000;52:115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 52.Muske LE, Fernald RD. Control of a teleost social signal. I. Neural basis for differential expression of a color pattern. J Comp Physiol [A] 1987;160:89–97. doi: 10.1007/BF00613444. [DOI] [PubMed] [Google Scholar]
- 53.Muske LE, Fernald RD. Control of a teleost social signal. II. Anatomical and physiological specializations of chromatophores. J Comp Physiol [A] 1987;160:99–107. doi: 10.1007/BF00613445. [DOI] [PubMed] [Google Scholar]
- 54.Nuñez SC, Jenssen TA, Ersland K. Female activity profile of a polygynous lizard (Anolis carolinensis): Evidence of intersexual asymmetry. Behaviour. 1997;134:205–223. [Google Scholar]
- 55.Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AV. Social modulation of androgen levels in male teleost fish. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- 56.Oliveira RF, Lopes M, Carneiro LA, Canario AV. Watching fights raises fish hormone levels. Nature. 2001;409 doi: 10.1038/35054128. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira RF, McGregor PK, Latruffe C. Know thine enemy: fighting fish gather information from observing conspecific interactions. Proc R Soc Lond B Biol Sci. 1998;265:1045–1049. [Google Scholar]
- 58.Orrell KS, Jenssen TA. Heterosexual signalling by lizard Anolis carolinensis, with intersexual comparisons across contexts. Behaviour. 2003;140:603–634. [Google Scholar]
- 59.Øverli Ø, Korzan WJ, Larson ET, Winberg S, Lepage O, Pottinger TG, Renner KJ, Summers CH. Behavioral and neuroendocrine correlates of displaced aggression in trout. Horm Behav. 2004;45:324–329. doi: 10.1016/j.yhbeh.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Øverli Ø, Pottinger TG, Carrick TR, Overli E, Winberg S. Brain monoaminergic activity in rainbow trout selected for high and low stress responsiveness. Brain Behav Evol. 2001;57:214–224. doi: 10.1159/000047238. [DOI] [PubMed] [Google Scholar]
- 61.Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- 62.Plavicki J, Yang E-J, Wilczynski W. Dominance status predicts response to nonsocial forced movement stress in the green anole lizard (Anolis carolinensis) Physiol Behav. 2004;80:547–555. doi: 10.1016/j.physbeh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Rand AS, Ryan MJ, Wilczynski W. Signal redundancy and receiver permissiveness in acoustic mate recognition by the tungara frog, Physalaemus pustulosus. Am Zool. 1992;32:81–90. [Google Scholar]
- 64.Ruby DE. Male breeding success and differential access to females in Anolis carolinensis. Herpetelogica. 1984;40:272–280. [Google Scholar]
- 65.Shannon NJ, Gunnet JW, Moore KE. A comparison of biochemical indices of 5-hydroxytryptaminergic neuronal activity following electrical stimulation of the dorsal raphe nucleus. J Neurochem. 1986;47:958–965. doi: 10.1111/j.1471-4159.1986.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 66.Stamps JA, Krishnan VV. Territory acquisition in lizards. IV. Obtaining high status and exclusive home ranges. Anim Behav. 1998;55:461–472. doi: 10.1006/anbe.1997.0612. [DOI] [PubMed] [Google Scholar]
- 67.Sugerman RA. Observer effects in Anolis sagrei. J Herpetol. 1990;24:316–317. [Google Scholar]
- 68.Summers CH. Mechanisms for quick and variable responses. Brain Behav Evol. 2001;57:283–292. doi: 10.1159/000047246. [DOI] [PubMed] [Google Scholar]
- 69.Summers CH. Social interaction over time, implications for stress responsiveness. Integ and Comp Biol. 2002;42:591–599. doi: 10.1093/icb/42.3.591. [DOI] [PubMed] [Google Scholar]
- 70.Summers CH, Forster GL, Korzan WJ, Watt MJ, Larson ET, Overli O, Hoglund E, Ronan PJ, Summers TR, Renner KJ, Greenberg N. Dynamics and mechanics of social rank reversal. J Comp Physiol A. 2005;191:241–252. doi: 10.1007/s00359-004-0554-z. [DOI] [PubMed] [Google Scholar]
- 71.Summers CH, Greenberg N. Somatic correlates of adrenergic activity during aggression in the lizard, Anolis carolinensis. Horm Behav. 1994;28:29–40. doi: 10.1006/hbeh.1994.1003. [DOI] [PubMed] [Google Scholar]
- 72.Summers CH, Greenberg N. Activation of central biogenic amines following aggressive interaction in male lizards, Anolis carolinensis. Brain Behav Evol. 1995;45:339–349. doi: 10.1159/000113561. [DOI] [PubMed] [Google Scholar]
- 73.Summers CH, Korzan WJ, Lukkes JL, Øverli Ø, Höglund E, Watt MJ, Larson ET, Forster GL, Ronan PJ, Summers TR, Renner KJ, Greenberg N. Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol Biochem Zool. 2005;78:679–694. doi: 10.1086/432139. [DOI] [PubMed] [Google Scholar]
- 74.Summers CH, Larson ET, Ronan PJ, Hofmann PM, Emerson AJ, Renner KJ. Serotonergic responses to corticosterone and testosterone in the limbic system. Gen Comp Endocrinol. 2000;117:151–159. doi: 10.1006/gcen.1999.7408. [DOI] [PubMed] [Google Scholar]
- 75.Summers CH, Larson ET, Summers TR, Renner KJ, Greenberg N. Regional and temporal separation of serotonergic activity mediating social stress. Neuroscience. 1998;87:489–496. doi: 10.1016/s0306-4522(98)00144-4. [DOI] [PubMed] [Google Scholar]
- 76.Summers CH, Summers TR, Moore MC, Korzan WJ, Woodley SK, Ronan PJ, Höglund E, Watt MJ, Greenberg N. Temporal patterns of limbic monoamine and plasma corticosterone response during social stress. Neuroscience. 2003;116:553–563. doi: 10.1016/s0306-4522(02)00708-x. [DOI] [PubMed] [Google Scholar]
- 77.Summers CH, Watt MJ, Ling TJ, Forster GL, Carpenter RE, Korzan WJ, Lukkes JL, Øverli Ø. Glucocorticoid interaction with aggression in non-mammalian vertebrates: Reciprocal action. Eur J Pharmacol. 2005;526:21–35. doi: 10.1016/j.ejphar.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 78.Summers CH, Winberg S. Interactions between the neural regulation of stress and aggression. J Exp Biol. 2006;209:4581–4589. doi: 10.1242/jeb.02565. [DOI] [PubMed] [Google Scholar]
- 79.Summers TR, Hunter AL, Summers CH. Female social reproductive roles affect central monoamines. Brain Res. 1997;767:272–278. doi: 10.1016/s0006-8993(97)00604-5. [DOI] [PubMed] [Google Scholar]
- 80.Tokarz RR. Body size as a factor determining dominance in staged agonistic encounters between male brown anoles (Anolis sagrei) Anim Behav. 1985;33:746–753. [Google Scholar]
- 81.Umegaki H, Munoz J, Meyer RC, Spangler EL, Yoshimura J, Ikari H, Iguchi A, Ingram DK. Involvement of dopamine D2 receptors in complex maze learning and acetylcholine release in ventral hippocampus of rats. Neuroscience. 2001;103:27–33. doi: 10.1016/s0306-4522(00)00542-x. [DOI] [PubMed] [Google Scholar]
- 82.van der Vegt BJ, Lieuwes N, van de Wall EH, Kato K, Moya-Albiol L, Martinez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci. 2003;117:667–674. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- 83.Vaughan GL, Greenberg N. Propranolol, a β-adrenergic antagonist, retards response to MSH in skin of Anolis carolinensis. Physiol Behav. 1987;40:555–558. doi: 10.1016/0031-9384(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 84.Winberg S, Nilsson GE. Induction of social dominance by L-dopa treatment in Arctic charr. Neuroreport. 1992;3:243–246. doi: 10.1097/00001756-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Winberg S, Nilsson GE. Roles of brain monoamine transmitters in agonistic behaviour and stress reactions, with particular reference to fish. Comp Biochem Physiol. 1993;106C:597–614. [Google Scholar]
- 86.Wingfield JC, Lynn S, Soma KK. Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evol. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- 87.Yang EJ, Wilczynski W. Social experience organizes parallel networks in sensory and limbic forebrain. Dev Neurobiol. 2007;67:285–303. doi: 10.1002/dneu.20347. [DOI] [PubMed] [Google Scholar]
- 88.Yang E-J, Phelps SM, Crews D, Wilczynski W. The effects of social experience on aggressive behavior in Anolis carolinensis. Ethology. 2001;107:777–793. [Google Scholar]