Abstract
Purpose
To identify patient characteristics that influence tacrolimus individual dose requirement in kidney transplant recipients.
Methods
Data on forty-four 12-h pharmacokinetic profiles from 29 patients and trough concentrations in 44 patients measured during the first 70 days after transplantation (1,546 tacrolimus whole blood concentrations) were analyzed. Population pharmacokinetic modeling was performed using NONMEM 7.2®.
Results
Standardization of tacrolimus whole blood concentrations to a hematocrit value of 45 % improved the model fit significantly (p < 0.001). Fat-free mass was the best body size metric to predict tacrolimus clearance and volume of distribution. Bioavailability was 49 % lower in expressers of cytochrome P450 3A5 (CYP3A5) than in CYP3A5 nonexpressers. Younger females (<40 years) showed a 35 % lower bioavailability than younger males. Bioavailability increased with age for both males and females towards a common value at age >55 years that was 47 % higher than the male value at age <40 years. Bioavailability was highest immediately after transplantation, decreasing steeply thereafter to reach its nadir at day 5, following which it increased during the next 55 days towards an asymptotic value that was 28 % higher than that on day 5.
Conclusions
Hematocrit predicts variability in tacrolimus whole blood concentrations but is not expected to influence unbound (therapeutically active) concentrations. Fat-free mass, CYP3A5 genotype, sex, age and time after transplant influence the tacrolimus individual dose requirement. Because hematocrit is highly variable in kidney transplant patients and increases substantially after kidney transplantation, hematocrit is a key factor in the interpretation of tacrolimus whole blood concentrations.
Electronic supplementary material
The online version of this article (doi:10.1007/s00228-013-1584-7) contains supplementary material, which is available to authorized users.
Keywords: Tacrolimus, Hematocrit, Fat-free mass, Population pharmacokinetics, Target concentration intervention, Kidney transplantation
Introduction
Tacrolimus is an immunosuppressive drug widely used after kidney transplantation. Applying a target concentration strategy is recommended to ensure adequate immunosuppression [1]. The initial dose of tacrolimus is typically calculated in proportion to total body weight, with subsequent doses adjusted on the basis of whole blood trough concentration measurements. The target concentration for initial and subsequent dosing varies from center to center with no detectable relationship between achieved whole blood concentrations and the risk of acute rejection [2]. Predicting the individual dose requirement to achieve and maintain the desired target whole blood concentration of tacrolimus is challenging due to considerable variability in apparent whole blood clearance (CL/F) between patients [coefficient of variance (CV) 30–42 %] as well as between dosing occasions within patients (CV 40–71 %) [3]. In addition, dose-normalized whole blood concentrations of tacrolimus tend to increase during the first months after kidney transplantation, which has led to the suggestion of time-dependent pharmacokinetics [4, 5]. Both under- and overdosing frequently occur during this period, and several dose adjustments are typically performed [6].
Tacrolimus distributes into and binds to erythrocytes, showing a blood-to-plasma ratio of approximately 50 with a hematocrit of 45 % [7], and the unbound fraction in the blood is <1 % [8]. The drug is routinely measured as total concentrations in whole blood, although the unbound concentration is expected to mediate the pharmacological effect. Hebert et al. [9] have recently reviewed the pharmacological principles underlying the interpretation of whole blood concentrations and pointed out the potential dangers of adjusting tacrolimus doses based on whole blood concentration alone. Since tacrolimus is a low-extraction-ratio drug [10], whole blood concentrations are expected to increase in proportion to erythrocyte binding (proportional to hematocrit), while the unbound, therapeutically active concentration remains unchanged. At the time of kidney transplant, hematocrit is generally low, and it usually increases during the first months after transplantation as the recipients recover from kidney failure and erythrocyte production normalizes [11]. Because changes in hematocrit are expected to change the concentration of bound tacrolimus without modifying the unbound concentration [9], this trend should not be interpreted as the need for dose adjustment. It is currently unclear how much of the apparent time-dependent pharmacokinetics of tacrolimus during the first post-transplant months are explained by changes in hematocrit.
In this study, a population pharmacokinetic model for tacrolimus was developed using data from adult kidney transplant recipients. The purpose was to identify patient characteristics that influence tacrolimus individual dose requirement. Measured whole blood concentrations of tacrolimus were standardized to a normal hematocrit value (45 %). This led to the development of a pharmacokinetic model that can be used to individualize tacrolimus dosing to achieve a target concentration that better reflects the unbound pharmacologically active drug.
Materials and methods
Patients and data
Data on a total of 1,546 tacrolimus whole blood concentrations were obtained from 69 adult patients who underwent kidney transplantation at Oslo University Hospital, Rikshospitalet and who received tacrolimus as part of their immunosuppressive therapy. Patients who received multiple organs or who received drugs that were known to pharmacokinetically interact with tacrolimus were excluded. However, if such drugs were used by >5 % of the patients, the patients were allowed in the analysis, and the drug was analyzed as a covariate. The patients contributed data to an Intensive Group or a TDM (therapeutic drug monitoring) Group. Four patients contributed data to both groups. The Intensive Group consisted of 12-h concentration–time profiles from 29 patients in a stable phase after transplantation (range 26 days–18 years). The data were collected on a total of 44 dosing occasions as described in three previously published studies [12–14]. The TDM Group consisted of routine trough concentrations (C0) measured in 44 patients during the first 70 days following kidney transplantation. Dosing at 12-h intervals and full compliance were presumed unless missed doses were recorded in the patient chart. Patients were told to be fasting at the time of drug intake.
The following data were collected for covariate analysis: cytochrome P450 3A5 (CYP3A5) genotype, hematocrit, sex, age, total body weight, height, serum albumin, serum creatinine, C-reactive protein, aspartate aminotransferase, alanine aminotransferase, total serum bilirubin, alkaline phosphatase, acute rejection episodes, oral prednisolone dose, intravenous methylprednisolone dose, and use of other potentially interactive drugs. Covariates with time-dependent values were updated for each recorded tacrolimus measurement. Covariates missing on the day of transplantation were imputed by carrying back the first known value. Covariates missing at any later time point were imputed by carrying forward the last known value. However, if more than two hematocrit values were missing in sequence and the hemoglobin level was known, hematocrit values were derived by linear regression between all known hematocrit and hemoglobin values within the patient. A summary of the study designs is listed in Table 1.
Table 1.
Characteristics of tacrolimus pharmacokinetic studies
| Study and reference | Number of tacrolimus whole blood concentrations for analysis | Patientsa (n) | Assay type | LLOQ (μg/L) | Assay CV (%) | Sampling times |
|---|---|---|---|---|---|---|
| 1. [12] | 134 | 5b | MEIAc | 3.0 | 13 at 5 μg/L | 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10 and 12 h after administration |
| 7 at 23 μg/L | ||||||
| 2. [13] | 164 | 7b | LC-MS/MSd | 1.1 | ≤5.2 | 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10 and 12 h after administration |
| 3. [14] | 216 | 19b | CMIAe | 1.0 | <6 at 2.3 μg/L | 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 23 and 24 h after administration |
| <9 at 7.0 μg/L | ||||||
| 4. NPP | 1,032 | 44 | CMIAe | 1.0 | <6 at 2.3 μg/L | Predose (trough concentrations) |
| <9 at 7.0 μg/L |
CMIA, Chemiluminescent microparticle immunoassay; CV, between-series coefficient of variation; LC-MS/MS, liquid chromatography/tandem mass spectrometry; LLOQ, lower limit of quantification; MEIA, microparticle enzyme immunoassay; NPP, not previously published data
aSix patients contributed data to more than one study. These were identified and their data were associated with the same ID in the data set, with an increase in the number of occasions. The total number of individuals was 69
bNumber of patients is different from number in original study due to exclusion criteria of the present study
cAnalyzed on the IMx® instrument (Abbott Laboratories, Abbott Park, IL)
dConverted to corresponding immunoassay concentrations: CM = (LC − 0.19)/0.80; see main text for details
eAnalyzed on the Architect® instrument (Abbott Laboratories)
The study was approved by the Regional Committee for Medical Research Ethics and by the Norwegian Medicines Agency. All patients gave written informed consent.
Immunosuppressive therapy
All patients received oral tacrolimus (Prograf® capsules; Astellas Pharma US Inc., Northbrook, IL). According to the center transplant protocol, initial dosage for the TDM Group was 0.04 mg/kg total body weight (0.05 mg/kg for high-risk patients) twice daily, adjusted to the nearest 0.5 mg dose. The dosage was subsequently adapted by measuring whole blood trough concentrations to maintain concentrations within the range of 3–7 μg/L in standard risk patients or 8–12 μg/L in high-risk patients. High-risk patients were defined by panel reactive antibody of >20 % and/or presence of donor-specific antibodies. Trough concentrations of tacrolimus were initially measured three to four times per week. Depending on clinical status and graft function, this frequency decreased in parallel with increasing time since transplantation.
In addition to tacrolimus, the immunosuppressive regimen for the TDM Group consisted of 0.75 g mycophenolate mofetil twice daily (without target concentration intervention), induction therapy with 20 mg of basiliximab on the day of transplant (day 1) and on day 5, an intravenous bolus of 250 mg methylprednisolone on day 1 and oral prednisolone once daily according to the following tapering regimen: 20 mg on days 2–15, 15 mg on days 16–29, 10 mg on days 30–61, 7.5 mg on days 62–180 and 5 mg from day 181 onwards. High-risk patients received induction therapy with 500 mg/kg intravenous human immune globulins daily on days 1–6 and 375 mg/m2 rituximab on day 1, intravenous boluses of 500 mg and 80 mg methylprednisolone on day 1 and 2, respectively, and oral prednisolone at a total dose of 80 mg on day 3, followed by tapering during days 4–7 to 30 mg daily, continued with once-daily doses of 20 mg on days 8–29, 15 mg on days 30–61, 10 mg from day 62 and in selected patients tapered to 5 mg from day 181 onwards. For the Intensive Group, prednisolone dose was the only recorded concomitant immunosuppressive drug on which information was provided (range 0–25 mg daily).
Analytical assays
Table 1 shows the analytical assays used to measure tacrolimus concentrations in each sub-study. Chemiluminescent microparticle immunoassay (CMIA) is currently used as a standard analytical method at our center. Of the concentrations available for analysis, 80 % were determined by CMIA, 9 % by microparticle enzyme immunoassay (MEIA) and 11 % by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Tacrolimus concentrations determined by CMIA and MEIA correlate well at concentrations of >5 μg/L [15]. As only 1 % of the concentrations in the present analysis determined by MEIA were <5 μg/L, no conversion was performed between immunoassay measurements. On the contrary, tacrolimus concentrations determined by immunoassay tend to be higher than those determined by mass spectrometry, mainly due to cross-reactivity with tacrolimus metabolites [1]. In order to use tacrolimus concentrations determined by LC-MS/MS in the present analysis, we converted LC-MS/MS measurements into CMIA-equivalent measurements by the means of a conversion equation (Eq. 1) derived from linear regression between 43 tacrolimus whole blood concentrations determined by both LC-MS/MS (range 2.7–13.0 μg/L) and CMIA (range 3.6–14.4 μg/L) (R 2 = 0.94). The regression analysis was available from routine procedures performed at our laboratory during transition between analytical assays.
| 1 |
In Eq. 1, CM is the CMIA tacrolimus concentration in micrograms per liter and LC is the LC-MS/MS tacrolimus concentration in micrograms per liter.
CYP3A5 genotyping
DNA was extracted from EDTA anti-coagulated whole blood using the MagNA Pure instrument (Roche Applied Science, Penzberg, Germany). CYP3A5 genotyping (rs776746; NG_007938.1:g.12083G > A, A = CYP3A5*1 and G = CYP3A5*3) was performed by real-time PCR and melt curve analysis with hybridization probes on the LightCycler® 480 instrument (Roche Applied Science) or by a previously reported PCR-restriction fragment length polymorphism assay [16]. Primers for the melt curve assay were designed using LightCycler Probe Design software version 2 (Roche Applied Science), and hybridization probe sequences were derived from Cheung et al. [17].
Population pharmacokinetic modeling
Structural model
Models with one or two compartments, zero or first order absorption and with or without an absorption lag time were investigated. Pharmacokinetic parameters were estimated in terms of clearance (CL), intercompartmental clearance (Q) and volumes of distribution (V1, V2). The population value of bioavailability (F) was defined as 1, and subgroup values and individual values of F were estimated relative to the population value. Because only data following oral administration were available, apparent disposition parameter estimates were obtained (e.g. CL/F, V1/F). Random effects in the pharmacokinetic parameters were modeled in terms of between subject variability (BSV) and between occasion variability (BOV). BSV and BOV were initially estimated for all pharmacokinetic parameters except lag time. Random effects were modeled exponentially (Eq. 2):
| 2 |
where Pik is the value of parameter P for the i-th individual at the k-th occasion, PGRP is the group value of P in the population, eηi is the deviation between PGRP and the individual value Pi and eηk is the deviance between the individual value Pi and the occasion-specific value Pik [18]. For the Intensive Group, one hospital visit was defined as one occasion. For the TDM Group, a new occasion was defined when a dose change took place. Equation 3 shows the residual error model used throughout the analysis:
| 3 |
where Yij and Concij are the model-predicted drug concentrations with and without residual error, respectively, for the i-th individual at the j-th measurement, θstudyi is a study-specific fixed effect, ε1 is a residual error term proportional to the measured concentrations, ε2 is an additive error term and eηerri is the individual random difference in residual error for the i-th individual [19]. The random variables ηi, ηk, ε1, ε2 and ηerri were assumed to be normally distributed with means of zero and variances ω2 P, π2 P , σ1 2, σ2 2 and ωerr 2, respectively.
Covariate model
Tacrolimus total concentrations (Ctot) in whole blood are the sum of the unbound (Cu), plasma bound (Cp) and erythrocyte bound (Cb) concentrations. Assuming a single binding site, Ctot can be described as a function of the unbound concentration (Eq. 4):
| 4 |
where Cbmax is the maximum concentration bound to erythrocytes and Cu50 is the unbound concentration leading to half maximum binding. With a blood:plasma ratio of approximately 50 [7], Ctot will mainly reflect Cb. By assuming that Cu is small relative to Cu50 and by standardizing Cbmax to a hematocrit value of 45 %, Eq. 4 can be written as Eq. 5:
| 5 |
where Cstd is the standardized concentration proportional to Cu, and R is the ratio between Cbmax and Cu50 at a hematocrit of 45 %. Equation 5 was included in the model by relating the pharmacokinetic parameters and predictions to Cstd rather than to Ctot. Potential concentration-dependent binding of tacrolimus to erythrocytes was investigated using Eq. 6:
| 6 |
where Cstdmax and Cstd50 are estimable parameters reflecting maximum binding capacity of tacrolimus to erythrocytes and the standardized concentration associated with half maximum binding, respectively. Similar principles to those described for hematocrit were used to investigate the standardization of plasma protein binding using serum albumin concentrations.
The pharmacokinetic disposition parameters were related to fat-free mass using theory-based allometric coefficients of 3/4 for clearances and 1 for volumes of distribution [20]. Fat-free mass was predicted from total body weight, height and sex (Eqs. 20 and 21 in [21]). Potential additional effects of fat mass (i.e. total body weight—fat-free mass) to the effect of fat-free mass on the pharmacokinetic parameters were estimated as described by Anderson and Holford [20].
All available patient characteristics were evaluated as covariates on parameters where an effect was biologically plausible or if an effect was found in previous studies. Regarding covariates for which little is known about the mechanism of influence on tacrolimus pharmacokinetics (age, time after transplantation, liver function test values), continuous covariate effects were initially investigated by binning observed concentrations by covariate value (e.g. age bins of 20–29 years, 30–39 years and so on). The early post-transplant period was binned by dividing it into 12 time intervals with similar number of observations in each interval: day 2, 3, 4–5, 6–7, 8–10, 11–14, 15–21, 22–28, 29–42, 43–56, 57–70 and >70 days. A mean pharmacokinetic parameter (CL or F) was estimated within each bin relative to a reference bin. Empirical models (linear, power, exponential or sigmoid functions) were subsequently used to match the discrete distribution of parameter values as a function of the continuous covariate and of time. This method helps to identify and obtain initial estimates of continuous functions that describe the data when there is little prior information on what relationship to expect [22]. Binary covariate effects (e.g. sex) were investigated by estimating the fractional change in one group compared to the other.
Model evaluation and statistical analysis
Model selection was guided by biological plausibility and the likelihood ratio test [difference in objective function value (ΔOFV)]. A decrease in OFV of >6.6 was considered to be statistically significant for the addition of one parameter (p < 0.01) [23]. Prediction-corrected visual predictive checks (pcVPCs) were generated to compare observed data with 90 % prediction intervals constructed from 100 simulated subsets of the original dataset [24]. Time after dose, time after transplantation and other continuous covariates were used as independent variables in pcVPCs. Covariates were included stepwise and subsequently independently deleted from the full model (backward elimination) to evaluate their final statistical contribution [25]. Confidence intervals (CIs) of the parameter estimates were generated from 1,000 nonparametric bootstrap replicates [26]. These were used to guide model simplification. Random effects were retained if the lower 2.5th percentile did not approach zero. Covariates were retained if the 95 % CI of the covariate coefficient estimate did not include the value equivalent to no effect. To describe variability in hematocrit between and within patients, hematocrit was modeled by BSV and BOV (considering each hematocrit measurement as a single occasion). The time course of hematocrit after transplantation was described by a sigmoid Emax function.
Modeling was performed in NONMEM® v. 7.2 [23] using the first order conditional estimation method with interaction. Models and bootstrap replicates were run using Wings for NONMEM® [27]. Statistical and graphical analyses were performed using the statistical package R® (v. 2.15.0; R Foundation for Statistical Computing, Vienna, Austria). Means for normally distributed covariates were compared using two-tailed t tests with assumption of equal variance. If not stated otherwise, descriptive statistics are expressed as the mean ± standard deviation.
Results
Patients and data
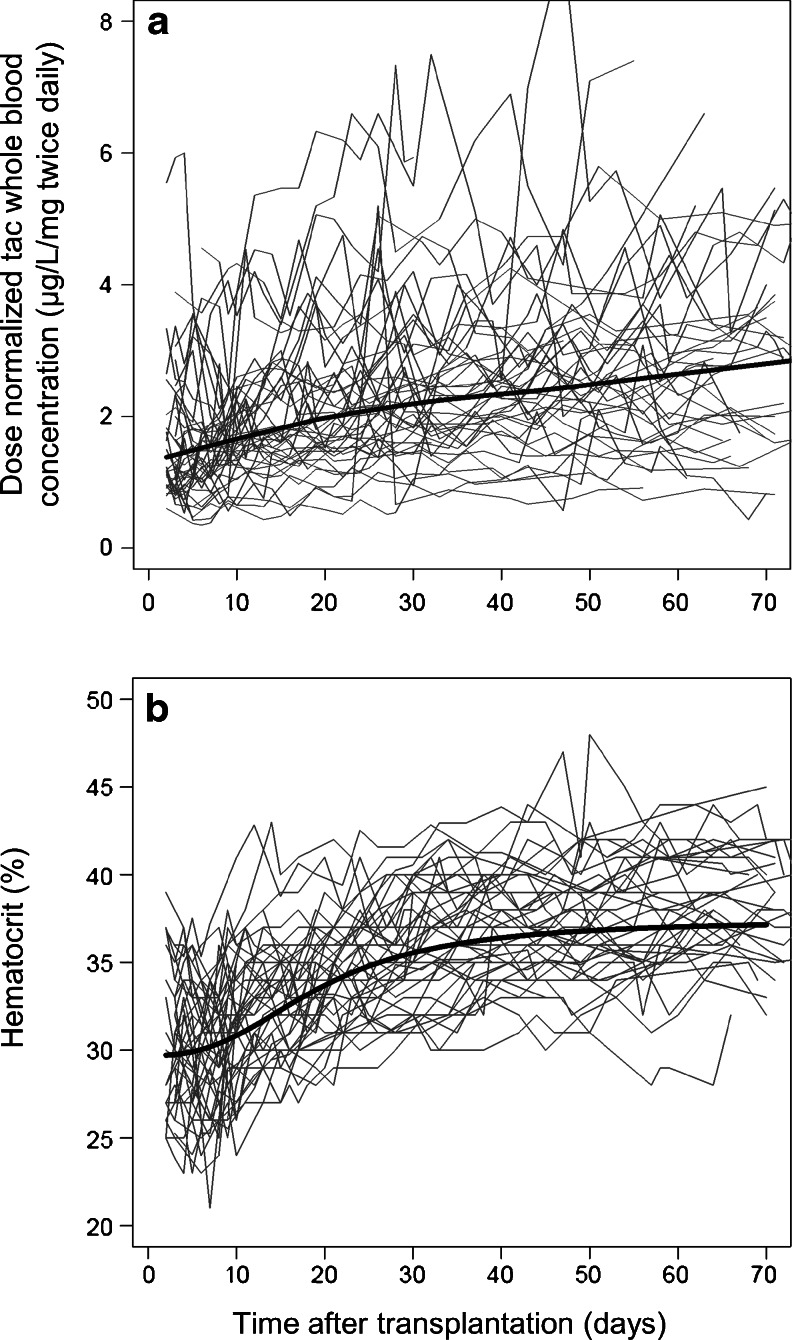
Demographic and clinical characteristics, details about tacrolimus treatment and the number of missing covariate values are presented in Table 2. Patients using carbamazepine (n = 2) were excluded because of potential drug interaction with tacrolimus. Patients using nifedipine (n = 26), lansoprazole (n = 12) and/or cinacalcet (n = 5) were retained. Figure 1a shows the evolution of the dose-normalized tacrolimus trough whole blood concentrations after transplantation. In total, 41 % of the measured trough concentrations in the TDM group were outside the target concentration ranges defined by the hospital protocol. The percentage of measured concentrations outside these ranges for week 1, 2, 3, 4, 5–6, 7–8 and 9–10 after transplantation were 42, 41, 47, 42, 43, 37 and 36 %, respectively. It was however not known if the clinician voluntarily had deviated from the protocol.
Table 2.
Demographic and clinical data
| Data | Number | Meana | 95 % observation interval | Change in TDM Group after transplantation | Number of missing data points | |||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 70 | |||||||
| Mean | 95 % observation interval | Mean | 95 % observation interval | |||||
| Demographic data | ||||||||
| Patients | 69 | |||||||
| Sex (male /female) | 50/19 | 0/69 | ||||||
| CYP3A5 genotype (*1/*1, *1/*3, *3/*3) | 0/10/59 | 0/69 | ||||||
| Age (years) | 43 | 22–73 | 0/69 | |||||
| Height (cm) | 176 | 151–197 | 0/69 | |||||
| Total body weight (kg) | 81 | 51–131 | 83 | 53–140 | 80 | 49–128 | 142/1,546 | |
| Predicted fat-free mass (kg) | 59 | 40–82 | 60 | 41–84 | 55 | 39–82 | 142/1,546 | |
| Hematocrit (%) | 36 | 29–43 | 31 | 25–39 | 38 | 32–42 | 109/1,546 | |
| Serum creatinine (μmol/L) | 147 | 73–347 | 409 | 123–1,079 | 120 | 78–208 | 2/1,546 | |
| C-reactive protein (mg/L) | 7 | <0.6–24 | 12 | <0.6–34 | 3 | <0.6–10 | 49/1,546 | |
| Serum albumin (g/L) | 42 | 33–47 | 37 | 27–45 | 45 | 37–50 | 498/1,546 | |
| Total serum bilirubin (μmol/L) | 7 | 3–13 | 5 | 1–14 | 6 | 3–15 | 500/1,546 | |
| Aspartate aminotransferase (IU/L) | 23 | 15–40 | 35 | 13–111 | 20 | 14–29 | 498/1,546 | |
| Alanine aminotransferase (IU/L) | 31 | 10–91 | 50 | 13–170 | 22 | 11–43 | 498/1,546 | |
| Alkaline phosphatase (IU/L) | 66 | 30–106 | 60 | 34–95 | 70 | 41–105 | 499/1,546 | |
| Pharmacokinetic data (TDM Group) | ||||||||
| Samples | 1,032 | |||||||
| Samples per patient | 23 | 13–29 | ||||||
| Tacrolimus trough concentration (μg/L) | 7.0 | 2.7–12.4 | ||||||
| Pharmacokinetic data (Intensive Group) | ||||||||
| Samples | 514 | |||||||
| Samples per patient | 18 | 9–39 | ||||||
| Tacrolimus concentration (μg/L) | 11.3 | 3.4–26.7 | ||||||
CYP3A5, Cytochrome P450 3A5; TDM, therapeutic drug monitoring
aCalculated from mean value across all observation times in each patient
Fig. 1.
a Dose-normalized whole blood trough concentrations of tacrolimus (tac) observed in the TDM Group during the first 70 days after transplantation. Thin lines 44 individual patients, thick line a smoother to show the trend with time. b Time course of hematocrit (%) during the first 70 days after transplantation. Thin lines 44 individual patients that were followed during this period, thick line sigmoid Emax model fit to the data to describe changes in hematocrit as a function of time
Hematocrit increased during the first 70 days after transplantation (Fig. 1b). The population baseline hematocrit on day 1 was estimated to be 29.7 %, increasing towards an asymptotic value of 37.4 % with half maximum increase on day 19 post-transplant. BSV and BOV of the hematocrit were estimated to be 8 and 11 %, respectively. Average hematocrit did not vary by sex (p = 0.28) or CYP3A5 genotype (p = 0.51). Average total body weight was 19 kg higher in males (86 ± 21 kg) than in females (67 ± 15 kg) (p < 0.001). Average fat-free mass was 18 kg higher in males (64 ± 10 kg) than in females (46 ± 5 kg) (p < 0.001).
Population pharmacokinetic modeling
Structural model
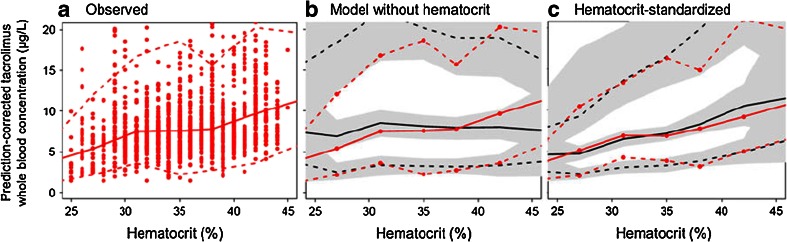
Tacrolimus pharmacokinetics were best described by a two-compartment model and first order absorption with a lag time. A study-specific absorption rate and lag time improved the description of substudy 2. When the BOV was estimated for F, the lower 2.5th percentile of the bootstrap distribution of BOV in the four disposition parameters (CL/F, V1/F, Q/F and V2/F) approached zero. BOV in these parameters were therefore removed (ΔOFV + 7.3 for four parameters, p = 0.12). For the absorption rate constant (ka), BSV was removed and BOV was retained (ΔOFV + 3.4 by removing BSV, p = 0.07; ΔOFV + 28.7 by removing BOV, p < 0.001), indicating that the variability in absorption rate is mainly dependent on dosing occasion rather than being a subject-specific process. We chose to estimate a full correlation matrix between CL/F, V1/F, Q/F and V2/F (ΔOFV = −59.0 for six parameters, p < 0.001) rather than to estimate BSV in F (ΔOFV = −32.4 for one parameter, p < 0.001). The pcVPC over the range of hematocrit revealed a systematic prediction error at this stage (Fig. 2b).
Fig. 2.
a Prediction-corrected tacrolimus whole blood concentrations over the range of hematocrit, b prediction-corrected visual predictive check (pcVPC) over the range of hematocrit using the base model without covariates, c pcVPC over the range of hematocrit using the base model with hematocrit standardized concentrations. Solid red line Median observed tacrolimus concentration (prediction-corrected), dashed red lines 90 % observation interval (5th to 95th percentile), solid black line median model-predicted concentrations, dashed black lines 90 % prediction interval. Gray-shaded areas 95 % confidence intervals (CIs) for each model-predicted percentile
Covariate model
The model fit improved significantly by linearly standardizing whole blood concentrations to a hematocrit of 45 % (ΔOFV = −78.3, p < 0.001). The estimated value for the parameter R in Eq. 5 was 0.99, and this parameter was removed without worsening the model fit (ΔOFV = +1.2, p = 0.27). By standardizing for hematocrit, the systematic prediction error over the range of hematocrit values was no longer present (Fig. 2c). Accounting for the possibility of saturated erythrocyte proteins (Eq. 6) or including similar standardization of the concentrations using serum albumin did not improve the model fit.
The parameters describing the effects of fat mass in addition to the effect of fat-free mass on the disposition parameters were estimated to be close to zero [bootstrap means of 0.1 (95 % CI −0.7 to 1.9) for clearances and 0.3 (95 % CI −0.7 to 2.4) for volumes of distribution]. This indicated that fat-free mass is a more predictive body size metric for tacrolimus pharmacokinetics than total body weight. The OFV did not increase by fixing these parameters to zero. CYP3A5 genotype significantly influenced both CL and F (p < 0.001).
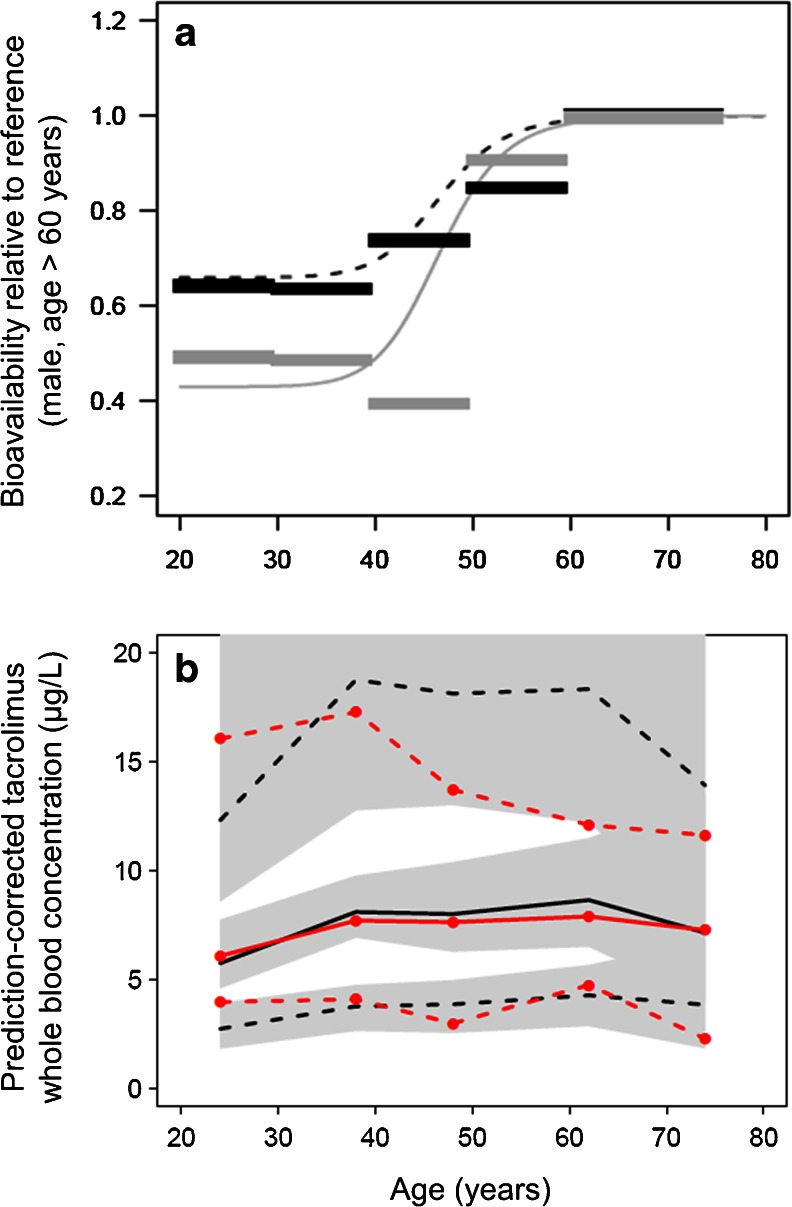
After including body size in the model, it was found that F was significantly lower in females (ΔOFV = −9.8, p = 0.002). When an age-specific value of F was estimated in each of the five age categories, it became apparent that F changed between age 40 and 50 years and was essentially constant at a lower relative value in patients aged <40 years and constant at a higher relative value in patients aged >55 years. A sigmoid function of age was useful to describe these changes and improved the model further (ΔOFV = −18.1 for three parameters, p < 0.001) (Eq. 7):
| 7 |
where Fminage is the asymptotic value of F at a low age relative to the highest value of F with increasing age, and Fage50 is the age of the half maximum change in F with the associated shape coefficient HillFage. The estimates of Fage50 and HillFage were similar for males and females, whereas Fminage was different between males and females. These results indicate that the sex difference in F is greatest in younger patients (<40 years) and that the difference diminishes at age >55 years (Fig. 3a).
Fig. 3.
a Thick horizontal lines Age interval-specific average values of bioavailability (F) estimated in males (black) and females (gray) relative to males aged >60 years (defined as F = 1). Lines Model-estimated functions for changes in bioavailability with age in males (black dashed) and females (gray solid). b pcVPC using the final model as a function of age. Solid red line Median observed tacrolimus concentration (prediction-corrected), dashed red lines 90 % observation interval (5th to 95th percentile), solid black line median model-predicted concentrations, dashed black lines 90 % prediction interval. Gray-shaded areas 95 % CI for each model-predicted percentile
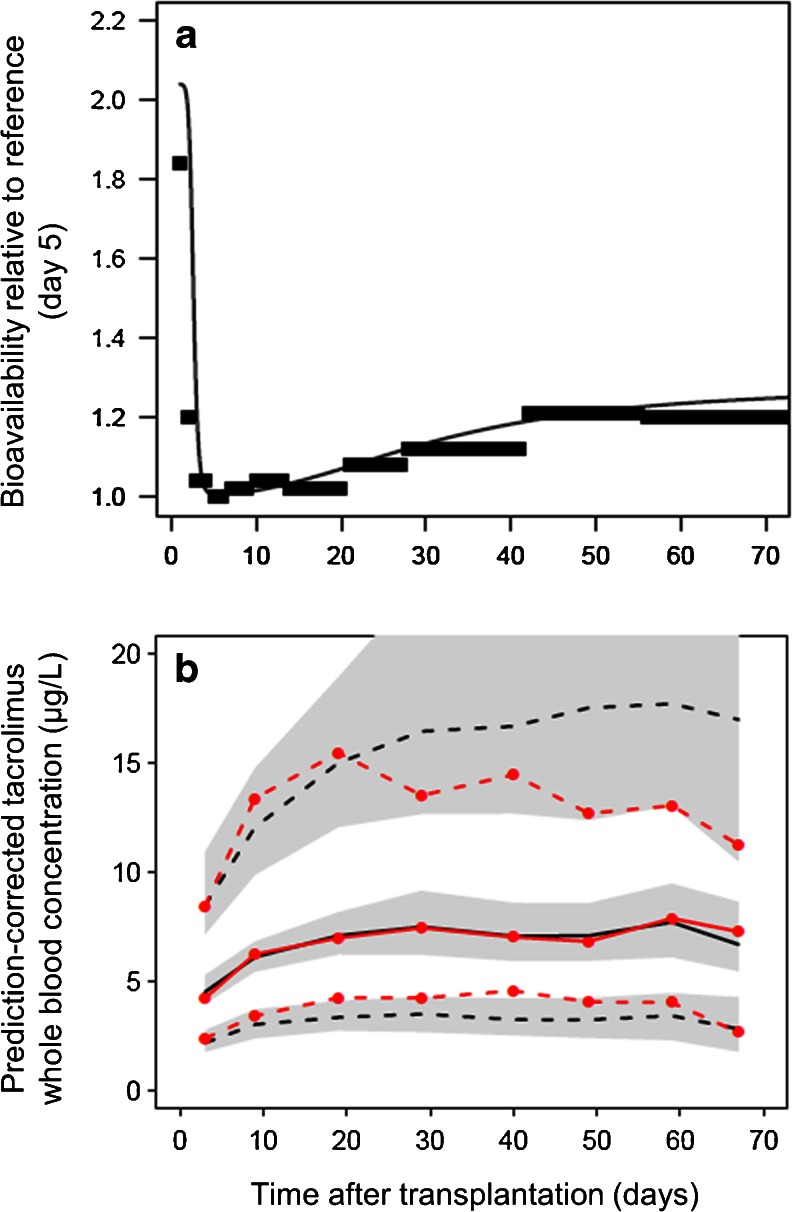
The time interval-specific value of F changed systematically with time after transplantation. These changes could be described using two distinct sigmoid functions of time after transplant (Fig. 4a and Eq. 8). A random effect was included to describe BSV in the extent of change at a late time point:
| 8 |
where Fi is the individual value of F, Fmaxearly is the maximum value of F immediately after transplantation, Fearly50 is the day with half maximum early effect on F with the associated shape coefficient HillFearly describing the steepness of this change, Flate50 is the day with half maximum later effect on F with the associated steepness coefficient HillFlate, Fmaxlate is the asymptotic maximum value of F with increasing post-transplant time and eηi is the difference between the individual asymptote and the population asymptote, where ηi’s are assumed to be normally distributed with mean zero and variance ωFlate 2. The day of transplant was defined as day 1. The six sigmoid model parameters replaced the 12 time interval parameters without loss in model goodness of fit (ΔOFV = −64.8 vs. −69.5, respectively, compared to a model without time after transplantation as a covariate). Estimation of BSV in Fmaxlate resulted in a further decrease (ΔOFV = −40.7 for one parameter, p < 0.001). The data did not support estimation of BSV in any of the remaining parameters in Eq. 8. No time-varying covariate, including prednisolone dose and liver function test values, replaced the empirical time model for F without a loss in model goodness of fit.
Fig. 4.
a Thick horizontal lines Time interval-specific average values of bioavailability after transplantation, estimated relative to the value at day 5 (defined as F = 1). Thin continuous line Model-estimated time course of relative bioavailability during the first 70 days after transplantation. b pcVPC using the final model as a function of the first 70 days after transplantation. Solid red line Median observed tacrolimus concentration (prediction-corrected), dashed red lines 90 % observation interval (5th to 95th percentile), solid black line median model predicted concentrations, dashed black lines 90 % prediction interval. Gray-shaded areas 95 % CI for each model-predicted percentile
Model reduction and evaluation
The study-specific residual errors for substudies 1 and 4 were similar and joined (ΔOFV + 0.9 for one parameter, p = 0.34). The lower 2.5th percentile of the bootstrap distribution of the additive residual error term and BSV in the residual error approached zero. Therefore, only the proportional error term was retained. All random effects describing correlations between disposition parameters except from between CL/F and Q/F were removed because their 95 % CIs included zero. The effect of CYP3A5 genotype on CL was removed because the 95 % CI of 0.81–1.46 did not support its inclusion in addition to an effect on F, which had a 95 % CI not including 1 (0.39–0.83) (ΔOFV +0.01 for one parameter, p = 0.91). Removing the remaining covariates significantly worsened the goodness of fit.
Expressed as bootstrap averages of the final model (Table 3), we found that F was 49 % lower in CYP3A5 expressers than in CYP3A5 nonexpressers. Furthermore, F was reduced to 0.43 and 0.66 for females and males, respectively, at the lowest ages compared to the plateau value set to 1 at highest age in males. The plateau value for females with increasing age was estimated as 1 (not different from males). The steepest change in F with age occurred around the age of 47 years for both sexes. Moreover, F was 104 % higher immediately after transplantation relative to its lowest value, followed by a steep decrease (HillF early = 9.4) to a nadir (the reference baseline where F is defined as 1), with half of the change at day 2.5 after transplantation. F then increased more slowly (HillF late = 2.5) to an asymptotic value 28 % higher than the nadir (BSV = 113 %) with half of the change after 31 days. PcVPCs indicated that the model was able to predict tacrolimus concentrations over the range of all the included covariates (shown for age in Fig. 3b and for time after transplantation in Fig. 4b).
Table 3.
Original data estimates and bootstrap average parameter estimates with 95 % confidence intervals
| Parameter | Unit | Original data estimatea | Bootstrap average | Bootstrap 95 % CIb |
|---|---|---|---|---|
| CL/Fn | L/h | 20.5 | 20.5 | 17.3–24.6 |
| V1/Fn | L | 105 | 107 | 87–136 |
| Q/Fn | L/h | 35.8 | 37.3 | 21.5–48.5 |
| V2/Fn | L | 450 | 424 | 297–583 |
| ka | h−1 | 1.14 | 1.18 | 0.80–1.78 |
| ka, study 2 | h−1 | 0.37 | 0.38 | 0.31–0.48 |
| Lag time | h | 0.21 | 0.22 | 0.20–0.24 |
| Lag timestudy 2 | h | 0.82 | 0.81 | 0.65–0.90 |
| Covariates on F | ||||
| CYP3A5 *1/*3 | factor | 0.51 | 0.51 | 0.42–0.62 |
| Age and sex | ||||
| Fminage, females | 0.44 | 0.43 | 0.23–0.60 | |
| Fminage, males | 0.68 | 0.66 | 0.36–0.89 | |
| Fage50 | years | 44 | 47 | 39–70 |
| HillF age | 12.2 | 14.0 | 3.5–24.9 | |
| Time, early | ||||
| Fmaxearly | 1.87 | 2.04 | 1.60–3.9 | |
| Fearly50 | days | 2.6 | 2.5 | 1.7–3.0 |
| HillF early | 10.0c | 9.4 | 3.7–10.0c | |
| Time, late | ||||
| Fmaxlate | 0.27 | 0.28 | 0.14–0.49 | |
| Flate50 | days | 30 | 31 | 24–40 |
| HillF late | 2.3 | 2.5 | 1.6–4.7 | |
| BSV | ||||
| CL/Fn | % | 33 | 31 | 21–40 |
| V1/Fn | % | 14 | 14 | 0.2–37 |
| Q/Fn | % | 91 | 86 | 51–114 |
| V2/Fn | % | 52 | 52 | 32–74 |
| HillF late | % | 117 | 113 | 78–146 |
| Correlation | ||||
| CL/Fn ∼ Q/Fn | 0.75 | 0.74 | 0.41–0.92 | |
| BOV | ||||
| Fn | % | 16 | 16 | 13–18 |
| ka | % | 63 | 60 | 41–84 |
| Residual variability | ||||
| Proportional error | % | 16.7 | 16.7 | 15.2–18.4 |
| Study 2 | factor | 0.57 | 0.56 | 0.41–0.72 |
| Study 3 | factor | 0.73 | 0.72 | 0.57–0.90 |
CI, Confidence interval, Fn, bioavailability at baseline nadir (5 days after transplantation); CL/Fn, apparent clearance; V1/Fn, apparent central volume of distribution; Q/Fn, apparent intercompartmental clearance; V2/Fn, apparent peripheral volume of distribution; ka, absorption rate constant; FFM, fat-free mass; Fminage, the minimum value of F at decreasing age relative to reference (male > 60 years); Fage50, the age with half maximum effect on F; HillF age, shape coefficient for the change in F with age; Fmaxearly, the maximum increase in F immediately after transplantation relative to Fn; Fearly50, the day with half maximum early effect on F; HillF early, shape coefficient for the change in F at early time; Fmaxlate, maximum increase in F at later time point, relative to Fn; Flate50, the day with half maximum later effect on F; HillF late, shape coefficient for the change in F at later time; BSV, between subject variability; BOV, between occasion variability
The final model was parameterized as follows: CL/Fn = 20.5 × (FFM /60)3/4 L/h; V1/Fn = 107 × (FFM /60) L; Q/Fn = 37.3 × (FFM /60)3/4 L/h; V2/Fn = 424 × (FFM /60) L
F = [2.04 + (1 − 2.04)/(1 + (TXT/2.5)−9.4)] × [1 + 0.28 / (1+(TXT/31)−2.5)] × [Fminage + (1 − Fminage)/(1 + (AGE/47)−14)] × FCYP where Fminage = 0.43 in females and 0.66 in males, FCYP is 0.51 in CYP3A5 expressers and 1 in CYP3A5 nonexpressers and TXT is the time after transplantation in days
aStandardized to 40-year-old male, CYP3A5 nonexpresser with fat-free mass of 60 kg and hematocrit of 45 %, at a time point with the lowest estimated bioavailability (Fn, day 5 post-transplant)
b2.5–97.5 percentile obtained from 500 bootstrap replicates
cUpper bound
Discussion
In this study, a population pharmacokinetic model was developed for tacrolimus by combining trough concentration data from the early post-transplant phase with rich sampling from patients in a stable phase after kidney transplantation. Hematocrit influenced the whole blood concentrations, while fat-free mass, CYP3A5 genotype, sex, age and time post-transplant were identified as factors influencing tacrolimus individual dose requirement to achieve a target concentration proportional to the unbound active drug. Although the importance of hematocrit has been highlighted in several population pharmacokinetic analyses of tacrolimus in kidney transplant patients [28–32], these analyses used hematocrit as an empirical predictor only of clearance or volume of distribution, but not both simultaneously. A novel approach of our study was the modeling of hematocrit as a covariate to adjust measured whole blood concentrations based on the theory that all whole blood-based pharmacokinetic disposition parameters are equally influenced by hematocrit for a low extraction ratio drug such as tacrolimus. When not accounting for differences in hematocrit, the pharmacokinetic model showed a clear systematic prediction error across the range of observed hematocrit values. The systematic prediction error was, however, no longer present after correction for the predictions to account for the actual hematocrit value (Fig. 2). This can be understood in terms of pharmacokinetic theory for a low-extraction-ratio drug, with elimination and distribution processes being independent of unbound drug concentration. Of note, the same theory described for hematocrit also applies to the binding of tacrolimus to albumin, α1-glycoprotein and lipoproteins in plasma [8, 9]. Although we did not identify any influence of albumin, it might have been obscured by the correlation between increases in albumin and hematocrit following kidney transplantation.
The challenge of reaching and maintaining a target concentration of tacrolimus during the first months after kidney transplantation has been described previously by Ekberg et al. who observed that approximately 50 % of tacrolimus trough concentrations during the first 8 post-transplant weeks were outside the intended range (3–7 μg/L) [6]. In our study, the percentage outside the target range was somewhat lower, but it was not reduced during the first 6 weeks after transplantation (42 % week 1, 43 % week 5–6). These challenges are, at least in part, related to the substantial increase in hematocrit following kidney transplantation (Fig. 1b) and high hematocrit within-subject variability (CV 11 %). Based on pharmacological theory and confirmation that hematocrit standardization is consistent with unbound concentration-independent pharmacokinetics, we recommend that tacrolimus whole blood concentrations be standardized to a hematocrit (Hct) of 45 % (Cstd = Ctot × 45 %/Hct) to reflect the unbound (active) drug more closely. This recommendation is based on the same well-known concepts that have guided the interpretation of phenytoin plasma concentration measurements when plasma proteins differ from normal [33, 34].
There is no consistent appreciation of the role of body size as a determinant of tacrolimus distribution and elimination [35], despite its biological basis and clinical use for predicting initial doses. This may be partly explained by small ranges of size, but also by not recognizing the role of body composition (fat-free mass and fat mass) as a determinant of functional body size. We investigated a large range of weights and identified fat-free mass as a predictor of tacrolimus pharmacokinetics, which to our knowledge is a novel finding. It is reasonable to accept fat-free mass as a predictor of tacrolimus clearance because fat mass is not expected to influence metabolic capacity directly [20]. The fact that volume of distribution of tacrolimus appeared to be closer related to fat-free mass than to total body weight indicates that the extent of tissue distribution of tacrolimus may be determined mainly by binding to proteins in lean tissues. For the patients in the current dataset with the lowest and highest total body weights (males of 43 kg and 160 kg with predicted fat-free masses of 39 and 95 kg, respectively), the model predicts apparent clearances of 16 and 27 L/h, respectively (given otherwise equal conditions; see Table 3). These calculations show that although total body weight may differ by fourfold in this patient group, the required dose differs by less than twofold and that the currently applied initial dose algorithm increases risk of overtreatment in obese patients. Indeed, previous studies have reported that high tacrolimus concentrations were more frequently observed in patients with higher weights [36, 37]. The proposed model provides a more rational use of body size for tacrolimus dose selection.
After appropriately accounting for body size differences in the model, we then identified sex differences in the pharmacokinetics of tacrolimus. The model fit was slightly better using sex as a covariate on bioavailability rather than on clearance, and there was little evidence for an additional effect on clearance. This result is somewhat supported by previously reported results [37, 38], although most population studies on tacrolimus did not establish a relationship with sex [29, 35, 39]. The difference between males and females was most apparent in younger patients, and as age increased, bioavailability increased for both sexes while the sex difference diminished. It has been previously described that sex differences in the pharmacokinetics of CYP3A substrates seem to be more pronounced at younger ages compared with in the elderly [40]. The effect may alternatively be caused by sex- and/or age-related differences in intestinal P-glycoprotein (ABCB1) activity. Previous reports have also described higher tacrolimus concentrations in elderly patients [37, 41, 42], which is consistent with our findings. It should be noted that only four females in our study were aged >50 years. Further studies are needed to recommend appropriate initial dosing of tacrolimus based on sex and age.
A major limitation of our study was the use of a high proportion (67 %) of trough concentrations. Concentrations measured at trough provide only modest pharmacokinetic information. Another limitation was that seven patients who received simultaneous pancreas–kidney transplantation and two patients who used carbamazepine were excluded from the analysis. This may have led to underprediction of the variability seen across patients in a true clinical setting. Furthermore, conversion between multiple analytical assay techniques for tacrolimus measurements may have introduced additional overall variability to the analysis. Hematocrit-related bias in concentration determinations by analytical immunoassays should however not be a relevant limitation because CMIA is not known to be affected by hematocrit [1]. All samples analyzed with MEIA had corresponding hematocrit values not associated with bias (25–45 %) [43]. Finally, the 95 % interval of the ordered observed hematocrit values was 29–43 %. In general, extrapolation of results to patients with covariate values outside of the investigated range should be done with caution. Yet, because the hematocrit model has a mechanistic basis, extrapolation is more likely to be reliable.
The whole blood concentration–effect relationship of tacrolimus is poorly characterized, and there is no consensus among transplant centers on an optimal tacrolimus concentration [1, 2]. Although a trough concentration range of 3–7 μg/L has been proposed [44] and is used at our center, ranges of 5–10 or 10–15 μg/L are targeted at other centers [45]. An increasing hematocrit leads to a decrease in therapeutically active unbound concentrations with time after transplantation if doses are adjusted to maintain unchanged total whole blood concentrations [9]. Differences in active unbound concentrations not reflected by whole blood concentrations will be a confounding factor in studies attempting to characterize the whole blood concentration-effect relationship. In a study in liver transplant patients, the estimated unbound concentrations of tacrolimus were significantly lower in patients experiencing acute rejection than in stable patients (p < 0.001), without a difference in whole blood concentrations (p = 0.5) [8]. Standardizing whole blood concentrations to hematocrit in future studies should improve our understanding of the concentration–effect relationship for tacrolimus and allow a more consistent target concentration to be achieved.
In conclusion, a population pharmacokinetic model was developed for tacrolimus in kidney transplant patients. Fat-free mass, CYP3A5 genotype, sex, age and time post-transplant were identified as predictors of individual tacrolimus dose requirement. Allometric scaling to fat-free mass provides a more rational use of body size than linear scaling to total body weight. Hematocrit is a predictor of variability in tacrolimus whole blood concentrations, but it is not expected to influence unbound (therapeutically active) concentrations. Because hematocrit is highly variable in kidney transplant patients and increases substantially after kidney transplantation, hematocrit is a key factor for interpretation of tacrolimus whole blood concentrations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Observed tacrolimus whole blood concentrations during a dosing interval (12 h) at steady state. A total of 514 tacrolimus measurements in 29 patients at 44 dosing occasions are shown [12–14]. (JPEG 22 kb)
Acknowledgments
We wish to thank the biomedical laboratory scientists at the Nephrology Laboratory and at the Department of Medical Biochemistry at Oslo University Hospital for excellent assistance in sampling, analysis and CYP3A5-genotype data management. We also wish to thank all kidney transplanted patients contributing data that enabled this analysis to be performed. Elisabet Størset received grants from The Norwegian Pharmaceutical Society, the Meltzer Fund and Shipowner Tom Wilhelmsens Foundation.
Conflict of interest
None.
References
- 1.Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31(2):139–152. doi: 10.1097/FTD.0b013e318198d092. [DOI] [PubMed] [Google Scholar]
- 2.Bouamar R, Shuker N, Hesselink DA, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials. Am J Transplant. 2013;13:1253–1261. doi: 10.1111/ajt.12191. [DOI] [PubMed] [Google Scholar]
- 3.Barraclough KA, Isbel NM, Kirkpatrick CM, et al. Evaluation of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients. Br J Clin Pharmacol. 2011;71:207–223. doi: 10.1111/j.1365-2125.2010.03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholten EM, Cremers SC, Schoemaker RC, et al. AUC-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005;67:2440–2447. doi: 10.1111/j.1523-1755.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 5.Saint-Marcoux F, Woillard J-B, Jurado C, Marquet P. Lessons from routine dose adjustment of tacrolimus in renal transplant patients based on global exposure. Ther Drug Monit. 2013;35:322–327. doi: 10.1097/FTD.0b013e318285e779. [DOI] [PubMed] [Google Scholar]
- 6.Ekberg H, Mamelok RD, Pearson TC, et al. The challenge of achieving target drug concentrations in clinical trials: experience from the Symphony Study. Transplantation. 2009;87:1360–1366. doi: 10.1097/TP.0b013e3181a23cb2. [DOI] [PubMed] [Google Scholar]
- 7.Jusko WJ, Piekoszewski W, Klintmalm GB, et al. Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther. 1995;57:281–290. doi: 10.1016/0009-9236(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 8.Zahir H, McCaughan G, Gleeson M, et al. Factors affecting variability in distribution of tacrolimus in liver transplant recipients. Br J Clin Pharmacol. 2004;57:298–309. doi: 10.1046/j.1365-2125.2003.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert MF, Zheng S, Hays K, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95:908–915. doi: 10.1097/TP.0b013e318278d367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Möller A, Iwasaki K, Kawamura A, et al. The disposition of 14C-labeled tacrolimus after intravenous and oral administration in healthy human subjects. Drug Metab Dispos. 1999;27:633–636. [PubMed] [Google Scholar]
- 11.Malyszko J, Oberbauer R, Watschinger B. Anemia and Erythrocytosis in patients after kidney transplantation. Transpl Int. 2012;25:1013–1023. doi: 10.1111/j.1432-2277.2012.01513.x. [DOI] [PubMed] [Google Scholar]
- 12.Falck P, Vethe NT, Åsberg A, et al. Cinacalcet’s effect on the pharmacokinetics of tacrolimus, cyclosporine and mycophenolate in renal transplant recipients. Nephrol Dial Transplant. 2008;23:1048–1053. doi: 10.1093/ndt/gfm632. [DOI] [PubMed] [Google Scholar]
- 13.Amundsen R, Åsberg A, Robertsen I, et al. Rimonabant affects cyclosporine A, but not tacrolimus pharmacokinetics in renal transplant recipients. Transplantation. 2009;87:1221–1224. doi: 10.1097/TP.0b013e31819f1001. [DOI] [PubMed] [Google Scholar]
- 14.Midtvedt K, Jenssen T, Hartmann A, et al. No change in insulin sensitivity in renal transplant recipients converted from standard to once-daily prolonged release tacrolimus. Nephrol Dial Transplant. 2011;26:3767–3772. doi: 10.1093/ndt/gfr153. [DOI] [PubMed] [Google Scholar]
- 15.Marubashi S, Nagano H, Kobayashi S, et al. Evaluation of a new immunoassay for therapeutic drug monitoring of tacrolimus in adult liver transplant recipients. J Clin Pharmacol. 2010;50:705–709. doi: 10.1177/0091270009352188. [DOI] [PubMed] [Google Scholar]
- 16.Falck P, Åsberg A, Byberg KT, et al. Reduced elimination of cyclosporine A in elderly (>65 years) kidney transplant recipients. Transplantation. 2008;86:1379–1383. doi: 10.1097/TP.0b013e31818aa4b6. [DOI] [PubMed] [Google Scholar]
- 17.Cheung CY, Op den Buijsch RA, Wong KM, et al. Influence of different allelic variants of the CYP3A and ABCB1 genes on the tacrolimus pharmacokinetic profile of Chinese renal transplant recipients. Pharmacogenomics. 2006;7:563–574. doi: 10.2217/14622416.7.4.563. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21:735–750. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 19.Kerbusch T, Wahlby U, Milligan PA, Karlsson MO. Population pharmacokinetic modelling of darifenacin and its hydroxylated metabolite using pooled data, incorporating saturable first-pass metabolism, CYP2D6 genotype and formulation-dependent bioavailability. Br J Clin Pharmacol. 2003;56:639–652. doi: 10.1046/j.1365-2125.2003.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 21.Janmahasatian S, Duffull SB, Ash S, et al. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 22.Størset E, Staatz C, Hennig S, et al (2013) Identification of continuous covariate relationships. Presented at the annual meeting of Population Approach Group of Australia and New Zealand (PAGANZ). Available at: http://www.paganz.org/abstracts/identification-of-continuous-covariate-relationships
- 23.Beal SL, Sheiner LB, Boeckmann AJ (1989–2006) NONMEM user’s guides. Icon Development Solutions, Ellicott City, MD
- 24.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlby U, Jonsson EN, Karlsson MO. Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS PharmSci. 2002;4:E27. doi: 10.1208/ps040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7:1–26. doi: 10.1214/aos/1176344552. [DOI] [Google Scholar]
- 27.Holford NHG (2011) Wings for NONMEM, v. 720 for NONMEM 7.2. Available at: http://wfn.sourceforge.net
- 28.Han N, Yun HY, Hong JY, et al. Prediction of the tacrolimus population pharmacokinetic parameters according to CYP3A5 genotype and clinical factors using NONMEM in adult kidney transplant recipients. Eur J Clin Pharmacol. 2012 doi: 10.1007/s00228-012-1296-4. [DOI] [PubMed] [Google Scholar]
- 29.Woillard JB, de Winter BC, Kamar N, et al. Population pharmacokinetic model and Bayesian estimator for two tacrolimus formulations–twice daily Prograf and once daily Advagraf. Br J Clin Pharmacol. 2011;71:391–402. doi: 10.1111/j.1365-2125.2010.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benkali K, Premaud A, Picard N, et al. Tacrolimus population pharmacokinetic-pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin Pharmacokinet. 2009;48:805–816. doi: 10.2165/11318080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.de Jonge H, de Loor H, Verbeke K, et al. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther. 2012;92:366–375. doi: 10.1038/clpt.2012.109. [DOI] [PubMed] [Google Scholar]
- 32.Musuamba FTF, Mourad MM, Haufroid VV, et al. A simultaneous d-optimal designed study for population pharmacokinetic analyses of mycophenolic acid and tacrolimus early after renal transplantation. J Clin Pharmacol. 2012;52:1833–1843. doi: 10.1177/0091270011423661. [DOI] [PubMed] [Google Scholar]
- 33.Reidenberg MM, Odar-Cederlöf I, von Bahr C, et al. Protein binding of diphenylhydantoin and desmethylimipramine in plasma from patients with poor renal function. N Engl J Med. 1971;285:264–267. doi: 10.1056/NEJM197107292850506. [DOI] [PubMed] [Google Scholar]
- 34.Musteata FM. Calculation of normalized drug concentrations in the presence of altered plasma protein binding. Clin Pharmacokinet. 2012;51:55–68. doi: 10.2165/11595650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Press RR, Ploeger BA, den Hartigh J, et al. Explaining variability in tacrolimus pharmacokinetics to optimize early exposure in adult kidney transplant recipients. Ther Drug Monit. 2009;31:187–197. doi: 10.1097/FTD.0b013e31819c3d6d. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigo E, de Cos MA, Sanchez B, et al. High initial blood levels of tacrolimus in overweight renal transplant recipients. Transplant Proc. 2005;37:1453–1454. doi: 10.1016/j.transproceed.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 37.Stratta P, Quaglia M, Cena T, et al. The interactions of age, sex, body mass index, genetics, and steroid weight-based doses on tacrolimus dosing requirement after adult kidney transplantation. Eur J Clin Pharmacol. 2012;68:671–680. doi: 10.1007/s00228-011-1150-0. [DOI] [PubMed] [Google Scholar]
- 38.Kuypers DR, Claes K, Evenepoel P, et al. Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet. 2004;43:741–762. doi: 10.2165/00003088-200443110-00005. [DOI] [PubMed] [Google Scholar]
- 39.Antignac M, Barrou B, Farinotti R, et al. Population pharmacokinetics and bioavailability of tacrolimus in kidney transplant patients. Br J Clin Pharmacol. 2007;64:750–757. doi: 10.1111/j.1365-2125.2007.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson PA, Schladt D, Oetting WS, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12:3326–3336. doi: 10.1111/j.1600-6143.2012.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Passey C, Birnbaum AK, Brundage RC, et al. Dosing equation for tacrolimus using genetic variants and clinical factors. Br J Clin Pharmacol. 2011;72:948–957. doi: 10.1111/j.1365-2125.2011.04039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomita T, Homma M, Hasegawa Y, et al. Use of sample hematocrit value to correct blood tacrolimus concentration derived by microparticle enzyme immunoassay. Biol Pharm Bull. 2008;31:1250–1253. doi: 10.1248/bpb.31.1250. [DOI] [PubMed] [Google Scholar]
- 44.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 45.Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2:374–384. doi: 10.2215/CJN.03791106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Observed tacrolimus whole blood concentrations during a dosing interval (12 h) at steady state. A total of 514 tacrolimus measurements in 29 patients at 44 dosing occasions are shown [12–14]. (JPEG 22 kb)