Abstract
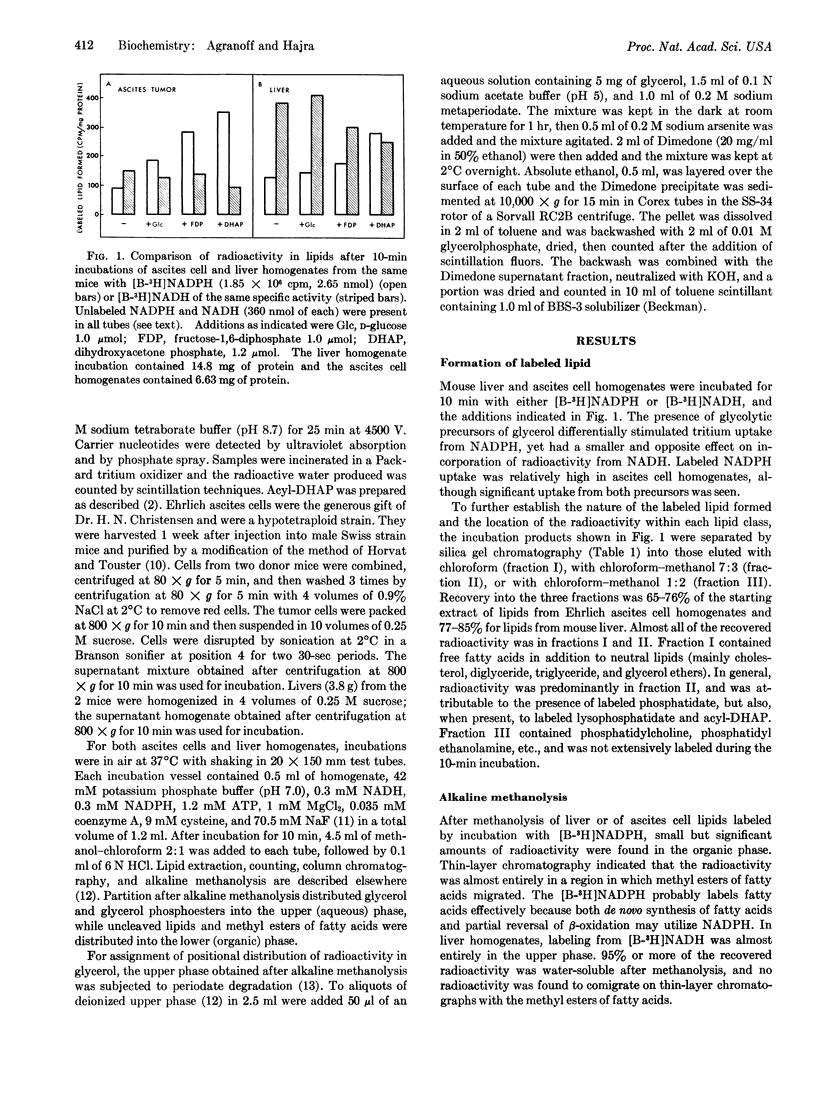
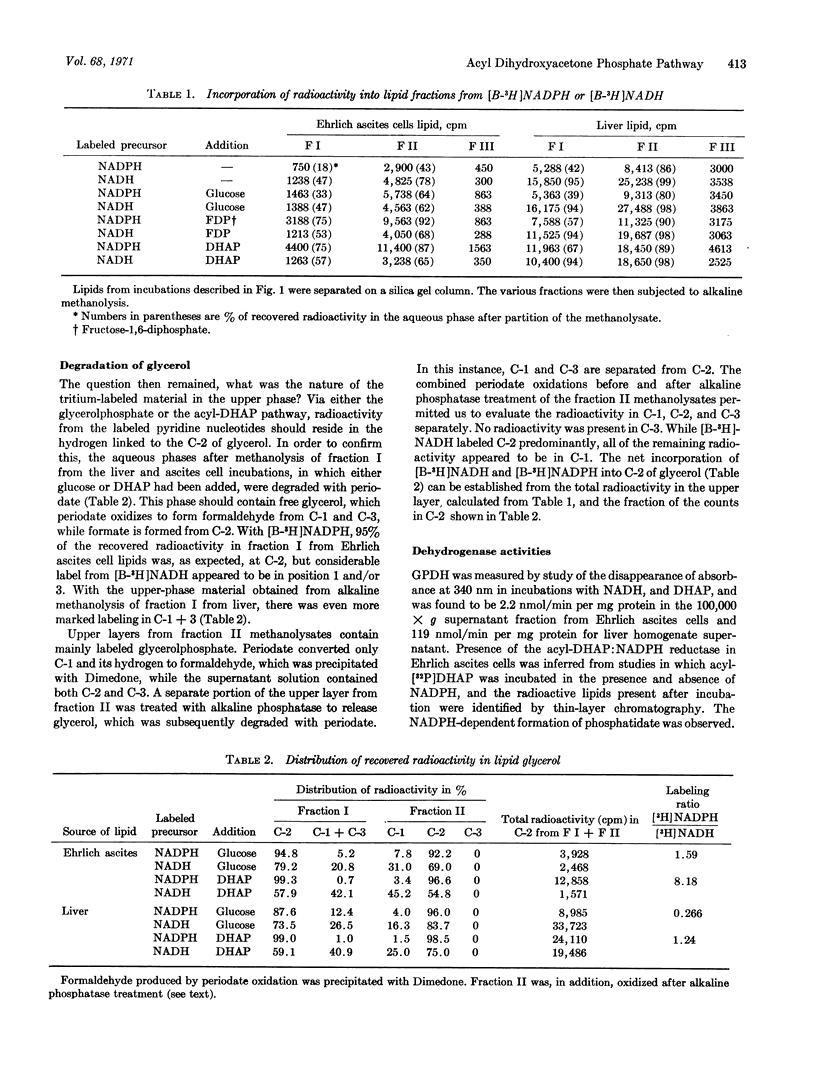
The glycerol portion of lipids may be derived biosynthetically by reduction of dihydroxyacetone phosphate to glycerolphosphate, and then be acylated with fatty acids or, alternatively, dihydroxyacetone phosphate may first be acylated and then reduced to 1-acyl sn glcerol-3-phosphate. Since the former pathway utilizes NADH for reduction of the C-2 carbonyl, while the latter requires NADPH, we were able to compare the relative participation of the two pathways for phospholipid synthesis by measuring the incorporation of radioactivity from tritiumlabeled NADH and NADPH into C-2 of lipid glycerol. The acyl-dihydroxyacetone phosphate pathway plays a significant role in glycerolipid synthesis in mouse liver homogenates and a clearly dominant one in Ehrlich ascites tumor cell homogenates. This finding is related to a reported lack of glycerol-3-phosphate dehydrogenase in tumor cells and to their high glycerol ether lipid content.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Benjamin J. A., Hajra A. K. Biosynthesis of phosphatidylinositol. Ann N Y Acad Sci. 1969 Oct 17;165(2):755–760. doi: 10.1111/j.1749-6632.1970.tb55954.x. [DOI] [PubMed] [Google Scholar]
- BOXER G. E., SHONK C. E. Low levels of soluble DPN-linked alpha-glycerophosphate dehydrogenase in tumors. Cancer Res. 1960 Jan;20:85–91. [PubMed] [Google Scholar]
- Bollinger J. N. The isolation and tentative identification of diacylglyceryl ethers from the walker 256 carcinoma of the rat and a human lymphosarcoma. Lipids. 1967 Mar;2(2):143–148. doi: 10.1007/BF02530914. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., OLLEY J. N. Chemical nature of monophosphoinositides. J Biol Chem. 1958 Apr;231(2):813–828. [PubMed] [Google Scholar]
- Hajra A. K. Acyl dihydroxyacetone phosphate: precursor of alkyl ethers. Biochem Biophys Res Commun. 1970;39(6):1037–1044. doi: 10.1016/0006-291x(70)90663-7. [DOI] [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Acyl dihydroxyacetone phosphate. A rapidly labeled lipid in guinea pig liver mitochondria. J Biol Chem. 1967 Mar 10;242(5):1074–1075. [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Acyl dihydroxyacetone phosphate. Characterization of a 32P-labeled lipid from guinea pig liver mitochondria. J Biol Chem. 1968 Apr 10;243(7):1617–1622. [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Reduction of palmitoyl dihydroxyacetone phosphate by mitochondria. J Biol Chem. 1968 Jun 25;243(12):3542–3543. [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of acyl dihydroxyacetone phosphate in guinea pig liver mitochondria. J Biol Chem. 1968 Jun 25;243(12):3458–3465. [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of phosphatidic acid from dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1968 Dec 30;33(6):929–935. doi: 10.1016/0006-291x(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Hajra A. K., Seguin E. B., Agranoff B. W. Rapid labeling of mitochondrial lipids by labeled orthophosphate and adenosine triphosphate. J Biol Chem. 1968 Apr 10;243(7):1609–1616. [PubMed] [Google Scholar]
- Hill E. E., Lands W. E. Formation of acyl and alkenyl glycerol derivatives in Clostridium butyricum. Biochim Biophys Acta. 1970 Feb 10;202(1):209–211. doi: 10.1016/0005-2760(70)90239-0. [DOI] [PubMed] [Google Scholar]
- Letnansky K., Klc G. M. Glycerolphosphate oxidoreductases and the glycerophosphate cycle in ehrlich ascites tumor cells. Arch Biochem Biophys. 1969 Mar;130(1):218–226. doi: 10.1016/0003-9861(69)90027-7. [DOI] [PubMed] [Google Scholar]
- PASTORE E. J., FRIEDKIN M. The chromatographic separation and recovery of reduced and oxidized pyridine nucleotides. J Biol Chem. 1961 Aug;236:2314–2316. [PubMed] [Google Scholar]
- Plackett P., Rodwell A. W. Glycerolipid biosynthesis by Mycoplasms strain Y. Biochim Biophys Acta. 1970 Jul 14;210(2):230–240. doi: 10.1016/0005-2760(70)90167-0. [DOI] [PubMed] [Google Scholar]
- RAPPORT M. M., NORTON W. T. Chemistry of the lipids. Annu Rev Biochem. 1962;31:103–138. doi: 10.1146/annurev.bi.31.070162.000535. [DOI] [PubMed] [Google Scholar]
- Snyder F., Cress E. A., Stephens N. An unidentified lipid prevalent in tumors. Lipids. 1966 Nov;1(6):381–386. doi: 10.1007/BF02532540. [DOI] [PubMed] [Google Scholar]
- Sánchez de Jiménez E., Cleland W. W. Studies of the microsomal acylation of L-glycerol-3-phosphate. I. The specificity of the rat brain enzyme. Biochim Biophys Acta. 1969 Jun 10;176(4):685–691. doi: 10.1016/0005-2760(69)90248-3. [DOI] [PubMed] [Google Scholar]
- Wallach D. F. Generalized membrane defects in cancer. N Engl J Med. 1969 Apr 3;280(14):761–767. doi: 10.1056/NEJM196904032801406. [DOI] [PubMed] [Google Scholar]



