Abstract
Currently, the Bovigam assay is used as an official supplemental test within bovine tuberculosis control programs. The objectives of the present study were to evaluate two Mycobacterium bovis-specific peptide cocktails and purified protein derivatives (PPDs) from two sources, liquid and lyophilized antigen preparations. PPDs and peptide cocktails were also used for comparison of a second-generation gamma interferon (IFN-γ) release assay kit with the currently licensed first-generation kit (Bovigam; Prionics AG). Three strains of M. bovis were used for experimental challenge: M. bovis 95-1315, M. bovis Ravenel, and M. bovis 10-7428. Additionally, samples from a tuberculosis-affected herd (i.e., naturally infected) were evaluated. Robust responses to both peptide cocktails, HP (PC-HP) and ESAT-6/CFP10 (PC-EC), and the PPDs were elicited as early as 3 weeks after challenge. Only minor differences in responses to Commonwealth Serum Laboratories (CSL) and Lelystad PPDs were detected with samples from experimentally infected animals. For instance, responses to Lelystad M. avium-derived PPD (PPDa) exceeded the respective responses to the CSL PPDa in M. bovis Ravenel-infected and control animals. However, a 1:4 dilution of stimulated plasma demonstrated greater separation of PPDb from PPDa responses (i.e., PPDb minus PPDa) with the use of Lelystad PPDs, suggesting that Lelystad PPDs provide greater diagnostic sensitivity than CSL PPDs. The responses to lyophilized and liquid antigen preparations did not differ. Responses detected with first- and second-generation IFN-γ release assay kits (Bovigam) did not differ throughout the study. In conclusion, antigens may be stored in a lyophilized state without loss in potency, PC-HP and PC-EC are dependable biomarkers for aiding in the detection of bovine tuberculosis, and second-generation Bovigam kits are comparable to currently used kits.
INTRODUCTION
Mycobacterium bovis is the primary causative agent of bovine tuberculosis (TB) in cattle. M. bovis is a member of the Mycobacterium tuberculosis complex (MTC), which also includes M. tuberculosis, M. caprae, M. microti, M. africanum, M. canettii, and M. pinnipedii (1). MTC species are similar to one another in their ability to cause tuberculosis infections; however, their host range and their virulence between hosts vary. M. bovis has the largest host range of the MTC species, infecting wildlife as well as alternative and domestic livestock, which makes it difficult to control (2). In many developed countries, cattle herds are monitored for bovine tuberculosis using slaughterhouse surveillance and antemortem testing. Antemortem testing is primarily based upon measures of cell-mediated immunity, such as the tuberculin skin test (e.g., caudal fold test [CFT] or single intradermal comparative cervical test [SICCT]) and gamma interferon (IFN-γ) release assays (e.g., Bovigam; Prionics AG, Schlieren, Switzerland) (3). In regions where annual skin testing is largely absent, such as tuberculosis-free states within the United States, most infected cattle go unnoticed until a lesioned animal is detected by slaughterhouse surveillance (4). After infection is detected, the movement history is investigated to determine other contact herds and the origin of infection (3). Affected herds may be depopulated, or a test and slaughter approach may be applied using skin tests and/or the IFN-γ release assay. Additionally, emerging serologic tests (i.e., antibody-based tests) are being evaluated for use in tuberculosis control programs. Although these tests are relatively accurate, improvement of antemortem diagnostic methods is needed.
The IFN-γ release assay was developed to aid in the diagnosis of bovine tuberculosis and is currently used mainly as a supplemental assay to the skin test in most TB eradication/control programs. IFN-γ is produced in high quantities in vitro and is not readily consumed during short-term culture (5, 6), making it a good biomarker for use in tuberculosis diagnostic tests (7). The standard use of the IFN-γ assay is performed by stimulating whole blood for 16 to 24 h with M. bovis purified protein derivative (PPDb), M. avium PPD (PPDa), medium alone (no stimulation), and a mitogen control for cell viability (6). IFN-γ produced within the stimulated samples is then measured by an enzyme-linked immunosorbent assay (ELISA). Infection status is determined by comparing differential amounts of IFN-γ produced in response to PPDb and PPDa stimulation.
Compared to the skin test, the IFN-γ assay has increased sensitivity and a slightly lower specificity, requires a single visit to the farm, and prevents observational variability associated with assessing skin test reactions (3, 8, 9). With the use of PPDs, the sensitivity and specificity of the IFN-γ assay are estimated at 73 to 100% and 87.7 to 99.2%, respectively (4, 9). Implementing TB-specific antigens into the IFN-γ assay may increase the accuracy of the test (10) and provide differentiation of infected from vaccinated animals (DIVA) (11). Early secretory antigen target 6 (ESAT-6) and culture filtrate protein 10 (CFP10) are TB-specific proteins that have been extensively studied for use in the detection of bovine tuberculosis (10, 12, 13, 14). When used as recombinant proteins or peptide cocktails, ESAT-6 and CFP10 are known to elicit immune responses, both in vitro (13, 14, 15) and in vivo (16), in M. bovis-infected cattle. Aagaard and colleagues (10) demonstrated decreased sensitivity (86% versus 97%) yet increased specificity (99% versus 94%) when comparing an ESAT-6 and CFP10 peptide cocktail to PPDs as antigens for use in the IFN-γ assay. This increased specificity elicited by the ESAT-6 and CFP10 peptide cocktail is promising; however, more research is needed to determine methods to increase sensitivity (17). For instance, use of ESAT-6 and CFP10 in combination with Rv3615 in the IFN-γ assay has been shown to increase sensitivity without loss of specificity (18).
Objectives of the current study were to compare IFN-γ responses elicited by Commonwealth Serum Laboratories (CSL) and Lelystad PPDs (Prionics AG) and two MTC-specific peptide cocktails, as well as liquid and lyophilized preparations. Studies were performed using samples from three groups of cattle, each experimentally infected with a different strain of M. bovis, as well as samples from a tuberculosis-affected herd (i.e., natural infection) within the United States. Strains for experimental infection included M. bovis Ravenel (a laboratory-adapted strain attenuated in cattle), M. bovis 95-1315 (a white-tailed deer isolate), and M. bovis 10-7428 (a cattle field isolate from a Colorado dairy herd). PPDs and peptide cocktails were also used to compare a second-generation Bovigam kit to the currently available kit.
MATERIALS AND METHODS
Calves, aerosol challenge, and necropsy.
For the first study, 15 9-month-old Holstein castrated male cattle were housed in a biosafety level 3 (BSL-3) containment facility in Ames, IA, at the National Animal Disease Center (NADC). All animal care and use procedures were reviewed and approved by the NADC Animal Care and Use Committee. Treatment groups included cattle inoculated with M. bovis 95-1315 (105 CFU, n = 5), cattle inoculated with M. bovis Ravenel (105 CFU, n = 5) (ATCC strain 35720, kind gift from John Chan, Albert Einstein College of Medicine, NY), and a noninfected control group (n = 5). For the second study, 23 6-month-old Holstein castrated male cattle were housed as described above. Treatment groups included cattle inoculated with M. bovis 95-1315 (5 × 104 CFU, n = 8), cattle inoculated with M. bovis 10-7428 (5 × 104 CFU, n = 8), and a noninfected control group (n = 7). Strains were prepared using standard procedures (19) in Middlebrook 7H9 liquid medium (Becton Dickinson, Franklin Lakes, NJ) supplemented with 10% oleic acid-albumin-dextrose-catalase complex (OADC) plus 0.05% Tween 80 (0.5% glycerol was included for strain Ravenel only). Enumeration of M. bovis challenge inocula, necropsy procedures (∼3.5 months after challenges), gross and microscopic assessment of lesions, and mycobacterial culture of M. bovis from tissues were performed as described previously (15, 20). The inoculation of each of the strains in both studies was via aerosol as described previously (20). Additionally, whole-blood samples were obtained from animals from a TB-affected herd in Colorado. Animals were categorized as infected based on positive culture of M. bovis (infected, n = 56).
Antigens.
PPDs and peptide cocktails were provided by Prionics AG (Schlieren, Switzerland) in liquid and lyophilized forms. RPMI 1640 medium (Life Technologies, Grand Island, NY) was used as a negative control and pokeweed mitogen (PWM; 5 μg/ml; Sigma-Aldrich, St. Louis, MO) was included as a positive control of cell viability. The two peptide cocktails consisted of ESAT-6 and CFP10 (PC-EC; Prionics AG) and ESAT-6, CFP10, Rv3615, and three other mycobacterium antigens (PC-HP; Prionics AG). Aliquots of liquid preparations of PC-HP and PC-EC were kept frozen at −20°C until the day of use. PC-HP and PC-EC were used according to the manufacturer's instructions. CSL PPDa and CSL PPDb were used at 20 μg/ml concentrations and Lelystad PPDa and Lelystad PPDb were used at 200 IU and 250 IU, respectively, as optimized and suggested by the manufacturers. All antigens were diluted in RPMI 1640 medium.
Whole-blood stimulation.
For the first study, whole blood was collected in sodium-heparinized tubes 2 weeks prior to aerosol challenge and at 3, 6, 8, 11, and 12 weeks postchallenge. On the day of blood collection, 250 μl of blood was added to 96-well plates for each animal. Twenty-five microliters of each antigen (PPDs and peptide cocktails) and controls (e.g., RPMI 1640 and PWM) was added in duplicate to selected wells. Plates were incubated at 39°C with 5% CO2 for 18 to 22 h. Plasma samples were harvested from each stimulation for each animal and placed in individual microtubes (VWR Scientific, Radnor, PA) and stored at −80°C until analyzed for IFN-γ concentrations via the Bovigam assay. For the second study, blood was collected at 2 weeks prechallenge as well as 2, 3, 4, 6, 8, and 12 weeks postchallenge. Whole-blood stimulation procedures were the same as those used in the first study.
IFN-γ detection.
In the first study, IFN-γ assays were performed using the first-generation Bovigam kit (Prionics AG). In the second study, first- and second-generation Bovigam kits were used. All IFN-γ release assays were performed according to kit procedures. Each plasma sample and standard was tested in duplicate. Recombinant bovine IFN-γ (Thermo Scientific, Rockford, IL) was used as a standard at 25 ng/ml, 12.5 ng/ml, 6.25 ng/ml, 5 ng/ml, 2.5 ng/ml, 1.5 ng/ml, 1.25 ng/ml, 0.625 ng/ml, and 0.3125 ng/ml, along with the positive and negative controls supplied by the manufacturer. Samples were read at a wavelength of 450 nm using a SPECTRAmax 340 microplate reader (Molecular Devices, Sunnyvale, CA) and analyzed using SOFTmax PRO software (Molecular Devices, Sunnyvale, CA) to calculate optical density (OD). Responses to the negative control (RPMI 1640) for each animal were subtracted from all antigen and mitogen responses. Results were considered positive if responses to PPDb (OD at 450 mm [OD450]) minus responses to PPDa (OD450) were >0.1 OD.
Tuberculin skin test procedures.
Skin tests were performed as specified in the circular on uniform methods and rules for the eradication of bovine tuberculosis in the United States (Animal and Plant Health Inspection Service [APHIS] circular 91-45-011) (21). At 10 weeks (study 1) or 12 weeks (study 2) postchallenge, skin thickness was measured with calipers immediately before intradermal injection of PPDa and PPDb (National Veterinary Services Laboratories, Ames, IA). The skin thickness was measured again after 72 h. The skin test was performed in the midcervical region (i.e., comparative cervical test). Responses were recorded on a scattergram for the interpretation of comparative cervical tests to determine the outcome of the tests (i.e., negative, suspect, or reactor).
Cattle pathogenesis studies: bacterial recovery and assessment of lesions.
All calves were euthanized by intravenous administration of sodium pentobarbital ∼3.5 months after challenge. Tissues were observed for gross lesions and collected and processed for microscopic analysis and isolation of M. bovis. Tissues collected included palatine tonsil, lung, and liver and mandibular, parotid, medial retropharyngeal, mediastinal, tracheobronchial, hepatic, and mesenteric lymph nodes. Lymph nodes were sectioned at 0.5-cm intervals and examined. Each lung lobe was sectioned at 0.5-cm to 1.0-cm intervals and examined separately.
Tissues collected for microscopic analysis were fixed by immersion in 10% neutral buffered formalin. For microscopic examination, formalin-fixed tissues were processed by standard paraffin-embedment techniques, cut in 5-μm sections, and stained with hematoxylin and eosin. Adjacent sections from samples containing caseonecrotic granulomata suggestive of tuberculosis were excised and stained by the Ziehl-Neelsen technique for identification of acid-fast bacteria.
M. bovis isolates were obtained from cattle tissues as described previously (22). Briefly, tissues were macerated in phenol red nutrient broth using a blender (Oster, Shelton, CT). Homogenates were decontaminated with NaOH and then neutralized with HCl. Samples were centrifuged, supernatants were removed, and pellets were used to inoculate the media (Middlebrook 7H10, 7H11, and 7H11 with antibiotics). Plates were incubated at 37°C and examined weekly for growth.
Statistics.
Results were analyzed using Prism software (GraphPad, La Jolla, CA). Optical densities were converted to ng/ml using the standard curve calculated from standards observed on each plate for each date. Data were analyzed using repeated measures analysis of variances with the Bonferroni multiple comparisons test and the Mann-Whitney t test.
RESULTS
Experimental infection.
Ten to 12 weeks after challenge, all cattle receiving M. bovis, regardless of strain (i.e., Ravenel, 95-1315, or 10-7428), were classified as reactors based upon standard interpretation of the comparative cervical skin test. Delayed-type hypersensitivity responses were not detected (i.e., classified as negative) with any of the animals within the noninfected control group. Upon necropsy, only one M. bovis Ravenel-inoculated calf had a single small granuloma in the left caudal lung lobe. In contrast, all M. bovis 95-1315- and M. bovis 10-7428-inoculated cattle had granulomatous lesions in the lungs and lung-associated lymph nodes. M. bovis was isolated from 4 of the 5 cattle inoculated with M. bovis Ravenel and all cattle receiving M. bovis 95-1315 or M. bovis 10-7428. M. bovis was not isolated from cattle within the noninfected group. These findings are consistent with the observation that M. bovis Ravenel is attenuated in cattle (W. R. Waters and M. V. Palmer, personal observations) and M. bovis strains 95-1315 and 10-7428 are fully virulent.
Comparison of liquid and lyophilized antigen preparations.
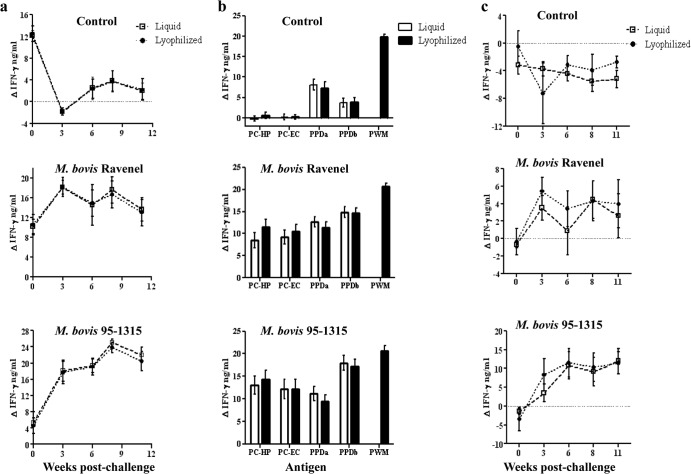
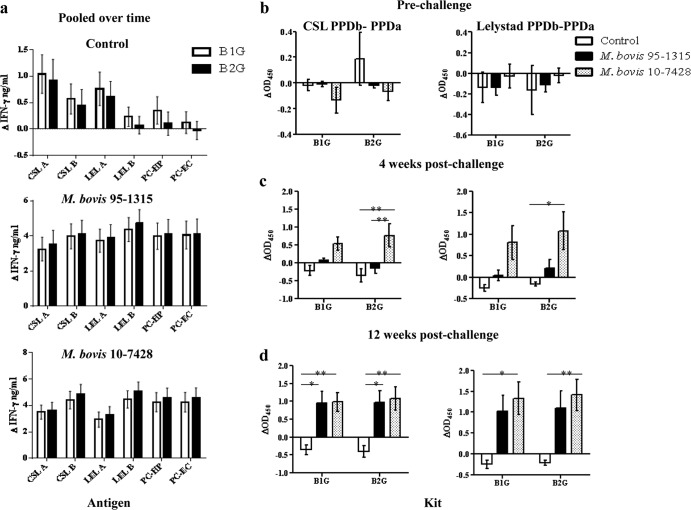
IFN-γ responses to liquid and lyophilized preparations were detected in M. bovis 95-1315- and M. bovis Ravenel-inoculated animals as early as 3 weeks after challenge (Fig. 1) (P < 0.05). When all time points were pooled, responses to liquid and lyophilized antigen preparations (PPDb, PC-HP, and PC-EC) by M. bovis Ravenel- and M. bovis 95-1315-challenged animals exceeded (P < 0.05) respective responses by control animals (Fig. 1b). Significant differences were not detected between liquid and lyophilized products for any antigens within each treatment group (Fig. 1b). As utilized for official use of the Bovigam test kit, responses to PPDb minus PPDa did not differ (P > 0.05) for liquid and lyophilized antigen preparations at any time point within each treatment group (Fig. 1c).
Fig 1.
Comparison of IFN-γ responses to liquid and lyophilized antigen preparations from control (n = 5) as well as M. bovis Ravenel- (n = 5) and M. bovis 95-1315-infected (n = 5) animals. (a) Response kinetics: PPDb (Lelystad) minus no stimulation. For control animals, the responses to liquid and lyophilized antigens were equivalent; thus, the lines on the graphs are overlapping. Responses to PPDb detected prior to challenge in all groups (including controls) were likely due to cross-reactive responses elicited by nontuberculous Mycobacteria spp. (b) Liquid and lyophilized antigen preparations pooled over time. Responses to pokeweed mitogen (PWM) are included as a positive controls for cell viability. (c) Response kinetics: PPDb minus PPDa (Lelystad PPDs).
IFN-γ responses to PPDa exceeded (P < 0.01) respective responses to PPDb for control animals (Fig. 1b), demonstrating prior sensitization to nontuberculous mycobacteria (NTM). Responses to MTC-specific antigens (PC-HP and PC-EC) were not detectable prior to challenge (Fig. 2a), and subtraction of PPDa responses from PPDb (PPDb minus PPDa) resulted in values of <0.1 ΔOD450 (i.e., negative) for all animals prior to challenge (Fig. 1c). Thus, responses to PPDb detected prior to challenge in all groups (Fig. 1a) were likely due to cross-reactive responses elicited by NTM.
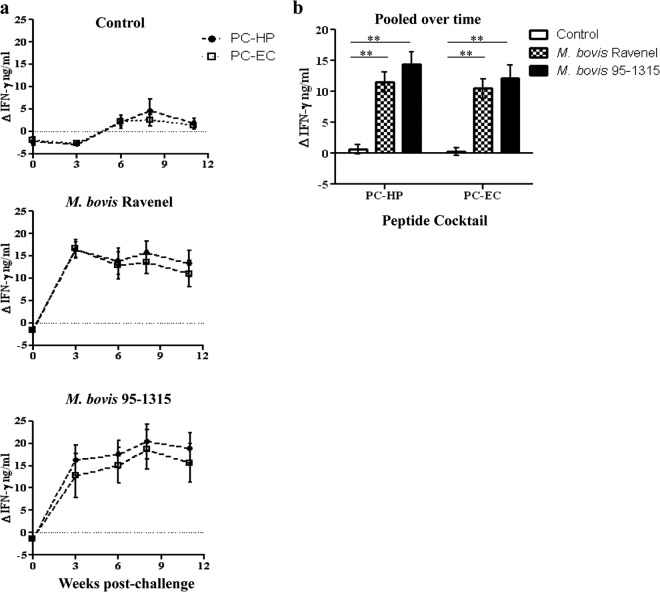
Fig 2.
Comparison of IFN-γ responses to PC-HP and PC-EC in control (n = 5) and M. bovis Ravenel- (n = 5) and M. bovis 95-1315-infected animals. (a) Responses over time and (b) responses pooled over time. **, responses differ between groups (P < 0.01).
Evaluation of peptide cocktails for the detection of bovine tuberculosis.
As early as 3 weeks after challenge, responses to PC-HP and PC-EC by M. bovis Ravenel- and M. bovis 95-1315-inoculated animals exceeded (P < 0.05) respective prechallenge responses and responses by control animals (Fig. 2a). When all time points were pooled, there was no difference (P > 0.05) between responses to PC-HP and PC-EC within treatment groups (Fig. 2b). Similar responses were detected in the second study using M. bovis 95-1315- and M. bovis 10-7428-challenged animals, and responses to PC-HP and PC-EC did not differ within treatment groups (see Fig. S1 in the supplemental material). These findings demonstrate that responses to PC-HP and PC-EC are similar for the early detection of M. bovis infection in cattle.
Comparison of IFN-γ responses to Lelystad and CSL PPDs in experimentally and naturally infected animals.
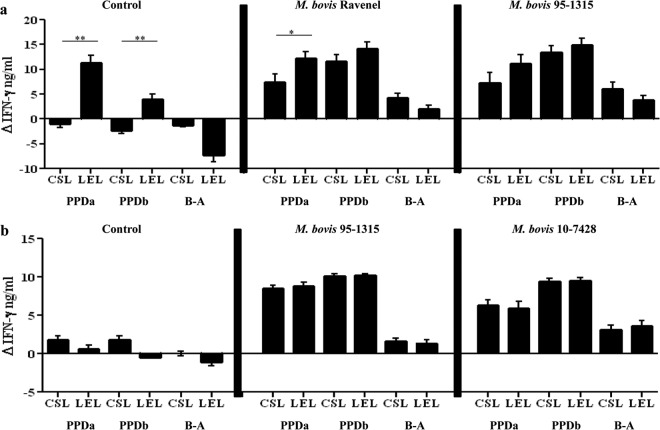
Lelystad and CSL PPDs are commonly used as antigens in the Bovigam assay for the detection of M. bovis infection in cattle (23, 24); however, uses of these antigens within IFN-γ release assays have yet to be directly compared. Experimental infection with M. bovis Ravenel, M. bovis 95-1315, and M. bovis 10-7428 elicited robust IFN-γ responses to both CSL and Lelystad PPDs (Fig. 3; also see Fig. S2 in the supplemental material). When pooled over time, responses to Lelystad PPDa exceeded (P < 0.05) responses to CSL PPDa within the control and M. bovis Ravenel treatment groups (Fig. 3a). Similarly, responses to Lelystad PPDb exceeded (P < 0.05) that of CSL PPDb in control animals when pooled (Fig. 3a).
Fig 3.
IFN-γ responses to Commonwealth Serum Laboratory (CSL) and Lelystad (LEL) PPDs in cattle upon experimental infection with M. bovis. (a) Comparison of IFN-γ responses to PPDs in control (n = 5) as well as M. bovis Ravenel- (n = 5), and M. bovis 95-1315-infected (n = 5) animals pooled over time. (b) Comparison of IFN-γ responses to PPDs in control (n = 7) as well as M. bovis 95-1315- (n = 8), and M. bovis 10-7428-infected (n = 8) animals pooled over time. PPDa refers to M. avium-derived PPD, PPDb refers to M. bovis-derived PPD, and B-A indicates values for PPDb minus PPDa. Asterisks indicate that responses differed (*, P < 0.05; **, P < 0.01).
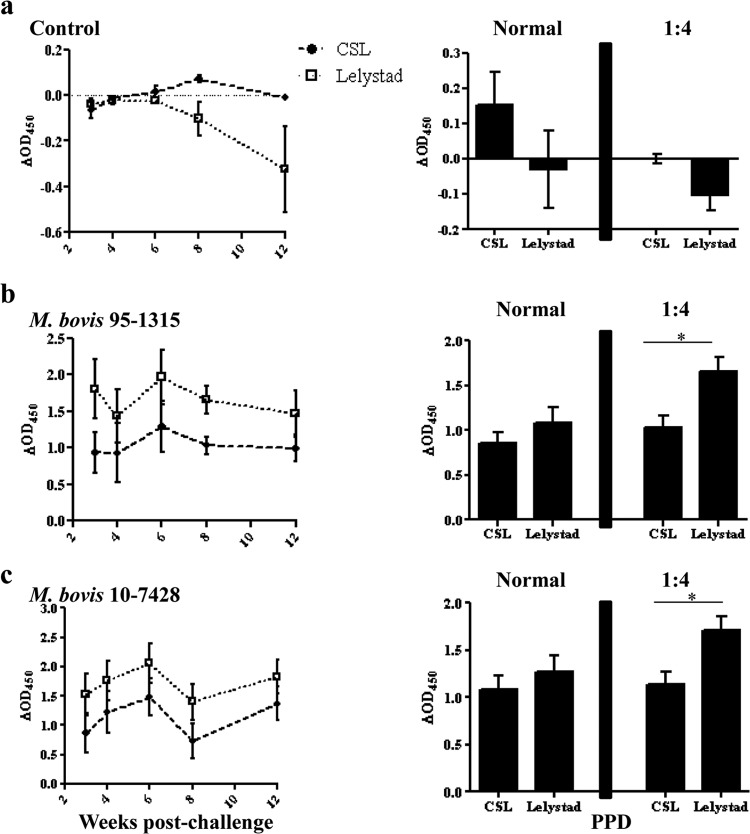
Experimental infection of cattle with M. bovis results in robust IFN-γ responses to both PPDb and PPDa (due to cross-reactivity of antigens) that are often at the maximum detection limit of the assay, thereby limiting the comparisons of PPD potencies and diagnostic potential. Thus, several dilutions of stimulated plasma were evaluated at selected time points to further evaluate the kinetics of the response and potency comparisons of the two PPDs (Table 1 and Fig. 4). When plasma from experimentally infected animals was diluted, it was determined that the majority of responses after challenge to PPDa were lower than the response to PPDb, giving a difference greater than 0.1 OD450, indicating a positive test result (Table 1 and Fig. 4). For diluted plasma samples, responses to Lelystad PPDb minus PPDa exceeded (P < 0.05) respective responses to CSL PPDb minus PPDa (Fig. 4). In general, diluting the plasma enabled improved accuracy for the detection of M. bovis infection in experimentally infected animals in addition to reducing the false-positive test results within the control group.
Table 1.
Number of positive animals per group after challenge using CSL and Lelystad PPDs when stimulated plasma samples were either not diluted or diluted 1:4
| Postchallenge time and dilution | PPD source | No. of positive animalsa/no. in group |
||
|---|---|---|---|---|
| Control | M. bovis 95-1315 | M. bovis 10-7428 | ||
| 3 wk | ||||
| Not diluted | CSL | 1/7 | 4/8 | 5/8 |
| Lelystad | 1/7 | 4/8 | 4/8 | |
| Diluted 1:4 | CSL | 0/7 | 7/8b | 7/8 |
| Lelystad | 0/7 | 7/8b | 8/8 | |
| 4 wk | ||||
| Not diluted | CSL | 1/7 | 6/8 | 7/8 |
| Lelystad | 0/7 | 4/8 | 5/8 | |
| Diluted 1:4 | CSL | 0/7 | 6/8b | 8/8 |
| Lelystad | 0/7 | 7/8b | 8/8 | |
| 6 wk | ||||
| Not diluted | CSL | 1/7 | 6/8 | 6/8 |
| Lelystad | 0/7 | 6/8 | 7/8 | |
| Diluted 1:4 | CSL | 1/7 | 7/8b | 8/8 |
| Lelystad | 0/7 | 8/8 | 8/8 | |
| 8 wk | ||||
| Not diluted | CSL | 6/7 | 6/8 | 6/8 |
| Lelystad | 0/7 | 7/8 | 4/8 | |
| Diluted 1:4 | CSL | 1/7 | 8/8 | 7/8 |
| Lelystad | 0/7 | 8/8 | 8/8 | |
| 12 wk | ||||
| Not diluted | CSL | 1/7 | 6/8 | 6/8 |
| Lelystad | 2/7 | 6/8 | 6/8 | |
| Diluted 1:4 | CSL | 0/7 | 8/8 | 8/8 |
| Lelystad | 0/7 | 8/8 | 8/8 | |
Animals were considered positive when PPDa responses were subtracted from PPDb responses and the difference in OD450 was >0.1.
Some animals within the experimentally infected groups were still at the maximum limit of detection, making it difficult to detect differences in responses to PPDb and PPDa.
Fig 4.
Comparison of PPDs using 1:4 dilutions of plasma stimulated with either Commonwealth Serum Laboratories (CSL) or Prionics (Lelystad) purified protein derivatives at select time points after challenge (left panel) and responses detected in normal (not diluted) and 1:4 diluted plasma pooled over time (right panel). (a) Control (n = 7), (b) M. bovis 95-1315-challenged animals (n = 8), and (c) M. bovis 10-7428-challenged animals. Data are presented as responses to PPDb minus PPDa. *, Responses differ (P < 0.05).
To further compare CSL and Lelystad PPDs, samples were obtained from a tuberculosis-affected dairy herd (Colorado, n = 56) in the United States. All animals were deemed M. bovis infected via mycobacterial culture. In contrast to experimental infection studies, in which samples were placed in culture with antigen within 2 h of blood collection, samples from naturally infected animals were shipped overnight and set up within 20 h of blood collection. Responses to Lelystad PPDs exceeded (P < 0.01) those to CSL PPDs, including PPDb minus PPDa (Fig. 5).
Fig 5.
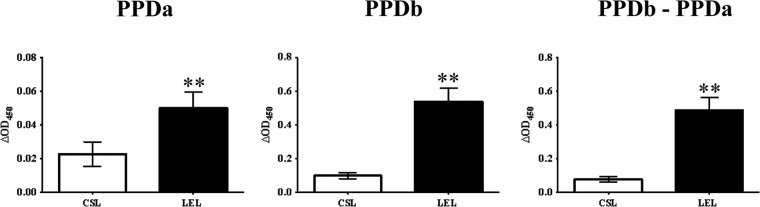
Evaluation of IFN-γ responses in samples obtained from dairy cattle within a tuberculosis-affected herd (i.e., natural infection). Comparison of CSL (Commonwealth Serum Laboratories) and Lelystad (Prionics AG) M. avium-derived PPD (PPDa) and M. bovis-derived PPD (PPDb). Responses from M. bovis-infected (n = 56) animals from a tuberculosis-affected dairy in Colorado. Animals were categorized as infected based on positive culture of M. bovis (infected, n = 56). **, responses to Lelystad PPDs exceeded (P < 0.01) respective responses to CSL PPDs.
Performances of second-generation Bovigam kits compared to those of the currently licensed kits.
The currently licensed Bovigam kits (B1G) and second-generation Bovigam kits (B2G) were compared using experimentally infected animals (Fig. 6). IFN-γ responses were not different (P > 0.05) between the two kits when time points were pooled (Fig. 6a). B2G kits were just as effective in the differentiation of control and infected groups when calculations of PPDb minus PPDa were performed throughout the course of the study (Fig. 6b, c, and d).
Fig 6.
Comparison of current (B1G) and new (B2G) Bovigam (Prionics AG) kits using responses to individual antigens and PPDb minus PPDa with samples from control (n = 7) as well as M. bovis 95-1315- (n = 8), and M. bovis 10-7428-infected (n = 8) animals. (a) IFN-γ responses pooled over time, (b) responses to prechallenge (Commonwealth Serum Laboratories) and Lelystad (Prionics AG) PPDb minus PPDa, (c) responses to 4-week-postchallenge CSL and Lelystad PPDb minus PPDa, and (d) responses to 12-week-postchallenge CSL and Lelystad PPDb minus PPDa.
DISCUSSION
IFN-γ is a reliable biomarker for use in the detection of M. bovis infection. With the current use of the Bovigam assay, antigens are provided within the kit as a liquid preparation. In this study, lyophilization had no effect on antigen performance. Responses were detected as early as 3 weeks postchallenge using both liquid and lyophilized antigen preparations. These findings indicate that lyophilized preparations may be implemented into the IFN-γ assay without loss in potency, thereby increasing the shelf life of the test kits. Additional TB-specific antigens are needed to further increase the accuracy of the IFN-γ release assay. Interestingly, although both PC-HP and PC-EC were not significantly different in the responses they elicited in infected animals throughout the study, responses to PC-HP were slightly higher than responses to PC-EC, which is consistent with findings by Sidders and colleagues (18). In experimentally infected animals, responses to PC-HP and PC-EC were detectable as early as 3 weeks postchallenge. PC-HP and PC-EC are commercially available products that may prove useful as antigens for bovine tuberculosis test kits, both for research and diagnostic purposes.
PPDs from differing manufacturers and lots are known to vary in potency (8). Commonwealth Serum Laboratories (CSL) has been the producer of PPDs for the Bovigam assay since the test was first approved for use in the United States. Due to manufacturing and marketing reasons, it is possible that CSL PPDs will not be available in the near future. Thus, it is critical to validate use of different PPDs within bovine tuberculosis diagnostic tests. Whipple and colleagues performed direct comparisons between CSL PPDs and PPDs prepared in the United States (USDA, APHIS, National Veterinary Services Laboratory) using the CFT and IFN-γ assay and found that the final interpretation of the test (i.e., positive and negative animals identified by each PPD) was usually the same (25). However, U.S. PPDs elicited a higher response (25). Recently, Lelystad PPDs (Prionics AG) were adopted for use in the skin test and Bovigam assay in the United Kingdom, Republic of Ireland, and various other European Union countries. Downs and colleagues reviewed data from field surveillance results of the single intradermal comparative cervical test (SICCT) using Lelystad and Weybridge (Central Veterinary Laboratory) PPDs in England, Scotland, and Wales from 2005 to 2009 (26). Due to financial reasons, Defra halted production of tuberculin at Weybridge and their tuberculin supply was exhausted in 2009 (26). Lelystad became the sole source of tuberculin in Great Britain; however, there were no data directly comparing the two sources of PPDs. Compilation and analysis of field surveillance results determined that Lelystad PPDs were superior to Weybridge PPDs in confirming infection within herds and individual animals when using the SICCT (26). Lelystad has been used in IFN-γ release assays since 2007 in the United Kingdom (27); however, direct comparisons to CSL PPDs have not been performed. In the present study, IFN-γ responses to Lelystad and CSL PPDs largely did not differ in undiluted samples from experimentally infected cattle (Fig. 3; also see Fig. S2 in the supplemental material). However, use of diluted sera (1:4) revealed greater differentiation of PPDb from PPDa responses with Lelystad PPDs versus CSL PPDs (i.e., PPDb minus PPDa) (Fig. 4) elicited after experimental M. bovis infection. With samples from a tuberculosis-affected herd, responses to Lelystad PPDs exceeded respective responses to CSL PPDs.
Experimental infection of cattle resulted in IFN-γ responses to both PPDb and PPDa that reached the maximum limit of detection, resulting in responses to PPDb minus PPDa of <0.1 ΔOD (i.e., negative), even though these animals were clearly infected as evidenced by robust IFN-γ responses to MTC peptide cocktails, delayed-type hypersensitivity responses to PPDb exceeding those to PPDa, and isolation of M. bovis from tissues at necropsy. Thus, differences between control and infected animals were not detectable using the analysis of PPDb minus PPDa with undiluted stimulated plasma. To address this issue, dilutions of plasma were evaluated to ascertain IFN-γ concentrations detectable within assay limits. After dilution (1:4), samples from infected animals had responses to PPDb that exceeded (P < 0.05) respective responses to PPDa, thus enabling an accurate diagnosis using calculations of PPDb minus PPDa. Dilution of plasma may be incorporated as a means to determine differences in IFN-γ responses to PPDb and PPDa. This may be particularly useful in regions where detection of bovine tuberculosis is hindered by high levels of exposure to NTM that elicit cross-reactive responses to PPDs. Additionally, evaluation of diluted samples may be useful when evaluating responses by animals with very robust responses, as seen with experimentally infected animals in this study. Indeed, use of diluted plasma with samples from experimentally infected cattle revealed responses to Lelystad PPDs exceeding those to CSL PPDs. These findings suggest that the use of Lelystad PPDs will provide greater diagnostic sensitivity than CSL PPDs.
In the current study, three strains of M. bovis were used to compare IFN-γ responses to PPDs and peptide cocktails elicited after experimental infection. With the use of the IFN-γ release assay, robust responses were detected using PPDs from the two different manufacturers as well as PC-HP and PC-EC. Strains examined were M. bovis 95-1315 (white-tailed deer field isolate), M. bovis Ravenel (laboratory-adapted strain), and M. bovis 10-7428 (cattle field isolate from a Colorado dairy). M. bovis strain 95-1315 has been used previously in experimental infection of cattle and has been shown to elicit robust immune responses as well as granulomatous lesions at necropsy (15). M. bovis strain Ravenel was isolated in the early 1900s, and although it is virulent in mice (28), rabbits (28, 29), and guinea pigs (13, 28, 30), it does not lead to progressive disease in cattle (W. R. Waters and M. V. Palmer, unpublished observations). M. bovis strain 10-7428 was isolated from a Holstein cow in a dairy herd in Colorado. This strain is speculated to be highly virulent given the high rate of progression of disease in this herd (Tolani Francisco, personal communication). In the first study, all M. bovis 95-1315 animals had granulomatous lesions, whereas only one M. bovis Ravenel animal had a small tuberculous lesion. In the second study, all animals in the M. bovis 95-1315 and M. bovis 10-7428 groups showed lesions upon necropsy as well as robust skin test responses. Thus, evaluation of IFN-γ responses to various antigen preparations in the present study were evaluated with samples from animals inoculated with 3 strains with varied virulence levels.
PPDs and peptide cocktails were also used for comparison of two generations of Bovigam kits. The second-generation kit requires a decreased number of repeated washes and combines the conjugate and chromogen into one solution; however, stimulation and incubation times within the test did not change. Although the new kit did not decrease the performance time, it did reduce the time for work labor. Present findings support the use of the second-generation Bovigam kit (B2G) for replacement of the current kit (B1G) in the detection of bovine tuberculosis.
In conclusion, lyophilized PPDs, PC-EC, and PC-HP are effective replacements for liquid antigens for use in the Bovigam assay. PC-HP and PC-EC are reliable biomarkers of bovine tuberculosis, and second-generation Bovigam kits perform similarly to first-generation kits. The present findings, while not definitive, are encouraging for replacement of CSL PPDs with Lelystad PPDs for use in the Bovigam assay to detect bovine tuberculosis in the United States. These findings, along with future evaluation of naturally infected animals, have the potential to increase the accuracy of current antemortem diagnostic methods in the detection of bovine tuberculosis.
Supplementary Material
ACKNOWLEDGMENTS
This research was carried out under a cooperative research and development agreement (no. 58-3K95-2-1551) between Prionics AG and USDA/ARS/NADC.
We thank Jessica Pollock, Emma Frimml-Morgan, Shelly Zimmerman, Mayara Maggioli, Molly Stafne, Allen Jensen, and Tracy Porter for their excellent technical assistance, as well as Rebecca Madison, Doug Ewing, Katie Pille, Jay Steffen, David Lubbers, Robin Zeisness, and David Panthen for the excellent care and handling of animals. We also thank Alecia L. Naugle, Kathleen A. Orloski, M. Celia Antognoli, Jeffery T. Nelson, C. William Hench, Mark A. Schoenbaum, Tom Brignole, Tolani I. Francisco, and Robert M. Meyer for collection of samples from tuberculosis-affected herds and assistance with these studies.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print 16 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00519-13.
REFERENCES
- 1.Cousins DV, Bastida R, Cataldi A, Quse V, Redrobe S, Dow S, Duigan P, Murray A, Dupont C, Ahmed N, Collins DM, Butler WR, Dawson D, Rodriguez D, Loureiro J, Romano MI, Alito A, Zumarraga M, Bernardelli A. 2003. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int. J. Syst. Evol. Microbiol. 53:1305–1314 [DOI] [PubMed] [Google Scholar]
- 2.Good M, Duignan A. 2011. Perspectives on the history of bovine TB and the role of tuberculin in bovine TB eradication. Vet. Med. Int. 2011:410470. 10.4061/2011/410470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinso RG, Christiansen KH, Clifton-Hadley RS. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 81:190–210 [DOI] [PubMed] [Google Scholar]
- 4.Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP, Waters WR. 2010. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 57:205–220 [DOI] [PubMed] [Google Scholar]
- 5.Wood PR, Jones SL. 2001. BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinb.) 81:147–155 [DOI] [PubMed] [Google Scholar]
- 6.Wood PR, Corner LA, Plackett P. 1990. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res. Vet. Sci. 49:46–49 [PubMed] [Google Scholar]
- 7.Mihret A, Bekele Y, Bobosha K, Kidd M, Aseffa A, Howe R, Walzl G. 2013. Plasma cytokines and chemokines differentiate between active disease and non-active tuberculosis infection. J. Infect. 66:357–365 [DOI] [PubMed] [Google Scholar]
- 8.Schiller I, Vordermeier HM, Waters WR, Kyburz A, Cagiola M, Whelan A, Palmer MV, Thacker T, Meijlis J, Carter C, Gordon S, Egnuni T, Hardegger R, Marg-Haufe B, Raeber A, Oesch B. 2010. Comparison of tuberculin activity using the interferon-γ assay for the diagnosis of bovine tuberculosis. Vet. Rec. 167:322–326 [DOI] [PubMed] [Google Scholar]
- 9.Vordermeier HM, Whelan A, Ewer K, Goodchild T, Clifton-Hadley R, Williams J, Hewinson RG. 2006. The Bovigam assay as ancillary test to the tuberculin skin test. Gov. Vet. J. 16:72–80 [Google Scholar]
- 10.Aagaard C, Govaerts M, Meikle V, Vallecillo AJ, Gutierrez-Pabello JA, Suarez-Güemes F, McNair J, Cataldi A, Espita C, Andersen P, Pollock JM. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 44:4326–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vordermeier M, Jones GJ, Whelan AO. 2011. DIVA reagents for bovine tuberculosis vaccines in cattle. Expert Rev. Vaccines 10:1083–1091 [DOI] [PubMed] [Google Scholar]
- 12.Waters WR, Palmer MV, Thacker TC, Bannantine JP, Vordermeier HM, Hewinson RG, Greenwald R, Esfandiari J, McNair Pollock JM, Andersen P, Lyashchenko KP. 2006. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin. Vaccine Immunol. 13:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waters WR, Palmer MV, Nonnecke BJ, Thacker TC, Scherer CF, Estes DM, Hewinson RG, Vordermeier HM, Barnes SW, Federe GC, Walker JR, Glynne RJ, Hsu T, Weinrick B, Biermann K, Larsen MH, Jacobs WR., Jr 2009. Efficacy and immunogenicity of Mycobacterium bovis DeltaRD1 against aerosol M. bovis infection in neonatal calves. Vaccine 27:1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vordermeier HM, Whelan A, Cockle PJ, Farrant L, Palmer N, Hewinson RG. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters WR, Whelan AO, Lyashchenko KP, Greenwald R, Palmer MV, Harris BN, Hewinson RG, Vordermeier HM. 2010. Immune responses in cattle inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii. Clin. Vaccine Immunol. 17:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelan AO, Clifford D, Upadhyay B, Breadon EL, McNair J, Hewinson GR, Vordermeier MH. 2010. Development of a skin test for bovine tuberculosis for differentiating infected from vaccinated animals. J. Clin. Microbiol. 48:3176–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vordermeier HM, Gordon SV, Hewinson RG. 2011. Mycobacterium bovis antigens for the differential diagnosis of vaccinated and infected cattle. Vet. Microbiol. 15:8–13 [DOI] [PubMed] [Google Scholar]
- 18.Sidders B, Pirson C, Hogarth PJ, Hewinson RG, Stoker NG, Vordermeier HM, Ewer K. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76:3932–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen MH, Biermann K, Jacobs WR., Jr 2007. Laboratory maintenance of Mycobacterium tuberculosis. Curr. Protoc. Microbiol. Chapter 10:Unit 10A.1. 10.1002/9780471729259.mc10a01s6 [DOI] [PubMed] [Google Scholar]
- 20.Palmer MV, Waters WR, Whipple DL. 2003. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis (Edinb.) 82:275–282 [DOI] [PubMed] [Google Scholar]
- 21.USDA Animal Plant and Health Inspection Service 2005. Bovine tuberculosis eradication uniform methods and rules (APHIS 91-45-011). U.S. Government Printing Office, Washington, DC [Google Scholar]
- 22.Robbe-Austerman S, Bravo DM, Harris B. 2013. Comparison of the MGIT 960, BACTEC 460 TB and solid media for isolation of Mycobacterium bovis in United States veterinary specimens. BMC Vet. Res. 9:74. 10.1186/1746-6148-9-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller I, Waters WR, Vordermeier HM, Nonnecke B, Welsh M, Keck N, Whelan A, Sigafoose T, Stamm C, Palmer M, Thacker T, Hardegger R, Marg-Haufe B, Raeber A, Oesch B. 2009. Optimization of a whole-blood gamma interferon assay for detection of Mycobacterium bovis-infected cattle. Clin. Vaccine Immunol. 16:1196–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood PR, Corner LA, Rothel JS, Baldock C, Jones SL, Cousins DB, McCormick BS, Francis BR, Creeper J, Tweddle NE. 1991. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68:286–290 [DOI] [PubMed] [Google Scholar]
- 25.Whipple DL, Bolin CA, Davis AJ, Jarnagin JL, Johnson DC, Nabors RS, Payeur JB, Saari DA, Wilson AJ, Wolf MM. 1995. Comparison of the sensitivity of the caudal fold skin test and a commercial gamma-interferon assay for diagnosis of bovine tuberculosis. Am. J. Vet. Res. 56:415–419 [PubMed] [Google Scholar]
- 26.Downs SH, Clifton-Hadley RS, Upton PA, Milne IC, Ely ER, Gopal R, Goodchild AV, Sayers AR. 2013. Tuberculin manufacturing source and breakdown incidence rate of bovine tuberculosis in British cattle, 2005–2009. Vet. Rec. 172:98. 10.1135/jvr.100679 [DOI] [PubMed] [Google Scholar]
- 27.Denis M, Wedlock DN, McCarthy AR, Parlane NA, Cockle PJ, Vordermeier HM, Hewinson RG, Buddle BM. 2007. Enhancement of the sensitivity of the whole-blood gamma interferon assay for diagnosis of Mycobacterium bovis infections in cattle. Clin. Vaccine Immunol. 14:1483–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., III 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76:2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Converse PJ, Dannenberg AM, Shigenaga T, McMurray DN, Phalen SW, Stanford JL, Rook GAW, Koru-Sengul T, Abbey H, Estep JE, Pitt MLM. 1998. Pulmonary bovine-type tuberculosis in rabbits: bacillary virulence, inhaled dose effects, tuberculin sensitivity, and Mycobacterium vaccae immunotherapy. Clin. Diagn. Lab. Immunol. 5:871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North RJ, Ryan L, LaCource R, Mogues T, Goodrich ME. 1999. Growth rate of mycobacteria in mice as an unreliable indicator of mycobacterial virulence. Infect. Immun. 67:5483–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.